Abstract
Trichomonas vaginalis is a parasite from the human urogenital tract that causes trichomonosis, the most prevalent non-viral sexually transmitted disease. The neutrophil infiltration has been considered to be primarily responsible for cytological changes observed at infection site, and the chemoattractants can play an important role in this leukocytic recruitment. Nitric oxide (NO) is one of the most widespread mediator compounds, and it is implicated in modulation of immunological mechanisms. Extracellular nucleotides and nucleosides are signaling molecules involved in several processes, including immune responses and control of leukocyte trafficking. Ectonucleoside triphosphate diphosphohydrolase members, ecto-5′-nucleotidase, and adenosine deaminase (ectoADA) have been characterized in T. vaginalis. Herein, we investigated the effects of purinergic system on NO production by neutrophils stimulated with T. vaginalis. The trophozoites were able to induce a high NO synthesis by neutrophils through iNOS pathway. The extracellular nucleotides ATP, ADP, and ATPγS (a non-hydrolyzable ATP analog) showed no significant change in NO secretion. In contrast, adenosine and its degradation product, inosine, promoted a low production of the compound. The immunosuppressive effect of adenosine upon NO release by neutrophils occurred due to adenosine A2A receptor activation. The ecto-5′-nucleotidase activity displayed by T. vaginalis was shown to be important in adenosine generation, indicating the efficiency of purinergic cascade. Our data suggest the influence of purinergic signaling, specifically adenosinergic system, on NO production by neutrophils in T. vaginalis infection, contributing to the immunological aspects of disease.
Keywords: Trichomonas vaginalis, Neutrophils, Nitric oxide, Purinergic signaling, Ectonucleotidases, Adenosine
Introduction
Trichomonas vaginalis is a flagellated protozoan that causes trichomonosis, the most common non-viral sexually transmitted disease (STD) in humans. There are 160–180 million cases occurring each year worldwide [1]. Trichomonosis has been associated with serious health consequences for women, including adverse pregnancy outcomes [2], infertility [3], predisposition to cervical cancer [4], and pelvic inflammatory disease [5]. The infection among men is a recognized cause of urethritis, and complications such as prostatitis, epididymitis, and infertility have been described [6]. Moreover, trichomonosis is a co-factor in human immunodeficiency virus (HIV) transmission and acquisition [7]. Considering all these aspects and the importance of this STD on public health, it is important to investigate biochemical and cellular mechanisms that contribute to host infection and pathogenesis.
In women, T. vaginalis causes a wide spectrum of symptoms, ranging from a relatively asymptomatic state to severe inflammation and irritation with foul-smelling vaginal discharge, low abdominal pain, and dysuria [8, 9]. Importantly, the infection is often recurrent, with no lasting immunity, suggesting the importance of innate immunity [10]. In this way, neutrophil infiltration has been considered to be primarily responsible for cytological change, but little is known about the exact mechanism of how neutrophils accumulate or mediate the initial inflammatory response after an acute T. vaginalis infection [11]. Nevertheless, it is generally believed that chemoattractants, such as interleukin-8 (IL-8), reactive nitrogen intermediates, and leukotrienes generated at reaction sites, may have an important role. Previous studies have already demonstrated that the production of IL-8 by neutrophils [12] and monocytes [13] is stimulated in response to T. vaginalis. In addition, nitric oxide (NO) is an effector of macrophage-mediated cytotoxicity against the parasite [14], and leukotriene B4 (LTB4) is a neutrophil-activating factor released by T. vaginalis [15]. These molecules are found in the vaginal discharges of symptomatic patients [16] and provide the induction of a rapid accumulation of neutrophils at the affected site in order to eliminate the invading mucosal pathogen [17].
Nucleotides such as ATP, ADP, and AMP, and their nucleoside derivative, adenosine, are found in all animal organ systems where they produce effects by intracellular and extracellular mechanisms [18]. Extracellular nucleotides are signaling molecules involved in several processes, including immune responses and control of leukocyte trafficking between the blood and tissues [19]. ATP's role in immunity is closely related to its breakdown product, the adenosine. The nucleoside has an already established role in immunity, in which it may contribute to the engineering of inflammation and immune responses by providing a suppressive tissue-protecting signal in a delayed, negative feedback manner [20]. The notion of an interrelation between ATP and adenosine is firmly based on the presence of a large family of purinergic receptors (P1 and P2 receptors for adenosine and ATP, respectively) [21]. Ectonucleotidases are a group of ecto-enzymes involved in a purinergic cascade by which extracellular purine levels and the ensuing purinergic signaling can be dynamically controlled during inflammatory and immune responses. These enzymes include the ectonucleoside triphosphate diphosphohydrolase (E-NTPDase) family, which hydrolyze nucleoside tri- and diphosphates, and the ecto-5′-nucleotidase, which hydrolyzes nucleoside monophosphates [22]. Together, ecto-5′-nucleotidase and adenosine deaminase (ADA), another ectoenzyme that is involved in purine salvage pathways and converts adenosine to inosine, closely regulate local, extracellular, and plasma concentrations of adenosine [23]. Previous studies from our group have already characterized NTPDase [24], ecto-5′-nucleotidase [25], and ecto-ADA [26] activities in trophozoites of T. vaginalis.
