Abstract
Background
We have previously documented that central Angiotensin type 2 receptors (AT2R) negatively modulate sympathetic outflow and arterial blood pressure (BP). In the current study, we determined the effects of intracerebroventricular (icv) infusion of Compound 21 (C21), the first selective non-peptide AT2R agonist, on norepinephrine (NE) excretion and BP in rats.
Methods
C21 was icv infused by a Micro-osmotic pump for 7 days. Urinary NE concentration was measured using the Norepinephrine Enzyme Immunoassay kit. BP was recorded by radiotelemetry. After 7 days, the rats were euthanized and three sympathetic relevant brain regions and cerebral cortex were micro-punched to measure nNOS protein expression by Western Blot. In addition, the influence of C21 on neuronal potassium current (IKv) was determined by whole cell patch-clamp in a neuron cell-line, CATH.a.
Results
(1) icv treatment of C21 significantly decreased NE concentration and amount in nighttime urine but had no effect in daytime urine. (2) C21 treated rats exhibited a slight but significant decrease in BP. (3) The effects of C21 on NE excretion and BP were abolished by AT2R antagonist, PD123319, and nitric oxide synthase (NOS) inhibitor, L-NAME. (4) C21 treatment significantly up regulated nNOS expression in the PVN and RVLM, but not in the NTS and cerebral cortex. (5) In CATH.a neurons, C21 treatment significantly increased IKv, which was completely abolished by PD123319 and L-NAME.
Conclusion
These results demonstrate a central inhibitory influence of C21 on sympathetic outflow via a nNOS dependent mechanism, which might be mediated by facilitating neuronal potassium channel.
Keywords: Angiotensin type 2 receptor, central nervous system, norepinephrine, blood pressure, potassium current
Introduction
Even though it is widely accepted that Angiotensin II (Ang II) primarily activates two receptor subtypes, the Angiotensin type 1 receptor (AT1R) and type 2 receptor (AT2R), our understanding of the functional significance of AT2R has been overshadowed by volumes of work on AT1Rs’1, 2. It has been well documented that AT1R mediate the majority of Ang II effects in both physiological and pathological conditions. These include the regulation of sympathetic outflow and cardiovascular function, water and electrolyte balance, thirst, and hormone secretion3, 4. The AT2R on the other hand, has long been viewed as having its major function during early stage of animal development and growth, due to the observation of its ubiquitous expression at very high levels in the fetus and its rapid regression to low levels shortly after birth1, 2. The latter notion on AT2R has dampened enthusiasm for the exploration of this receptor’s function especially in adult animals.
Some studies, however, insistently imply an involvement of AT2R in physiological significance. For example, in vitro experiments demonstrated that activation of AT2R stimulation increases potassium current (IKv) and therefore decreases neuronal excitability5, 6. Siragy et al.7 reported that AT2R-null mice had slightly elevated systolic blood pressure compared with wild-type control mice, and infusion of a subpressor dose of Ang II induced no change in blood pressure in wild-type mice but significantly increased blood pressure in AT2R knock-out mice. Moreover, Li et al.8 found that injection of Ang II into the cerebral ventricle evoked a larger increase in blood pressure in AT2R knock-out mice compared to wild type mice. These investigators further demonstrated that, in wild type mice, central injection of Ang II plus the AT2R antagonist PD123319 initiated a greater pressor response than that induced by Ang II alone. These results suggest a negative influence of AT2R on neuronal function and cardiovascular activity, which opposes that due to AT1R stimulation. Indeed, we recently found that AT2R protein expression was significantly down regulated in the rostral ventrolateral medulla (RVLM) of rats with chronic heart failure, and that this decreased AT2R expression played a critical role in sympatho-excitation in this syndrome9. In conscious normal rats, on the other hand, over expression of AT2R in the RVLM evoked a transient but significant decrease in arterial blood pressure (AP) and norepinephrine (NE) excretion10. Interestingly, in recent data from this laboratory we clearly demonstrated that, in the brainstem, liver, and kidney, adult rats exhibit a significantly higher AT2R protein expression than do fetuses and neonates11, suggesting a potential function of AT2R not only in early life but also in adult animals.
Compound 21 (C21) is a recently created non-peptide AT2R agonist, which is metabolically stable, orally effective with potential therapeutic efficacy12. C21 has been shown to have beneficial effects in animal models of hypertension and myocardial infarction when administered systemically12, 13. Moreover, C21 evoked dose-dependent vasorelaxation in isolated aortic and mesenteric vessels14. However, there have been no reports concerning its effects in the central nervous system on sympathetic outflow and AP. In the current experiment we hypothesized that central administration of C21 would have sympatho-inhibitory and depressor effects. We therefore determined the influences of central administration of C21 on NE excretion and AP in conscious normal rats.
