Abstract
Glioblastoma is the most aggressive tumor in the CNS and is characterized by having a cancer stem cell (CSC) subpopulation essential for tumor survival. The purinergic system plays an important role in glioma growth, since adenosine triphosphate (ATP) can induce proliferation of glioma cells, and alteration in extracellular ATP degradation by the use of exogenous nucleotidases dramatically alters the size of gliomas in rats. The aim of this work was to characterize the effect of the purinergic system on glioma CSCs. Human U87 glioma cultures presented tumor spheres that express the markers of glioma cancer stem cells CD133, Oct-4, and Nanog. Messenger RNA of several purinergic receptors were differently expressed in spheres when compared to a cell monolayer not containing spheres. Treatment of human gliomas U87 or U343 as well as rat C6 gliomas with 100 μM of ATP reduced the number of tumor spheres when grown in neural stem cell medium supplemented with epidermal growth factor and basic fibroblast growth factor. Moreover, ATP caused a decline in the number of spheres observed in culture in a dose-dependent manner. ATP also reduces the expression of Nanog, as determined by flow cytometry, as well as CD133 and Oct-4, as analyzed by flow cytometry and RT-PCR in U87 cells. The differential expression of purinergic receptor in tumor spheres when compared to adherent cells and the effect of ATP in reducing tumor spheres suggest that the purinergic system affects CSC biology and that ATP may be a potential agonist for differentiation therapy.
Electronic supplementary material
The online version of this article (doi:10.1007/s11302-011-9252-9) contains supplementary material, which is available to authorized users.
Keywords: Glioma, Cancer stem cells, CD133, Purinergic system, ATP
Introduction
Glioblastomas are the most common brain tumors, characterized by its high invasivity and recurrence, with patients presenting a median survival that does not exceed 15 months, despite multiple treatments [1]. The concept of cancer stem cells (CSCs) brought a change of paradigm in cancer biology and therapeutics [2, 3], suggesting that specifically targeting these cells for destruction [4] or differentiation [5] may be the best therapeutic strategy to follow for several aggressive types of cancers. This, however, may not be easy, since CSCs have shown to present less reactive oxygen species [6] and to be more resistant to ionizing radiation [7], vincristine [8], hypoxia, and other chemotherapeutics [9] when compared to non-CSCs. Treatment with some of these agents increased the proportion of CSCs, which may be an explanation for more aggressive recurrence [7]. On the other hand, preferential elimination of CSC population may be a part of the effectiveness of temozolomide, the most effective pharmacologic agent used in glioma treatment [10].
Extracellular purines have been implicated in several aspects of glioblastoma biology, such as proliferation [11], migration [12], and death [13]. Models of glioma tumor growth suggest that endogenous adenosine triphosphate (ATP) is liberated and plays important tumorigenic roles. We have previously shown that injection of the glioma cells together with apyrase, an enzyme that degrades ATP and adenosine diphosphate (ADP) to adenosine monophosphate (AMP), significantly reduced tumor growth [14], whereas expression of NTPDase2, an enzyme that degrades ATP to produce mainly ADP, strongly increased tumor size due to the pro-angiogenic effects of ADP [15].
ATP-mediated signaling was shown to be important in the differentiation of the murine embryonal carcinoma cell line P19 [16], but no study has addressed the impact of nucleotides and nucleosides in glioma CSCs. Here we show that ATP reduces the sphere formation in human and rat glioma CSCs and that purinergic receptors are differently expressed in tumor spheres when compared to adherent cells.