In this sense, considering (1) the importance of purinergic signaling on immune responses and (2) the necessity to elucidate how neutrophils mediate the initial inflammatory response in trichomonosis, the present study investigated the involvement of purinergic signaling through ectonucleotidases, nucleotides, nucleoside, and purinoceptors on NO production by neutrophils stimulated with T. vaginalis.
Materials and methods
Chemicals
Lipopolysaccharide (LPS), nucleotides (ATP, ADP, AMP), adenosine 5′-(3-thiotriphosphate) tetralithium salt (ATPγS), adenosine, inosine, erythro-9-(2-hydroxy-3-nonly) adenine (EHNA), α,β-methylene adenosine diphosphate (AMPCP), NG-methyl-l-arginine acetate salt (l-NMMA), CGS21680 and caffeine were purchased from Sigma Chemical Co (St. Louis, MO, USA).
Culture of T. vaginalis
T. vaginalis isolates, 30236 (from the American Type Culture Collection) and TV-LACM2, a fresh clinical isolate (from Laboratório de Análises Clínicas, Faculdade de Farmácia, UFRGS, Brazil, (project with ethical approval by UFRGS Ethical Committee, number 18923), were used in this study. Trichomonads were cultured axenically in vitro on trypticase-yeast extract-maltose (TYM) medium, (pH 6.0), supplemented with 10% (v/v) heat-inactivated bovine serum, and incubated at 37°C (±0.5) [27]. Organisms in the logarithmic phase of growth exhibiting motility and normal morphology were harvested, centrifuged, washed three times with phosphate buffered saline 1× (PBS), and resuspended on new TYM medium for the experiments.
Enzyme assays
Trophozoites were harvested and washed three times with 0.9% (w/v) NaCl solution, and the viability was checked using trypan blue (0.2%) dye exclusion. The parasites' suspension was diluted to a final protein concentration of approximately 0.6 mg/mL (for both isolates) to ensure linearity in the enzyme assays. Protein was measured by the Coomassie Blue method [28], using bovine serum albumin as standard.
Intact organisms were added to the NTPDase reaction mixture (50 mM Tris buffer, pH 7.2, and 5.0 mM CaCl2) for measuring ATP and ADP hydrolysis [24]. The same density of parasites was added to the ecto-5′-nucleotidase reaction mixture (50 mM Tris buffer, pH 7.5, and 33.0 mM MgCl2) [25]. The samples were preincubated for 5 min at 37°C in 200 μL of the reaction mixture. The reaction started with the addition of substrates: ATP and ADP (final concentration 1.0 mM), for NTPDase assay, and AMP (final concentration 3.0 mM), for ecto-5′-nucleotidase assay. The reaction was terminated by adding 200 μL of 10% (v/v) trichloroacetic acid (TCA). The samples were chilled on ice for 10 min before the release of inorganic phosphate (Pi) measurement [29]. Controls included intact organisms added to the reaction mixtures containing TCA in order to correct non-enzymatic hydrolysis of substrates, and the averages of control values were subtracted from the test samples. Specific activity was expressed as nanomoles Pi released per minute per milligram of protein. All samples were run in triplicate at least in three independent experiments.
Isolation of human neutrophils
Human neutrophils were isolated as previously described [30], with some modifications. Briefly, venous blood of healthy volunteers (ethical approved by UFRGS Ethical Committee, number 19346) was collected on heparin anticoagulant solution, centrifuged (250×g, 10 min, 24°C), and the resulting platelet-rich plasma discarded. Leukocytes were obtained following erythrocyte sedimentation in 2% Dextran T-500 and centrifuged (525×g, 20 min, 24°C) through a layering on Histopaque 1077 (Sigma, St. Louis, MO, USA). The neutrophil-enriched pellet was subjected to a 15-s hypotonic lysis to remove the remaining erythrocytes and centrifuged (1,000×g, 5 min, 24°C). The purified neutrophils were resuspended in RPMI 1640 supplemented with 10% fetal bovine serum and 10 mM HEPES for the next experiments. The purity of neutrophils was confirmed morphologically (>95%) and examined by flow cytometry (FACSCalibur, BD Bioscience, Franklin Lakes, NJ, USA). The phenotypic analysis were realized by Cell Quest BD and Paint a Gate Pro BD softwares, after staining with fluorescein isothiocyanate (FITC)-conjugated anti-CD45, anti-CD15, anti-CD8 antibodies and phycoerythrin (PE)-conjugated anti-CD14, anti-CD22, anti-CD3, and anti-CD4 antibodies (BD Bioscience, Franklin Lakes, NJ, USA).