Methods
1. Animal Experiments
35 male Sprague-Dawley rats weighing between 290 to 380 g were used in this experiment. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center and were carried out under the guidelines of the American Physiological Society and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Under isoflurane anaesthesia, rats were implanted with radiotelemetry devices (model TA11PA-C40, Physiotel, Data Sciences International), which were secured in the inguinal area. The sensing catheter was inserted into the left femoral artery against blood flow for the measurement of pulsatile and mean arterial blood pressure (MAP).
The rat was then placed on a stereotaxic frame (Stoelting Instruments, Inc.). The skull was exposed through a midline scalp incision. A small burr hole was made above the right cerebral ventricle (coordinates: 0.8 mm posterior to the bregma and 1.5 mm lateral from midline). ALZET Brain Infusion Kits and 1007D Micro-Osmotic Pumps (DURECT Corporation, ALZET Osmotic Pumps, Cupertino, CA) were used for the intracerebroventricular (icv) infusion of reagents for 7 days. Based on the infused reagents, the rats were assigned to one of following four groups: vehicle control group (aCSF 1 μl/hr), C21 group (0.5μg/μl/hr), C21 (0.5μg/μl/hr) + PD123319 (0.5μg/μl/hr) group, and C21 (0.5μg/μl/hr) + N-omega-Nitro-L-Arginine Methyl Ester (L-NAME, 50μg/μl/hr) group. C21 was gifted from Vicore Pharma AB (Goteborg, Sweden). PD123319 and L-NAME were purchased from Sigma-Aldrich (St. Louis, MO, USA).
After surgery, rat was placed into a metabolism cage to collect urine, and at the same time the AP was recorded. The urine was harvested twice a day: at 8:00 am (night urine) and at 8:00 pm (day urine), and then frozen (−80°C) until it was analyzed for NE.
Urinary NE was measured using a Norepinephrine Enzyme Immunoassay kit (Labor Diagnostika Nord GmbH & Co KG, Germany). A 50 μl urine sample was taken from the urine collected from each rat, and diluted with 950 μl ddH2O to obtain a 20:1 dilution, from which 10 μl was then used for NE measurements following the instructions provided by the company. Each sample was measured in duplicate. NE excretion was determined by multiplying the urinary NE concentration by the urine volume collected over 12 h. The data are expressed as μg/12 h.
MAP was recorded 10 min/h for 24 h/d, using a macro written for Chart (version 5.4.2) software (ADInstruments, Inc).
2. Preparation of brain tissue samples and Western blot analyses
After 7 days of icv infusion rats were euthanized. The brains were removed, frozen on dry ice, blocked in the coronal plane, and sectioned at 100 μm thicknesses in a cryostat. The visual cortex, paraventricular hypothalamic nucleus (PVN), rostroventrolateral medulla (RVLM), and nucleus of the solitary tract (NTS) were punched out based on the technique of Palkovits and Brownstein15. The punch sites for each nucleus in each section was determined based on the description by Paxinos and Watson16. The coordinates of punched brain regions were: PVN 0.9 – 1.9 mm caudal to bregma, 0.0 – 0.6 mm lateral to midline, and 6.8 – 8.2 mm ventral to skull; NTS 13.7 – 14.6 mm caudal to bregma, 0.4 – 1.2 mm lateral to midline, and 0.2 – 0.8 mm from the dorsal surface of the medulla; RVLM 11.8 – 12.7 mm posterior to bregma, 2.0 – 2.6 mm midline to lateral, and 2.8 – 3.6 mm from the dorsal surface of the medulla. Tissue was homogenized in RIPA buffer. Protein extraction from the homogenate was used to analyze neuronal nitric oxide synthase (nNOS) expression by Western blot. The samples were boiled for 5 min following by loading on a 7.5% SDS-PAGE gel (20 μg protein/30 μL per well) for electrophoresis using a Bio-Rad mini gel apparatus at 40 mA/gel for 45 min. The fractionized protein on the gel was transferred onto a PVDF membrane (Millipore) and electrophoresed at 300 mA for 90 min. The membrane was probed with primary antibodies (nNOS rabbit polyclonal antibody, Santa Cruz, 1:1000) and secondary antibody (goat anti-rabbit IgG-HRP, Santa Cruz, 1:2500), and then treated with enhanced chemi-luminescence substrate (Pierce; Rockford, IL) for 5 min at room temperature. The bands in the membrane were visualized and analyzed using UVP BioImaging Systems. After obtaining the nNOS blot density, the membrane was then treated using Restore Western Blot Stipping Buffer (Thermo Scientific) to remove the nNOS signal, followed by probing with GAPDH primary antibodies (GAPDH mouse monoclonal IgG, sc-32233, Santa Cruz Biotechnology Inc, 1:1000) using the same process as the nNOS antibodies to get the GAPDH blot densities. The final reported data are the nNOS band density normalized to GAPDH.
