Abstract
Signs of Parkinson’s disease (PD) are augmented by speech and repetitive motor tasks. The neurophysiological basis for this phenomenon is unknown, but may involve augmentation of β (13–30Hz) oscillations within the subthalamic nucleus (STN). We hypothesized that speech and motor tasks increase β power in STN, and propose a mechanism for clinical observations of worsening motor state during such behaviors. Subjects undergoing DBS surgery performed tasks while STN local field potential (LFP) data were collected. Power in the β frequency range was analyzed across the entire recording to observe slow shifts related to block design, and during time epochs synchronized to behavior to evaluate immediate fluctuations related to task execution. Bilaterally symmetric β event related desynchronization was observed in analysis time-locked to subject motor and speech tasks. We also observed slow shifts of β power associated with blocks of tasks. Repetitive combined speech and motor, and isolated motor blocks were associated with the highest bilateral β power state. Overt speech alone and imagined speech were associated with a low bilateral β power state. Thus, changing behavioral tasks is associated with bilateral switching of β power states. This offers a potential neurophysiologic correlate of worsened PD motor signs experienced during clinical examination with provocative tasks: switching into a high β power state may be responsible for worsening motor states in PD patients when performing unilateral repetitive motor tasks and combined speech and motor tasks. Beta state changes could be chronically measured and potentially used to control closed loop neuromodulatory devices in the future.
Keywords: Subthalamic Nucleus, Parkinson’s Disease, Deep Brain Stimulation, Local Field Potentials, Oscillations, Speech
Parkinson’s disease (PD) is a neurodegenerative disorder with motor signs of tremor, rigidity and bradykinesia that are augmented by speech tasks and alternate limb motor tasks (Levy et al., 2000, Powell et al., 2011). The neurophysiologic basis for this phenomenon is unknown, but may involve interaction of distinct processing circuits of the basal ganglia and cortex.
The basal ganglia, and the subthalamic nucleus (STN) in particular, is involved in generating meaningful speech. Reading tasks modulate STN unit activity prior to voice onset, and in a pattern that is syllable specific during speech (Watson and Montgomery, 2006). Further, pre- and post-operative neuropsychological assessment reveals mild impairment of verbal fluency scores with STN deep brain stimulation (DBS) (Witt et al., 2008, Follett et al., 2010). The STN may be subdivided into sensorimotor, associative and limbic regions (Temel et al., 2005), but it is unclear if speech function resides fully within sensorimotor or partly in an adjacent functional zone.
Local field potential (LFP) recordings have been used in humans to characterize activity within cortical regions and subcortical nuclei (Engel et al., 2005). LFPs represent coherent activity of small cell assemblies on the ~300µm scale, based on cortical studies using 2-dimensional microarrays rather than macroelectrodes that are typically used for LFP measurement (Katzner et al., 2009). Time-frequency analysis of motor cortex electrocorticography (ECoG) (Miller et al., 2009, Miller et al., 2010) and STN LFPs (Cassidy et al., 2002, Levy et al., 2002, Amirnovin et al., 2004, Kempf et al., 2007) have revealed suppression of β (13–30Hz) frequency spectral power during motor tasks. Thus, β oscillations have been coined “anti-kinetic” as they are synchronized in the rest state and desynchronized with activity (Crone et al., 1998, Pfurtscheller and Lopes da Silva, 1999, Kühn et al., 2004, Foffani et al., 2005). While several groups have characterized the motor-related modulation of STN β power (Cassidy et al., 2002, Amirnovin et al., 2004, Alegre et al., 2005, Devos et al., 2006, Kempf et al., 2007), and others have characterized β change with speech production and audition in cortex (Crone et al., 2001, Edwards et al., 2009) there is limited information on LFP modulation with speech in STN.
To investigate speech and motor task modulation of STN LFPs, we conducted a behavioral paradigm during continuous recording of STN LFPs in patients undergoing DBS for PD. Due to the constraint of the intraoperative environment, the paradigm was intentionally designed with basic tasks to facilitate reliable subject performance, and robust, simple, analysis.
We hypothesized that both speech and motor tasks would independently induce β modulation in the STN. This hypothesis is based on clinical observations that both speech tasks, such as counting and reciting the months of the year, and motor tasks, such as finger tapping, worsen the motor signs of PD. Thus, our experimental paradigm was modeled after these clinical examination tools: speech tasks of reciting the months of the year and counting, and a thumb-press task to model finger tapping. We speculated that these behaviors separately or combined would augment β power in the STN. In particular, we examined both sustained overall “state changes” in β modulation over entire blocks of behavior, and also trial-by-trial, transient, suppression in β power (event-related desynchronization, ERD). In our analysis of β spectral power, we discovered that β modulation occurs on different time scales. Whereas each speech or motor action is associated with transient β suppression, there is a slower, sustained, upward shift of β power during a repetitive motor task that may explain worsening of contralateral PD signs during the behavior.
Methods
SUBJECTS
Seven subjects (Table 1) undergoing DBS as standard of care for treatment of idiopathic PD were enrolled in this study. All subjects provided informed consent for participation in this research study, in a manner approved by the internal review board of the University of Washington. Eleven independent recordings/lateralities were measured from the seven participants. Two subjects underwent sequential recordings from each side, another two had simultaneous bilateral recording, and another two subjects were recorded with a second electrode design and amplifier system in the same hemisphere. There were 7 left, 2 right, and 2 simultaneous bilateral recordings (9 left and 4 right), for a total of 13 recordings for analysis (Table 2).
Table 1.
Subject Characteristics. PD: Parkinson’s Disease, H&Y: Hoehn and Yahr scale, UPDRS: Unified Parkinson’s Disease Rating Scale Motor (III) score off medication.
| Subject | Age | Gender | Diagnosis | Handedness | H&Y | UPDRS- III (Off) |
|---|---|---|---|---|---|---|
| 1 | 58 | M | PD | L | 3 | 42 |
| 2 | 47 | M | PD | R | 2 | 37 |
| 3 | 54 | F | PD | R | 2 | 27 |
| 4 | 53 | M | PD | R | 2 | 28 |
| 5 | 47 | F | PD | R | 2 | 35 |
| 6 | 63 | M | PD | L | 2 | 36 |
| 7 | 51 | F | PD | R | 2 | 33 |
Table 2.
Research recordings. Fs: sampling frequency, pME: paired microelectrode recording, DBS: deep brain stimulation lead recording.
| Recording | Subject | Side | Design | Fs (Hz) | Band Pass (Hz) |
Total Speech Trials |
Total Motor Trials |
|---|---|---|---|---|---|---|---|
| 1 | 1 | Lt | pME | 4000 | 5–300 | 111 | 58 |
| 2 | 1 | Rt | pME | 4000 | 5–300 | 107 | 55 |
| 3 | 2 | Lt | pME | 4000 | 5–300 | 111 | 60 |
| 4 | 2 | Rt | pME | 4000 | 5–300 | 81 | 56 |
| 5 | 3 | Lt | pME | 4000 | 5–300 | 107 | 59 |
| 6 | 3 | Lt | DBS | 5000 | 1–1000 | 58 | 29 |
| 7 | 4 | Lt | DBS | 5000 | 1–1000 | 118 | 58 |
| 8 | 5 | Lt | pME | 4000 | 5–300 | 53 | 22 |
| 9 | 5 | Lt | DBS | 5000 | 1–1000 | 117 | 53 |
| 10 | 6 | Bi | DBS | 5000 | 1–1000 | 54 | 59 |
| 11 | 7 | Bi | DBS | 5000 | 1–1000 | 75 | 60 |
DBS SURGERY AND RECORDING DESIGN
Subjects underwent DBS surgery per clinical routine. All subjects were in the off medication state. Surgery was performed with a Leksell (Elekta, Sweden) stereotactic head frame and Medtronic (Minneapolis, MN) Framelink targeting software. Targeting of the dorsolateral STN was based on a combination of formula-based and indirect coordinates. We used an a priori, formula-based target of [x,y,z]: [±12mm, −3mm, −4mm] with respect to the mid-commissural point and AC-PC plane. Targeting was then adjusted based on indirect targeting from the borders of the red nucleus (RN) for [x,y,z]: [3mm lateral to RN border, at the anterior border of RN, 2mm inferior to superior border of RN]. The prescribed sagittal trajectory angle was 60 degrees from the AC-PC plane, and the coronal angle was 15 degrees from a parasagittal plane, with minor adjustments for cortical sulci. Microelectrodes were positioned in the center and posterior positions of a BenGun trajectory guide, with a parallel separation of 2mm. LFP recording was carried out using paired microelectrodes or the DBS lead, or both sequentially (Figure 1, 2). For the paired microelectrode (pME) design, we recorded from the macro ring electrodes (reference contact, Figure 1) of a pair of dual-channel microelectrodes (Alpha Omega, Israel). The macro ring electrode is stainless steel, has a surface area of 2.2mm2 and impedance of 3.2kΩ (mean; 95% CI = 1.7 – 4.8kΩ). As the macroelectrode of the dual-channel microelectrode lags behind the micro tip by 3mm, the clinical microelectrode recording (MER) was paused at the point where the microtip was advanced 3mm beyond the region of first-encountered proprioceptive STN neurons, as defined by modulation of firing frequencies with passive joint movement.