Culture condition of neutrophils and T. vaginalis
The NO release curve was performed with neutrophils (2.0 × 105) and 100 ng/mL LPS (used as positive control) into 24 h (5, 30, 60, 360, and 1,440 min) in order to choose the best condition for the next experiments. Thus, the neutrophils were cocultured with live trophozoites (1.0 × 104) on 96-well microplate, for 24 h, in presence of 100 μM nucleotides (ATP, ADP, AMP), 100 μM ATPγS (a non-hydrolyzable ATP analog), 100 μM adenosine, 100 μM inosine, or ecto-5′-nucleotidase, ADA, and inducible nitric oxide synthase (iNOS) inhibitors (100 μM AMPCP, 50 μM EHNA, and 200 μM l-NMMA, respectively). In addition, 1.0 μM CGS21680 and 12.3 μM caffeine, adenosine A2A receptor agonist and antagonist, respectively, were added or not along with neutrophils and T. vaginalis.
After incubation time, the cell-free culture supernatants were collected and subjected to quantification of nitrite immediately. The results are representative of at least three independent experiments.
Measurement of nitrite production
The concentration of NO in culture supernatants was determined as nitrite using Griess reagent (Sigma, St. Louis, MO, USA) in accordance with the manufacturer's instructions.
Statistical analysis
Data were expressed by mean ± standard deviation (S.D.) and analyzed by one-way analysis of variance (ANOVA) followed by Tukey's multiple range test or Student's t test, considering p < 0.05 as significant. The analyses were performed using the Statistical Package for the Social Sciences (SPSS) software version 18.
Results
We have evaluated the effect of purinergic signaling on NO production by human neutrophils stimulated with T. vaginalis. The viability of trophozoites and neutrophils were assessed by the motility and trypan blue exclusion dye before the assays. The cellular integrity was not affected by any of the conditions. Also, as revealed by phenotypic analysis, 93% of the isolated immune cells corresponded to neutrophils (data not shown).
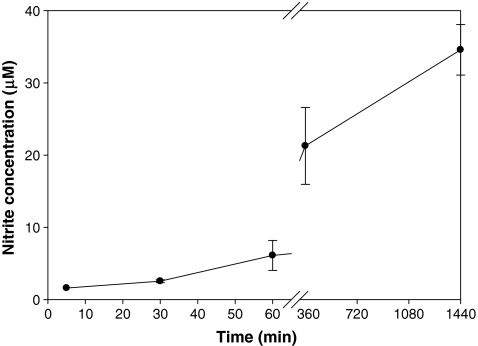
Figure 1 reveals that LPS, an important inducer of host responses, was unable to promote an efficient NO release by neutrophils in the first 60 min. However, the secretion has increased over the time, showing the highest nitrite synthesis in 24 h. Due to this observation, the 24-h period was chosen for the next assays.
Fig. 1.
Dependence of NO secretion by LPS-stimulated neutrophils on time (5 to 1,440 min). The NO secretion has increased over the time, showing the highest nitrite synthesis in 24 h. Line represents the mean ± S.D. of at least three independent experiments
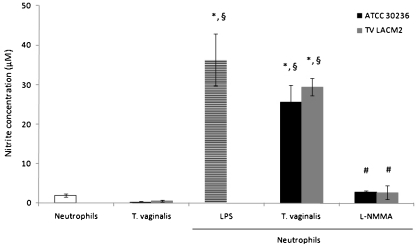
The effects of ectonucleotidases and adenine nucleotides/nucleoside on NO production by neutrophils were investigated after stimuli with T. vaginalis trophozoites. Figure 2 shows that neutrophils alone produced low levels of NO (1.98 ± 0.35 μM); however, when stimulated with LPS, the concentration increased more than 18 times (36.31 ± 6.57 μM). Similarly, the trophozoites when alone were also unable to release large nitrite amounts (0.305 ± 0.16 μM and 0.603 ± 0.22 μM for ATCC 30236 and TV-LACM2, respectively). On the other side, as expected, the coculture among neutrophils and T. vaginalis isolates produced high NO amount. In order to confirm the NO production through iNOS pathway, we have incubated the neutrophils and parasites with l-NMMA, an iNOS inhibitor. The results showed that l-NMMA reversed the anterior effect, demonstrating the iNOS involvement on NO production by neutrophils.
Fig. 2.
Effects of LPS (positive control), T. vaginalis isolates (ATCC 30236 and TV-LACM2) and iNOS inhibitor (L-NMMA) on NO production by neutrophils. Neutrophils and parasites alone did not promote activation in NO production. LPS and T. vaginalis isolates were able to increase the NO release, while l-NMMA reversed the effect. Bars represent the mean ± S.D. of at least three independent experiments. Data were analyzed by ANOVA followed by Tukey's test (p < 0.05). Symbols represent difference when compared with: (asterisk) neutrophils alone (control); (sectionsign) T. vaginalis isolates; (number sign) neutrophils stimulated with T. vaginalis isolates
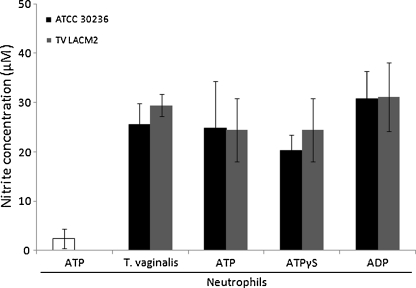
The influence of extracellular nucleotides on NO synthesis can be observed in Fig. 3. The presence of ATP on neutrophils (with no T. vaginalis) did not promote alteration on NO release when compared with neutrophils alone in Fig. 2. The nucleotides (ATP or ADP) were not able to increase the NO secretion by neutrophils when compared to the control (neutrophils and T. vaginalis). In the sense to eliminate a possible interference of ATP hydrolysis by enzymatic action of T. vaginalis and neutrophils NTPDases, we evaluated the effect of ATPγS, a non-hydrolyzable ATP analog. The substance also did not promote alteration compared to the control or to the ATP condition, showing that adenine nucleotides as well as NTPDase activities, responsible for ATP and ADP hydrolysis, are not involved in NO release by T. vaginalis-stimulated neutrophils.