3. Cell Culture and Patch-Clamp Experiments
CATH.a cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA) and grown in RPMI 1640 containing 8% horse serum, 4% fetal bovine serum, 1% penicillin-streptomycin, at 37°C in a humidified atmosphere equilibrated with 5% CO2. After incubated for 3–5 days, cells were subcultured and differentiated with N 6,2′-O-dibutyryladenosine 3′,5′-monophosphate (dbcAMP 1 mmol/L, Sigma). The differentiated CATH.a cells were then used in the following experiments to determine the effect of C21 on delayed rectifier K+ current (IKv).
The IKv was recorded by the whole cell patch-clamp technique. Briefly, differentiated CATH.a cells were bathed in a solution containing (in mmol/L) NaCl 137, KCl 5.4, CaCl2 1.35, MgSO4 2, NaH2PO4 0.3, dextrose 10, HEPES 10, pH 7.4 (NaOH) with CdCl2 (0.3mM) and TTX (1.5μM) added to block Ca2+ and Na+ channels, respectively. The patch pipette microelectrodes were heat-polished before use and had resistances of 3–4 MΩ when filled with an internal pipette solution containing (in mmol/L) KCl 130, CaCl2 0.25, MgCl2 2, ATP 1.0, GTP 0.1, EGTA 5, dextrose 8 and HEPES 10, pH 7.2 (KOH). Cell capacitance was calculated by integrating the area under the uncompensated capacitive transient evoked by a voltage step of 5 mV and dividing this area by the voltage step. All experiments were performed at room temperature (23–24 °C) with an Axopatch 200B amplifier and a Digitdata 1322A interface (Axon Instruments). Cell capacitance was cancelled electronically and the series resistance (<10MΩ) was compensated by 75–80 %. Currents were measured and analyzed by pCLAMP 8.0 software system. Standard recording conditions for IKv were achieved by stepping from a holding potential of −80 to +10 mV for 300 ms. IKv current was measured at 50 ms after the initiation of the test pulse. Current density was expressed as pA/pF.
4. Statistical analyses
All data are reported as the mean ± SEM. A two way ANOVA followed by Student-Newman-Keuls was used for the statistical analysis of NE concentration, NE volume, and MAP during the daytime and nighttime from control and C21 treated rats. A paired t-test was used to statistically analyze the IKv between C21 treatment and its control. A one way ANOVA followed by Student-Newman-Keuls was used for the statistical analysis of all other data. Statistical analysis was done with the aid of the SigmaPlot software. P < 0.05 was considered statistically significant.
Results
Effects of C21 on NE excretion
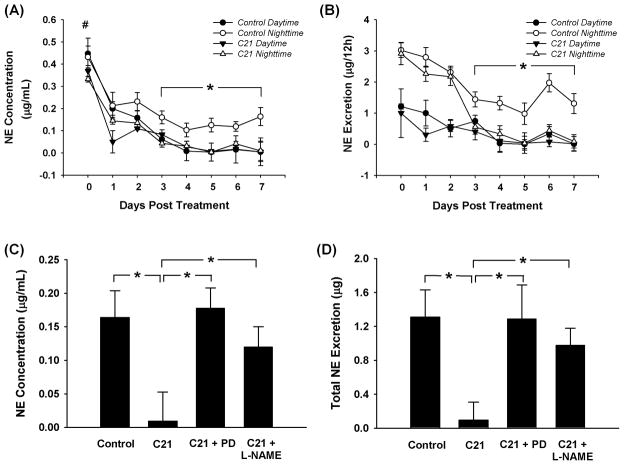
Figure 1 shows the NE concentration (panel A) and NE volume (panel B) in daytime and nighttime urine in control and C21 treated rats. From panel A we can see that all rats exhibited a significantly higher NE concentration in both daytime and nighttime urine at day 0 (surgery day) compared to the following 7 days (P < 0.05), most likely stress-induced. From days 3 to 7, control rats exhibited a significantly higher NE concentration in nighttime urine than that in daytime urine. C21 treated rats however, showed no significant difference in NE concentration between nighttime and daytime urine. In other words, C21 prevented the nocturnal increases in NE excretion. Moreover, NE concentration in nighttime urine of C21-treated rats was lower than that in nighttime urine of control rats, but was the same as that in the daytime urine of control rats. On the other hand, there were no differences in daytime urine NE concentration between these two groups. Panel B shows 12 hour NE volume in control and C21 treated rats. As was the case for NE concentration, 12 hour NE volume showed the same tendency to be reduced during the nighttime hours following C21 infusion.