Figure 1.
Schematic representation of recording electrodes used for LFP recordings. A: Medtronic 3389 DBS lead (reprinted with the permission of Medtronic, Inc © 2008). B: Alpha Omega Neuroprobe microelectrode. Recordings were obtained from the labeled reference contact (reprinted with the permission of Alpha Omega Co. USA © 2011).
Figure 2.
Collage demonstrating LFP spectral power changes seen for all overt speech tasks (with and without button press are combined). Upper panel, from recording 1 (see table 2) with paired mER electrode design. A: Power spectral density (PSD) plot for entire recording (All), Speech (1000ms epoch), and pre-Speech baseline (1000ms epoch). Note the depression of PSD in the β band with speech. Also, β power is augmented in the immediate pre-speech period (baseline) relative to the entire recording session. B: Frequency-time representation of PSD for speech onset (left panel, time 0) and speech offset (right panel, time 0). Again, note depression of β band power with speech. C: Averaged β band power over speech trials, synchronized to speech onset (time 0, left panel) and speech offset (time 0, right panel), overlaid on averaged audio track (grey). Red emphasis indicated region that was significant on bootstrap permutation test (BS*). D: Atlas representation demonstrating paired mER recording electrodes (red) in dorsal STN (yellow). Sagittal atlas, lateral 11mm, adopted from Fig 4.31 in (Morel, 2007) with permission. Lower panel, from recording 10 with DBS lead design. E: Similar PSD plot demonstrating β power depression with speech and augmentation of β in the pre-speech baseline period. F: Frequency-Time representation. G: β band power with speech onset and offset. H: Atlas representation demonstrating DBS lead design. Sagittal atlas, lateral 10mm, adopted from Fig 4.30 in (Morel, 2007) with permission. For B, C, F, G, data was baselined to adjust the mean power in 1000ms period before the behavioral cue to zero. For B, F, data was Z-scored and baselined to adjust the mean power in 1000ms period before the behavioral cue to zero; blue and red represent negative and positive relative change, respectively, to baseline.
Moderate propofol anesthesia was used without a protected airway only during placement of the burr hole. MER was performed from 25mm above to approximately 5mm below target. Whereas subjects may have had residual effects from propofol during the initial MER though thalamic nuclei, we did not proceed with recording in the STN region until patients were fully awake and conversant. All research recordings where obtained after clinician testing for proprioceptive modulation of firing rates had begun.
After optical isolation and amplification (MicroGuide, AlphaOmega, Nazareth, Israel), the signals were digitized (4kHz) and combined with event markers and subject response signals (PowerLab, ADInstruments, New South Wales, Australia). The MER guide tube was used as common reference, and the LFP channels were bipolar re-referenced prior to analysis.
For the DBS lead recording design, we recorded from each of the 4 contacts of the DBS lead Medtronic 3389, Minneapolis, MN, see Figure 1). Although primarily designed for stimulation, these electrodes have been used for LFP recording in humans as it does not require modification of standard surgical practice, for example, see (Lopez-Azcarate et al., 2010). The DBS lead contact is platinum/iridium, has a surface area of 6.0mm2 and impedance of 1.7kΩ (mean; 95% CI = 1.1 – 2.4kΩ). Signals were amplified, digitized (5kHz), and combined with event markers and subject response signals (SynAMPS2, Neuroscan, Victoria, Australia). A linked-mastoid common reference was used for recording, and the LFP channels were subsequently bipolar re-referenced (0–1, 1–2, 2–3) prior to analysis.
BEHAVIOURAL STUDY
Behaviours included motor, speech, and combination tasks. The motor task block consisted of 15 cued repetitions of a button press using either the ipsilateral or contralateral thumb. There were 4 variants of a speech initiation task: naming the months of the year, counting upwards from 1, naming the months of the year with a simultaneous button press marking the first month, counting with a simultaneous button press marking the first number. To determine if there was an effect with “silent speech,” two subjects repeated the naming tasks, but only with mental rehearsal of speech. Speech tasks were also completed in a block of 15 repetitions. Upon completion of a full set of speech and motor blocks, the entire paradigm was repeated as a second set of blocks in identical order. For task initiation and completion, subjects received an audio cue from a presentation laptop computer running E-Prime 2.0 (Psychology Software Tools, Sharpsburg, PA). A random time factor was programmed into task length to reduce any effect of anticipation.
ANALYSIS
Time series data of subject response channels were reviewed to mark motor onset and speech onset and offset times for overt tasks. In all recordings, voice was recorded using a microphone, and motor movement was recorded using a digital channel input from a button press. Forearm EMG was recorded in the DBS recording design only. LFP data was re-referenced using adjacent electrode bipolar montage. All data were downsampled to 2kHz prior to spectral analysis using linear interpolation. Subject response channels, such as EMG and audio, were also downsampled to allow creation of event-synchronized matrices for visualization (Figures 2–5).
Figure 5.
Comparison of β suppression with speech alone versus speech plus button press across recordings. A: For recording 8, β depression with speech alone (orange) and speech plus button press (blue). Overlaid on averaged audio channel data. This composite is formed from appending speech onset and offset matrices (see text and Figure 2). Dotted horizontal red and blue lines indicate the average β power for the final 3000ms of speech, chosen to prevent direct influence of button press-related β rebound on the mean value. Data was baselined to adjust the mean power in 1000ms period before the behavioral cue to zero. B: Mean β suppression for last 3000ms of speech across all recordings and for all recordings combined. For this analysis, the entire length of the recording was z-scored (within-subject) using the overall mean and standard deviation of the log transformed β power. Group difference is significant (−2.8 versus −2.1 log units, p=0.02, 2-tailed t-test). C: Mean β suppression for last 3000ms of speech across all recordings combined. For this analysis, the recording was z-scored within each block of tasks (set of 15 repetitive tasks). This within-subject/within-task normalization reduced the effect size of the added button press on β suppression in the final 3000ms of speech (−3.1 versus −2.9 log units, p=0.60, 2-tailed t-test).
Time-dependent power spectral density estimates were calculated using both wavelet analysis and a fixed-window technique using Welch’s method (Matlab, Mathworks, Natick, MA). Wavelet analysis utilized complex Morlet wavelets and operated on time-x-trial event matrices based on subject response time markers. Wavelet analysis results were used in this study to create illustrations (Figures 2–4), but were not used for statistical analyses. The power spectral density over the 13–30Hz frequency band was calculated using Welch’s method for the entire experimental session (approximately 30 minutes) in fixed time windows of 512ms masked with a Hann window, stepping forward by 20ms increments. These data were interpolated to create a β power vector with an apparent sampling frequency of 2kHz. Dual behavior and baseline power spectral plots were calculated using Welch’s method applied to power of 2 length concatenated behavior and pre-cue segments, respectively, windowed with a Hann window segment of equal or lesser (by a factor of 2) length. The length of the Hann window was constructed to be greater than twice the wavelength of a 10Hz oscillation at the native sampling frequency of the record.
Figure 4.