Fig. 3.
Effects of adenine nucleotides and a non-hydrolyzable ATP analog (ATPγS) on NO production by neutrophils stimulated with T. vaginalis. ATP, ADP, and ATPγS caused no significant changes compared to neutrophils stimulated with the parasites. Bars represent the mean ± S.D. of at least three independent experiments. Data were analyzed by ANOVA followed by Tukey's test (p < 0.05)
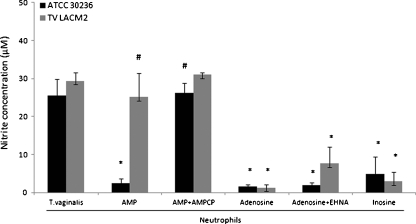
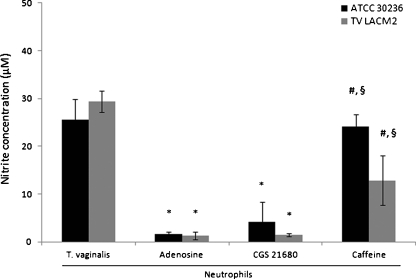
Figure 4 displays the capacity of AMP and the degradation products, adenosine and inosine, in interfering on NO production by neutrophils. The addition of AMP caused a significant decrease in NO levels on 30236 isolate when compared with control or clinical isolate, TV-LACM2. This finding can be justified by the action of ecto-5′-nucleotidase, the enzyme able to hydrolyze AMP. Importantly, while the 30236 isolate presented a specific ecto-5′-nucleotidase activity of 0.321 ± 0.018 nmol/Pi/min/mg protein, the TV-LACM2 isolate showed an activity more than seven times lower (0.044 ± 0.030) (Table 1). Thus, the 30236 isolate produced higher amounts of adenosine, which could explain the anti-inflammatory effect observed by the decrease on NO production. To verify the action of ecto-5′-nucleotidase, we tested the inhibitor AMPCP, and it was observed a reversion of the previous effect, strongly suggesting the involvement of the enzyme in the NO secretion. In same way, the nucleoside adenosine also promoted reduction of NO levels on both isolates. In addition, the ADA inhibitor, EHNA, and inosine, the product of ADA activity, maintained a low NO production. All together, these results indicate that the last step of the enzymatic cascade, involving the action of the parasite ecto-5′-nucleotidase and ADA, and consequent formation of adenosine and inosine, is able to modulate the NO secretion by neutrophils stimulated with T. vaginalis. In addition, to further support the adenosine anti-inflammatory effect, we have tested CGS21680 and caffeine, A2A receptor agonist and antagonist, respectively. As expected, the NO production by T. vaginalis-stimulated neutrophils was inhibited by CGS21680, whereas it was increased by caffeine, indicating the adenosine effect through activation of adenosine A2A receptor (Fig. 5).
Fig. 4.
Effects of AMP, adenosine, inosine, ecto-5′-nucleotidase and ADA inhibitors (AMPCP and EHNA, respectively) on NO production by neutrophils stimulated with T. vaginalis. The ecto-5′-nucleotidase plays an important role on nucleoside generation. Adenosine and inosine triggered low levels on NO secretion. Bars represent the mean ± S.D. of at least three independent experiments. Data were analyzed by ANOVA followed by Tukey's test (p < 0.05). Symbols represent difference when compared with: (asterisk) neutrophils stimulated with T. vaginalis isolates; (number sign) effect of AMP
Table 1.
T. vaginalis NTPDase and ecto-5′-nucleotidase specific hydrolytic activities
| Isolate | ATP | ADP | AMP |
|---|---|---|---|
| ATCC 30236 | 1.07 ± 0.04 | 0.65 ± 0.01 | 0.32 ± 0.01 |
| TV LACM2 | 0.96 ± 0.21 | 0.49 ± 0.04 | 0.04 ± 0.03a |
Data represent the mean ± SD for at least three experiments and were analyzed statistically by Student's t test. Specific activities are expressed in nanomole Pi per min per milligram protein
aSignificant difference between the isolates
Fig. 5.