Figure 1.
Panels A and B show NE concentration and NE volume in daytime and nighttime urine. #P < 0.05 compared with days 1 to 7; *P < 0.05 compared with the daytime urine of control rats and the nighttime urine of C21 treated rats, n = 8 for the control group and 10 for the C21 treated group. Panels C and D show the blockade of AT2R antagonist and NOS blocker on C21-induced effects at the day 7. *P < 0.05. n = 8 for control, 10 for C21, 9 for C21 plus PD123319, and 8 for C21 plus L-NAME.
To explore the potential mechanisms underlying the C21 effect on nighttime NE excretion, we carried out additional experiments. C21 plus PD123319 and C21 plus L-NAME were infused icv in separate groups of rats. Panels C and D in figure 1 show the nighttime NE concentration and volume at the day 7 after treatment. Both PD123319 and L-NAME completely abolished the C21-induced decrease in NE concentration and amount.
Effects of C21 on arterial blood pressure
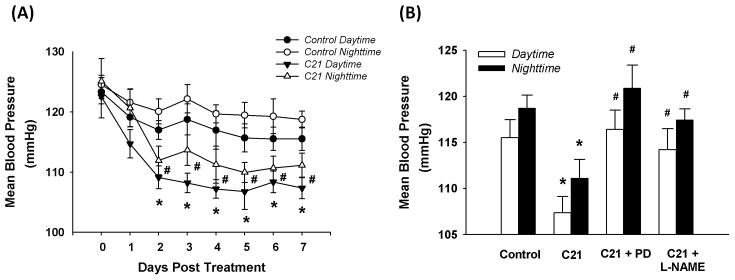
Panel A in Figure 2 shows the daytime and nighttime MAP in control and C21 treated rats. MAP in both groups exhibited a daily rhythmicity, with higher MAP at night and lower MAP during the daytime hours. However, C21 treated rats exhibited a lower MAP during both daytime and nighttime hours than did control rats. Panel B of figure 2 shows the daytime and nighttime MAP at day 7 after treatment for control, C21, C21 plus PD123319, and C21 plus L-NAME groups. Both PD123319 and L-NAME completely abolished the effect of C21 on MAP. In three separate rats, we also observed the effects of PD123319 alone and L-NAME alone on MAP. PD123319 did not alter blood pressure, but L-NAME evoked a slight, but non-significant, increase in blood pressure.
Figure 2.
Daytime and nighttime mean arterial pressure (MAP). Panel A: MAP in control (n = 8) and C21 treated rats (n = 10). *P < 0.05 compared with control daytime; #P < 0.05 compared with control nighttime. Panel B: MAP at the day 7 after treatment of control (n = 8), C21 (n = 10), C21 plus PD123319 (n = 9), and C21 plus L-NAME (n =8). *P < 0.05 compared with control, #P < 0.05 compared with C21.
Effects of C21 on nNOS expression
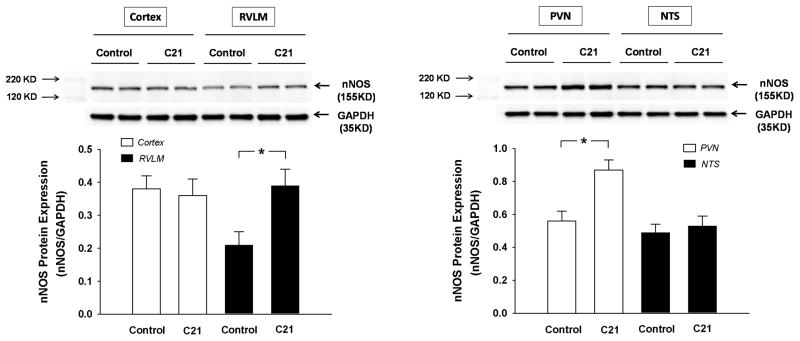
Figure 3 shows nNOS protein expression in the cortex, RVLM, PVN, and NTS from control and C21 treated rats at day 7 post-treatment. There was no difference in nNOS expression in the cortex and NTS between control and C21 treated rats. However, in the RVLM and PVN, C21 treated rats exhibited a significantly higher nNOS protein expression than that in control rats.
Figure 3.
nNOS protein expression in the cortex, RVLM, PVN, and NTS from control (n = 8) and C21 treated rats (n = 10) at the day 7 post treatment. *P < 0.05.