Material and methods
Cell culture
Human glioma cell line U87 was obtained from the American Type Culture Collection (Rockville, MD, USA) and maintained in Dulbecco’s modified Eagle’s medium (DMEM) low glucose (Gibco BRL) containing 2% (w/v) l-glutamine and 5% (v/v) fetal bovine serum (FBS—Gibco BRL), 5% CO2/95% air at 37°C. Fungizone and penicillin/streptomycin (Gibco BRL) were added to the medium. Additionally, cells were also maintained in defined, serum-free, neural stem cell (NSC) medium containing DMEM/F12 supplemented with human recombinant epidermal growth factor (EGF) (20 ng/ml; Sigma), basic fibroblast growth factor (FGF) (20 ng/ml; Upstate), leukemia inhibitory factor (LIF) (10 ng/ml; Sigma), and B27 (1×; Gibco). Spheres of cells grown on FBS were obtained from cells grown on 1% agar layer for 7–10 days with medium change every 2–3 days and isolated from single cells by sedimentation. In order to evaluate the number of spheres formed as an indication of the presence of colony-forming cells, a sphere formation assay was performed [17]. For cells grown on FBS, cells were plated at 250, 500, 750, and 1,000 cells per well in a 96-well plate with 200 μl/well of DMEM + 5% FBS and allowed to grow for 7 days. ATP or temozolomide (Sigma Chemical Co., St. Louis, MO, USA) were added at the moment of plating, and spheres were counted from days 3 until 7. Groups of cells loosely attached that presented a diameter of more than 60 μm were considered spheres (Fig. 1a, arrows). For the ATP titration assay, a sphere formation assay was performed, with 1,000 cells plated per well in a 96-well plate with 200 μl/well of NSC medium, and ATP was added to the final concentrations of 1, 10, and 100 μM for 7 days [8].
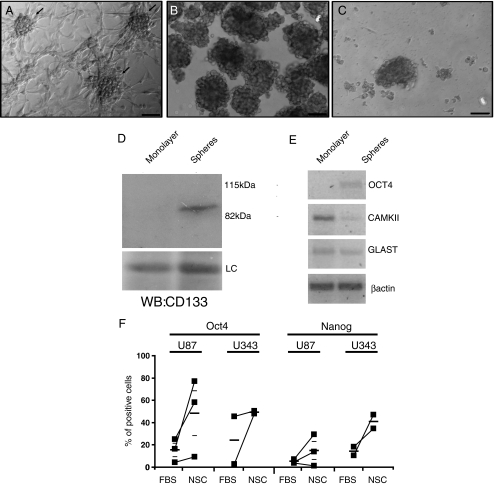
Fig. 1.
Characterization of tumor spheres. U87 human gliomas form spheres in culture under adherent conditions (arrows) (a), when grown for 7 days on soft agar in medium with 5% fetal bovine serum (b) and when grown on NSC medium (c). Spheres grown on soft agar and adhered cells, obtained from subconfluent cultures which did not contain spheres, were analyzed by western blotting with anti-CD133 (d) and RT-PCR for markers of undifferentiated (Oct-4) and differentiated cells (GLAST and CaMKII) (e). LC loading control. Bar size = 100 μm. Proportion of Oct-4 and Nanog-positive cells was evaluated by flow cytometry in U87 (n = 3) and U343 (n = 2) human gliomas grown on 5% FBS and on NSC medium. Paired experiments are linked by lines. Average ± SEM are shown as small lines (f)
Flow cytometry
For CD133 flow cytometry, cells were mechanically dissociated, washed twice in a solution of ethylenediamine tetraacetic acid (EDTA) 2 mM in phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA), and then incubated with anti-CD133/1 (AC133)-PE antibody (Miltenyi Biotec, Germany) 1:10 for 10 min at 4°C in the dark. For Oct-4 and Nanog flow cytometry, cells were harvested, washed twice in PBS, and fixed with formaldehyde to a final concentration of 4%. Cells were washed and permeabilized with methanol to a final concentration of 90%. For immunostaining, cells were incubated in incubation buffer (5 mg/ml BSA in PBS), and Nanog (D73G4) XP™ rabbit mAb or Oct-4 Rabbit Ab primary antibodies (Cell Signaling Technology, MA, USA) were added to a final concentration of 1:200 and incubated for 2 h. A new washing step followed, and secondary antibody anti-rabbit IgG Fab2 Alexa Fluor® 488 was added to a final concentration of 1:1,000. Cells were incubated for 1 h, washed, and resuspended in PBS for analysis. Cells were then analyzed by flow cytometry in a PCA-96 System machine (Guava Technologies, Hayward, CA, USA).