Influence of A2A receptor agonist (CGS 21680) and antagonist (caffeine) on NO production by neutrophils stimulated with T. vaginalis. The presence of agonist CGS 21680 caused a low NO production, as well as adenosine. The antagonist, caffeine, reversed the effect of the nucleoside. Bars represent the mean ± S.D. of at least three independent experiments. Data were analyzed by ANOVA followed by Tukey's test (p < 0.05). Symbols represent difference when compared with: (asterisk) neutrophils stimulated with T. vaginalis isolates; (number sign) effect of adenosine; (section sign) effect of CGS 21680
Discussion
A better understanding of how neutrophils mediate initial immune response against mucosal parasites is important to investigate the mechanisms and pathways involved in the production of chemoattractants. In the same direction, extracellular nucleotides are signaling molecules in inflammatory processes, and recent studies have shown that the purinergic system is closely related to the activation and recruitment of neutrophils [31–33]. In this current report, we showed the influence of adenine nucleotides and nucleoside, inosine, ectonucleotidases, and purinoceptors on NO production by neutrophils stimulated with T. vaginalis.
NO is one of the most widespread signaling molecules in mammals. The compound is implicated in modulating a variety of physiological reactions, including vasodilatation and smooth muscle relaxation associated with the circulation, neurotransmission in a variety of neural processes, and immunological mechanisms. The neutrophil response, fundamental component of the non-specific immune defense, is characterized by adhesion to the vascular endothelium, followed by migration into tissues, oxygen radical-dependent killing of microorganisms, and elimination of parasites and tissue debris by phagocytosis. NO seems to be involved in most of these processes and can also ultimately lead to tissue injury [34, 35]. The involvement of NO in immune responses can be evidenced due its intense production after LPS stimuli, as observed in our study. The LPS is a potent agent in inflammatory process that promotes the NO fluxes as result of oxide nitric synthase activation, mainly due to the lipid A portion from LPS molecule [36].
Previous studies have investigated the NO role in immunity to trichomonosis. Park et al. [14] showed that macrophage cytotoxicity against T. vaginalis is regulated by NO, with inhibition of trichomonicidal activity after macrophages exposure to l-NMMA. In other work, the parasites induced macrophage to produce proinflammatory cytokines, such as IL-1, IL-6, TNF-α, and NO, via NF-κB [37]. In vivo, it was observed the increase in polymorphonuclears (PMNs), vaginal epithelial cells, and reactive nitrogen intermediate (RNI) levels in mice infected with isolates from symptomatic patients as compared to asymptomatic subjects [38]. Several studies have reported NO production by neutrophils, but others have claimed little or no NO production by these cells [34]. Our results demonstrate, for the first time, that the presence of T. vaginalis trophozoites was able to increase the NO production by human neutrophils. The inducible form of NOS is synthesized upon cell activation, and this was originally described in macrophages [39]. However, despite that immune cells are major producers of NO, we showed that neutrophils also generate high levels of the compound in response to the presence of trichomonads due to iNOS activity, since the enzyme inhibitor, l-NMMA, reversed the observed effect.
Extracellular nucleotides are powerful signaling molecules that contribute to the regulation of a variety of biological processes [18]. After tissue damage, or during inflammation or infection, many cells release ATP, which acts as a proinflammatory immunomediator by triggering multiple immunological effector cell types including neutrophils, macrophages, dendritic cells, and lymphocytes. In neutrophils, extracellular ATP has been shown to induce the production of reactive oxygen species (ROS), stimulate degranulation, promote adhesion to endothelial cells, and contribute to the delay in neutrophil apoptosis [20]. An important study showed that P2Y2 receptors represent a critical link in the signaling process, resulting in the activation of PMNs by infectious or inflammatory mediators [33]. However, our findings suggest that adenine nucleotides are not involved in NO secretion by neutrophils in trichomonosis. To better understand the results obtained when neutrophils were incubated with ATP and T. vaginalis, we have tested ATPγS, a non-hydrolyzable ATP analog. The levels for NO secretion found in these two situations were similar, indicating that ATP enzymatic hydrolysis was not decisive for NO release. However, CD39/NTPDase 1 is expressed on numerous different types of normal leukocytes, including neutrophils [40]. In this study, we could not neglect the nucleotide hydrolysis by the neutrophil NTPDase considering that NTPDase1 efficiently hydrolyzes ATP. However, we could not completely inhibit ATP hydrolysis from neutrophils, since one of the most used general ectonucleotidase inhibitors, ARL-67156, is a weak competitive inhibitor of NTPDase1 and NTPDase3, which does not affect NTPDase2 [41].
