Abstract
In cardiac resynchronisation therapy, failure to implant a left ventricular lead in a coronary sinus branch has been reported in up to 10% of cases. Although surgical insertion of epicardial leads is considered the standard alternative, this is not without morbidity and technical limitations. Endocardial left ventricular pacing can be an alternative as it has been associated with a favourable acute haemodynamic response compared with epicardial pacing in both animal and human studies. In this paper, we discuss left ventricular endocardial pacing and compare it with epicardial surgical implantation. Ease of application and procedural complications and morbidity compare favourably with epicardial surgical techniques. However, with limited experience, the most important concern is the still unknown long-term risk of thromboembolic complications. Therefore, for now endovascular implants should remain reserved for severely symptomatic heart failure patients and patients at high surgical risk of failed coronary sinus implantation.
Keywords: Cardiac resynchronization therapy, Left ventricular pacing, Endocardial stimulation, Transseptal catheterization
Introduction
Cardiac resynchronisation therapy (CRT) is an established treatment modality for patients with systolic heart failure, left ventricular (LV) dyssynchrony and intraventricular conduction disturbances [1–4]. The standard approach requires inserting an LV pacing lead into one of the coronary sinus tributaries. However, failure to deliver a coronary sinus lead has been reported in up to 10% of attempts [5, 6]. Post-implant dislocation of the lead, non-capture or phrenic nerve stimulation also reduces effective LV stimulation [5].
Until now, surgical epicardial lead implantation was considered the standard alternative for failed coronary sinus implants [7–12]. In this paper we will introduce endocardial LV stimulation and compare it with the surgical alternatives.
Why endocardial stimulation?
In a previously published report, we demonstrated that in a non-responder to coronary sinus LV pacing, endocardial LV pacing delivered both a substantial acute haemodynamic improvement (measured by LV dP/dt max) and long-term clinical amelioration [13]. This favourable effect of endocardial pacing has also been shown in animal studies: after the induction of left bundle branch block, endocardial stimulation yielded better acute haemodynamic improvement than pacing from the opposing epicardial site in dogs [14]. This was also demonstrated by Rademakers et al. in a heart failure model of myocardial infarction and induced left bundle branch block [15]. In patients, Derval et al. demonstrated that LV pacing in non-ischaemic cardiomyopathy was significantly better at the best endocardial site than (single site) coronary sinus pacing [16]. Spragg et al. obtained similar results in ischaemic cardiomyopathy [17]. However, both authors evaluated a single epicardial coronary sinus pacing site only and noted that endocardial stimulation at an opposing site from the coronary sinus lead pacing location was not haemodynamically better in these acute studies. Therefore, although stimulation at endocardial and epicardial sites opposite from each other has not been investigated for multiple locations, it seems that in patients the ability to stimulate often inaccessible sites with coronary sinus pacing is a main advantage of endocardial stimulation [13]. Thus evidence shows that endocardial pacing is at least a good alternative route for failed coronary sinus implantation, but may also be very useful in non-responders to standard CRT in case of suboptimal lead positioning.
How to stimulate the LV endocardium?
LV endocardial pacing was first described by Jais et al. [18]. They inserted a guidewire transseptally from the femoral vein into the left atrium, and used a retriever inserted via the right internal jugular vein to recover the proximal end of the guidewire. A sheath was then advanced from the jugular vein over the guidewire into the left atrium enabling the implantation of a pacing wire at the LV endocardium. However, the procedure was complicated due to the easy loss of the guidewire position in the left atrium.
Others attempted a transseptal puncture from the right jugular or even the subclavian vein, but this demands special ability from the operator as this approach lacks the traditional landmarks of a standard femoral transseptal puncture. Also the transseptal needle needs to be grossly modified according to the patient’s anatomy [19, 20].
These approaches are not suited for routine application. An ideal method should use standard techniques and materials, be within the capabilities of the average operator and have a high predictable success rate. Further, it should preferably be executable in the same procedure as a failed coronary sinus implantation. In the following paragraphs, we describe the evolution of our approach to obtain these goals.