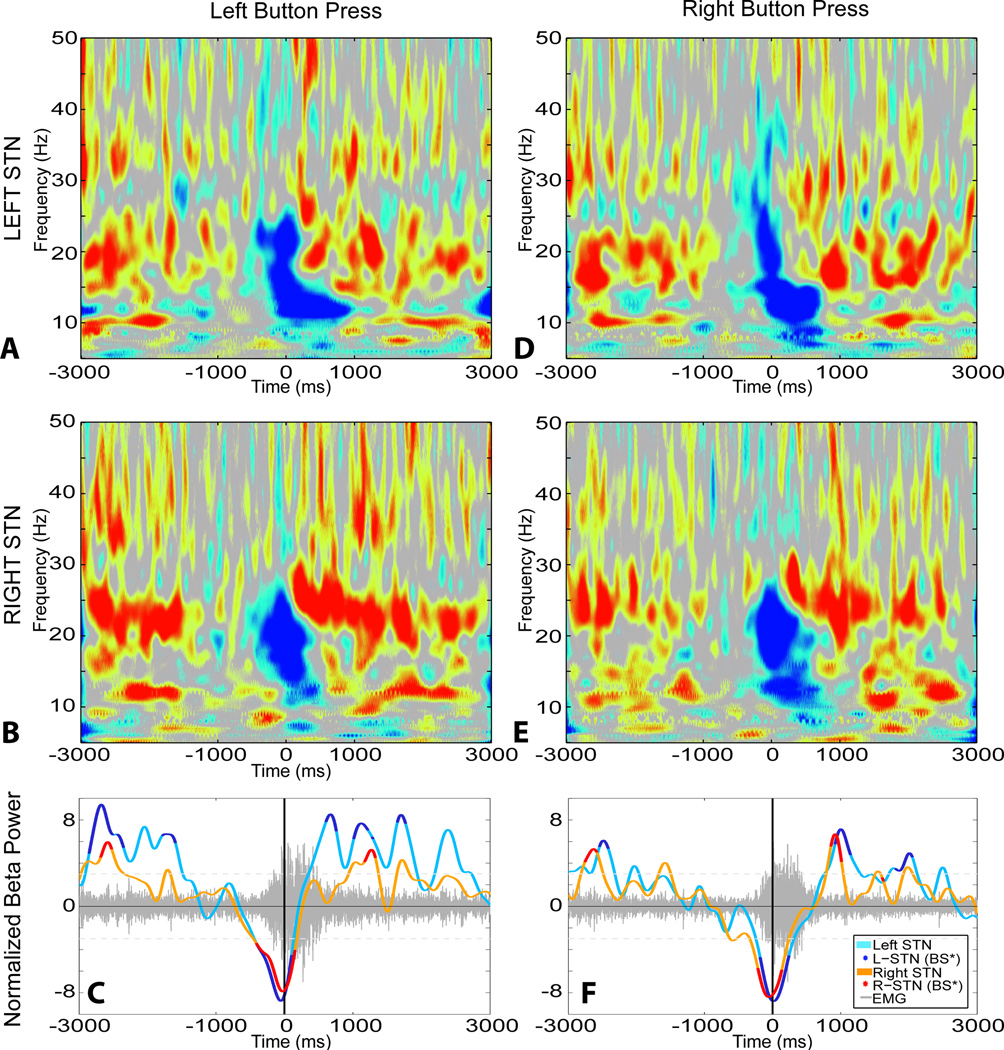
Power spectral density (PSD) modulation with button press. A, B: Frequency-Time representation of STN LFP recorded synchronously for left button press. C: β power modulation from left and right STN overlaid on average EMG activity (grey). D, E: Frequency-Time representation of STN LFP recorded synchronously for right button press. F: β power modulation from left and right STN overlaid on average EMG activity (grey). Note the bilateral symmetry in LFP modulation for left and right button press in left and right STN. Data from recording 10. A, B, C were recorded synchronously, and D, E, F were recorded synchronously. Data was baselined to adjust the mean power in 1000ms period before the behavioral cue to zero. For A, B, D, E, blue and red represent negative and positive relative change, respectively, to baseline.
For overt speech trials, we utilized the recorded audio channel to mark both onset and offset of speech for each trial. This allowed creation of a [trial X time] speech onset matrix of β power with time epoch range [−3000ms, +3000ms], with speech onset at time zero, and a similar speech offset matrix with time epoch range [−3000ms, +3000ms] with speech offset at time zero. These two matrices were concatenated to create a single large [trial X time] matrix with time epoch range of [−3000ms, +9000ms], with speech onset at time zero and speech offset at time +6000ms. Actual duration of speech ranged from 4500 to 6500ms for each trial due to the random variation of trial length. Therefore, the matrix near +3000ms either contained duplication of data, or up to 500ms of data was excluded, depending on the exact duration of speech for the particular trial. In practice, speech onset lagged the cue by greater than 500ms, so no data were excluded from the concatenated matrices. This concatenation approach was a convenient tool for both quantitative comparisons across trials and visual presentation of the data.
As the time-course of β power vector was observed to be log-normal distributed, the β power data was log transformed prior to normalization. In the process of analysis, we observed that comparison of trial-by-trial, transient, relative suppression of β power across task conditions might be confounded by “state changes” in β power. That is, the degree of transient β-suppression appeared different if the β time-course was normalized (by z-score) with respect to the entire experimental run, or the 15 trials of the same type within each block. Two methods of within-subject z-score were applied independently for comparison. First, the entire length of the recording was z-scored using the overall mean and standard deviation of the log transformed β power. Alternatively, the recording was z-scored within each block of tasks (set of 15 repetitive behaviors). For creation of time-by-trial event matrices, task baseline was defined as 1000ms of data prior to the subject’s cue.
To visualize slow shifts of the β spectral power across blocks of tasks, the β power vector created using Welch’s method was low-pass filtered using a Butterworth IIR filter design. Two levels of filtering were performed as illustrated in Figure 6. The state-related, sustained, shifts of β spectral power are illustrated using the β power time series low-pass filtered with cutoff frequency of 0.04Hz. However, to quantify power within each task state, the unfiltered Z-z-scored log β power was averaged for each cued task repetition, in order to avoid any filter effects. To facilitate comparison across subjects, β power for each task was divided by the mean β power for the entire record. Behavioral categories included motor task alone, speech with a motor task, speech alone, silent speech with a motor task, and silent speech alone.
Figure 6.
Slow β state changes across blocks of tasks for 30-minute recordings in simultaneous right and left STN recordings for subject 6. These results demonstrate that β power is correlated across bilateral STN and varies with the type of behavioral task. Task blocks of 15 repetitive tasks are color coded. A, B: Plot of smoothed time vector of instantaneous β power (Black, low-pass cutoff frequency 0.04Hz) for left and right STN recordings. The highlighted region is expanded in Figure 7. C: Bar graph representation of average β power within each task group for two subjects with bilateral simultaneous STN recording and silent speech trials (subjects 6 and 7). Average β power for entire task period (cue-to-cue) as well as specifically for pre-cue baseline period (1000ms) are presented. D: Bar graph representation of β power for all subjects for common tasks. The unilateral repetitive motor task produced the greatest β state in bilateral STN. Both the overt speech alone and the silent speech alone tasks yield low β states. Combined tasks created an intermediate β state. There is general agreement between β power in the entire task period (cue-to-cue) and for the pre-cue baseline period with the exception of the combined speech + motor task, which has a high pre-cue baseline average β power but intermediate power for entire task period. ** denotes statistical significant difference from all other tasks (p<0.001 one-way ANOVA between conditions).
STATISTICS
Permutation (bootstrap) analyses were applied to the β power time-by-trial response matrices to confirm that the averaged β power response was representative of all trials. These matrices were built using entire-record normalized log transformed data, baselined on a trial-by-trial basis. A permutation “sign-test” was performed using randomly sampled trials without replacement using a custom Matlab script. For each permutation, the sign of half of the trials was inverted, and a new average generated. For the null hypothesis (that β suppression is not significantly different from zero), inversion of the sign would be expected to increase the absolute value of the average for a substantial number of permutations. For the alternate hypothesis (significantly different from zero), inversion would be expected to decrease the absolute value of the average.
