Abstract
Lung cancer is the leading cause of cancer mortality worldwide. Helicobacter pylori (H. pylori) is a risk factor for distal stomach cancer, and a few small studies have suggested that H. pylori may be a potential risk factor for lung cancer. To test this hypothesis, we conducted a study of 350 lung adenocarcinoma cases, 350 squamous cell carcinoma cases, and 700 controls nested within the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (ATBC) cohort of male Finnish smokers. Controls were one-to-one matched by age and date of baseline serum draw. Using enzyme-linked immunosorbent assays to detect immunoglobulin G antibodies against H. pylori whole-cell and cytotoxin-associated gene (CagA) antigens, we calculated odds ratios (ORs) and 95% confidence intervals (95% CIs) for associations between H. pylori seropositivity and lung cancer risk using conditional logistic regression. H. pylori seropositivity was detected in 79.7% of cases and 78.5% of controls. After adjusting for pack-years and cigarettes smoked per day, H. pylori seropositivity was not associated with either adenocarcinoma (OR: 1.1, 95% CI: 0.75–1.6) or squamous cell carcinoma (OR: 1.1, 95% CI: 0.77–1.7). Results were similar for CagA-negative and CagA-positive H. pylori seropositivity. Despite earlier small studies suggesting that H. pylori may contribute to lung carcinogenesis, H. pylori seropositivity does not appear to be associated with lung cancer.
Introduction
Lung cancer kills more people worldwide (over 1 million each year) than any other cancer [1]. Although smoking is the primary cause, most smokers (≥80%) never develop lung cancer [2], suggesting that oncogenesis requires additional co-factors. Infections and immune responses that mediate inflammation may contribute to lung carcinogenesis [3], [4]. Evidence supporting this hypothesis includes associations of lung cancer with 1) elevated inflammatory markers, such as C-reactive protein, interleukin (IL)-6, and IL-8 [5], [6]; 2) chronic obstructive pulmonary disease, to which infections can contribute [7], [8]; 3) human leukocyte antigen polymorphisms in genome-wide association studies [9], [10]; and 4) overt infections like tuberculosis and pneumonia [3], [7]. In addition, Jaagsiekte sheep retrovirus causes ovine pulmonary adenocarcinoma (OPA), a malignancy occurring in sheep [4]. OPA has similar histology to human lung adenocarcinoma, bronchiolar-alveolar adenocarcinoma in particular. In humans, lung adenocarcinoma occurs at younger ages more often than squamous cell carcinoma [4], which is consistent with an infectious origin since some infection-related cancers occur at younger ages [11], [12].
One microbe postulated to play a role in lung cancer is Helicobacter pylori (H. pylori) [13], [14]. H. pylori is a key etiologic agent in the development of distal stomach cancer [15]. A gram-negative spiral-shaped bacterium, H. pylori colonizes the gastric mucosa, inducing local inflammation and a systemic immune response [16]. H. pylori has been classified as a group 1 carcinogen for stomach cancer by the International Agency for Research on Cancer [17]. H. pylori can be broadly categorized into two groups: type I strains, which express the cytotoxin-associated gene (cagA), and type II strains, which do not [18]. CagA-positive strains affect gastric epithelial cell signal transduction, which can affect the cytoskeleton, inflammatory cascades, and mitogenic pathways [16], [18].
H. pylori could potentially affect the lungs in several ways. Lipopolysaccharide is the major component of the cell wall of gram-negative bacteria like H. pylori. Lipopolysaccharide stimulates the production of pro-inflammatory cytokines including IL-1, IL-6, and tumor necrosis factor (TNF)-alpha. This local inflammation can have systemic effects [16], [19], [20]. H. pylori persistence leads to chronic inflammation and immune stimulation, which could contribute to carcinogenesis or conditions associated with lung cancer, such as chronic bronchitis [16]. The lungs develop embryologically from the same endodermal cells that line the gastrointestinal (GI) tract and contain cells that produce peptide hormones like gastrin [14]. Therefore, higher plasma levels of gastrin due to H. pylori in the stomach might promote cellular proliferation in the lungs as well [14].
It is also possible that gastric H. pylori colonization could decrease the risk of lung cancer. H. pylori prevalence has declined over the last 70 years, accompanied by a marked decrease in noncardia gastric cancer and an increase in esophageal adenocarcinoma [21]. Similar to the esophagus where the proportion of cancers due to adenocarcinomas are increasing in relation to squamous cell cancers, the relative proportion of lung adenocarcinoma has been increasing [22]. With the observed inverse association between H. pylori and esophageal adenocarcinoma in Western countries and the lack of association with esophageal squamous cell carcinoma, an inverse association between H. pylori and lung adenocarcinoma but no association with lung squamous cell carcinoma also could be hypothesized.
