Abstract
Introduction
Although the life expectancy for patients with cystic fibrosis (CF) has increased dramatically in the preceding decades, often the final therapeutic option for patients with end-stage CF is lung transplantation. Prior to transplantation, patients with severe disease may require mechanical ventilation. Those refractory to mechanical ventilation may require extracorporeal membrane oxygenation (ECMO). The purpose of this case report is to describe the physical therapy management of a patient who received ECMO as a bridge to lung transplantation.
Case Presentation
A 16-year-old girl with severe acute respiratory failure due to a CF exacerbation eventually required ECMO to maintain adequate gas exchange. While on ECMO, she received physical therapy interventions ranging from therapeutic exercise, manual therapy, and integumentary protection techniques in addition to airway clearance techniques. Prior to her transplant, she was standing multiple times per day with moderate assistance, was sitting on the edge-of-bed, as well as taking steps to transfer to/from a chair. She successfully received a bilateral lung transplant after 8 days on ECMO.
Conclusion
Physical therapy interventions, including out-of-bed mobility, can be safely provided to patients on portable ECMO as a bridge to lung transplantation. These interventions were focused on preventing the negative sequelae of bed rest, increasing her strength and endurance, as well as improving her level of consciousness and psychological well being in preparation for lung transplantation.
Key Words: extracorporeal membrane oxygenation, lung transplantation, physical therapy, exercise
BACKGROUND AND PURPOSE
Cystic fibrosis (CF) is no longer a disease of children; projected life expectancy for patients with CF has increased into the late 30s, and continues to grow. The genetic defect, affecting the cystic-fibrosis transmembrane conductance regulator (CFTR), leads to mucosal obstruction in multiple tissues, especially the lung. Associated lung pathology is the primary contributor to mortality in patients with CF.1 Treatment of the pulmonary involvement typically includes inhaled medications [eg, hypertonic saline, tobramycin (an antibiotic), and dornase alfa (a mucolytic)], systemic antibiotics, airway clearance, and exercise.2 As the disease progresses, pulmonary exacerbations become more frequent and severe and are associated with increased morbidity and mortality.3 Although there are many new exciting therapies for CF in the pipeline,1,2 for patients with frequent exacerbations and severe disease, lung transplantation is often the final therapeutic option.2
Cystic fibrosis is the third leading indication for lung transplantation.4 Although transplantation of patients on mechanical ventilation was previously discouraged, the current US lung allocation system assigns high scores to ventilator-dependent patients, since they have a high “medical urgency.”4 Patients with CF who undergo lung transplantation have similar outcomes whether they are mechanically ventilated or nonmechanically ventilated at the time of transplantation.5 Another potentially controversial group for lung transplantation are patients requiring preoperative extracorporeal membrane oxygenation (ECMO).4 Although the early postoperative risk of death is almost 2.6 times higher in patients requiring ECMO prior to lung transplant compared to the unsupported patient, survival after 9 months is fairly similar.6
Extracorporeal membrane oxygenation is used to maintain adequate gas exchange in patients with severe respiratory failure that is refractory to even maximal mechanical ventilatory support, including patients who are waiting for a lung transplant. Most patients on ECMO are sedated and on bed rest, but there are a few reports of ambulatory patients on ECMO as a bridge to lung transplantation.7,8 If ECMO is to be a successful bridge to lung transplantation, then prevention of the many sequelae of bed rest and a continuation of pretransplant rehabilitation is needed. The purpose of this case report is to describe the physical therapy management of a patient who received ECMO as a bridge to lung transplantation. Consent of the patient and permission of her family were provided to present the case.
CASE DESCRIPTION
“Jane,” a 16-year-old female with CF, was admitted to a tertiary-care children's hospital for worsening shortness of breath. Over the next week, her condition continued to deteriorate until she eventually went into severe respiratory failure, requiring mechanical ventilation. The following day Jane was transferred to the pediatric intensive care unit (PICU) at our hospital for management of her respiratory failure and evaluation for lung transplantation (hospital day [HD) 0]. On admission, her respiratory failure was refractory to traditional mechanical ventilation (see Table 1), and required that she be placed on a high-frequency percussive ventilator [(The Percussionaire® VDR®-4) with peak inspiratory pressure 30 cm H2O, frequency 510 cycles/min, inspiratory time 3.0 sec, positive end expiratory pressure (PEEP) 10 cm H20, FiO2 1.00]. The high-frequency percussive ventilation improved her hypercapnia and acid-base balance, but did little for her oxygenation (Table 1).