Search in T. vaginalis genome reveals four genes encoding hypothetical proteins with NTPDase characteristics (locus tags TVAG_063220, TVAG_167570, TVAG_351590, and TVAG_397320) [42]. The presence of multigene families for expression of proteins is often observed in T. vaginalis genome [43]. As a mucosal pathogen, T. vaginalis should efficiently adapt to different environments such as alterations in extracellular nucleotide concentrations, hence more than one NTPDase gene sequence could collaborate to this aim. The adaptability of T. vaginalis to challenging environmental pressures may be explained by the fact that trichomonads possess signal transduction pathways that link changes in the environment with appropriate changes in transcriptional and post-transcriptional regulatory mechanisms [9]. In addition, T. vaginalis presented heterogeneity in nucleotide hydrolysis with different ATP:ADP hydrolysis ratios [44]. In that previous study, we have observed that fresh clinical isolates presented higher NTPDase activities when compared to the activities from long-term-grown isolates. However, this is not the case in the present study. The TV-LACM2 fresh clinical isolate is metronidazole-resistant and exhibits an amoebic morphological behavior, which suggests high virulence; however, it shows similar ATP and ADP hydrolysis to the long-term-grown isolate, ATCC 30236. Future reports may indicate molecules other than NO and pathways that interact with the purinergic signaling promoting the activation of immune cells in T. vaginalis infection. Moreover, the next investigations will be conducted with the human cervical and epithelial cells, which represent the main physiologic model for the study of trichomonosis because they are the host cells supporting the parasite adherence, survival, and replication in the genital tract [10].
In contrast to ATP, the anti-inflammatory role of adenosine is able to reverse all the effects mentioned above [45]. Thus, ectonucleotidases are important modulators of immune response, since they control the degradation of ATP to adenosine through the purinergic cascade [18]. Inosine, produced by adenosine deamination, also exhibits potent immunomodulatory effects, suppressing synthesis of proinflammatory mediators by macrophages, lymphocytes, and neutrophils [46]. In this sense, we verified that adenosine and inosine did influence the decrease on NO production by neutrophils. These suppressive effects may generate consequences on the parasitism, contributing to the establishment of infection by T. vaginalis trophozoites. In Fig. 4, the significant difference observed on NO production between the isolates, in the presence of AMP, shows the importance of ecto-5′-nucleotidase in providing adenosine. The 30236 isolate displays higher enzyme activity than the clinical isolate, TV-LACM2, producing ultimately higher rates of adenosine and decreasing the NO levels. Tasca et al. [44] reported that some isolates has little or no ecto-5′-nucleotidase activity. This finding is surprising since the parasite is dependent on salvage pathways to generate de novo nucleotides [47, 48]. Furthermore, the low enzyme activity may cause outcome for both host and parasite during infection, considering that decreased amounts of adenosine as an anti-inflammatory agent could result in acute symptoms due to leukocytic infiltration among patients infected with these organisms [44].
All inhibitory effects of adenosine on neutrophil functions are mediated by A2A receptors [49], which increase intracellular cAMP levels and decrease the proinflammatory action of PMNs [50]. In an important way, the activation of this receptor subtype occurs when the nucleoside is found in high concentrations. Therefore, once neutrophils have arrived at a site where there is significant tissue injury, the high amounts of adenosine generated by damaged tissues or cells, acts as a feedback inhibitor of inflammatory neutrophil functions [38]. In this study, the effects promoted by A2A agonist and antagonist prove the receptor involvement on immunosuppressive events of adenosine.
In summary, the present report demonstrated the influence of purinergic system, specifically the enzyme ecto-5′-nucleotidase and adenosine through activation of A2A receptors on NO production by neutrophils in T. vaginalis infection. Herein, possible mechanisms related with chemoattractants production and finally with leukocytic infiltration were investigated, contributing to the host–parasite interactions as well as the immunity in trichomonosis.
Acknowledgements
A.P.F. is recipient of fellowship from CNPq. This study received financial support from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) grant #477348/2008-4 awarded to T.T., and from the NANOBIOTEC-Brazil program from CAPES (Brazil). The authors thank Dr. Christina Bittar and Hospital de Clínicas de Porto Alegre for neutrophil phenotypic analysis.
References
- 1.Global prevalence and incidence of selected curable sexually transmitted infections. Overview and estimates. Geneva: World Health Organization; 2001. [PubMed] [Google Scholar]