Effect of C21 on neuronal potassium current
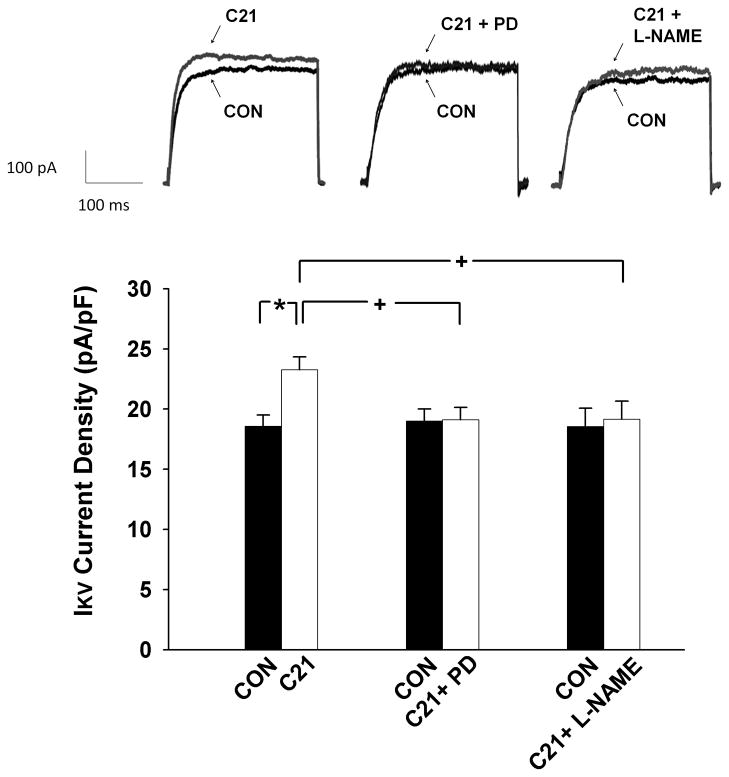
The top panel of figure 4 shows representative current tracings of Ikv and the bottom panel is the group data. CATH.a neurons exhibited a small but significantly higher IKv after C21 treatment compared to control, suggesting that C21 facilitates potassium channel function. In addition, the AT2R antagonist, PD123319, and the NOS blocker, L-NAME, completely abolished the effects of C21 on IKv, demonstrating that the influence of C21 on potassium channel function was mediated by AT2R via the nitric oxide (NO) signaling pathway. PD123319 or L-NAME alone did not alter IKv and washout of C21 completely reversed the effects on IKv.
Figure 4.
IKv in CATH.a cells measured by whole cell patch clamp. Top: representative current tracings; Bottom: mean data of IKv current densities. *P < 0.001 and +P < 0.05. n = 14 for C21, 10 for C21 plus PD123319, and 9 for C21 plus L-NAME treated cells.
Discussion
Brain Ang II exhibits a markedly influence on autonomic function and circulation activity. Activation of central AT1R evokes an increase in sympathetic nerve activity and blood pressure17, but selectively stimulating AT2R in the RVLM evoked an opposite effect to AT1R9. In the current experiment, we demonstrate a negative influence of chronic icv infusion of C21, the first selective non-peptide AT2R agonist, on NE excretion (a surrogate for sympathetic nerve activity) and MAP in normal, conscious rats. We found that C21 treatment significantly decreased nighttime NE excretion and mean arterial blood pressure. We further demonstrated that these inhibitory influences of C21 were completely abolished by PD123319, an AT2R antagonist, and L-NAME, a NOS inhibitor. Finally, we found that icv infusion of C21 significantly up regulated nNOS protein expression in the PVN and RVLM. These data strongly suggest a central suppressive effect of C21 on sympathetic drive and blood pressure mediated by AT2R via a nNOS/NO pathway. In addition, we also observed the influence of C21 on potassium current in a neuronal cell line, the CATH.a, and found that C21 facilitates the neuronal potassium current, suggesting an inhibitory effect of C21 on neuronal excitability.
C21 is a newly created orally effective non-peptide AT2R agonist, which permits selective stimulation of the AT2R under many experimental conditions12. Previous experiments have demonstrated beneficial effects of this compound in hypertension or heart failure models. In spontaneously hypertensive rat (SHR), intravenous treatment of C21 significantly decreased MAP12. On the other hand, intraperitoneal injection of C21 led to a pronounced improvement of systolic and diastolic cardiac function coinciding with a smaller scar volume in rats with permanent coronary ligation, probably via an anti-apoptotic mechanism13. It has recently been demonstrated that C21 evokes vasorelaxation in mouse and SHR aorta and in normotensive rat mesenteric arteries14, providing further evidence at the organ level for the above-mentioned C21-induced hypotension. The data presented in the current study is the first, to our knowledge, demonstrating a central effect of C21 on sympathetic outflow and MAP. As expected, we further found that the C21-evoked decrease in NE excretion and MAP were abolished by PD123319, demonstrating that the AT2R was the exclusive mediator of the C21 effects. It is not clear why central C21-treatment only suppressed nightime NE excretion, but exhibited no effects on diurnal NE excretion, in normal rats. As a nocturnal rodent, rats are characterized with a higher locomotor activity and sympathetic tone at nighttime than that during the daytime18. We therefore assumed that the lowered neuronal activity during the daytime may not be further suppressed by C21. However, Takekoshi et al.19 demonstrated that activation of the AT2R can reduce tyrosine hydroxylase activity and thus NE biosynthesis, providing biochemical evidence for our findings.