To correct for multiple comparisons, we conservatively assumed that each 60ms segment could be considered an independent sample. Thus, our 6000ms time window requires statistical correction for 100 multiple comparisons. We calculated the number of bootstraps such that the resolution of our p-value was ¼ of the corrected significance value for an alpha error of 5%. This calculation was 4*( 5% / 100)−1 = 8000 permutations. Our corrected p-value resolution was 1/8000. To calculate our p-value, we counted the number of permutations that created a new mean greater than the original mean, and multiplied this count by the resolution of our p value, producing a statistical time vector. Thus, if the permutation mean was greater than the original mean at least 5/8000 times, it was deemed non-significant, and the null hypothesis adopted for that time point.
Significant modulation of the LFP power was defined as the combined criteria of a Z-score greater than 3 and significance based on the permutation statistic. Analysis was restricted to > 400ms segments of significant data after the cue. Up to three sequential segments with equal polarity that were separated by gaps less than 100ms were combined as a continuous segment. Subsequent segments with opposite polarity meeting the duration threshold of 400ms were also included, excluding the intervening segment. This allowed us to include both β power suppression as well as subsequent rebound in the statistical analysis. To quantify the timing and magnitude of the significant β LFP response, latency (ms from time zero) and duration (total ms of significant time) were calculated. The two-sample Wilcoxon rank-sum test was performed on these measures to determine any differences in latency and duration of response between ipsilateral and contralateral button press, and between speech with button press and speech alone.
Quantification of the permutation analyses measures of latency and duration allows for analysis of the robustness of a response to a behavioral task. However, secondary statistical analyses are likely to be confounded. Therefore, analysis of the difference between speech alone and speech plus button press was repeated using a traditional t-test across trials. For comparison of the magnitude of β suppression for speech with a button press to speech alone, the time-by-trial appended event matrices were averaged across the time dimension from 3000ms to 6000ms of the appended event matrix for both tasks, equivalent to the final 3000ms of speech for each trial. Independent sample grouped t-tests were performed both within subjects and across all subjects, comparing the β suppression related to “Speech with a Button press” versus “Speech alone”. As described above, the β time vector was normalized by two critically distinct methods. First, the log-transformed β time vector was Z-scored with the mean and standard deviation of the entire recording, typically 30 minutes in length. Secondly, the log-transformed β time vector was Z-scored within each task block, to the task-block specific mean and standard deviation. These two different methods were used to assess whether the difference between speech alone and speech and button press was due to a sustained state-change in the β power, or to transient changes in response to single-actions.
To determine if there were unique “β states” corresponding to a particular behavior, the ratio of log transformed β power for each task to that subject’s overall mean β power was categorized by task. A one-way ANOVA with subsequent Bonferroni multiple-comparison test was performed to determine statistical difference of β state between these behavioral conditions.
RECORDING LOCATION
STN recording locations were estimated for illustration. For the paired MER design recordings, the trajectory through STN was calculated based on the surgical target coordinates and the angle of the approach trajectory with respect to the AC-PC plane. Target coordinates and trajectory were plotted on a sagittal Morel standardized brain atlas (Morel, 2007) that best fit the microelectrode recording. As the recording location was typically past “target” along the linear trajectory, we corrected for this added depth by measuring the recording location depth past the dorsal STN border for the anterior electrode (Figure 2).
For the DBS lead design recordings, the final location of the DBS leads were measured using a postoperative Extended Hounsfield Unit CT algorithm (Hebb and Poliakov, 2009), and a semi-automated algorithm to detect the active contacts of the DBS lead (Hebb and Miller, 2010). These target coordinates and approach trajectory were similarly plotted on the standardized atlas.
Results
All subjects were able to comply with the experimental paradigm. Across all subjects, 91% of attempted speech trials and 96% of attempted motor trials were included in analysis. The remainders were excluded due to failure of response. Subject 1 underwent a control paradigm to evaluate for LFP response to the audio cue. With equal volume settings and timing, there was no β power response to the audio cue (data not shown).
STN-LFP Power Spectra
Power spectra for STN LFPs were dominated by β power (Figure 2, 3). On task execution, there was marked β suppression during speech (Figure 2, 3) and button press (Figure 3). Power spectra of baseline time epochs, a period of 1000ms prior to task cue, demonstrated augmented β power during the combined speech and motor task block (Figure 3D), but was similar to the average for the entire recording for the speech only task block (Figure 3H).
Figure 3.
Power spectral density (PSD) modulation with either speech alone (right panel) or speech plus button press (left panel) from a single subject (recording 10). A, E: Frequency-Time representation for left STN. B, F: Right STN. C, G: averaged patient response activity for audio channel (upper trace) and EMG (lower trace). Note the strong EMG response for button press preceding speech onset for the combined task, but no EMG activity during the speech-only task. D, H: PSD plots for each condition. This demonstrates an increase of pre-speech (1000ms epoch) β power for the speech plus button press tasks (left panel), but no such increase of pre-speech β power for speech-only tasks (right panel), suggesting a unique β state. A, B, C were recorded synchronously, and E, F, G were recorded synchronously. For speech plus button press tasks, 50% of trials were left-handed button press, 50% right handed. For A, B, E, F, data was Z-scored and baselined to adjust the mean power in 1000ms period before the behavioral cue to zero; blue and red represent negative and positive relative change, respectively, to baseline.
MOTOR ONSET
For ipsilateral and contralateral button press, there was a characteristic response in 11/13 and 9/13 recordings, respectively. The β power was suppressed preceding and coincident with the motor task, followed by an over-shoot rebound after the motor arrest (Figure 4). Statistically significant β power change began (mean±SE) 261ms (± 190ms) and 61ms (± 135ms) after the button press for ipsi- and contralateral response respectively (Table 3). This mean included observations where the initial β suppression was not statistically significant, but the later rebound was. Thus, the distribution of the significant β change latency for button press tasks has a rightward skew (skewness = 0.86) but was normally distributed (skewness and kurtosis test for normality, p=0.16). The 25th percentile of latency for β change was 238ms prior to button press for both ipsi and contra combined, and 284ms and 238ms prior to button press for ipsi and contralateral button press respectively. There was no statistically significant difference between ipsilateral and contralateral button press for β power change latency, however the contralateral button press produced a longer duration of significance (832ms versus 515ms, p=0.04 Wilcoxon rank-sum). The signed area under the bootstrap significant portions of the curve was more positive for the contralateral button press, indicating that this increase of duration may have been due to increased β rebound, but this difference was not statistically significant. These findings indicate that the STN are bilaterally involved in unilateral motor movements.
Table 3.
Quantification of β band (13–30Hz) power spectral density modulation with respect to pre-cue baseline for each task condition. Time lag and duration of significant β modulation based on permutation (boot-strap) analysis. Time 0 defined as the subject response. nSR/tN: number of significant responses/total N. (* Significant difference in duration, Speech + Button versus Speech Alone (p=0.04), and Ipsi versus Contra button press (p=0.04), Wilcoxon rank-sum test.)
| CONDITION (Sync Response) |
RESPONSE Delay (ms): Mean (SD) |
RIGHT STN | LEFT STN | COMBINED LEFT & RIGHT STN | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Right: nSR/tN |
Lag (ms): Median Mean (SE) |
Duration (ms): Mean (SE) |
Left: nSR/tN |
Lag (ms): Median Mean (SE) |
Duration (ms): Mean (SE) |
L & R: nSR/tN |
Lag (ms): Median Mean (SE) |
Duration (ms): Mean (SE) |
||
| All Speech (S) |
788 (152) | 4/4 | −557 −579 (70) |
3498 (946) | 9/9 | −484 −186 (343) |
3441 (836) | 13/13 | −557 −307 (240) |
3459 (626) |
| Ipsi Button (B) |
372 (73) | 3/4 | −28 −75 (110) |
360 (99) | 8/9 | 246 388 (248) |
573 (163) | 11/13 | −28 261 (190) |
515* (122) |
| Contra Button (B) |
363 (97) | 2/4 | −242 −242 (160) |
667 (233) | 7/9 | −38 148 (157) |
879 (116) | 9/13 | −81 61 (135) |
832* (102) |
| Speech + B (S) |
909 (191) | 4/4 | −638 −616 (67) |
3350 (915) | 9/9 | −524 −525 (65) |
3557 (759) | 13/13 | −612 −553 (49) |
3507* (575) |
| Speech Alone (S) |
673 (117) | 3/4 | −174 −229 (102) |
956 (58) | 8/9 | −211 780 (771) |
1748 (810) | 11/13 | −174 505 (569) |
1532* (589) |
SPEECH ONSET
Overt speech was associated with β suppression for the duration of speech production (Figure 2, 3, 5). For combined tasks in which subjects performed a single brief button press on initiation of speech, this relative β suppression was often amplified for the entire duration of speech activity, well beyond the duration of effect seen with button press alone. For overt speech tasks without a button press, the overall mean duration of statistically significant β modulation was 1532ms (95% CI 353-2711ms), whereas for speech with a button press, this duration was 3507ms (95% CI 2355–4660ms) (p=0.04, Two-sample Wilcoxon rank-sum, Table 3). This indicates that a dual-task paradigm produced a far more robust relative β power change in the subthalamic nucleus than speech alone.