Femoral septum perforation but superior lead introduction
We started with a standard transseptal puncture from the femoral vein, but to facilitate the passage of the septum we dilated the puncture site first with a 6 mm angioplasty balloon. Subsequently, leaving the guidewire across the septum as a marker, we tried to pass the septum with a guidewire inserted from the subclavian vein through a deflectable guiding catheter. However, despite the additional use of curved inner sheaths, anatomical constraints often hamper aiming the guidewire at the septal puncture site. There were sometimes also problems in passing the sheath through the septum because of lack of backup support in the right atrium. However, once the sheath was advanced into the left atrium, it was often easy to point the tip through the mitral valve towards the desired basal posterolateral area of the left ventricle. Development of dedicated sheaths might facilitate the procedure, but until now it still does not satisfy the requirements for an ideal procedure. A detailed description of the procedure has been previously published [21].
Superior RF septum perforation and lead introduction
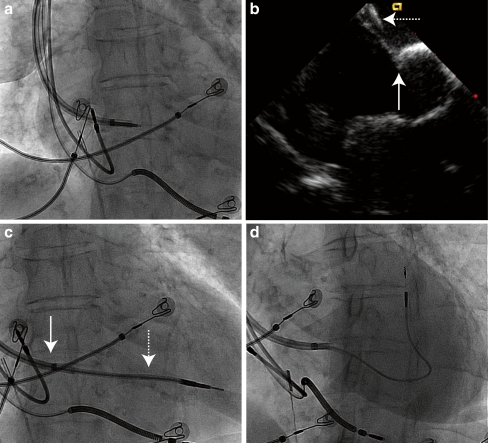
To avoid aiming a guidewire through an existing puncture site, we again tried to puncture the septum using a superior approach, but with the help of radiofrequency (RF) energy (Fig. 1). The latter has already been used to facilitate standard transseptal puncture and we modified the concept for a superior transseptal puncture [22]. For this purpose, a deflectable sheath loaded with an inner sheath and its dilator (Attain Select II, Medtronic Inc. Minneapolis, MN, USA) is pointed towards the septum from the subclavian vein and advanced until the septum is clearly ‘tented’ on intracardiac or transoesophageal echo (Fig. 1b). A standard 0.035″ guidewire is then protruded just outside the dilator of the inner sheath (Fig. 1a). With slight pressure on the guidewire, unipolar RF energy from a standard electrosurgical unit is applied on the proximal ending of the guidewire to ablate the tip through the septum. Then the dilator and inner sheath are advanced into the left atrium and ventricle whilst the deflectable sheath remains in the right atrium (Fig. 1c). A 110 cm long Select Secure lead (Medtronic 3830-110, Medtronic Inc. Minneapolis, MN, USA) is inserted into the LV myocardium. The extra length is necessary to accommodate for the length of the sheaths. The Attain Select II is withdrawn into the right atrium and both sheaths are subsequently split (Fig. 1d).
Fig. 1.
Atrial septal puncture by a superior approach with radiofrequency and introduction of the lead superiorly. a The assemblage of the deflectable sheath, the Attain Select II sheath and dilator positioned against the fossa ovalis with the guidewire just protruding from the dilator. b On intracardiac echocardiography, the tenting (solid arrow) of the inter-atrial septum by the sheaths (dashed arrow) is shown. c The Attain Select II with the dilator (dashed arrow) is advanced over the guidewire towards the left ventricle. The deflectable sheath (solid arrow) remains at the right atrial surface of the septum. d Final position of the Select Secure lead with the Attain Select catheter withdrawn in the steerable guiding catheter at the right side of the septum. See text for full discussion
Ablating through the septum is easy once the sheath is perpendicular to the septum and the latter properly tented. But this can prove to be more difficult than expected: the sheaths often slide from the septum thus missing the necessary tenting before RF energy can be applied.