Prior assessment of the association between H. pylori and lung cancer has been limited, with fewer than 75 cases in each of five case-control studies [13], [14], [23], [24], [25]. A recent meta-analysis including four of these studies calculated a pooled odds ratio (OR) of 3.2 [95% confidence interval (CI): 1.1–9.5], but the authors noted marked heterogeneity in the results from these studies [26]. Using existing H. pylori seropositivity data from previous studies in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (ATBC) cohort of male Finnish smokers [27], [28], [29], [30], we found suggestive evidence of an inverse association of H. pylori with lung adenocarcinoma, but not squamous cell carcinoma (see Methods section). Based on these intriguing preliminary data and the lack of well-powered, high-quality studies, we designed the first prospective study of H. pylori seropositivity and lung cancer in ATBC.
Materials and Methods
Study population
The ATBC Study, a randomized, double-blind, placebo-controlled, primary prevention trial, was designed to test the hypothesis that daily supplementation with alpha-tocopherol, beta-carotene, or both would reduce the incidence of lung or other cancers among male smokers. Details of this study have been published [31]. In brief, Finnish males between 50 and 69 years of age were identified through the Central Population Register. Questionnaires were mailed to those with available addresses. Men who smoked ≥5 cigarettes per day and who agreed to participate were mailed an invitation to a local field station for further evaluation. After excluding men with proven malignancy (other than nonmelanoma skin cancer or carcinoma in situ), severe angina on exertion, chronic renal insufficiency, liver cirrhosis, chronic alcoholism, current anticoagulant therapy, other medical problems that could limit participation for 6 years, or current use of supplements with vitamin E (>20 mg/d) or vitamin A (>20,000 IU/d = 2000 retinol equivalents) or beta-carotene (>6 mg/d), 29,246 men were randomized. Later, 113 men were found to be ineligible, leaving 29,133 eligible Finnish male smokers enrolled in ATBC between 1985 and 1988. Both the US National Cancer Institute and the Finnish National Public Health Institute institutional review boards approved the ATBC Study, and all participants provided written informed consent. The men continue to be followed as a cohort since the trial ended in 1993.
Preliminary evaluation using existing data
We used existing H. pylori seropositivity data from previous studies in ATBC [27], [28], [29], [30] to conduct a preliminary evaluation of the association between H. pylori and lung cancer. Enzyme-linked immunosorbent assay (ELISA) measurements of H. pylori seropositivity were available from 98 lung cancer cases (11 with documented adenocarcinoma and 33 with squamous cell carcinoma) and over 1500 subjects without lung cancer. The preliminary data revealed no apparent association between H. pylori seropositivity and lung cancer overall (OR: 0.96, 95% CI: 0.61–1.5). However, we observed a trend toward an inverse association with adenocarcinoma (OR: 0.46, 95% CI: 0.14–1.5), which was stronger for CagA-positive H. pylori strains (OR: 0.38, 95% CI: 0.07–2.0).
Case and control definition
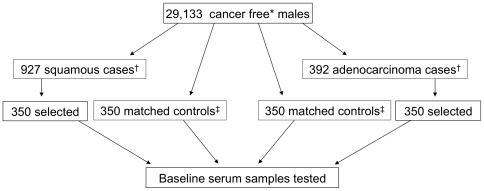
Lung cancer cases were identified using the International Classification of Diseases, 9th Revision (ICD-9) [32] code 162, through the Finnish Cancer Registry, which provides nearly 100% coverage of all cancer cases in Finland [33]. Histology was determined using the International Classification of Diseases for Oncology, 2nd edition (ICD-O-2), and 3rd edition (ICD-O-3). Squamous cell carcinoma included malignant ICD-O codes of 8070. Adenocarcinoma included malignant ICD-O codes of 8140 (adenocarcinoma), 8250 (bronchioloalveolar carcinoma), and 8260 (papillary carcinoma). Cases were selected from among all first primary lung squamous cell carcinoma or adenocarcinoma cases diagnosed at least 1 year after the baseline draw date through 30 July 2007. We randomly selected 350 from 927 eligible squamous cell carcinoma cases and 350 from 392 eligible adenocarcinoma cases (Figure 1). Controls were matched one-to-one by age at baseline serum draw (+/−5 years) and date of baseline serum draw (+/−30 days). Controls had to be alive and cancer free at the date that the corresponding case was diagnosed.
Figure 1. Lung cancer case and control selection for Helicobacter pylori serology testing within the ATBC cohort.
*Individuals with nonmelanoma skin cancer were not excluded. †Eligible cases included all first primary lung squamous cell carcinoma or adenocarcinoma cases diagnosed at least 1 year after the baseline draw date through 30 July 2007. ‡Controls were matched one-to-one by age at baseline serum draw (+/−5 years) and date of baseline serum draw (+/−30 days). Controls had to be alive and cancer free at the date that the corresponding case was diagnosed.