We then averaged the β suppression from 3000 to 6000ms for all trials in all recordings. For log-transformed β power normalized within-subject to the entire record, the additive effect of a heralding button press was significant: −2.8 log units for the combined task versus −2.1 log units for speech alone (p=0.02, 2-tailed t-test, Figure 5B). However, when the β power vector was normalized locally within each task block, rather than globally within-subject, the statistical significance was lost (−3.1 versus −2.9 log units, p=0.60, 2-tailed t-test, Figure 5C). Therefore, the difference is sensitive to temporal locality of normalization. This suggests that different tasks produce sustained “state changes” in β power within which transient, trial-to-trial, fluctuations also occur.
Left and right STN β power modulations were not statistically different for β modulation latency or duration of effect, suggesting that speech production is associated with bilateral STN β modulation.
SILENT SPEECH
In two subjects (6 and 7), the experimental paradigm was modified to include trials of “silent speech.” For half of these trials, subjects were asked to press a button at initiation of their mental rehearsal of the speech task, just as they had done in the overt speech case. The combined overt speech + button press was compared with the combined silent speech + button press, cued to the onset of the button press (as this was available in both conditions). The latency of β modulation was similar between both conditions, and was similar to button press alone trials (~200ms post-cue). However, the duration of the effect was markedly reduced during silent speech (Table 4). When comparing the combined silent speech + button press to button press only, the β rebound, seen after completion of the button press, was not seen if silent speech accompanied the button press (data not shown). This suggests a quenching of the β rebound by the mental rehearsal of speech. On examination of silent speech without a heralding button press, β suppression did not meet criteria for significance (Table 4).
Table 4.
Time lag and duration of significant β band (13–30Hz) power spectral density modulation based on permutation (boot-strap) analysis for subjects undergoing silent speech trials. Analysis is performed with the subject cue at time 0 (columns 2–4), and with the subject response at time 0 (columns 5–7). nSR/tN: number of significant responses/total N.
| CONDITION (Sync Response) |
AUDIO CUE SYNCHRONIZED ANALYSIS | SUBJECT RESPONSE SYNCHRONIZED ANALYSIS | ||||
|---|---|---|---|---|---|---|
| L & R: nSR/tN |
Lag (ms) Mean (SE) |
Duration (ms) Mean (SE) |
L & R: nSR/tN |
Lag (ms) Mean (SE) |
Duration (ms) Mean (SE) |
|
| All Speech (S) | 4/4 | 280 (76) | 1195 (488) | 4/4 | −584 (11) | 1779 (731) |
| Ipsi Button (B) | 4/4 | 159 (16) | 693 (127) | 4/4 | −190 (56) | 730 (208) |
| Contra Button (B) | 3/4 | 164 (19) | 891 (245) | 3/4 | −171 (67) | 873 (269) |
| Speech + B (S) | 4/4 | 214 (19) | 1302 (442) | 4/4 | −721 (48) | 1789 (743) |
| Speech Alone (S) | 3/4 | 484 (167) | 573 (111) | 4/4 | 330 (595) | 491 (262) |
| Speech + B (B) | 4/4 | 213 (19) | 1286 (436) | 4/4 | −177 (73) | 1516 (574) |
| Silent Speech (−) | 1/4 | 2992 (−) | 9 (−) | NO MEASUREABLE RESPONSE | ||
| Silent Speech + B (B) | 3/4 | 187 (26) | 589 (172) | 3/4 | −181 (63) | 544 (157) |
SLOW TASK-RELATED β STATE SHIFTS
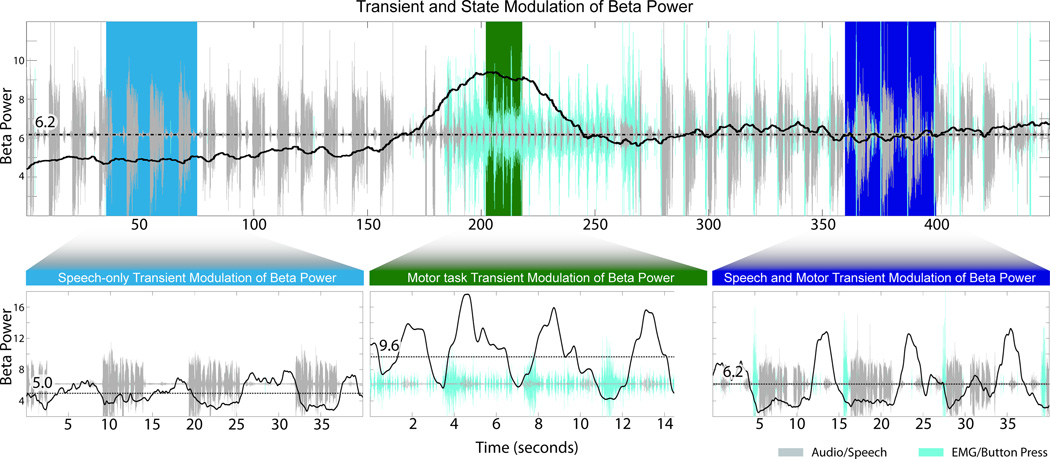
On inspection of the β power vector over the entire recording time, the average β power state is modulated, and is different between task blocks. This is apparent in Figure 3 D&H, where the pre-behavior β power is augmented above baseline in the one task (D, Speech + Button press), but is not augmented in a different task (H, Speech alone). Application of a low-pass filter to the β vector over the entire experimental paradigm (approximately 30 minutes) highlights the slow shift of the β power baseline with changing behavioral states (Figure 6A,B). Tasks were divided into 5 categories. The simple repetitive motor movement task (button press) was associated with the highest level of β activity despite β suppression linked with the onset of each movement. The “high β” states include repetitive motor task alone, speech plus motor task, and silent speech plus motor task. The “low β” states were those without a simple motor task: speech alone and silent speech alone. The β-power for each behavioral category was statistically significant from all others (Figure 6D, p ≤ 0.001, one-way ANOVA with Bonferroni multiple-comparison test). Therefore, while each speech or motor action is associated with transient β suppression, there is a slower, sustained shift of the β baseline power during blocks of repeated tasks. The magnitude of this β baseline power is specific to behavioural task (Figure 7).
Figure 7.
Transient and state modulation of β power over three distinct task blocks. Upper panel demonstrates slow, state modulation of β power over a time scale of minutes, shifting from a relatively low β state (speech only, light blue), to a high β state (motor only, green), then to an intermediate β state (speech + motor, dark blue). Each colored block is expanded in a lower panel to demonstrate transient modulation of β on a task-by-task level on the time scale of seconds/milliseconds. Dotted black line represents the mean β power in that panel. Numerical value on the dotted line represents the mean beta power in arbitrary units. The baseline for audio and EMG data serves as a reference marker for the mean β power in the upper panel. The region of data presented here is highlighted in the upper panel of Figure 6.
Discussion
We observed bilateral and symmetric STN β suppression during simple motor movement and speech production tasks in patients with PD. When speech production was combined with a heralding motor task (button press), relative β-suppression appeared to be magnified for the duration of the speech task. However, we found that this added motor task led to a sustained increase of average β power, or a heightened β state. This high β state contributed to the greater relative β suppression during the combined speech and movement task. This study therefore supports bilateral motor and speech related STN β modulation and introduces the concept of a bilateral task dependent β power state in the STN.