Table 1.
Arterial Blood Gas Values
| Hospital day | FiO2 | pH | PCO2 (mm Hg) | pO2 (mm Hg) | HCO3 (mmol/L) | SaO2 (%) | PaO2/FiO2 |
|---|---|---|---|---|---|---|---|
| 0 | 1.00 | 7.09 | 187 | 63 | 54 | 87.8 | 63 |
| High-frequency jet ventilation initiated | |||||||
| 1 | 0.85 | 7.30 | 102 | 63 | 49 | 91.3 | 74 |
| 5 | 0.70 | 7.28 | 94 | 68 | 42 | 93.1 | 97 |
| ECMO initiated | |||||||
| 7 | 0.45 | 7.56 | 40 | 42 | 35 | 86.3 | 93 |
| 9 | 0.35 | 7.46 | 49 | 83 | 35 | 96.3 | 237 |
| 11 | 0.35 | 7.47 | 51 | 77 | 36 | 95.7 | 220 |
| 14 | 0.35 | 7.40 | 57 | 70 | 34 | 91.9 | 200 |
| Bilateral lung transplant | |||||||
| 16 | 0.21 | 7.55 | 32 | 91 | 28 | 94.8 | 433 |
| 19 | 0.21 | 7.54 | 37 | 75 | 32 | 93.8 | 357 |
Cardiovascular and pulmonary examination revealed mild sinus tachycardia (90–110 bpm) of normal rhythm (per EKG) and a blood pressure ranging from 90–125/64–74 mm Hg (per arterial BP monitor); her respiratory frequency was ventilator dependent (as noted above) and SpO2 ranged from 95% to 97%. The high-frequency percussive ventilator precluded performance of a valid chest examination due to the excessive noise and vibration from the ventilator. She also had severe digital clubbing and 4+ pitting edema in her right foot. Her skin was dry, but intact and without evidence of excessive pressure.
Shoulder flexion and abduction were limited bilaterally to ∼150° and 120°, respectively; otherwise, her upper extremity range of motion (ROM) was normal. Lower extremity ROM and flexibility were normal with the exception of ankle dorsiflexion to neutral with her knees extended. Muscle bulk was quite diminished. She was hypotonic but demonstrated right greater than left ankle clonus.
Past Medical History
Over the course of the past year, Jane had repeated lung infections requiring repeated intravenous (IV) antibiotics. Her last exacerbation was 3 months prior to this episode. Jane was also pancreatic insufficient and had lost approximately 14 kg (30 lbs) over the last several months. Her family reported that she had been active and fairly athletic until her recent exacerbations.
Physical Examination (Hospital Day 6)
Physical therapy was consulted on HD 6, while Jane was in the PICU sedated and nonresponsive. On observation, she was on mechanical ventilation via oral endotracheal tube, and connected to a left radial arterial line, left brachial double-lumen peripheral-inserted central catheter, two right peripheral intravenous catheters, naso-gastric tube, Foley catheter, pulse oximeter, and ECG monitor. Her body mass and height were 38 kg (84 lbs) and 152.5 cm (5“0”) respectively, giving her a BMI of 16.3 kg/m2.
Clinical Impression
Jane was severely hypercapnic, requiring high levels of mechanical ventilation, high FiO2, and medical sedation. Given her current medical status, we did not address any activity goals at this time. Rather, we focused our goals on preventing anticipated problems9 associated with bed rest and immobility such as joint contractures at the shoulder, elbow, hip, knee, and ankle that can limit activity and often persist through discharge from the hospital.10 Pressure sores also frequently occur in the ICU. Underweight patients, like Jane, have a 5-fold greater risk for developing pressure sores primarily at the sacrum and heel.11,12
Interventions (HD 6)
Initial interventions included passive range of motion (PROM) to all major upper and lower extremity joints, stretching of plantar flexors, and application of pressure relieving ankle foot orthoses (PRAFO®) to maintain the feet in neutral dorsiflexion, hip in neutral rotation, and keep her heels elevated off the bed (Table 2). Airway clearance was not a PT intervention as respiratory therapy administered intrapulmonary percussive ventilator treatments every 4 hours.13
Table 2.