Helicobacter pylori testing
We tested fasting serum specimens collected at baseline and stored in aliquots at −70°C for immunoglobulin G antibodies against H. pylori whole-cell and CagA antigens using ELISAs [34], [35]. Eleven samples (four from cases and seven from controls) lacked sufficient serum for testing. We calculated optical density ratios relative to co-analyzed laboratory standards and classified optical density ratios ≥1.0 for the whole-cell antigen assay and ≥0.35 for the CagA antigen assay as positive. We evaluated H. pylori seropositivity in two ways. First, we examined any H. pylori seropositivity (whole-cell or CagA seropositivity) compared to seronegativity for both whole-cell and CagA antigens. Second, we split H. pylori seropositivity into whole-cell and CagA seropositivity. Twelve individuals were classified as seropositive for CagA and seronegative for antibodies against the whole-cell antigen. A previous study comparing serology with gastric biopsy culture found that individuals who are negative for H. pylori whole-cell antibodies but positive for CagA antibodies had CagA-positive H. pylori organisms present in their stomach by culture [36]. Therefore, we classified individuals who were seronegative for both whole-cell and CagA antibodies as H. pylori negative, individuals seropositive for whole-cell but seronegative for CagA as H. pylori-seropositive/CagA seronegative (CagA-negative strains), and individuals seropositive for CagA, regardless of whole-cell serostatus, as CagA seropositive (CagA-positive strains).
In addition to the 1389 samples from lung cancer cases and controls, 3 quality control serum samples aliquoted from a single large pool were equally distributed among 37 different batches. All samples were assayed in duplicate in the same batch by experienced technicians blinded to case-control status. In the case of indeterminate results (i.e., if the values from the duplicate sample straddled the seropositivity threshold), a repeat analysis was conducted on the residual of the original aliquot and averaged across all results, excluding obvious outliers. 95.9% of the samples were resolved with two runs in the whole-cell ELISA, and 94.9% of the samples were resolved with two runs in the CagA ELISA. More than 99% of samples were resolved with two or three runs (99.5% for whole-cell and 99.4% for CagA). The remainders were resolved with four runs.
Statistical analysis
Based on the observed prevalence of H. pylori seropositivity in a prior ATBC study with a prevalence of 45% among controls [27], we designed this study to have 90% power to detect ORs of 0.70 or 1.42 for CagA and all lung cancers combined. Assuming an overall H. pylori exposure prevalence of 75% among the controls, the minimal detectable ORs at 90% power were 0.68 or 1.53 for all lung cases compared to controls. We calculated within-batch coefficients of variation (CV) for the whole-cell and CagA antigen assays based on continuous optical density values from the 111 pooled QC samples (three within each batch). The within batch CVs were 12.9% for the whole-cell assay and 25.5% for the CagA assay, similar to the CVs of 15% and 20%, respectively, in a previous study of H. pylori and gastric cancer in ATBC using the same laboratory [27].
We used conditional logistic regression to calculate separate ORs and 95% CIs for the association of lung adenocarcinoma and squamous cell carcinoma with H. pylori seropositivity, using the categories described above. Results for adenocarcinoma and squamous cell carcinoma were similar according to the Wald test for homogeneity [37] (P = 0.8), so we also evaluated all lung cancer patients combined.
Potential confounders included ATBC treatment group (placebo, alpha-tocopherol, beta-carotene, or both), age at randomization (continuous), baseline pack-years (continuous), baseline number of cigarettes per day (continuous), tooth loss (continuous), dentures (yes/no), obesity (ordinal body mass index <25, 25–30, 30+), asthma (yes/no), emphysema (yes/no), bronchitis (yes/no), and education (<elementary, elementary, partial junior high, junior high, senior high, graduate). We evaluated the importance of these variables by removing each covariate from the model one by one through backwards modeling and determining whether the ORs for CagA-negative strains and CagA-positive strains changed by at least 10% in comparison with the full model. No covariate met this criterion. However, we included pack-years and smoking amount (cigarettes per day) in all final models to adjust for smoking exposure, in accord with several recent studies [8], [38], [39], [40]. Adjusting for pack-years or amount of smoking had little effect on the ORs (e.g., 2–3% for any H. pylori seropositivity).
Sensitivity analyses
A previous study of H. pylori seropositivity in Finland found that antibody levels remain relatively stable over time in this population [41], and in ATBC the association between H. pylori and gastric cancer was similar when stratified by time to diagnosis [27]. However, to evaluate the effect of potential changes in H. pylori antibody levels over time, we stratified by median time since blood draw. Since microbe-related cancers often occur at younger ages than those not related to infection [11], [12], we also stratified by median age at diagnosis and evaluated whether the association between any H. pylori seropositivity and lung cancer varied by age at diagnosis (i.e., whether there were departures from multiplicative joint effects) using the likelihood ratio test (LRT) for interaction terms [37], [42].