- 2.Cotch MF, Pastorek JG, Nugent RP, Hillier SL, Gibbs RS, Martin DH, Eschenbach DA, Edelman R, Carey JC, Regan JA, Krohn MA, Klebanoff MA, Rao AV, Rhoads GG. The vaginal infections and prematurity study group. Sex Transm Dis. 1997;24:353–360. doi: 10.1097/00007435-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein F, Goldman MB, Cramer DW. Relation of tubal infertility to a story of sexually transmitted diseases. Am J Epidemiol. 1993;137:577–584. doi: 10.1093/oxfordjournals.aje.a116711. [DOI] [PubMed] [Google Scholar]
- 4.Viikki M, Pukkala E, Nieminen P, Hakama M. Gynaecological infections as risk determinants of subsequent cervical neoplasia. Acta Oncol (Madr) 2000;39:71–75. doi: 10.1080/028418600431003. [DOI] [PubMed] [Google Scholar]
- 5.Cherpes T, Wiesenfeld H, Melan M, Kant JA, Consentino LA, Meyn LA, Hillier SL. The associations between pelvic inflammatory disease, Trichomonas vaginalis infection, and positive herpes simplex virus type 2 serology. Sex Transm Dis. 2006;33:747–752. doi: 10.1097/01.olq.0000218869.52753.c7. [DOI] [PubMed] [Google Scholar]
- 6.Johnston VJ, Mabey DC. Global epidemiology and control of Trichomonas vaginalis. Curr Opin Infect Dis. 2008;21:56–64. doi: 10.1097/QCO.0b013e3282f3d999. [DOI] [PubMed] [Google Scholar]
- 7.Pol B, Kwok C, Pierre-Louis B, Rinaldi A, Salata RA, Chen PL, Wijgert J, Miro F, Mugerwa R, Chipato T, Morrison CS. Trichomonas vaginalis infection and human immunodeficiency virus acquisition in African women. J Infect Dis. 2008;197:548–554. doi: 10.1086/526496. [DOI] [PubMed] [Google Scholar]
- 8.Petrin D, Delgaty K, Bhatt R, Garber G. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev. 1998;11:300–317. doi: 10.1128/cmr.11.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehker MW, Alderete JF. Biology of trichomonosis. Curr Opin Infect Dis. 2000;13:37–45. doi: 10.1097/00001432-200002000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Fichorova RN. Impact of T. vaginalis infection on innate immune responses and reproductive outcome. J Reprod Immunol. 2009;83:185–189. doi: 10.1016/j.jri.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Escario A, Gómez Barrio A, Simons Diez B, Escario JA. Immunohistochemical study of the vaginal inflammatory response in experimental trichomoniasis. Acta Trop. 2010;114:22–30. doi: 10.1016/j.actatropica.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Ryu JS, Kang JH, Jung SY, Shin MH, Kim JM, Park H, Min DY. Production of interleukin-8 by human neutrophils stimulated with Trichomonas vaginalis. Infect Immun. 2004;72:1326–1332. doi: 10.1128/IAI.72.3.1326-1332.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaio MF, Lin PR, Liu JY, Yang KD. Generation of interleukin-8 from human monocytes in response to Trichomonas vaginalis stimulation. Infect Immun. 1995;63:3864–3870. doi: 10.1128/iai.63.10.3864-3870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park GC, Ryu JS, Min DY. The role of nitric oxide as an effector of macrophage-mediated cytotoxicity against Trichomonas vaginalis. Korean J Parasitol. 1997;35:189–195. doi: 10.3347/kjp.1997.35.3.189. [DOI] [PubMed] [Google Scholar]
- 15.Shaio MF, Lin PR, Lee CS, Hou SC, Tang P, Yanga KD. Novel neutrophil-activating factor released by Trichomonas vaginalis. Infect Immun. 1992;60:4475–4482. doi: 10.1128/iai.60.11.4475-4482.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaio MF, Lin PR. Leukotriene B4 levels in the vaginal discharges from cases of trichomoniasis. Ann Trop Med Parasitol. 1995;89:85–88. doi: 10.1080/00034983.1995.11812934. [DOI] [PubMed] [Google Scholar]
- 17.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 18.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 19.Salmi M, Jalkanen S. Cell-surface enzymes in control of leukocyte trafficking. Nat Rev Immunol. 2005;5:760–771. doi: 10.1038/nri1705. [DOI] [PubMed] [Google Scholar]
- 20.Bours MJL, Swennen ELR, Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Therapeut. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 22.Zimmermann H. Ectonucleotidases: some recent developments and a note on nomenclature. Drug Develop Res. 2001;52:44–56. doi: 10.1002/ddr.1097. [DOI] [Google Scholar]
- 23.Robson SC, Sévigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matos JAA, Borges FP, Tasca T, Bogo MR, Carli GA, Fauth MG, Dias RD, Bonan CD. Characterization of an ATP diphosphohydrolase (Apyrase, EC 3.6.1.5) activity in Trichomonas vaginalis. Int J Parasitol. 2001;31:770–775. doi: 10.1016/S0020-7519(01)00191-6. [DOI] [PubMed] [Google Scholar]
- 25.Tasca T, Bonan CD, Carli GA, Battastini AMO, Sarkis JJF. Characterization of an ecto-5′-nucleotidase (EC 3.1.3.5) activity in intact cells of Trichomonas vaginalis. Exp Parasitol. 2003;105:167–173. doi: 10.1016/j.exppara.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Weizenmann M, Frasson AP, Barros MP, Vieira Pde B, Rosemberg DB, Carli GA, Bogo MR, Bonan CD, Tasca T. Kinetic characterization and gene expression of adenosine deaminase in intact trophozoites of Trichomonas vaginalis. FEMS Microbiol Lett. 2011;319:115–124. doi: 10.1111/j.1574-6968.2011.02283.x. [DOI] [PubMed] [Google Scholar]