Medication List on Initial Examination (HD 6)
| Drug (trade name) | Drug class |
|---|---|
| tobramycin | aminoglycoside antibiotic |
| meropenem | carbapenem antibiotic |
| cefepime | cephalosporin antibiotic |
| ciprofloxacin (Cipro) | fluoroquinolone antibiotic |
| vancomycin | glycopeptide antibiotic |
| voriconazole (Diflucan) | triazole antifungal |
| midazolam (Versed) | anxiolytic-sedative (benzodiazepine) |
| methylprednisolone (Solu-Medrol) | corticosteriod |
| pancrelipase (Ultrase) | digestive enzyme |
| ketamine | general anesthetic |
| famotidine (Pepcid) | histamine-2 blocker |
Re-examination (HD 7)
Despite high FiO2 and high-frequency percussive ventilation, Jane remained significantly hypoxic and hypercapnic (see Table 1) resulting in her placement on veno-venous ECMO via double lumen cannulation of the right internal jugular vein, as well as placement on the lung transplantation list (HD 6). On the morning of HD 7, she received a tracheostomy and was converted to synchronized intermittent mechanical ventilation (tidal volume 4.2 ml/kg, frequency 16 breaths/min, PEEP 10 cm H2O, FiO2 0.45) with much improved gas exchange (see Table 1). She remained medically sedated.
Interventions (HD 7–8)
Jane's physicians were consulted regarding her ability to participate in physical therapy now that she was on ECMO. Given that Jane's pulmonary status was stabilized, the physicians planned to decrease her sedation to allow her to progress to active exercise and begin mobility training. Jane's right upper extremity ROM was limited to prevent placing stress on her internal jugular catheters, but this did not preclude glenohumeral joint mobilization. In addition, active assistive ROM was begun as she became more alert. Consults for occupational therapy (OT) and speech therapy were recommended as Jane attempted to mouth words but became frustrated with her inability to communicate.
Re-examination (HD 9)
Team discussions resulted in agreement that the goal for Jane would be that she would be cognitively alert and weight bearing/ambulatory prior to receiving a lung transplant. While Jane was awake and following commands, she remained lethargic. She had gross muscle atrophy consistent with strength of ∼2/5 in all key upper and lower extremity muscle groups, but her strength diminished with multiple repetitions. She was able to maintain stable vital signs when positioned in a semi-upright position (bed in the “chair position”).
Clinical Impression
Jane's most significant impairments included decreased muscle strength, power, and endurance that limited her ability to perform simple bed mobility tasks such as rolling, bridging, or scooting in bed. Goals included sitting edge of bed without assistance and transferring from bed to chair and walking in the room with assistance.
Interventions (HD 9–14)
Jane's PT interventions are described in Table 3, which included a progression from active exercises in bed, to resistive and task-specific exercise as Jane improved in strength and endurance. She sat up with the in bed in the “chair position” (∼60°) twice on HD 9 with PT, OT, nursing, and respiratory therapy. From sitting upright with the bed in the “chair position,” Jane was then able to sit on the edge-of-bed (HD 10) (Figure 1). She initially required maximal assistance for sit-to-stand (HD 11) but was able to come to stand with moderate assistance, and maintain standing with minimum assistance. As she progressed, the interventions were progressed to having Jane perform higher numbers of repetitions of the tasks and to maintain upright sitting and standing for longer periods of time. In addition, PT and OT continued to provide PROM and joint mobilization as well as active assistive ROM exercises in sitting.
Table 3.