Results
The case and control series were similar with regard to age, median cigarettes per day, and other characteristics (Table 1). As expected based on the primary results of the trial [43], [44], squamous cell cases were slightly less likely to have received placebo and slightly more likely to have received beta-carotene than controls. Cases had a higher number of pack-years than controls, but adjusting for pack-years did not affect the association between H. pylori and lung cancer, as described in the Methods section. Among the 696 cases and 693 controls with sufficient sample available, 555 cases (79.7%) and 544 controls (78.5%) had antibodies against either H. pylori whole-cell or CagA antigens (Table 2). Thirty-seven percent of cases (N = 258) and 34.3% of controls (N = 238) were seropositive for CagA-negative strains of H. pylori, and 42.7% of cases (N = 297) and 44.2% of controls (N = 306) were seropositive for CagA-positive strains. Of the 603 CagA-positive individuals, 591 [289 out of 297 cases (97.3%) and 302 out of 306 controls (98.7%)] also were positive for antibodies against the whole-cell antigens.
Table 1. Characteristics of randomly selected lung adenocarcinoma and squamous cell carcinoma cases and matched cancer-free controls from the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study.
| Characteristic | Subgroup | Adenocarcinoma | Squamous cell carcinoma | ||
| Cases | Controls* | Cases | Controls* | ||
| Median age at baseline serum draw | 58.0 | 58.0 | 59.8 | 59.6 | |
| Median date of baseline serum draw | 1/23/1987 | 1/23/1987 | 1/22/1987 | 1/23/1987 | |
| Treatment group, N (%) | |||||
| Placebo | 84 (24.0) | 94 (26.9) | 75 (21.4) | 97 (27.7) | |
| Beta-carotene | 88 (25.1) | 85 (24.3) | 110 (31.4) | 87 (24.9) | |
| Alpha-tocopherol | 89 (25.4) | 83 (23.7) | 80 (22.9) | 86 (24.6) | |
| Alpha-tocopherol+beta-carotene | 89 (25.4) | 88 (25.1) | 80 (22.9) | 85 (24.3) | |
| Education level, N (%) | |||||
| Primary school or lower | 282 (80.6) | 275 (78.6) | 291 (83.1) | 288 (82.3) | |
| High school or higher | 68 (19.4) | 75 (21.4) | 59 (16.9) | 62 (17.7) | |
| Obesity, N (%) | |||||
| BMI† <25 | 178 (50.9) | 136 (38.9) | 157 (44.9) | 129 (36.9) | |
| BMI† 25–<30 | 144 (41.1) | 160 (45.7) | 141 (40.3) | 165 (47.1) | |
| BMI† ≥30 | 28 (8.0) | 54 (15.4) | 52 (14.9) | 56 (16.0) | |
| Median cigarettes per day (range) | 20 (5–55) | 20 (5–75) | 20 (5–60) | 20 (5–60) | |
| Median pack-years (range) | 42 (1–121) | 35 (1–101) | 42 (4–111) | 35 (1–123) | |
| Asthma, N (%) | 14 (4.0) | 8 (2.3) | 19 (5.4) | 7 (2.0) | |
| Emphysema, N (%) | 32 (9.1) | 24 (6.9) | 36 (10.3) | 21 (6.0) | |
| Bronchitis, N (%) | 41 (11.7) | 27 (7.7) | 47 (13.4) | 25 (7.1) | |
| Median number of lost teeth (range) | 4 (1–5) | 4 (1–5) | 4 (2–5) | 4 (1–5) | |
| Dentures, N (%) | 238 (68.0) | 216 (61.9) | 261 (74.8) | 241 (68.9) | |
*Controls matched with cases on age at baseline serum draw and date of baseline serum draw.
BMI = body mass index.
Table 2. Association of Helicobacter pylori (H. pylori) seropositivity with risk of lung adenocarcinoma and squamous cell carcinoma in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study.