Femoral lead introduction with lead retrieval to the pocket
Transseptal puncture from the femoral vein has been largely unaltered since the description by Brockenbrough et al. 50 years ago, and this remains the most predictable way to cross the atrial septum [23]. Additionally, transfemoral implantation of pacemaker leads has also been used for more than 30 years [24, 25]. Therefore, inserting a permanent LV endocardial lead via a classic transseptal catheterisation seems an obvious solution, with the only obstacle being retrieval of the lead to the subpectoral area. We have developed two solutions for this problem: a subcutaneous and an endovascular route.
We first modified a standard 8F Mullins transseptal sheath into a slittable one: the native hub-valve is cut away and replaced with the valve from a standard 9 F peel away SafeSheath (Pressure Products, San Pedro, CA, USA) which is secured by applying Dermabond skin adhesive (Ethicon, Somerville, NJ, USA).
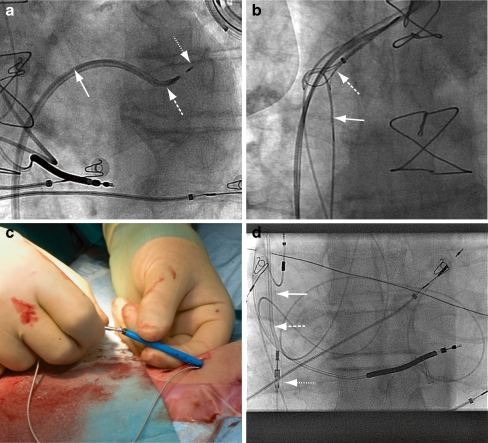
We start with a standard transseptal puncture with this modified Mullins sheath. Once the Mullins sheath is in the left atrium we direct an Attain Select II inner sheath with 130º tip towards the left ventricle (Fig. 2a). With this sheath, we can rove the LV endocardium to select the pacing site. A 110 cm long 4.1 F active fixation Select Secure lead is implanted, the length being necessary to accommodate for the length of the sheaths and to allow enough slack while retrieving the sheaths.
Fig. 2.
Transfemoral introduction of the lead with transvenous tunnelling towards the generator pocket. a The Mullins sheath (solid arrow) has passed the septum and the Attain Select guiding system (long dashed arrow) has been directed over a guidewire into the left ventricle. The tip of the Attain Select is rotated towards the basal posterolateral wall of the left ventricle. The Select Secure lead (short dashed arrow) is positioned against the myocardium. b The stiff guidewire (solid arrow), introduced via the Mullins sheath towards the superior vena cava, is captured by a snare (dashed arrow), which was introduced via the subclavian vein. c The lead secured into the angiocatheter is introduced into the 12 F sheath that emerges retrogradely from the femoral vein through the same puncture site as the previously introduced lead. d The 12F sheath (solid arrow) and the angiocatheter (long dashed arrow) with the Select Secure lead (short dashed arrow) are pulled back through the venous system. The tip of the sheath is still in the vena cava inferior, but the lead has already been pulled into the vein
With a subcutaneous approach both the Attain Select and the Mullins sheath are withdrawn towards the right atrium and slit in a standard fashion. The lead is then tunnelled subcutaneously with a technique similar to an axillofemoral bypass towards the device pocket in the subclavicular area. As the 110 cm Select Secure lead is not long enough to directly bridge this distance, it is first tunnelled towards an incision just below the ribcage. From there on the lead is extended with a 35 cm long bipolar lead extension and further tunnelled towards the subpectoral pocket. The whole procedure is performed under local anaesthesia with additional sedation with midazolam and fentanyl as required.