The findings of symmetric STN β suppression add to the existing evidence for functional connectivity between the left and right STN. Extracellular multi-unit activity (MUA) is increased during contralateral STN high frequency stimulation (HFS) using clinical DBS systems (Novak et al., 2009, Walker et al., 2011). Unilateral HFS of STN leads to an entrainment of contralateral STN neurons in a temporally specific firing pattern with increased mean firing rates(Walker et al., 2011). This entrainment of neuronal firing to HFS pulses is similar to results obtained in GPi MUA during HFS of the ipsilateral STN in a primate model of PD(Hashimoto et al., 2003) and a single patient clinical report(Reese et al., 2011). Although we can draw parallels between the GPi response to ipsilateral STN HFS and the STN response to contralateral STN HFS, there are well known anatomic pathways to explain the former but not the latter results. Simultaneous recordings of bilateral STN LFP oscillations also support the concept of a bilaterally connected basal ganglia network. In a study of 12 subjects with bilateral STN LFP recordings, STN oscillations were found to be coherent with maximal coherence peaks in the β range, between 11Hz to 34Hz (mean 24.7Hz) across subjects (de Solages et al., 2010). Transient, single trial, bilateral STN β suppression with movement has been found previously, with differing reports of it being either symmetric (Alegre et al., 2005, Kempf et al., 2007) or earlier contralaterally (Devos et al., 2006). In contrast to the cerebral cortex, where β suppression is asymmetric in response to motor movement (Devos et al., 2006, Zanos et al., 2008, Darvas et al., 2010), and like the studies of Alegre et. al. and Kempf et. al., we found that the transient movement associated β ERD within the STN is symmetric. Beyond this, we found symmetric β ERD during individual speech trials (on a timescale of seconds), as well as bilateral sustained shifts in average β power during whole task blocks (on a timescale of minutes).
Anatomic evidence for direct connectivity between bilateral STN is absent. Carpenter et al. recorded degenerated fibers entering the contralateral medial segment of globus pallidus (GP) via the dorsal supraoptic decussation after an ipsilateral STN lesion in non-human primates (Carpenter and Strominger, 1967). With the exception of a few questionably degenerated fibers in contralateral STN, there was no evidence for an STN-to-STN connection. A subsequent anterograde tracing study using radioactively labeled amino acids failed to demonstrate any contralateral efferent projections from the STN (Carpenter et al., 1981). This study confirmed ipsilateral efferent projections of the STN to both segments of GP and to substantia nigra (SN), with an absence of projections to pedunculopontine nuclei (PPN) or other brainstem nuclei. This study refutes evidence for direct connectivity between STN and PPN, with only sparse retrograde labeling of PPN from STN injection. Therefore, connections between bilateral STN must be multisynaptic and initially via the efferent connected structures. The anatomical decussation may be multiple, via decussation of projection fibers of STN-connected subcortical nuclei such as SN (Beckstead and Frankfurter, 1982) or GP (Hazrati and Parent, 1991), and via cortical loops such as bilateral cortico-striatal projections (Inase et al., 1999) or corpus callosum. As the corpus callosum provides an order of magnitude greater number of decussating fibers than that of subcortical commissures (Lamantia and Rakic, 1990), and recent evidence of direct ipsilateral cortical-STN projections (Nambu et al., 2002), the cortex is likely to be a major node of this crossed basal ganglia network with asymmetric cortical β modulation remaining unexplained.
Reconciliation of the experimental findings of bilaterally coherent STN LFP in the β range together with entrainment of contralateral STN unit activity to a DBS pulse train may require consideration of different anatomical pathways for each. Theories should also incorporate recent findings of unilateral cortical transcranial magnetic stimulation (TMS) suppression of β oscillations in bilateral STN (Gaynor et al., 2008). Walker et al. argue for the presence of decussating STN neurons in the region of the stimulation probe to explaining their short-latency contralateral STN response to HFS (Walker et al., 2011). Although there is no anatomic evidence for decussating STN neurons, there is evidence for decussating GP neurons which may be stimulated as axons en passage in the region of the DBS lead. In explanation of bilateral LFP coherence, oscillatory networks allow for multi-synaptic pathways with differing delays between nodes (Jaeger and Kita, 2011) and would not require a short latency connection between bilateral STN. This model would also support experimental findings of improved Parkinsonian symptoms related stimulation input into the circuit at non-traditional nodes, such as motor cortex (Arle et al., 2008) or GPe (Vitek et al., 2004). Further, suppression of bilateral STN β oscillations by unilateral TMS and by movement and speech tasks in the current study may be explained by the presence of bilateral cortico-striatal projections (Inase et al., 1999). Thus, the STN resides within a network that is bilaterally connected at both the subcortical and cortical level. Explanation of clinical and experimental findings may require evoking one or both of these connectivities.
Pre-activity β power (sustained baseline shift) was observed to be greater during speech task blocks that included a repetitive motor task compared to those without (Figure 3, D & H, Figure 6D), contributing to a significantly greater relative magnitude effect for the speech tasks combined with a button press (Figure 5B). To correct for this heightened β state, the β power time series was normalized within each task block. This normalization method reduced the effect of the task dependent β state, and the β suppression during the dual task was no longer significantly different from the speech alone task (Figure 5C).
How a repetitive motor task leads to a heightened β state is not clear as each individual movement causes β suppression. Two elementary hypotheses include 1) prolonged β rebound effect after movement is completed and 2) a task-dependent anticipatory β power increase for the next cue. Anticipatory changes of β power have been observed in sensorimotor cortex (Babiloni et al., 2006, van Ede et al., 2011); however these studies demonstrated anticipatory decrease of β power. This suggests that more complex dynamics are responsible for the heightened β state, requiring consideration of the network within which STN is located.
Reciprocal and mutually opposite connections between basal ganglia nuclei provide the network required to create oscillatory neural activity. Computer models of the GPe ↔ STN circuit suggest that these structures are sufficient to produce β oscillations (Holgado et al., 2010). The major inputs into this sub-network include the classic indirect pathway (cortex → striatum → GPe) and the hyperdirect pathway (cortex → STN) (Nambu et al., 2002, Jaeger and Kita, 2011). Loss of dopamine in advancing PD may lead to increased synaptic strength between striatum and GPe (Holgado et al., 2010) from the loss of D2 receptor activation, whose role is to attenuate cortical glutamateric signaling in striatal medium spiny neurons (MSNs) projecting to GPe (Surmeier et al., 2007). Alternatively, chronic loss of DA leads to pruning of dendritic spines on these MSNs (Surmeier et al., 2007), which may lead to isolation of the GPe ↔ STN circuit from its striatal input and dysregulation between this GABAergic input and its glutamateric direct cortical input. In the 6-OHDA rat model of PD, chronic loss of DA was required for the emergence of STN β oscillations suggesting that neural plasticity is involved in its development (Mallet et al., 2008). Thus, pruning of dendritic spines on MSNs related to overstimulation of glutamatergic synapses on these spines due to chronic DA loss with subsequent isolation of the GPe ↔ STN circuit from its corticostriatal influence may be required for the generation of excessive oscillatory power. These pathological changes may facilitate increased oscillatory drive, and hence the increased β oscillations in PD, by direct cortico-STN input resulting from a repetitive motor task.
These results suggest how clinical examination findings of PD may be explained by β spectral power fluctuations in the STN LFP. Our clinically driven hypothesis was that motor and speech tasks would augment β power. We have demonstrated that a unilateral repetitive motor task drives the β power in bilateral STN into a heightened state. As PD has been associated with a pathological increase of β power(Hammond et al., 2007), and this increase of power is related to motor signs of PD(Kuhn et al., 2009), shift of bilateral β power into a higher state could directly result in increased rigidity.
Speech tasks employed in this paradigm, reciting the months of the year and counting, are commonly used to increase motor signs of PD during clinical examination. In the current study, speech induced bilaterally symmetric STN β suppression. Although novel in the STN, this β ERD has previously been demonstrated in cortex (Miller et al., 2011). Speech-alone tasks were also associated with a β state that was below the mean for the entire recording (Figure 6D). However, one should not interpret this as a suppression of β power or a negative power state since power is a positive metric by definition. Global normalization of the β power time series was naïve to subject behavior. This method of normalization was chosen as β power is a dynamic value (Figure 6A, B), and in exploration of our data, we could not define a gold standard resting period where β power was in a relatively stable range over repeated rest periods during our 30 minute paradigm. Given that speech tasks augment clinical signs of PD, we had expected that speech would also produce a heightened state. In our 2 subjects that performed a silent speech task, speech alone was associated with a β power state greater than silent speech alone (Figure 6C). Therefore, speech alone could be considered a heightened β state with respect to silent speech and further work may be required to adequately define resting state for β power in the STN.