| Model | H. pylori serostatus | Histology | Cases, N* (%) | Controls, N* (%) | OR (95% CI)† |
| 1 | H. pylori seronegative | Adenocarcinoma | 74 (21.2) | 79 (22.8) | 1.0 (referent) |
| H. pylori seropositive | 275 (78.8) | 268 (77.2) | 1.1 (0.75–1.6) | ||
| 2‡ | H. pylori seronegative | 74 (21.2) | 79 (22.8) | 1.0 (referent) | |
| CagA-seronegative‡ | 125 (35.8) | 119 (34.3) | 1.1 (0.71–1.7) | ||
| CagA-seropositive‡ | 150 (43.0) | 149 (42.9) | 1.1 (0.73–1.7) | ||
| 1 | H. pylori seronegative | Squamous cell carcinoma | 67 (19.3) | 70 (20.2) | 1.0 (referent) |
| H. pylori seropositive | 280 (80.7) | 276 (79.8) | 1.1 (0.77–1.7) | ||
| 2‡ | H. pylori seronegative | 67 (19.3) | 70 (20.2) | 1.0 (referent) | |
| CagA-seronegative ‡ | 133 (38.3) | 119 (34.4) | 1.3 (0.82–1.9) | ||
| CagA-seropositive ‡ | 147 (42.4) | 157 (45.4) | 1.0 (0.65–1.6) | ||
| 1 | H. pylori seronegative | All lung cancer | 141 (20.3) | 149 (21.5) | 1.0 (referent) |
| H. pylori seropositive | 555 (79.7) | 544 (78.5) | 1.1 (0.86–1.5) | ||
| 2‡ | H. pylori seronegative | 141 (20.3) | 149 (21.5) | 1.0 (referent) | |
| CagA-seronegative ‡ | 258 (37.1) | 238 (34.3) | 1.2 (0.87–1.6) | ||
| CagA-seropositive ‡ | 297 (42.7) | 306 (44.2) | 1.1 (0.79–1.5) |
*N's do not sum to total due to missing values.
Odds ratios (ORs) and 95% confidence intervals (CIs) were adjusted for baseline pack-years and total number of cigarettes per day.
Single model for CagA-seropositive and CagA-seronegative versus no H. pylori seropositivity.
H. pylori seropositivity was not associated with lung cancer in ATBC (Table 2). The OR for any H. pylori seropositivity and all lung cancers combined was 1.1 (95% CI: 0.86–1.5). The ORs did not vary by histology (OR: 1.1, 95% CI: 0.75–1.6 for adenocarcinoma and 1.1, 95% CI: 0.77–1.7 for squamous cell carcinoma). In addition, there was no notable variability in the associations for CagA-negative or CagA-positive H. pylori seropositivity (Table 2). Results were similar when limited to heavy smokers with more than 35 pack-years (e.g., OR: 0.99, 95% CI: 0.63–1.6; LRT p-value: 0.7 for any H. pylori seropositivity and all lung cancers combined).
Sensitivity analyses
Despite concerns that changes in H. pylori seropositivity over time may weaken the association between H. pylori seropositivity and cancer risk, associations were similar by median time between baseline serum draw and diagnosis in this study (Table 3). ORs tended to be slightly elevated for cases diagnosed more than 9 years after baseline serum draw, but there was no significant association. In addition, there was little difference in the association between H. pylori seropositivity and lung cancer by median age at diagnosis (age 68). For example, the ORs for any H. pylori seropositivity and any lung cancer were 1.1 (95% CI: 0.73–1.6) for cases diagnosed at or before age 68 compared to their matched controls and 1.2 (95% CI: 0.79–1.8) for cases diagnosed after age 68 (LRT p-value: 0.7). Similarly, the ORs for CagA-negative H. pylori seropositivity and CagA-positive H. pylori were 1.0 (95% CI: 0.68–1.6) and 1.1 (95% CI: 0.72–1.7), respectively, for cases diagnosed at or before age 68 and 1.3 (95% CI: 0.83–2.0) and 1.1 (95% CI: 0.69–1.7) for cases diagnosed after age 68 (LRT p-value: 0.7). Results were similar for adenocarcinoma and squamous cell carcinoma separately.
Table 3. Association of Helicobacter pylori (H. pylori) seropositivity with risk of lung adenocarcinoma and squamous cell carcinoma by time to diagnosis in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study.
| Comparison | Time to diagnosis | OR (95% CI)* | ||
| Adenocarcinoma | Squamous cell carcinoma | All lung cancer | ||
| Any H. pylori seropositivity versus none | ||||
| ≤9 years | 0.95 (0.54–1.7) | 1.1 (0.64–1.9) | 1.0 (0.70–1.5) | |
| >9 years | 1.3 (0.74–2.2) | 1.3 (0.72–2.4) | 1.3 (0.86–1.9) | |
| CagA-negative seropositivity versus none | ||||
| ≤9 years† | 0.96 (0.52–1.8) | 1.2 (0.64–2.1) | 1.1 (0.70–1.6) | |
| >9 years‡ | 1.2 (0.68–2.2) | 1.5 (0.79–2.9) | 1.4 (0.88–2.1) | |
| CagA-positive seropositivity versus none | ||||
| ≤9 years† | 0.93 (0.49–1.8) | 1.0 (0.57–1.9) | 0.99 (0.64–1.5) | |
| >9 years‡ | 1.3 (0.72–2.3) | 1.0 (0.53–2.1) | 1.2 (0.77–1.9) | |
*Odds ratios (ORs) and 95% confidence intervals (CIs) were adjusted for baseline pack-years and total number of cigarettes per day.
Derived from a single model for CagA-seropositive and CagA-seronegative versus no H. pylori seropositivity for all cases diagnosed ≤9 years after baseline blood draw and their paired controls.