Summary of Physical Therapy Episode of Care
| HD | Key examination findings | Physical Therapy Interventions |
|---|---|---|
| 6 | Medically sedated and non responsive | PROM to all major upper extremity (UE) and lower extremity (LE) joints, stretching of plantar flexors, and application of pressure relieving ankle foot orthoses (PRAFO®) for positioning and pressure relief. |
| ECMO initiated | ||
| 7 | Medically sedated, now on ECMO, SIMV, and s/p tracheostomy | PROM continued, mother instructed in PROM and donning/doffing the PRAFOs. Right glenohumeral joint mobilization. |
| 8 | Awake, mouthing words | Active assistive range of motion (AAROM) of all major UE and LE muscle groups. Recommendation for speech therapy and occupational therapy consults. |
| 9 | Awake and following commands, but lethargic. Bleeding from trach site. Bilateral UE and LE muscle strength −2/5 | AM: Patient “sat up” with the bed in the “chair position” (∼60°) for ∼10 min. AAROM in “sitting.” PM: Sitting with bed in “chair position” for ∼15 min. Bilateral planar flexor stretches, active assisted ankle, knee, and hip flexion and extension while in sitting. |
| 10 | Alert, ready to sit up. Brief complaint of dizziness, in sitting, but MAP 87–93 mm Hg, HR 80–90 bpm, and SpO2 92–95% on FiO2 0.35. Knee extensor strength 3-/5. | Transfer from supine to sitting edge-of-bed (EOB) with maximal assistance (2 persons to assist the patient and 3 more to guide her ventilator tubes, ECMO cannulae, and other arterial and venous lines). Minimal/moderate assistance required to maintain upright sitting (more as patient fatigued). Sat edge of bed for ∼40 min, intermittently performing UE and LE AAROM. |
| 11 | Eager to sit and stand. No adverse signs or symptoms related to intervention except pain at trach site and fatigue at end of session (MAP 90–108 mm Hg, HR 68–95 bpm, SpO2 mid-90s). | UE and LE AAROM in supine. Transferred to EOB with maximal assistance. Sat EOB for 15 min with contact guard-to-minimal assistance (see Fig 1). Worked on weight shifting, reaching and scooting while in sitting. She stood twice with maximal assistance (15 to 20 sec each). Knees did not buckle in standing, but she required assistance to stand erect, presumably due to hip extensor weakness. |
| 12 | AM: Anxious and medically sedated PM: Drowsy but motivated. | Transferred to EOB with maximal assistance. Stood twice with maximal assistance, but was able to remain standing for almost 2 min each time. Required assistance to block her knees and help facilitate hip extension. |
| 13 | Stood twice for 60–90 sec. Required only moderate assistance for sit-to-stand, then only minimal assistance to maintain standing. Sat EOB ∼45 min with contact guard/minimal assistance. Performed active (gravity resisted) UE and LE exercises in sitting. | |
| 14 | Off ventilator, on trach collar trial for first time (FiO2 0.40). No complaints of trach site pain, dizziness, or dyspnea. Patient excited to be out of bed. Vital signs stable throughout. | Sit-to-stand with moderate assistance. Practiced weight shifting in standing. Took 5 steps from bed and pivoted to sit in chair for the first time. Repeated 2 more sit-to-stand trials, with standing durations of ∼45 sec. Sat upright in chair for ∼30 min and then with feet elevated for another 90 min. Transferred back to bed, again taking 5–6 steps. |
| Bilateral lung transplant | ||
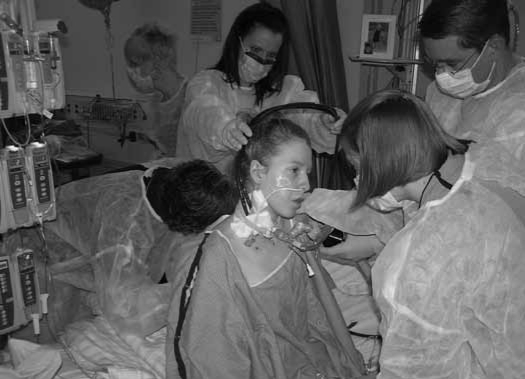
Figure 1.
Jane sitting edge-of-bed on HD 11 with PT and a respiratory therapist in the foreground and another respiratory therapist and nurse behind her.
Clinical Impression
Continued close attention was paid to any signs and symptoms of poor cardiac output, including orthostatic intolerance (decreasing arterial BP or SpO2, increased HR, diaphoresis, pallor, and complaints of dizziness, fatigue, or shortness of breath) as well as ensuring the integrity of the ECMO cannula. Also, a respiratory therapist continuously monitored her ECMO flow. Even in response to vertical postures (sitting and standing), there were never any instances in which either her ECMO flows diminished or she appeared to have inadequate systemic oxygen delivery.