Patch-clamp and extracellular single unit discharge data have demonstrated that stimulation of AT2R facilitated neuronal potassium current and decreased neuronal spontaneous discharge. Kang et al20 reported that activation of AT2R increased neuronal IKv in cultured neurons from newborn rat hypothalamus and brainstem. They further indicated that the third intracellular loop of the AT2R is a key component in the stimulation of neuronal IKv elicited by activation of this receptor21. Martens et al22 documented that activation of AT2R by CGP42112 modulates rat hypothalamus and brainstem neuronal whole-cell K+ current by increasing their open probability. Matsuura et al.23, on the other hand, demonstrated an AT2R-mediated hyperpolarization and decrease in firing rate in bulbospinal RVLM neurons. These data imply that activation of AT2R facilitates potassium channel current and therefore suppresses neuronal excitability. Indeed, in the current experiment, we found that C21 significantly increased the neuronal potassium current in cultured neurons, which was completely abolished by AT2R antagonist. These data further imply that the facilitatory influence of C21 on neuronal potassium channels may be underlying the central effects of C21 on NE excretion and arterial pressure observed in the current study.
Matsubara24 and Noute25 have summarized at least three major transduction mechanisms responsible for AT2R-mediated intracellular signaling: (1) regulation of the nitric oxide-guanosine 3′, 5′ –cyclic monophosphate (NO-cGMP) system; (2) stimulation of PLA2 with subsequent release of arachidonic acid; and (3) activation of various protein phosphatases causing protein dephosphorylation. Among these three pathways, the NO-cGMP pathway is mostly expected to be potentially involved in the about observed effects of AT2R on sympathetic outflow. An AT2R coupled increase in NO generation has been found in neuronal cell lines PC12W26 and NG-108-1527, 28. On the other hand, it has been shown that NO regulates several types of K+ channels, including ATP-dependent K+ channels and Ca2+-activated K+ channels29. Han et al.30 recently reported that, in mouse neocortical neurons, low concentrations of NO donor SNAP or an NO solution enhanced whole cell delayed rectifier K+-current (Ik) and left the fast inactivating A (IA) current unchanged. In cell-attached experiments, a significant increase in channel open probability was observed when using low concentrations of SNAP or NO. The increase in channel activities by low concentrations of SNAP was abolished in the presence of either inhibitors of soluble guanylate cyclase or inhibitors of cGMP-dependent protein kinase G, suggesting a link to the NO-cGMP signaling cascade. These results suggest that AT2R-induced activation of NO/cGMP might be responsible for the AT2R-evoked neuronal potassium current alteration. Indeed, in the current experiment, we found that the L-NAME, a NOS blocker, completely abolished the C21 evoked inhibition of NE excretion and hypotension in the conscious rats and facilitated potassium current in a neuronal cell line.
In order to determine which brain region(s) are involved in the C21 effects observed in this study, we measured nNOS protein expressions in three sympathetically relevant brain nuclei and in the cortex as a control area. We found that, nNOS expressions in the PVN and RVLM, but not in the NTS or cortex, of C21 treated rats were significantly higher than that in control rats. These data demonstrated that C21 up regulated nNOS protein expression, suggesting that activation of the NO pathway in PVN and RVLM is involved in icv treatment of C21 induced suppression of NE excretion and blood pressure.
In summary, we found that directly central administration of C21 significantly decreases the nighttime NE excretion and blood pressure in conscious normal rats, and these effects were completely abolished by an AT2R blocker and NOS inhibitor. In the current study, we further demonstrated that C21 increases potassium current in a neuronal cell line. These results suggest a central inhibitory influence of C21 on sympathetic outflow exclusively through AT2R via a NO signaling pathway.
Acknowledgments
We would like to acknowledge the expert technical assistance of Li Yu, Phyllis Anding, Johnnie F. Hackley, and Pamela Curry. We also thank Drs. Mingqi Zheng and George J. Rozanski for their help with the patch clamp experiments. The authors appreciate critical suggestions for these experiments and valuable revision of the manuscript by Dr. Irving H. Zucker. The authors thank Vicore Pharma AB for the generous donation of Compound 21.