Derived from a single model for CagA-seropositive and CagA-seronegative versus no H. pylori seropositivity for all cases diagnosed >9 years after baseline blood draw and their paired controls.
Discussion
Given our preliminary evidence from existing data in ATBC supporting an inverse association between H. pylori seropositivity and lung adenocarcinoma on the one hand, and previous published studies supporting a positive association on the other, we carefully designed a large nested case-control study to thoroughly address the hypothesis that H. pylori is associated with lung cancer. In this well-powered nested case-control study, we found no evidence of an association between H. pylori and lung cancer. Neither overall H. pylori seropositivity nor CagA-specific H. pylori seropositivity were associated with lung cancer. Results were equally null for lung adenocarcinoma and squamous cell carcinoma. Sensitivity analyses by time since diagnosis and age had little effect.
The results of previous studies of H. pylori seropositivity and lung cancer have been mixed. Of the five previous small case-control studies, three found moderate to strong increased risk of lung cancer associated with H. pylori seropositivity [13], [14], [25], and two found only weak, non-statistically significant evidence of a positive association [23], [24]. Two studies provided no information regarding smoking status and lung histology [14], [23], and three provided limited information on smoking status [13], [24], [25]. Only one previous small study of 22 squamous cell carcinoma cases and 21 adenocarcinoma cases provided data on H. pylori seropositivity by lung histology; the association in that study did not vary by lung histology [13]. None of these studies evaluated factors like smoking that might affect the association between H. pylori seropositivity and lung cancer. Given this lack of adjustment for potential confounders and the fact that small studies are more likely to be affected by chance [45] and less likely to publish null findings [46], it unsurprising that previous, small studies found positive (albeit imprecise) associations.
This study improves on previous studies in a number of ways. First, as a case-control study nested within the ATBC cohort, this study provides the only prospective data on H. pylori seropositivity and subsequent risk of lung cancer. With 700 lung cancer cases, this study is nearly 10 times larger than the next largest study and is well powered to assess differences by histology (adenocarcinoma and squamous cell carcinoma). In addition, all participants were smokers at baseline, so there is less variation in smoking habits and therefore less potential for confounding by smoking. As a well-characterized epidemiologic cohort, ATBC has extensive information about potential confounders, which allowed us to carefully control for cumulative smoking exposure and thoroughly assess other potential confounders. Finally, the association between H. pylori and gastric cancer has already been established in this population using these same assays, demonstrating the adequacy of these assays.
Although the inclusion of only Finish male smokers is beneficial in limiting the potential for confounding, it also limits the generalizability of these findings. This study could not address the association between H. pylori seropositivity and subsequent risk of lung cancer in women. In addition, never smokers may have a different risk of lung cancer associated with H. pylori seropositivity than ever smokers. Although unlikely, it is possible that the potential biologic mechanisms described in the Introduction (e.g., systemic inflammation from gastric H. pylori infection) may be gender specific or may only be evident in never smokers.
This study provides the most definitive findings for H. pylori seropositivity and lung cancer to date. In this population of Finish male smokers, H. pylori seropositivity was not associated with lung cancer. Although these findings are not entirely generalizable to other populations (e.g., never smokers), it seems unlikely that H. pylori is involved in lung carcinogenesis.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by General Funds from the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics. The Division of Cancer Epidemiology and Genetics reviewed and approved the ATBC study and cleared the manuscript for publication but had no role in the analysis, decision to publish, or preparation of the manuscript.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Thun MJ, Henley SJ, Calle EE. Tobacco use and cancer: an epidemiologic perspective for geneticists. Oncogene. 2002;21:7307–7325. doi: 10.1038/sj.onc.1205807. [DOI] [PubMed] [Google Scholar]
- 3.Engels EA. Inflammation in the development of lung cancer: epidemiological evidence. Expert Rev Anticancer Ther. 2008;8:605–615. doi: 10.1586/14737140.8.4.605. [DOI] [PubMed] [Google Scholar]