OUTCOME
Jane received a bilateral orthotopic lung transplant on HD 15. When seen by PT on postoperative day 2 (HD 17), she was already off the ventilator breathing humidified room air (FiO2 0.21) through a trach collar, started taking her first steps the following day (HD 18), and was discharged from the ICU on postoperative day 7 (HD 22). By hospital discharge (HD 45), she was walking over 365 m independently on room air with SpO2 ≥ 96% with minimal dyspnea and no pain.
DISCUSSION
Several recent articles in the physical therapy literature have discussed the feasibility and safety of providing PT interventions, including out-of-bed mobility, for patients with invasive lines, tubes, monitors, and cardiac support devices.14,15,16,17,18 This case demonstrates that patients on ECMO may be safely mobilized by physical therapy and benefit from physical and occupational therapy in the pretransplantation phase that may include mechanical ventilation and ECMO. While this is not the first case report of ambulatory ECMO used as a bridge to lung transplantation,7,8 it appears to be the first pediatric case, the first in a patient with CF, and the first to describe the physical therapy management of a patient on ECMO. Two additional patients at our facility with CF and acute respiratory failure have recently been successfully mobilized out-of-bed with portable ECMO prior to lung transplantation, with one patient walking over 200 m less than two weeks after being placed on ECMO.
Continuous Examination
Due to the severe acuity of Jane's condition, every PT session was a re-examination. Typically, she had improved since the last visit and the intervention could be progressed, but often her condition had worsened or other complications had developed (eg, bleeding or anxiety), requiring a change in plans. Even during the course of a session, her response to exercise and activity was continuously monitored by the team to ensure she was hemodynamically stable and safe. All of the PTs and OTs involved in her care had extensive experience working with critically ill patients and were accustomed to being flexible, prepared for the unexpected, and able to think on their feet as they continuously re-examined her response to treatment.
Interventions and Rationale
Often patients in the ICU are seen as lower priority patients, but this attitude may be changing.19 Since the medical team wanted to make sure she was cognitively and physically able to participate in post-transplant rehabilitation, Jane was considered a high priority patient, receiving 12 physical therapy sessions during her 8 days on ECMO prior to receiving her lung transplant. As seen in Figure 1, Jane required the assistance of numerous health care professionals during mobility activities. The interdisciplinary team spent additional time coordinating schedules to accommodate nursing and respiratory therapy interventions.
Specific physical therapy interventions ranged from therapeutic exercise, manual therapy, and integumentary protection techniques to airway clearance techniques. As noted in Table 3, therapeutic exercise encompassed the bulk of interventions. As soon as Jane was able, we progressed her exercises from active exercise in bed, to resistive, task-specific performance training to increase her lower extremity strength, power, and endurance. Since she initially (HD 11) required maximal assistance for sit-to-stand, this represented “high-intensity” resistance training for her lower extremity extensors. As she became stronger and required less physical assistance to stand, her “relative intensity” of resistance training was lower, allowing us to increase the number of repetitions and increase her muscular and cardiovascular endurance.
Sitting upright, initially with the bed in the “chair position,” and then later sitting on the edge-of-bed benefitted Jane by allowing her to maximize ventilation/perfusion matching, ease her work of breathing, and mobilize her secretions.20 Sitting on the edge-of-bed was also a therapeutic exercise that strengthened Jane's core/trunk muscles and improved balance while reconditioning her cardiovascular system by being in a more vertical posture.20 Continued close attention was paid to any signs and symptoms of poor cardiac output, including orthostatic intolerance (ie, decreasing arterial BP; increased HR; decreasing SpO2, diaphoresis, pallor, and complaints of dizziness, fatigue, or shortness of breath).
We did not specifically work on rolling in bed and supine-to-sit transfers. In order to protect the ECMO cannula and reduce sheer stress on her sacrum while moving in bed, the transition from supine to sit was a maximal assist to dependent transfer. Also in an effort to protect the ECMO cannula while maintaining or increasing glenohumeral capsular extensibility, joint mobilization was substituted for full shoulder PROM on the right.
Airway clearance is always a concern in patients with CF. In this case, an intervention, intrapulmonary percussive ventilation, with similar efficacy of postural drainage, percussion, and vibration,13 was provided by respiratory therapy on a frequent basis throughout the day. This allowed PT to concentrate on exercise interventions, which often required 60 to 90 minutes per day. In addition, the upright positioning, spontaneous deep breathing that occurred during physical activity, and coordinated deep breathing with upper extremity exercise provided a stimulus for mobilization of secretions.