Sources of Funding
This study was supported by a Scientist Development Grant from the American Heart Association 0635007N and NIH grants RO1HL093028 and P30HL101296.
Footnotes
Disclosure: None
References
- 1.Kaschina E, Unger T. Angiotensin AT1/AT2 receptors: regulation, signalling and function. Blood Press. 2003;12:70–88. doi: 10.1080/08037050310001057. [DOI] [PubMed] [Google Scholar]
- 2.de GM, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- 3.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 4.Touyz RM, Berry C. Recent advances in angiotensin II signaling. Braz J Med Biol Res. 2002;35:1001–1015. doi: 10.1590/s0100-879x2002000900001. [DOI] [PubMed] [Google Scholar]
- 5.Sumners C, Gelband CH. Neuronal ion channel signalling pathways: modulation by angiotensin II. Cell Signal. 1998;10:303–311. doi: 10.1016/s0898-6568(97)00133-2. [DOI] [PubMed] [Google Scholar]
- 6.Gelband CH, Sumners C, Lu D, Raizada MK. Angiotensin receptors and norepinephrine neuromodulation: implications of functional coupling. Regul Pept. 1997;72:139–145. doi: 10.1016/s0167-0115(97)01050-1. [DOI] [PubMed] [Google Scholar]
- 7.Siragy HM, Inagami T, Ichiki T, Carey RM. Sustained hypersensitivity to angiotensin II and its mechanism in mice lacking the subtype-2 (AT2) angiotensin receptor. Proc Natl Acad Sci U S A. 1999;96:6506–6510. doi: 10.1073/pnas.96.11.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z, Iwai M, Wu L, Shiuchi T, Jinno T, Cui TX, Horiuchi M. Role of AT2 receptor in the brain in regulation of blood pressure and water intake. Am J Physiol Heart Circ Physiol. 2003;284:H116–H121. doi: 10.1152/ajpheart.00515.2002. [DOI] [PubMed] [Google Scholar]
- 9.Gao L, Wang WZ, Wang W, Zucker IH. Imbalance of angiotensin type 1 receptor and angiotensin II type 2 receptor in the rostral ventrolateral medulla: potential mechanism for sympathetic overactivity in heart failure. Hypertension. 2008;52:708–714. doi: 10.1161/HYPERTENSIONAHA.108.116228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao L, Wang W, Wang W, Li H, Sumners C, Zucker IH. Effects of angiotensin type 2 receptor overexpression in the rostral ventrolateral medulla on blood pressure and urine excretion in normal rats. Hypertension. 2008;51:521–527. doi: 10.1161/HYPERTENSIONAHA.107.101717. [DOI] [PubMed] [Google Scholar]
- 11.Yu L, Zheng M, Wang W, Rozanski GJ, Zucker IH, Gao L. Developmental Changes in AT1 and AT2 Receptor Protein Expression in Rats. Journal of the Renin-Angiotensin-Aldosterone System. 2010;11:214–221. doi: 10.1177/1470320310379065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wan Y, Wallinder C, Plouffe B, Beaudry H, Mahalingam AK, Wu X, Johansson B, Holm M, Botoros M, Karlen A, Pettersson A, Nyberg F, Fandriks L, Gallo-Payet N, Hallberg A, Alterman M. Design, synthesis, and biological evaluation of the first selective nonpeptide AT2 receptor agonist. J Med Chem. 2004;47:5995–6008. doi: 10.1021/jm049715t. [DOI] [PubMed] [Google Scholar]
- 13.Kaschina E, Grzesiak A, Li J, Foryst-Ludwig A, Timm M, Rompe F, Sommerfeld M, Kemnitz UR, Curato C, Namsolleck P, Tschope C, Hallberg A, Alterman M, Hucko T, Paetsch I, Dietrich T, Schnackenburg B, Graf K, Dahlof B, Kintscher U, Unger T, Steckelings UM. Angiotensin II type 2 receptor stimulation: a novel option of therapeutic interference with the renin-angiotensin system in myocardial infarction? Circulation. 2008;118:2523–2532. doi: 10.1161/CIRCULATIONAHA.108.784868. [DOI] [PubMed] [Google Scholar]
- 14.Bosnyak S, Welungoda I, Hallberg A, Alterman M, Widdop R, Jones E. Stimulation of angiotensin AT(2) receptors by the non-peptide agonist, Compound 21, evokes vasodepressor effects in conscious spontaneously hypertensive rats. Br J Pharmacol. 2010;159:709–716. doi: 10.1111/j.1476-5381.2009.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palkovits M, Brownstein MJ. Maps and Guide to Microdissection of the Rat Btrain. Elsevier Science Publishing Co., Inc; 52 Vanderbilt Avenue, New York, New York 10017: 1988. [Google Scholar]