RT-PCRs
Messenger RNA (mRNA) was extracted from the two U87 populations (spheres and adherent cells) using Trizol LS® reagent (Life Technologies). Complementary DNA was synthesized with oligo(dT) primers and MMLV-RT® reverse transcriptase enzyme (Promega). Primers are shown in Supplementary Table 1. Products of the PCR reaction were analyzed on a 1% agarose gel stained with 0.5× SYBR Green (Molecular Probes). β-Actin was used as the internal control gene.
Immunoblotting analysis
For western blot, cells were lysed with lysis buffer (50 mM Tris pH 7.4, 100 mM NaCl, 1 mM DTT, 1 mM EGTA, 10 mM β-glycerophosphate, 1% Triton-X 100, and protease inhibitors without EDTA from Roche, Germany). Protein content was assessed by the Lowry method, and equal loading was accessed by staining the PVDF membrane with Coomassie blue. The primary antibody used was anti-CD133 (Cell Signaling, 1:1,000) and the secondary antibody was horseradish peroxidase-conjugated anti-rabbit antibody (1:2,000, Jackson Immune Research) both incubated at RT for 1 h and detection was as described [18].
Methylthiazolyltetrazolium bromide viability assay
After 7 days of treatment, culture medium in the 96-well plate used for the sphere formation assay was replaced by the methylthiazolyltetrazolium bromide (MTT) solution dissolved in PBS, and the plate was incubated at 37°C for 3 h. The MTT solution was then aspirated and the formazan crystals formed were dissolved in DMSO. Absorbance was read at 570 nm and the growth inhibition in treated cells was expressed as the percentage of the untreated control cells.
Protein determination
For assessing the amount of protein in samples, Pierce ® BCA protein assay kit (Thermo Scientific, Rockford, IL, USA) was used according to instructions from the manufacturer.
Statistical analysis
The level of significance was determined by a paired two-tailed Student’s t test (GraphPad InStat3 software). All quantitative data presented are the mean ± standard error of the mean (SEM).
Results
Characterization of a tumor stem cell population in the U87 cell line
The U87 human glioma cells grown on high density present two morphologically distinct populations: one that consists of cells attached to the plate and a population that grows as spheres that resemble neurospheres (Fig. 1a). Induction of tumor sphere growth in vitro is normally achieved by using specific growth factors in the culture medium instead of serum. In our hands, though, spheres were readily observed even when grown in the presence of 10% FBS. In order to optimize sphere formation in culture, we plated U87 cells with different concentrations of serum and observed that the amount of spheres formed was the highest at 5% FBS, whereas serum concentrations lower than 5% restricted cell culture. Spheres were isolated by growing cells on agar (Fig. 1b), and were separated from single cells by differential sedimentation. When U87 cells were grown on a neural stem cell medium, they formed spheres even when plated on normal culture dishes. The size of the spheres was smaller, and the number was much higher when compared to serum-containing medium (Fig. 1c—see also Figs. 2 and 3).
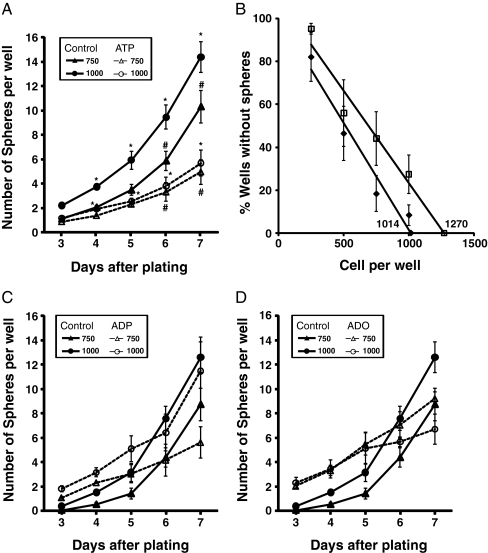
Fig. 2.