This study is limited by the absence of a control task for speech for most subjects. In two subjects, our control task consisted of silent speech. Otherwise, we did not measure the effect on β power of production of meaningless sounds, facial or tongue motor movements. Similarly, we did not vary the cognitive load of the language task. The rationale for excluding these measures were the time restrictions that were imposed by the operating room environment, and the need to create a task that would be reliably performed by our subjects during surgery. More focused lines of investigation into the effect of cognitive load and non-language facial motor tasks may now be pursued. Further, hand motor sequencing tasks would provide a motor control condition for facial movements and speech that is superior to button press alone.
As these recordings were all performed in the PD patient population, aspects of these results may be pathological. Given the absence of direct anatomical interconnectivity between bilateral STN, it is possible that the symmetry of β modulation is a pathological finding of PD. Stratifying the changes seen in the β state by PD severity may highlight pathological features. However, all but one subject in this study were the same clinical stage (Table 1), and therefore this stratification was not possible. A larger study with a greater spread of clinical PD stage would be required to address this question.
Conclusions
Overall, this study presents evidence for task-related switching of bilateral β power states in the STN during speech and motor tasks. Although we observed event related desychronization of β oscillations precisely synchronized to behavior, there was a sustained shift of the average β power between blocks of distinct tasks. Tasks that would be expected to worsen contralateral motor signs of PD such as repetitive simple motor tasks were associated with the greatest level of absolute β power. Combined speech and motor tasks were also associated with a high β power state. Speech and silent speech tasks were associated with the lowest β state. In clinical observations of motor signs of PD contralateral to a limb performing a reinforcement maneuver, the performing limb is enjoying a task-synchronized β power suppression, whereas the non-performing limb only experiences the heightened bilateral β power state and subsequent pathological rigidity. This bilateral β state in the STN may be a pathological finding of PD. As closed loop stimulation devices are developed, they will require a robust and relevant physiological signal to monitor. Thus, the β power state may provide an appropriate input variable for these closed loop neurostimulation systems.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Adam O. Hebb, University of Washington, Department of Neurological Surgery.
Felix Darvas, University of Washington, Department of Neurological Surgery.
Kai J. Miller, Stanford University, Department of Neurosurgery.
REFERENCES
- Alegre M, Alonso-Frech F, Rodriguez-Oroz MC, Guridi J, Zamarbide I, Valencia M, Manrique M, Obeso JA, Artieda J. Movement-related changes in oscillatory activity in the human subthalamic nucleus: ipsilateral vs. contralateral movements. Eur J Neurosci. 2005;22:2315–2324. doi: 10.1111/j.1460-9568.2005.04409.x. [DOI] [PubMed] [Google Scholar]
- Amirnovin R, Williams ZM, Cosgrove GR, Eskandar EN. Visually guided movements suppress subthalamic oscillations in Parkinson's disease patients. J Neurosci. 2004;24:11302–11306. doi: 10.1523/JNEUROSCI.3242-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arle JE, Apetauerova D, Zani J, Deletis DV, Penney DL, Hoit D, Gould C, Shils JL. Motor cortex stimulation in patients with Parkinson disease: 12-month follow-up in 4 patients. Journal of neurosurgery. 2008;109:133–139. doi: 10.3171/JNS/2008/109/7/0133. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Brancucci A, Vecchio F, Arendt-Nielsen L, Chen AC, Rossini PM. Anticipation of somatosensory and motor events increases centro-parietal functional coupling: an EEG coherence study. Clin Neurophysiol. 2006;117:1000–1008. doi: 10.1016/j.clinph.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Beckstead RM, Frankfurter A. The distribution and some morphological features of substantia nigra neurons that project to the thalamus, superior colliculus and pedunculopontine nucleus in the monkey. Neuroscience. 1982;7:2377–2388. doi: 10.1016/0306-4522(82)90202-0. [DOI] [PubMed] [Google Scholar]
- Carpenter MB, Carleton SC, Keller JT, Conte P. Connections of the subthalamic nucleus in the monkey. Brain research. 1981;224:1–29. doi: 10.1016/0006-8993(81)91113-6. [DOI] [PubMed] [Google Scholar]
- Carpenter MB, Strominger NL. Efferent fibers of the subthalamic nucleus in the monkey. A comparison of the efferent projections of the subthalamic nucleus, substantia nigra and globus pallidus. Am J Anat. 1967;121:41–72. doi: 10.1002/aja.1001210105. [DOI] [PubMed] [Google Scholar]
- Cassidy M, Mazzone P, Oliviero A, Insola A, Tonali P, Di Lazzaro V, Brown P. Movement-related changes in synchronization in the human basal ganglia. Brain. 2002;125:1235–1246. doi: 10.1093/brain/awf135. [DOI] [PubMed] [Google Scholar]
- Crone NE, Hao L, Hart J, Jr, Boatman D, Lesser RP, Irizarry R, Gordon B. Electrocorticographic gamma activity during word production in spoken and sign language. Neurology. 2001;57:2045. doi: 10.1212/wnl.57.11.2045. [DOI] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Sieracki JM, Wilson MT, Uematsu S, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. I. Alpha and beta event-related desynchronization. Brain : a journal of neurology. 1998;121(Pt 12):2271–2299. doi: 10.1093/brain/121.12.2271. [DOI] [PubMed] [Google Scholar]
- Darvas F, Scherer R, Ojemann JG, Rao RP, Miller KJ, Sorensen LB. High gamma mapping using EEG. Neuroimage. 2010;49:930–938. doi: 10.1016/j.neuroimage.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Solages C, Hill BC, Koop MM, Henderson JM, Bronte-Stewart H. Bilateral symmetry and coherence of subthalamic nuclei beta band activity in Parkinson's disease. Exp Neurol. 2010;221:260–266. doi: 10.1016/j.expneurol.2009.11.012. [DOI] [PubMed] [Google Scholar]
- Devos D, Szurhaj W, Reyns N, Labyt E, Houdayer E, Bourriez JL, Cassim F, Krystkowiak P, Blond S, Destee A, Derambure P, Defebvre L. Predominance of the contralateral movement-related activity in the subthalamo-cortical loop. Clin Neurophysiol. 2006;117:2315–2327. doi: 10.1016/j.clinph.2006.06.719. [DOI] [PubMed] [Google Scholar]
- Edwards E, Soltani M, Kim W, Dalal SS, Nagarajan SS, Berger MS, Knight RT. Comparison of time-frequency responses and the event-related potential to auditory speech stimuli in human cortex. Journal of neurophysiology. 2009;102:377. doi: 10.1152/jn.90954.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Moll CK, Fried I, Ojemann GA. Invasive recordings from the human brain: clinical insights and beyond. Nat Rev Neurosci. 2005;6:35–47. doi: 10.1038/nrn1585. [DOI] [PubMed] [Google Scholar]
- Foffani G, Bianchi AM, Baselli G, Priori A. Movement-related frequency modulation of beta oscillatory activity in the human subthalamic nucleus. J Physiol. 2005;568:699–711. doi: 10.1113/jphysiol.2005.089722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett KA, Weaver FM, Stern M, Hur K, Harris CL, Luo P, Marks WJ, Jr, Rothlind J, Sagher O, Moy C, Pahwa R, Burchiel K, Hogarth P, Lai EC, Duda JE, Holloway K, Samii A, Horn S, Bronstein JM, Stoner G, Starr PA, Simpson R, Baltuch G, De Salles A, Huang GD, Reda DJ. Pallidal versus subthalamic deep-brain stimulation for Parkinson's disease. N Engl J Med. 2010;362:2077–2091. doi: 10.1056/NEJMoa0907083. [DOI] [PubMed] [Google Scholar]