Potential Medical Complications
Although thrombus and infection are also risks of veno-venous ECMO, bleeding and decannulation are the major concerns when mobilizing patients on ECMO.21 Jane did have several episodes of bleeding at her tracheostomy site that disrupted her therapy schedule. During mobility interventions, great care was taken by the interdisciplinary team to manage her tubing to prevent tension from being placed on either her internal jugular cannula or her tracheostomy tube that could have further exacerbated her bleeding or led to decannulation.
Although Jane was not specifically diagnosed with a critical illness myopathy and/or neuropathy, neuromuscular abnormalities occur in approximately 50% of patients requiring prolonged mechanical ventilation, is more common in women, and may be associated with glucocorticoid use.22 Although her daily dose of methylprednisolone (20 mg per day) was not excessively high, glucocorticoids can impair muscle function by increasing protein degradation and decreasing protein synthesis,23 and could have resulted in her significantly impaired muscle strength.
CONCLUSION
There is increased interest in early mobilization for patients in the ICU.24,25 We report on a novel population of patients who benefit from PT in the ICU, those on portable ECMO. As portable ECMO becomes a more common bridge to lung transplantation, mobilization of patients in the ICU while on ECMO will be needed to maintain or increase patients’ physical function and psychological well-being while awaiting transplantation. This case demonstrates that a coordinated, interdisciplinary team effort can be safely used to meet these goals of patients on ECMO awaiting lung transplantation.
ACKNOWLEDGEMENTS
We thank our willing and dedicated patient and her family for blazing a new path for the many patients that will continue to follow in her footsteps at our facility and around the world. Her success was made possible by an innovative and committed interdisciplinary team of physicians, surgeons, nurses, respiratory therapists, occupational therapists, and physical therapists. In particular, we would like to thank the therapists from the Department of Physical and Occupational Therapy that frequently saw Jane: Whitney Diebolt, OTR/L; Erin Diebold, PT, DPT; Bethany Yoder, PT, DPT; and Heidi Pongracz, PT, MPH, as well as David Zaas, MD, MBA, Medical Director of Lung Transplantation, Duke University Health System.
This work was supported by the Cystic Fibrosis Foundation (RDP464) and the National Institutes of Health (P30 DK072482).
REFERENCES
- 1.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352(19):1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 2.O'Sullivan BP, Freedman SD. Cystic fibrosis. Lancet. 2009;373(9678):1891–1904. doi: 10.1016/S0140-6736(09)60327-5. [DOI] [PubMed] [Google Scholar]
- 3.Ferkol T, Rosenfeld M, Milla CE. Cystic fibrosis pulmonary exacerbations. J Pediatr. 2006;148(2):259–264. doi: 10.1016/j.jpeds.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Kotloff RM, Thabut G. Lung transplantation. Am J Respir Crit Care Med. 2011;184(2):159–171. doi: 10.1164/rccm.201101-0134CI. [DOI] [PubMed] [Google Scholar]
- 5.Spahr JE, Love RB, Francois M, Radford K, Meyer KC. Lung transplantation for cystic fibrosis: current concepts and one center's experience. J Cyst Fibros. 2007;6(5):334–350. doi: 10.1016/j.jcf.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Mason DP, Thuita L, Nowicki ER, Murthy SC, Pettersson GB, Blackstone EH. Should lung transplantation be performed for patients on mechanical respiratory support? The US experience. J Thorac Cardiovasc Surg. 2010;139(3):765–773. doi: 10.1016/j.jtcvs.2009.09.031. e761. [DOI] [PubMed] [Google Scholar]