Treatment with ATP but not ADP or adenosine reduces the tumor spheres and stem cell population. U87 cells (750 or 1,000) were grown in a 96-well plate in the presence or absence of 100 μM of ATP for 7 days, and spheres (average ± SEM) were counted from days 3 to 7 (n = 6). *p < 0.05 for 750 plated cells and #p < 0.05 for 1,000 plated cells (a). Regression analysis of the wells without spheres—numbers indicate the value of the X-axis intercept of the best linear fit (b). U87 cells were treated with 100 μM of ADP (c) or adenosine (d) and evaluated as in a. Bar size = 50 μm
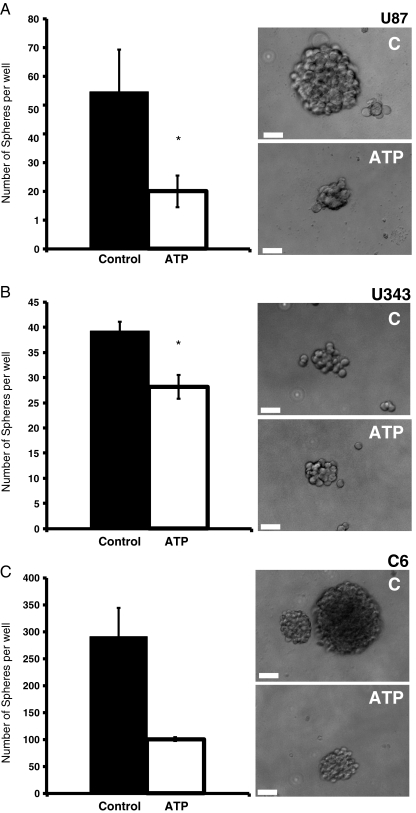
Fig. 3.
Treatment with ATP reduces the tumor spheres grown in neural stem cell (NSC) medium in different glioma cell lines. One thousand cells were grown in a 96-well plate for 7 days in the presence or absence of 100 μM of ATP in NSC medium. The number of spheres is shown as average ± SEM at day 7 in U87 (n = 4) (a), C6 (n = 2) (b), or U343 (n = 5) (c). Bars represent SEM. t test values: *p < 0.05. Right panel: representative images of tumor spheres of control and cultures treated for 7 days with ATP
Spheres were described to be richer in tumor stem cells when compared to cells directly attached to the culture flask (referred as monolayer). Accordingly, spheres presented more CD133+ cells when compared to attached cells, when analyzed by western blotting (Fig. 1d) or flow cytometry (Supplementary Fig. 1). The mRNA expression of markers of differentiation supported this observation, since expression of octamer-4 (Oct-4), a marker of embryonic stem cells and a transcription factor that is able to induce pluripotent cells [19], was observed only in spheres, whereas the attached population presented more expression of GLAST, a marker for differentiated glial cells and of CAMKII, a neuronal marker (Fig. 1e). Despite high inter-experiment variability, both U87 and U343 glioma cell lines grown in NSC medium presented higher proportion of Oct-4 and Nanog-positive cells when compared to cells grown in serum-containing medium in the same experiment (Fig. 1f).
ATP reduces sphere formation
In the sphere formation assay, cells were plated with and without agonist, and the number of spheres formed was counted from days 3 to 7 after plating. In the presence of 100 μM ATP, there were significantly fewer spheres both when 750 and 1,000 cells were plated (Fig. 2a). Another way of accessing the relative presence of sphere-forming cells in a population is to calculate a linear regression of the percentage of wells without spheres [17]. The mean x-intercept value of the graph indicates the number of cells needed to form one sphere per well, which was higher in the ATP-treated cells (1,270) when compared to the control (1,014), suggesting that ATP reduces the relative number of cells capable of forming spheres in the population (Fig. 2b).
Although U87 gliomas have a very low rate of ectonucleotide degradation [20], long treatments may produce some degradation of ATP to ADP or adenosine which could be responsible for the biological effect. Therefore, we tested ADP and adenosine, which had no significant effect on sphere formation (Fig. 2c, d). UTP, an agonist of P2Y2, P2Y4, and P2Y6 receptors [21] also did not lead to any change in tumor sphere formation (Table 1).