- 27.Diamond LS. The establishment of various Trichomonas of animals and man in axenic cultures. J Parasitol. 1957;43:488–490. doi: 10.2307/3274682. [DOI] [PubMed] [Google Scholar]
- 28.Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:218–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 29.Chan KM, Delfert D, Junger KD. A direct colorimetric assay for Ca2+-stimulated ATPase activity. Anal Biochem. 1986;157:375–380. doi: 10.1016/0003-2697(86)90640-8. [DOI] [PubMed] [Google Scholar]
- 30.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 31.Virgilio F. Purinergic signalling in the immune system. A brief update. Purinergic Signal. 2007;3:1–3. doi: 10.1007/s11302-006-9048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Junger GW. Purinergic regulation of neutrophil chemotaxis. Cell Mol Life Sci. 2008;65:2528–2540. doi: 10.1007/s00018-008-8095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Yao Y, Sumi Y, Li A, To UK, Elkhal A, Inoue Y, Woehrle T, Zhang Q, Hauser C, Junger WG. Purinergic signaling: a fundamental mechanism in neutrophil activation. Sci Signal. 2010;125:ra45. doi: 10.1126/scisignal.2000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armstrong R. The physiological role and pharmacological potential of nitric oxide in neutrophil activation. Int Immunopharmacol. 2001;1:1501–1512. doi: 10.1016/S1567-5769(01)00094-7. [DOI] [PubMed] [Google Scholar]
- 35.Wink DA, Mitchell JB. Chemical biology of nitric oxide: insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med. 1998;25:434–456. doi: 10.1016/S0891-5849(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 36.Zagryazhskaya NA, Lindner SC, Grishina ZV, Galkina SI, Steinhilber D, Sud'ina GF. Nitric oxide mediates distinct effects of various LPS chemotypes on phagocytosis and leukotriene synthesis in human neutrophils. Int J Biochem Cell Biol. 2010;42:921–931. doi: 10.1016/j.biocel.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 37.Han IH, Goo SY, Park SJ, Hwang SJ, Kim YS, Yang MS, Ahn MH, Ryu JS. Proinflammatory cytokine and nitric oxide production by human macrophages stimulated with Trichomonas vaginalis. Korean J Parasitol. 2009;47:205–212. doi: 10.3347/kjp.2009.47.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malla N, Yadav M, Gupta I. Kinetics of serum and local cytokine profile in experimental intravaginal trichomoniasis induced with Trichomonas vaginalis isolates from symptomatic and asymptomatic women. Parasite Immunol. 2007;29:101–105. doi: 10.1111/j.1365-3024.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- 39.Coleman J. Nitric oxide in immunity and inflammation. Int Immunopharmacol. 2001;1:1397–1406. doi: 10.1016/S1567-5769(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 40.Pulte ED, Broekman MJ, Olson KE, Drosopoulos JHF, Kizer JR, Islam N, Marcus AJ. CD39/NTPDase-1 activity and expression in normal leukocytes. Thromb Res. 2007;121:309–317. doi: 10.1016/j.thromres.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lévesque SA, Lavoie EG, Lecka J, Bigonnesse F, Sévigny J. Specificity of the ecto-ATPase inhibitor ARL 67156 on human and mouse ectonucleotidases. Br J Pharmacol. 2007;152:141–150. doi: 10.1038/sj.bjp.0707361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sansom FM, Robson SC, Hartland EL. Possible effects of microbial ecto-nucleoside triphosphate diphosphohydrolases on host–pathogen interactions. Microbiol Mol Biol Rev. 2008;72:765–781. doi: 10.1128/MMBR.00013-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alderete JF, Millsap KW, Lehker MW, Benchimol M. Enzymes on microbial pathogens and Trichomonas vaginalis: molecular mimicry and functional diversity. Cell Microbiol. 2001;3:359–370. doi: 10.1046/j.1462-5822.2001.00126.x. [DOI] [PubMed] [Google Scholar]
- 44.Tasca T, Bonan CD, Carli GA, Sarkis JJ, Alderete JF. Heterogeneity in extracellular nucleotide hydrolysis among clinical isolates of Trichomonas vaginalis. Parasitology. 2005;131:71–78. doi: 10.1017/S0031182005007377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haskó G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 46.Haskó G, Sitkovsky MV, Szabó C. Immunomodulatory and neuroprotective effects of inosine. Trends Pharmacol Sci. 2004;25:152–157. doi: 10.1016/j.tips.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Heyworth PG, Gutteridge WE, Ginger CD. Purine metabolism in Trichomonas vaginalis. FEBS Lett. 1982;141:106–110. doi: 10.1016/0014-5793(82)80026-4. [DOI] [PubMed] [Google Scholar]
- 48.Heyworth PG, Gutteridge WE, Ginger CD. Pyrimidine metabolism in Trichomonas vaginalis. FEBS Lett. 1984;176:55–60. doi: 10.1016/0014-5793(84)80910-2. [DOI] [PubMed] [Google Scholar]
- 49.Fortin A, Harbour D, Fernandes M, Borgeat P, Bourgoin S. Differential expression of adenosine receptors in human neutrophils: up-regulation by specific Th1 cytokines and lipopolysaccharide. J Leukoc Biol. 2006;79:574–585. doi: 10.1189/jlb.0505249. [DOI] [PubMed] [Google Scholar]
- 50.Thibault N, Burelout C, Harbour D, Borgeat P, Naccache PH, Bourgoin SG. Occupancy of adenosine A2A receptors promotes fMLP-induced cyclic AMP accumulation in human neutrophils: impact on phospholipase D activity and recruitment of small GTPases to membranes. J Leukoc Biol. 2002;71:367–377. [PubMed] [Google Scholar]