- Gaynor LM, Kuhn AA, Dileone M, Litvak V, Eusebio A, Pogosyan A, Androulidakis AG, Tisch S, Limousin P, Insola A, Mazzone P, Di Lazzaro V, Brown P. Suppression of beta oscillations in the subthalamic nucleus following cortical stimulation in humans. The European journal of neuroscience. 2008;28:1686–1695. doi: 10.1111/j.1460-9568.2008.06363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson's disease: networks, models and treatments. Trends Neurosci. 2007;30:357–364. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:1916–1923. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazrati LN, Parent A. Contralateral pallidothalamic and pallidotegmental projections in primates: an anterograde and retrograde labeling study. Brain research. 1991;567:212–223. doi: 10.1016/0006-8993(91)90798-z. [DOI] [PubMed] [Google Scholar]
- Hebb AO, Miller KJ. Semi-automatic stereotactic coordinate identification algorithm for routine localization of Deep Brain Stimulation electrodes. J Neurosci Methods. 2010;187:114–119. doi: 10.1016/j.jneumeth.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Hebb AO, Poliakov AV. Imaging of Deep Brain Stimulation Leads Using Extended Hounsfield Unit CT. Stereotact Funct Neurosurg. 2009;87:155–160. doi: 10.1159/000209296. [DOI] [PubMed] [Google Scholar]
- Holgado AJ, Terry JR, Bogacz R. Conditions for the generation of beta oscillations in the subthalamic nucleus-globus pallidus network. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:12340–12352. doi: 10.1523/JNEUROSCI.0817-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inase M, Tokuno H, Nambu A, Akazawa T, Takada M. Corticostriatal and corticosubthalamic input zones from the presupplementary motor area in the macaque monkey: comparison with the input zones from the supplementary motor area. Brain research. 1999;833:191–201. doi: 10.1016/s0006-8993(99)01531-0. [DOI] [PubMed] [Google Scholar]
- Jaeger D, Kita H. Functional connectivity and integrative properties of globus pallidus neurons. Neuroscience. 2011 doi: 10.1016/j.neuroscience.2011.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzner S, Nauhaus I, Benucci A, Bonin V, Ringach DL, Carandini M. Local Origin of Field Potentials in Visual Cortex. Neuron. 2009;61:35–41. doi: 10.1016/j.neuron.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf F, Kühn AA, Kupsch A, Brucke C, Weise L, Schneider GH, Brown P. Premovement activities in the subthalamic area of patients with Parkinson's disease and their dependence on task. Eur J Neurosci. 2007;25:3137–3145. doi: 10.1111/j.1460-9568.2007.05536.x. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Tsui A, Aziz T, Ray N, Brucke C, Kupsch A, Schneider GH, Brown P. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson's disease relates to both bradykinesia and rigidity. Exp Neurol. 2009;215:380–387. doi: 10.1016/j.expneurol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Kühn AA, Williams D, Kupsch A, Limousin P, Hariz M, Schneider GH, Yarrow K, Brown P. Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain. 2004;127:735–746. doi: 10.1093/brain/awh106. [DOI] [PubMed] [Google Scholar]
- Lamantia AS, Rakic P. Cytological and quantitative characteristics of four cerebral commissures in the rhesus monkey. The Journal of comparative neurology. 1990;291:520–537. doi: 10.1002/cne.902910404. [DOI] [PubMed] [Google Scholar]
- Levy R, Ashby P, Hutchison WD, Lang AE, Lozano AM, Dostrovsky JO. Dependence subthalamic nucleus oscillations on movement and dopamine in Parkinson's disease. Brain. 2002;125:1196–1209. doi: 10.1093/brain/awf128. [DOI] [PubMed] [Google Scholar]
- Levy R, Hutchison WD, Lozano AM, Dostrovsky JO. High-frequency synchronization of neuronal activity in the subthalamic nucleus of parkinsonian patients with limb tremor. J Neurosci. 2000;20:7766–7775. doi: 10.1523/JNEUROSCI.20-20-07766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Azcarate J, Tainta M, Rodriguez-Oroz MC, Valencia M, Gonzalez R, Guridi J, Iriarte J, Obeso JA, Artieda J, Alegre M. Coupling between beta and high-frequency activity in the human subthalamic nucleus may be a pathophysiological mechanism in Parkinson's disease. J Neurosci. 2010;30:6667–6677. doi: 10.1523/JNEUROSCI.5459-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Pogosyan A, Sharott A, Csicsvari J, Bolam JP, Brown P, Magill PJ. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J Neurosci. 2008;28:4795–4806. doi: 10.1523/JNEUROSCI.0123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, Abel TJ, Hebb AO, Ojemann JG. Rapid online language mapping with electrocorticography. J Neurosurg Pediatr. 2011;7:482–490. doi: 10.3171/2011.2.PEDS1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, Schalk G, Fetz EE, den Nijs M, Ojemann JG, Rao RP. Cortical activity during motor execution, motor imagery, and imagery-based online feedback. Proc Natl Acad Sci U S A. 2010;107:4430–4435. doi: 10.1073/pnas.0913697107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, Zanos S, Fetz EE, den Nijs M, Ojemann JG. Decoupling the cortical power spectrum reveals real-time representation of individual finger movements in humans. J Neurosci. 2009;29:3132–3137. doi: 10.1523/JNEUROSCI.5506-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel A. Stereotactic atlas of the human thalamus and basal ganglia: Informa Healthcare. 2007 [Google Scholar]
- Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamopallidal 'hyperdirect' pathway. Neuroscience research. 2002;43:111–117. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- Novak P, Klemp JA, Ridings LW, Lyons KE, Pahwa R, Nazzaro JM. Effect of deep brain stimulation of the subthalamic nucleus upon the contralateral subthalamic nucleus in Parkinson disease. Neurosci Lett. 2009;463:12–16. doi: 10.1016/j.neulet.2009.07.040. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Powell D, Hanson N, Threlkeld AJ, Fang X, Xia R. Enhancement of parkinsonian rigidity with contralateral hand activation. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2011;122:1595–1601. doi: 10.1016/j.clinph.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese R, Leblois A, Steigerwald F, Potter-Nerger M, Herzog J, Mehdorn HM, Deuschl G, Meissner WG, Volkmann J. Subthalamic deep brain stimulation increases pallidal firing rate and regularity. Experimental neurology. 2011;229:517–521. doi: 10.1016/j.expneurol.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Temel Y, Blokland A, Steinbusch HW, Visser-Vandewalle V. The functional role of the subthalamic nucleus in cognitive and limbic circuits. Prog Neurobiol. 2005;76:393–413. doi: 10.1016/j.pneurobio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- van Ede F, de Lange F, Jensen O, Maris E. Orienting attention to an upcoming tactile event involves a spatially and temporally specific modulation of sensorimotor alpha- and beta-band oscillations. J Neurosci. 2011;31:2016–2024. doi: 10.1523/JNEUROSCI.5630-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitek JL, Hashimoto T, Peoples J, DeLong MR, Bakay RA. Acute stimulation in the external segment of the globus pallidus improves parkinsonian motor signs. Movement disorders : official journal of the Movement Disorder Society. 2004;19:907–915. doi: 10.1002/mds.20137. [DOI] [PubMed] [Google Scholar]
- Walker HC, Watts RL, Schrandt CJ, Huang H, Guthrie SL, Guthrie BL, Montgomery EB., Jr Activation of subthalamic neurons by contralateral subthalamic deep brain stimulation in Parkinson disease. Journal of neurophysiology. 2011;105:1112–1121. doi: 10.1152/jn.00266.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P, Montgomery EB., Jr The relationship of neuronal activity within the sensori-motor region of the subthalamic nucleus to speech. Brain Lang. 2006;97:233–240. doi: 10.1016/j.bandl.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Witt K, Daniels C, Reiff J, Krack P, Volkmann J, Pinsker MO, Krause M, Tronnier V, Kloss M, Schnitzler A, Wojtecki L, Botzel K, Danek A, Hilker R, Sturm V, Kupsch A, Karner E, Deuschl G. Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson's disease: a randomised, multicentre study. Lancet Neurol. 2008;7:605–614. doi: 10.1016/S1474-4422(08)70114-5. [DOI] [PubMed] [Google Scholar]
- Zanos S, Miller KJ, Ojemann JG. Electrocorticographic spectral changes associated with ipsilateral individual finger and whole hand movement. IEEE Eng Med Biol Soc. 2008:5939–5942. doi: 10.1109/IEMBS.2008.4650569. [DOI] [PubMed] [Google Scholar]