- 4.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers–a different disease. Nat Rev Cancer. 2007;7:778–790. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi AK, Caporaso NE, Katki HA, Wong HL, Chatterjee N, et al. C-reactive protein and risk of lung cancer. J Clin Oncol. 2010;28:2719–2726. doi: 10.1200/JCO.2009.27.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pine SR, Mechanic LE, Enewold L, Chaturvedi AK, Katki HA, et al. Increased Levels of Circulating Interleukin 6, Interleukin 8, C-Reactive Protein, and Risk of Lung Cancer. J Natl Cancer Inst. 2011;103:1112–1122. doi: 10.1093/jnci/djr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner DR, McLaughlin JR, Hung RJ. Previous lung diseases and lung cancer risk: a systematic review and meta-analysis. PLoS ONE. 2011;6:e17479. doi: 10.1371/journal.pone.0017479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koshiol J, Rotunno M, Consonni D, Cecilia Pesatori AC, De Matteis S, et al. Chronic obstructive pulmonary disease and altered risk of lung cancer in a population-based case-control study. PLoS ONE. 2009;4:e7380. doi: 10.1371/journal.pone.0007380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohno T, Kunitoh H, Mimaki S, Shiraishi K, Kuchiba A, et al. Contribution of the TP53, OGG1, CHRNA3, and HLA-DQA1 Genes to the Risk for Lung Squamous Cell Carcinoma. J Thorac Oncol. 2011;6:813–817. doi: 10.1097/JTO.0b013e3181ee80ef. [DOI] [PubMed] [Google Scholar]
- 10.Kohno T, Kunitoh H, Shimada Y, Shiraishi K, Ishii Y, et al. Individuals susceptible to lung adenocarcinoma defined by combined HLA-DQA1 and TERT genotypes. Carcinogenesis. 2010;31:834–841. doi: 10.1093/carcin/bgq003. [DOI] [PubMed] [Google Scholar]
- 11.Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008;113:3036–3046. doi: 10.1002/cncr.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 13.Ece F, F Hatabay N, Erdal N, Gedik C, Guney C, et al. Does Helicobacter pylori infection play a role in lung cancer? Respir Med. 2005;99:1258–1262. doi: 10.1016/j.rmed.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 14.Gocyk W, Niklinski T, Olechnowicz H, Duda A, Bielanski W, et al. Helicobacter pylori, gastrin and cyclooxygenase-2 in lung cancer. Med Sci Monit. 2000;6:1085–1092. [PubMed] [Google Scholar]
- 15.Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol. 2009;472:467–477. doi: 10.1007/978-1-60327-492-0_23. [DOI] [PubMed] [Google Scholar]
- 16.Kanbay M, Kanbay A, Boyacioglu S. Helicobacter pylori infection as a possible risk factor for respiratory system disease: a review of the literature. Respir Med. 2007;101:203–209. doi: 10.1016/j.rmed.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 17.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- 18.Tegtmeyer N, Wessler S, Backert S. Role of the cag-pathogenicity island encoded type IV secretion system in Helicobacter pylori pathogenesis. FEBS J. 2011;278:1190–1202. doi: 10.1111/j.1742-4658.2011.08035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnold IC, Dehzad N, Reuter S, Martin H, Becher B, et al. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest. 2011;121:3088–3093. doi: 10.1172/JCI45041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang YJ, Kim HY, Albacker LA, Lee HH, Baumgarth N, et al. Influenza infection in suckling mice expands an NKT cell subset that protects against airway hyperreactivity. J Clin Invest. 2011;121:57–69. doi: 10.1172/JCI44845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Islami F, Kamangar F. Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prev Res (Phila Pa) 2008;1:329–338. doi: 10.1158/1940-6207.CAPR-08-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janssen-Heijnen ML, Coebergh JW. The changing epidemiology of lung cancer in Europe. Lung Cancer. 2003;41:245–258. doi: 10.1016/s0169-5002(03)00230-7. [DOI] [PubMed] [Google Scholar]
- 23.Najafizadeh K, Falah Tafti S, Shiehmorteza M, Saloor M, Jamali M. H pylori seroprevalence in patients with lung cancer. World J Gastroenterol. 2007;13:2349–2351. doi: 10.3748/wjg.v13.i16.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Philippou N, Koursarakos P, Anastasakou E, Krietsepi V, Mavrea S, et al. Helicobacter pylori seroprevalence in patients with lung cancer. World J Gastroenterol. 2004;10:3342–3344. doi: 10.3748/wjg.v10.i22.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Behroozian R, Moradkhan E. The assessment of probable relationship between lung cancer and Helicobacter pylori infection. Trop Gastroenterol. 2010;31:34–36. [PubMed] [Google Scholar]