To avoid the subcutaneous tunnelling and extension of the lead, we modified a technique first described by Dhillon et al. [26]. A 110 cm Select Secure lead is inserted from the femoral vein into the LV endocardium through the modified Mullins sheath as described above. After the sheaths have been withdrawn into the right atrium, the two procedures differ: only the Attain Select II is first removed, leaving the Mullins sheath in the right atrium. A 260 cm 0.038″ stiff guidewire is then introduced along the pacing lead into the Mullins sheath and advanced to the superior vena cava, from where it is retrieved from the subclavian vein with a snare (Fig. 2b). The Mullins sheath is then slit and a 85 cm long 12 F sheath is inserted over the guidewire from the subclavian vein and advanced until it emerges from the femoral vein, thus exiting through the same puncture site as the lead. To retrieve the pacing lead, the connector pin is fitted into the tip of a 6 F right Judkins angiography catheter inserted using a superior approach through the 12 F sheath (Fig. 2c). The angiography catheter with the Select Secure lead attached is then pulled into the 12 F sheath, and both are pulled back together until the sheath—and the lead—emerge from the subclavian vein (Fig. 2d). The lead is released from the angiography catheter and secured in the pocket.
Although the last technique seems to be quite complex (for a detailed description see Van Gelder et al. [27]) it comes closest to an ideal technique: (almost) standard equipment and techniques, and each individual step has a high probability of success. Until now we have applied this technique successfully in 13 patients.
Limitations of an endocardial approach
Thromboembolic complications are common in patients with severe LV dysfunction: excluding patients with atrial fibrillation, the incidence estimates range from 1.5 to 3.5% per 100 patient-years [28]. This risk may increase with indwelling LV leads. So far, thromboembolic complications have also been reported in inadequately anticoagulated CRT patients with LV endocardial leads. After mid- to long-term follow-up, Jais et al. and Pasquié et al. reported a transient ischaemic attack in 1 out of 11 and 1 out of 6 patients, respectively, both with anticoagulation interrupted at the time of the incident [29, 30]. Until now, we have encountered 3 thromboembolic complications in 42 patients, all 3 also inadequately anticoagulated at the time of the embolism (INR <1.5 in 2 patients, and <2.0 in one patient, respectively). In one patient this was shortly after electrical cardioversion for atrial fibrillation [13]. Two of the patients recovered completely, one suffered a severe stroke. Also from the experience with inadvertently placed LV endocardial leads, it is clear that life-long anticoagulation with coumarin derivatives is mandatory, but once installed the risk of embolism seems to be low [31, 32]. We target an INR between 3.5 and 4.5, similar to that with mechanical prosthetic valves. Therefore, if anticoagulation is contraindicated or not expected to be adequately maintained, one should refrain from LV endocardial lead insertion. Newer anticoagulant agents may prove to be superior for this indication [33]. To further minimise the risk for clotting, the use of polyurethane insulated leads is mandatory as they are less prone to thrombus formation than silicone insulated leads [34, 35]. As of now, the true risk increment from the LV leads still needs to be determined.
Secondly, limited experience shows that the LV leads do not seem to interfere significantly with mitral valve function, maybe due to the minimal scar tissue reaction in the left heart [35]. Also the use of a thin, floppy lead provided with enough slack may prevent valve dysfunction.
The surgical approach
Historically, surgical epicardial lead placement has become the standard alternative for coronary sinus implantation. Studies comparing both techniques revealed excellent long-term lead results and fewer LV-related complications for the surgical approach [10]. Different techniques have been described using mini-thoracotomy, video-assisted thoracoscopic surgery (VATS) and robotic surgery. Surgery has the theoretical advantage of no limitation as to the choice of pacing sites compared with a coronary sinus route. However, Koos et al. reported that leads were more often implanted too anteriorly compared with CS implants (44% vs. 5.4%), resulting in a smaller increase in ejection fraction and functional capacity [36]. To improve on this, malleable epicardial lead placement tools might be helpful to reach the preferred target area [7]. VATS might provide a better opportunity to reach the posterolateral wall; however, it requires expertise and is relatively expensive [8, 9, 11]. In our experience too, we have noticed that the posterolateral surface of the left heart is sometimes hard to reach, especially in patients who have undergone previous surgery or with grossly enlarged hearts. Four of our endocardially treated patients had a previous epicardial surgical implant with a position that is too anterior.