- 16.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 3. Academic Press, Inc; 525 B Street, Suite 1900, San Diego, California 92101–4495: 1997. [Google Scholar]
- 17.Lu N, Helwig BG, Fels RJ, Parimi S, Kenney MJ. Central Tempol alters basal sympathetic nerve discharge and attenuates sympathetic excitation to central ANG II. Am J Physiol Heart Circ Physiol. 2004;287:H2626–H2633. doi: 10.1152/ajpheart.00030.2004. [DOI] [PubMed] [Google Scholar]
- 18.Bowers CW, Baldwin C, Zigmond RE. Sympathetic reinnervation of the pineal gland after postganglionic nerve lesion does not restore normal pineal function. J Neurosci. 1984;4:2010–2015. doi: 10.1523/JNEUROSCI.04-08-02010.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takekoshi K, Ishii K, Isobe K, Nanmoku T, Kawakami Y, Nakai T. Angiotensin-II subtype 2 receptor agonist (CGP-42112) inhibits catecholamine biosynthesis in cultured porcine adrenal medullary chromaffin cells. Biochem Biophys Res Commun. 2000;272:544–550. doi: 10.1006/bbrc.2000.2821. [DOI] [PubMed] [Google Scholar]
- 20.Kang J, Sumners C, Posner P. Angiotensin II type 2 receptor-modulated changes in potassium currents in cultured neurons. Am J Physiol. 1993;265:C607–C616. doi: 10.1152/ajpcell.1993.265.3.C607. [DOI] [PubMed] [Google Scholar]
- 21.Kang J, Richards EM, Posner P, Sumners C. Modulation of the delayed rectifier K+ current in neurons by an angiotensin II type 2 receptor fragment. Am J Physiol. 1995;268:C278–C282. doi: 10.1152/ajpcell.1995.268.1.C278. [DOI] [PubMed] [Google Scholar]
- 22.Martens JR, Wang D, Sumners C, Posner P, Gelband CH. Angiotensin II type 2 receptor-mediated regulation of rat neuronal K+ channels. Circ Res. 1996;79:302–309. doi: 10.1161/01.res.79.2.302. [DOI] [PubMed] [Google Scholar]
- 23.Matsuura T, Kumagai H, Onimaru H, Kawai A, Iigaya K, Onami T, Sakata K, Oshima N, Sugaya T, Saruta T. Electrophysiological properties of rostral ventrolateral medulla neurons in angiotensin II 1a receptor knockout mice. Hypertension. 2005;46:349–354. doi: 10.1161/01.HYP.0000173421.97463.ac. [DOI] [PubMed] [Google Scholar]
- 24.Matsubara H. Pathophysiological role of angiotensin II type 2 receptor in cardiovascular and renal diseases. Circ Res. 1998;83:1182–1191. doi: 10.1161/01.res.83.12.1182. [DOI] [PubMed] [Google Scholar]
- 25.Nouet S, Nahmias C. Signal transduction from the angiotensin II AT2 receptor. Trends Endocrinol Metab. 2000;11:1–6. doi: 10.1016/s1043-2760(99)00205-2. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y, Biermann T, Luther C, Unger T, Culman J, Gohlke P. Contribution of bradykinin and nitric oxide to AT2 receptor-mediated differentiation in PC12 W cells. J Neurochem. 2003;85:759–767. doi: 10.1046/j.1471-4159.2003.01719.x. [DOI] [PubMed] [Google Scholar]
- 27.Gendron L, Oligny JF, Payet MD, Gallo-Payet N. Cyclic AMP-independent involvement of Rap1/B-Raf in the angiotensin II AT2 receptor signaling pathway in NG108-15 cells. J Biol Chem. 2003;278:3606–3614. doi: 10.1074/jbc.M202446200. [DOI] [PubMed] [Google Scholar]
- 28.Cote F, Laflamme L, Payet MD, Gallo-Payet N. Nitric oxide, a new second messenger involved in the action of angiotensin II on neuronal differentiation of NG108-15 cells. Endocr Res. 1998;24:403–407. doi: 10.3109/07435809809032622. [DOI] [PubMed] [Google Scholar]
- 29.Prast H, Philippu A. Nitric oxide as modulator of neuronal function. Prog Neurobiol. 2001;64:51–68. doi: 10.1016/s0301-0082(00)00044-7. [DOI] [PubMed] [Google Scholar]
- 30.Han NL, Ye JS, Yu AC, Sheu FS. Differential mechanisms underlying the modulation of delayed-rectifier K+ channel in mouse neocortical neurons by nitric oxide. J Neurophysiol. 2006;95:2167–2178. doi: 10.1152/jn.01185.2004. [DOI] [PubMed] [Google Scholar]