- 7.Mangi AA, Mason DP, Yun JJ, Murthy SC, Pettersson GB. Bridge to lung transplantation using short-term ambulatory extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg. 2010;140(3):713–715. doi: 10.1016/j.jtcvs.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 8.Garcia JP, Iacono A, Kon ZN, Griffith BP. Ambulatory extracorporeal membrane oxygenation: a new approach for bridge-to-lung transplantation. J Thorac Cardiovasc Surg. 2010;139(6):e137–139. doi: 10.1016/j.jtcvs.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Rothstein JM, Echternach JL, Riddle DL. The Hypothesis-Oriented Algorithm for Clinicians II (HOAC II): a guide for patient management. Phys Ther. 2003;83(5):455–470. [PubMed] [Google Scholar]
- 10.Clavet H, Hébert PC, Fergusson D, Doucette S, Trudel G. Joint contracture following prolonged stay in the intensive care unit. Can Med Assoc J. 2008;178(6):691–697. doi: 10.1503/cmaj.071056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fife C, Otto G, Capsuto EG, et al. Incidence of pressure ulcers in a neurologic intensive care unit. Crit Care Med. 2001;29(2):283–290. doi: 10.1097/00003246-200102000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Schue RM, Langemo DK. Pressure ulcer prevalence and incidence and a modification of the Braden Scale for a rehabilitation unit. J Wound Ostomy Continence Nurs. 1998;25(1):36–43. doi: 10.1016/s1071-5754(98)90011-0. [DOI] [PubMed] [Google Scholar]
- 13.Homnick DN, White F, de Castro C. Comparison of effects of an intrapulmonary percussive ventilator to standard aerosol and chest physiotherapy in treatment of cystic fibrosis. Pediatr Pulmonol. 1995;20(1):50–55. doi: 10.1002/ppul.1950200110. [DOI] [PubMed] [Google Scholar]
- 14.Ciesla N, Murdock K. Lines, tubes, catheters and physiologic monitoring in ICU. Cardiopulm Phys Ther J. 2000;11(1):16–25. [Google Scholar]
- 15.Senduran M, Malkoc M, Oto O. Physical therapy in the intensive care unit in a patient with biventricular assist device. Cardiopulm Phys Ther J. 2011;22(3):31–34. [PMC free article] [PubMed] [Google Scholar]
- 16.Nicholson C, Paz JC. Total artificial heart and physical therapy management. Cardiopulm Phys Ther J. 2010;21(2):13–21. [PMC free article] [PubMed] [Google Scholar]
- 17.McVey LW, Hillegass E. A nontraditional approach to cardiac rehabilitation in the dialysis center for a patient with end-stage renal disease following coronary artery bypass surgery: A case report. Cardiopulm Phys Ther J. 2010;21(4):14–21. [PMC free article] [PubMed] [Google Scholar]
- 18.Stiller K, Phillips A, Lambert P. The safety of mobilisation and its effect on haemodynamic and respiratory status of intensive care patients. Physiother Theory Practice. 2004;20(3):175–185. [Google Scholar]
- 19.Morris PE, Griffin L, Berry M, et al. Receiving early mobility during an intensive care unit admission is a predictor of improved outcomes in acute respiratory failure. Am J Med Sci. 2011;341(5):373–377. doi: 10.1097/MAJ.0b013e31820ab4f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dean E. Body Positioning. In: Frownfelter DL, Dean E, editors. Cardiovascular and Pulmonary Physical Therapy: Evidence and Practice. 4th ed. St. Louis: Mosby; 2006. [Google Scholar]
- 21.Arlt M, Philipp A, Zimmermann M, et al. Emergency use of extracorporeal membrane oxygenation in cardiopulmonary failure. Artif Organs. 2009;33(9):696–703. doi: 10.1111/j.1525-1594.2009.00860.x. [DOI] [PubMed] [Google Scholar]
- 22.Stevens R, Dowdy D, Michaels R, Mendez-Tellez P, Pronovost P, Needham D. Neuromuscular dysfunction acquired in critical illness: a systematic review. Intensive Care Med. 2007;33(11):1876–1891. doi: 10.1007/s00134-007-0772-2. [DOI] [PubMed] [Google Scholar]
- 23.Schakman O, Gilson H, Thissen JP. Mechanisms of glucocorticoid-induced myopathy. J Endocrinol. 2008;197(1):1–10. doi: 10.1677/JOE-07-0606. [DOI] [PubMed] [Google Scholar]
- 24.Jolley SE, Hough CL. Physician attitudes towards early mobilization in the medical intensive care unit. Am J Respir Crit Care Med. 2011;183:A3149. [Google Scholar]
- 25.Vazquez A, Arnold S, Johnson MM. Early mobilization of intensive care unit patients: staff attitudes and opinions. Am J Respir Crit Care Med. 2010;181:A3767. [Google Scholar]