In reports on surgical epicardial implantation, the complication rate appears to be low and procedural mortality has been reported, and Mair et al. reported on 16 patients, two of whom developed a pneumothorax and one an atelectasis [10]. Navia et al. mentioned renal failure not requiring haemodialysis and decompensated heart failure, both in two out of 34 patients [11]. However, as with endocardial implants, these are small studies. In a recent large retrospective study on postoperative mortality in heart failure patients with both ischaemic and non-ischaemic origin, patients who underwent noncardiac minor surgery experienced a 30-day mortality of 8% [37]. In our own experience with surgical implantation, one patient died after a periprocedural myocardial infarction We also encountered ventilation-related problems and haemodynamic instability necessitating inotropic support. Postoperative thoracotomy-related pain must be added to morbidity.
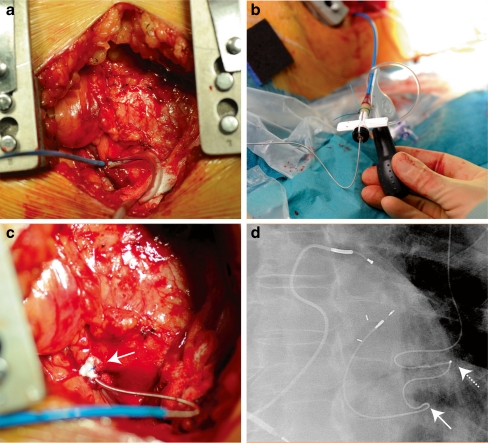
Finally, a combination of an endocardial implant and a surgical approach was first described by Kassai et al. [38]. A limited left lateral thoracotomy is made directly over of the LV apex and a purse string suture applied at the LV apex through which the left ventricle is punctured with a standard Seldinger technique, allowing the introduction of a deflectable sheath into the left ventricle (Fig. 3a). The sheath can be easily manoeuvred to the desired position and a 74 cm long Select Secure lead is screwed into the endocardium (Fig. 3b). Haemostasis is established by closing the purse string suture around the lead after slitting the sheath (Fig. 3c). From the intercostal incision, the lead is tunnelled subcutaneously to the generator pocket in the left pectoral region (Fig. 3d).
Fig. 3.
Transapical surgical insertion of a left ventricular endocardial lead. a The Select Secure guiding system is introduced through the LV apex and bleeding controlled by tightening the purse string around it. b The Select Secure lead is introduced into the guiding system, which can be deflected by turning the knob. c The Select Secure lead is implanted, the sheath withdrawn before splitting, and the pursue string fortified with additional pledge material tightened around the puncture site. d X-ray of final position of the lead. The solid arrow indicates the exit site of the lead from the left ventricle, the dashed arrow the anchoring site of the lead at the ribcage
This approach is useful when a transseptal implant is not possible or in the presence of a mitral valve prosthesis. It still necessitates general anaesthesia, but is technically less demanding then epicardial lead placement because the left ventricle may be approached from anteriorly.
How to choose between endocardial and surgical LV lead implantation?
There are no studies confirming haemodynamic superiority of one of the two approaches in humans. Although in the majority of patients it is possible to find a better acute haemodynamic response at an endocardial site compared with a single coronary sinus site, there is no proof of a difference in (either acute or chronic) haemodynamic effect when multiple endocardial and epicardial sites could be compared.
Therefore, as of now the choice has to be made on factors relating to the technical merits and reported complications. A cardiologist who is experienced with CRT should be able to perform an endocardial approach on site and under local anaesthesia (on the condition that he or she is familiar with transseptal catheterisation). In contrast, a surgical implant usually necessitates a second procedure in a centre with cardiothoracic surgery. Technically, it is quite easy to reach the preferred basal posterolateral implant area endocardially as this is the first area encountered with a transseptal approach when passing the mitral valve. Overall, the procedural risk is more limited with an endocardial approach and less morbidity is to be expected compared with surgery, especially in these severe heart failure patients. Also the hospital stay can be significantly shorter.