- 26.Zhuo WL, Zhu B, Xiang ZL, Zhuo XL, Cai L, et al. Assessment of the relationship between Helicobacter pylori and lung cancer: a meta-analysis. Arch Med Res. 2009;40:406–410. doi: 10.1016/j.arcmed.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Kamangar F, Dawsey SM, Blaser MJ, Perez-Perez GI, Pietinen P, et al. Opposing risks of gastric cardia and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity. J Natl Cancer Inst. 2006;98:1445–1452. doi: 10.1093/jnci/djj393. [DOI] [PubMed] [Google Scholar]
- 28.Stolzenberg-Solomon RZ, Blaser MJ, Limburg PJ, Perez-Perez G, Taylor PR, et al. Helicobacter pylori seropositivity as a risk factor for pancreatic cancer. J Natl Cancer Inst. 2001;93:937–941. doi: 10.1093/jnci/93.12.937. [DOI] [PubMed] [Google Scholar]
- 29.Cook MB, Dawsey SM, Diaw L, Blaser MJ, Perez-Perez GI, et al. Serum pepsinogens and Helicobacter pylori in relation to the risk of esophageal squamous cell carcinoma in the alpha-tocopherol, beta-carotene cancer prevention study. Cancer Epidemiol Biomarkers Prev. 2010;19:1966–1975. doi: 10.1158/1055-9965.EPI-10-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy G, Kamangar F, Dawsey SM, Stanczyk FZ, Weinstein SJ, et al. The relationship between serum Ghrelin and the risk of gastric and esophagogastric junctional adenocarcinomas. J Natl Cancer Inst. 2011;103:1123–1129. doi: 10.1093/jnci/djr194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The ATBC Cancer Prevention Study Group. The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. Ann Epidemiol. 1994;4:1–10. doi: 10.1016/1047-2797(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. International statistical classifi cation of diseases and related health problems. Geneva (Switzerland): World Health Organization; 1978. [Google Scholar]
- 33.Korhonen P, Malila N, Pukkala E, Teppo L, Albanes D, et al. The Finnish Cancer Registry as follow-up source of a large trial cohort–accuracy and delay. Acta Oncol. 2002;41:381–388. doi: 10.1080/028418602760169442. [DOI] [PubMed] [Google Scholar]
- 34.Limburg P, Qiao Y, Mark S, Wang G, Perez-Perez G, et al. Helicobacter pylori seropositivity and subsite-specific gastric cancer risks in Linxian, China. J Natl Cancer Inst. 2001;93:226–233. doi: 10.1093/jnci/93.3.226. [DOI] [PubMed] [Google Scholar]
- 35.Reibman J, Marmor M, Filner J, Fernandez-Beros ME, Rogers L, et al. Asthma is inversely associated with Helicobacter pylori status in an urban population. PLoS ONE. 2008;3:e4060. doi: 10.1371/journal.pone.0004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romero-Gallo J, Perez-Perez GI, Novick RP, Kamath P, Norbu T, et al. Responses of endoscopy patients in Ladakh, India, to Helicobacter pylori whole-cell and Cag A antigens. Clin Diagn Lab Immunol. 2002;9:1313–1317. doi: 10.1128/CDLI.9.6.1313-1317.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rothman K, Greenland S. Modern epidemiology. Philadelphia: Lippincott-Raven; 1998. [Google Scholar]
- 38.Lubin JH, Caporaso NE. Cigarette smoking and lung cancer: modeling total exposure and intensity. Cancer Epidemiol Biomarkers Prev. 2006;15:517–523. doi: 10.1158/1055-9965.EPI-05-0863. [DOI] [PubMed] [Google Scholar]
- 39.Lubin JH, Kogevinas M, Silverman D, Malats N, Garcia-Closas M, et al. Evidence for an intensity-dependent interaction of NAT2 acetylation genotype and cigarette smoking in the Spanish Bladder Cancer Study. Int J Epidemiol. 2007;36:236–241. doi: 10.1093/ije/dym043. [DOI] [PubMed] [Google Scholar]
- 40.Lubin JH, Virtamo J, Weinstein SJ, Albanes D. Cigarette smoking and cancer: intensity patterns in the alpha-tocopherol, beta-carotene cancer prevention study in Finnish men. Am J Epidemiol. 2008;167:970–975. doi: 10.1093/aje/kwm392. [DOI] [PubMed] [Google Scholar]
- 41.Kosunen TU, Aromaa A, Knekt P, Salomaa A, Rautelin H, et al. Helicobacter antibodies in 1973 and 1994 in the adult population of Vammala, Finland. Epidemiol Infect. 1997;119:29–34. doi: 10.1017/s0950268897007565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greenland S, Rothman KJ. Approximate statistics: the likelihood-ratio method. In: Rothman KJ, Greenland S, editors. Modern Epidemiology. Philadelphia: Lippincott Williams & Wilkins; 1998. pp. 218–220. [Google Scholar]
- 43.Albanes D, Heinonen OP, Taylor PR, Virtamo J, Edwards BK, et al. Alpha-Tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of base-line characteristics and study compliance. J Natl Cancer Inst. 1996;88:1560–1570. doi: 10.1093/jnci/88.21.1560. [DOI] [PubMed] [Google Scholar]
- 44.Albanes D, Heinonen OP, Huttunen JK, Taylor PR, Virtamo J, et al. Effects of alpha-tocopherol and beta-carotene supplements on cancer incidence in the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study. Am J Clin Nutr. 1995;62:1427S–1430S. doi: 10.1093/ajcn/62.6.1427S. [DOI] [PubMed] [Google Scholar]
- 45.Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997;315:1533–1537. doi: 10.1136/bmj.315.7121.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53:1119–1129. doi: 10.1016/s0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]