However, the main concern of endocardial leads remains thromboembolic complications. Given this uncertainty with the currently limited experience we reserve endocardial implants for patients with severely symptomatic class III or IV NYHA heart failure or other high surgical risk patients, as the surgical risk probably outweighs the thromboembolic risk in these patients. Lately, we have expanded the indication to NYHA class III or IV patients not responding to standard coronary sinus CRT, but only after establishing potential efficacy with LV dp/dt max measurements and temporary endocardial pacing [13].
Recently, the benefit of CRT has been shown in less symptomatic NYHA class I or II patients [39, 40]. These studies did not include either surgical epicardial or endocardial techniques, so it remains uncertain if these approaches will be beneficial in these patient categories. As improving prognosis but not acute symptomatic improvement is the primary goal in NYHA class I or II patients, it may be prudent to use the more accepted surgical epicardial implant technique if CRT is considered desirable when these patients fail coronary sinus implantation, at least until further data on thromboembolic complications of endocardial leads become available.
Conclusion
Endocardial left ventricular lead implantation has become a new valuable and efficient technique for providing LV stimulation to patients failing standard coronary sinus implants. Currently, the implementation should remain restricted to severely symptomatic, high surgical risk patients until more data are available concerning the risk of thromboembolism.
Contributor Information
F. A. Bracke, Phone: +31-40-2397004, FAX: +31-40-2447885, Email: f.bracke@me.com
J. F. ter Woorst, Phone: +31-40-2399111
J. A. Teijink, Phone: +31-40-2399111
References
- 1.Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–53. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 2.Cazeau S, Leclercq C, Lavergne T, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344:873–80. doi: 10.1056/NEJM200103223441202. [DOI] [PubMed] [Google Scholar]
- 3.Cleland JGF, Daubert J-C, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 4.Auricchio A, Stellbrink C, Sack S, et al. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol. 2002;39:2026–33. doi: 10.1016/S0735-1097(02)01895-8. [DOI] [PubMed] [Google Scholar]
- 5.León AR, Abraham WT, Curtis AB, et al. Safety of transvenous cardiac resynchronization system implantation in patients with chronic heart failure: combined results of over 2,000 patients from a multicenter study program. J Am Coll Cardiol. 2005;46:2348–56. doi: 10.1016/j.jacc.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 6.Gras D, Leclercq C, Tang ASL, et al. Cardiac resynchronization therapy in advanced heart failure the multicenter InSync clinical study. Eur J Heart Fail. 2002;4:311–20. doi: 10.1016/S1388-9842(02)00018-1. [DOI] [PubMed] [Google Scholar]
- 7.Doll N, Opfermann UT, Rastan AJ, et al. Facilitated minimally invasive left ventricular epicardial lead placement. Ann Thorac Surg. 2005;79:1023–5. doi: 10.1016/j.athoracsur.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 8.Gabor S, Prenner G, Wasler A, et al. A simplified technique for implantation of left ventricular epicardial leads for biventricular resynchronisation using video-assisted thoracoscopy (VATS) Eur J Cardio Thorac Surg. 2005;28:797–800. doi: 10.1016/j.ejcts.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 9.Jansens J-L, Jottrand M, Preumont N, et al. Robotic-enhanced biventricular resynchronization: an alternative to endovenous cardiac resynchronization therapy in chronic heart failure. Ann Thorac Surg. 2003;76:413–7. doi: 10.1016/S0003-4975(03)00435-1. [DOI] [PubMed] [Google Scholar]
- 10.Mair H, Sachweh J, Meuris B, et al. Surgical epicardial left ventricular lead versus coronary sinus lead placement in biventricular pacing. Eur J Cardiothorac Surg. 2005 1;27(2):235–42. [DOI] [PubMed]
- 11.Navia JL, Atik FA. Minimally invasive surgical alternatives for left ventricle epicardial lead implantation in heart failure patients. Ann Thorac Surg. 2005;80:751–4. doi: 10.1016/j.athoracsur.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Puglisi A, Lunati M, Marullo AGM, et al. Limited thoracotomy as a second choice alternative to transvenous implant for cardiac resynchronisation therapy delivery. Eur Heart J. 2004;25:1063–9. doi: 10.1016/j.ehj.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Bracke FA, Houthuizen P, Rahel BM, et al. Left ventricular endocardial pacing improves the clinical efficacy in a non-responder to cardiac resynchronization therapy: role of acute haemodynamic testing. Europace. 2010;12:1032–4. doi: 10.1093/europace/euq043. [DOI] [PubMed] [Google Scholar]
- 14.Deursen C, Geldorp IE, Rademakers LM, et al. Left ventricular endocardial pacing improves resynchronization therapy in canine left bundle-branch hearts. Circ Arrhythm Electrophysiol. 2009;2:580–7. doi: 10.1161/CIRCEP.108.846022. [DOI] [PubMed] [Google Scholar]
- 15.Rademakers LM, Kerckhoven R, Deursen CJM, et al. Myocardial infarction does not preclude electrical and hemodynamic benefits of cardiac resynchronization therapy in dyssynchronous canine hearts. Circ Arrhythm Electrophysiol. 2010;3:361–368. doi: 10.1161/CIRCEP.109.931865. [DOI] [PubMed] [Google Scholar]
- 16.Derval N, Steendijk P, Gula LJ, et al. Optimizing hemodynamics in heart failure patients by systematic screening of left ventricular pacing sites: the lateral left ventricular wall and the coronary sinus are rarely the best sites. J Am Coll Cardiol. 2010;55:566–75. doi: 10.1016/j.jacc.2009.08.045. [DOI] [PubMed] [Google Scholar]
- 17.Spragg DD, Dong J, Fetics BJ, et al. Optimal left ventricular endocardial pacing sites for cardiac resynchronization therapy in patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2010;56:774–81. doi: 10.1016/j.jacc.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Jaïs P, Douard H, Shah DC, et al. Endocardial biventricular pacing. Pacing Clin Electrophysiol. 1998;21:2128–31. doi: 10.1111/j.1540-8159.1998.tb01133.x. [DOI] [PubMed] [Google Scholar]
- 19.Leclercq F, Hager FX, Macia JC, et al. Left ventricular lead insertion using a modified transseptal catheterization technique: a totally endocardial approach for permanent biventricular pacing in end-stage heart failure. Pacing Clin Electrophysiol. 1999;22:1570–5. doi: 10.1111/j.1540-8159.1999.tb00374.x. [DOI] [PubMed] [Google Scholar]
- 20.Ji S, Cesario DA, Swerdlow CD, et al. Left ventricular endocardial lead placement using a modified transseptal approach. J Cardiovasc Electrophysiol. 2004;15:234–36. doi: 10.1046/j.1540-8167.2004.03431.x. [DOI] [PubMed] [Google Scholar]
- 21.Gelder BM, Scheffer MG, Meijer A, et al. Transseptal endocardial left ventricular pacing: an alternative technique for coronary sinus lead placement in cardiac resynchronization therapy. Heart Rhythm. 2007;4:454–60. doi: 10.1016/j.hrthm.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 22.McWilliams MJ, Tchou P. The use of a standard radiofrequency energy delivery system to facilitate transseptal puncture. J Cardiovasc Electrophysiol. 2009;20:238–40. doi: 10.1111/j.1540-8167.2008.01323.x. [DOI] [PubMed] [Google Scholar]
- 23.Brockenbrough EC, Braunwald E, Ross J. Transseptal left heart catheterization a review of 450 studies and description of an improved technique. Circulation. 1962;25:15–21. doi: 10.1161/01.cir.25.1.15. [DOI] [PubMed] [Google Scholar]
- 24.Gamal El M, Gelder B. Preliminary experience with the helifix electrode for transvenous atrial implantation. Pacing Clin Electrophysiol. 1979;2:444–54. doi: 10.1111/j.1540-8159.1979.tb05220.x. [DOI] [PubMed] [Google Scholar]
- 25.Bracke FA, Ozdemir I, Gelder B. The femoral route revisited: an alternative for pectoral pacing lead implantation. Neth Heart J. 2010;18:42–4. [PMC free article] [PubMed] [Google Scholar]
- 26.Dhillon PS, Gallagher MM. Femoral vein implantation with subclavian vein pullthrough for left ventricular lead placement. Europace. 2010;12:1193–4. doi: 10.1093/europace/euq088. [DOI] [PubMed] [Google Scholar]
- 27.van Gelder BM, Houthuizen P, Bracke FA. Transseptal left ventricular endocardial pacing: preliminary experience from a femoral approach with subclavian pull-through. Europace. 2011 published online May 11; doi: 0.1093/europace/eur136 [DOI] [PubMed]
- 28.Sirajuddin RA, Miller AB, Geraci SA. Anticoagulation in patients with dilated cardiomyopathy and sinus rhythm: a critical literature review. J Card Fail. 2002;8:48–53. doi: 10.1054/jcaf.2002.31907. [DOI] [PubMed] [Google Scholar]
- 29.Pasquié JL, Massin F, Macia JC, et al. Long-term follow-up of biventricular pacing using a totally endocardial approach in patients with end-stage cardiac failure. Pacing Clin Electrophysiol. 2007;30(Suppl 1):S31–3. doi: 10.1111/j.1540-8159.2007.00599.x. [DOI] [PubMed] [Google Scholar]
- 30.Jaïs P, Takahashi A, Garrigue S, et al. Mid-term follow-up of endocardial biventricular pacing. Pacing Clin Electrophysiol. 2000;23:1744–7. doi: 10.1111/j.1540-8159.2000.tb07010.x. [DOI] [PubMed] [Google Scholar]
- 31.Bordachar P, Derval N, Ploux S, et al. Left ventricular endocardial stimulation for severe heart failure. J Am Coll Cardiol. 2010;56:747–53. doi: 10.1016/j.jacc.2010.04.038. [DOI] [PubMed] [Google Scholar]
- 32.Gelder BM, Bracke FA, Oto A, et al. Diagnosis and management of inadvertently placed pacing and ICD leads in the left ventricle: a multicenter experience and review of the literature. Pacing Clin Electrophysiol. 2000;23:877–83. doi: 10.1111/j.1540-8159.2000.tb00858.x. [DOI] [PubMed] [Google Scholar]
- 33.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 34.Palatianos GM, Dewanjee MK, Panoutsopoulos G, et al. Comparative thrombogenicity of pacemaker leads. Pacing Clin Electrophysiol. 1994;17:141–5. doi: 10.1111/j.1540-8159.1994.tb01364.x. [DOI] [PubMed] [Google Scholar]
- 35.Reinig M, White M, Levine M, et al. Left ventricular endocardial pacing: a transarterial approach. Pacing Clin Electrophysiol. 2007;30:1464–8. doi: 10.1111/j.1540-8159.2007.00892.x. [DOI] [PubMed] [Google Scholar]
- 36.Koos R, Sinha A-M, Markus K, et al. Comparison of left ventricular lead placement via the coronary venous approach versus lateral thoracotomy in patients receiving cardiac resynchronization therapy. Am J Cardiol. 2004;94:59–63. doi: 10.1016/j.amjcard.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 37.Diepen S, Bakal JA, McAlister FA, et al. Mortality and readmission of patients with heart failure, atrial fibrillation, or coronary artery disease undergoing noncardiac surgery: an analysis of 38 047 patients. Circulation. 2011;124:289–96. doi: 10.1161/CIRCULATIONAHA.110.011130. [DOI] [PubMed] [Google Scholar]
- 38.Kassai I, Foldesi C, Szekely A, et al. Alternative method for cardiac resynchronization: transapical lead implantation. Ann Thorac Surg. 2009;87:650–2. doi: 10.1016/j.athoracsur.2008.04.080. [DOI] [PubMed] [Google Scholar]
- 39.Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–38. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 40.Tang ASL, Wells GA, Talajic M, et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363:2385–95. doi: 10.1056/NEJMoa1009540. [DOI] [PubMed] [Google Scholar]