Table 1.
Number of spheres and the amount of cells from U87, C6, and U343 cells at day 7
| Cell line | Cells plated | 750 cells | 1,000 cells | |||
|---|---|---|---|---|---|---|
| Medium | Treatment | Spheres | % cellsa | Spheres | % cellsa | |
| U87 | D+FCS | Control | 7.7 ± 1.5 (6) | 100 | 11.6 ± 1.4 (6) | 100 |
| U87 | D+FCS | ATP | 3.7 ± 0.9* (6) | 79 ± 9 (3) | 3.9 ± 0.8* (6) | 61 ± 14 (3) |
| U87 | D+FCS | ADP | 5.6 ± 1.2 (4) | 83 ± 10 (3) | 11.5 ± 1.2 (4) | 92 ± 20 (3) |
| U87 | D+FCS | ADO | 9.2 ± 0.4 (3) | 89 ± 19 (3) | 6.7 ± 1.0 (3) | 66 ± 7 (3) |
| U87 | D+FCS | UTP | 8.9 ± 1.1 (4) | 75 ± 2 (3) | 11.1 ± 1.1 (4) | 73 ± 5 (3) |
| U343 | NSC | Control | 39.3 ± 1.8 (5) | 100 | ||
| U343 | NSC | ATP | 36.4 ± 4.22 (5) | 91.9 ± 7.6 (5) | ||
| C6 | NSC | Control | 290.4 ± 53.3 (2) | 100 | ||
| C6 | NSC | ATP | 100.7 ± 3.8 (2) | 66.8 ± 1 (2) | ||
Values expressed as average ± SEM (n)
D + FSC DMEM + 5% FCS, NSC neural stem cell medium supplemented with EGF, FGF, LIF, and B27
*p < 0.05 test
aC6 and U343 cells were grown in NSC and cell amount was evaluated at day 7 using protein assay. U87 were grown in DMEM plus 5% FCS and cell amount was evaluated by MTT. Number of experiments are in parenthesis
We have previously shown that 24 h of treatment with ATP induces proliferation of U87 gliomas in serum-deprived conditions [11]. Since the number of cells directly affects the formation of spheres, we measured the amount of viable cells at the end of the sphere-forming assay. Chronic treatment with all purinergic agonists reduced the amount of viable cells at the end of a 7-day treatment. Even correcting for this reduction in proliferation induced by ATP, the number of spheres was reduced by 45% and 40% with 750 cells and 1,000 cells, respectively (Table 1). It is important to mention that the relation between cells plated and spheres formed is linear with an R2 of 0.98, considering 250, 500, 750, and 1,000 cells plated per well, indicating that there is no saturation occurring due to an excess of cells.
There are several evidence pointing to the importance of growing CSCs in a defined medium that does not contain serum. When experiments were performed in NSC medium, ATP presented a much larger effect in inhibiting U87 sphere growth when compared to serum-containing medium (Fig. 3a). It is also important to observe that for the same number of cells plated, much more spheres where observed in NSC medium when compared to serum-containing medium since the former contains factors that favor CSC proliferation. Additionally, another human glioma cell line, U343 as well as the C6 rat glioma cell line, which is widely used in animal studies and was recently shown to contain CSCs [22, 23], presented a drastic reduction in sphere formation when treated with 100 μM ATP in NSC medium (Fig. 3b, c).
In order to estimate from which concentration ATP would exert its effect in decreasing sphere formation, an ATP titration assay was performed. U87 and U343 cells were plated at a density of 1,000 cells per well in ATP concentrations ranging from 1 to 100 μM in NSC medium. ATP 1 μM already reduced the sphere number in U87, but not in U343 (Supplementary Fig. 2a). Sphere size was already reduced with 10 μM, and with 100 μM, only a few small spheres remained after 7 days (Supplementary Fig. 2b).
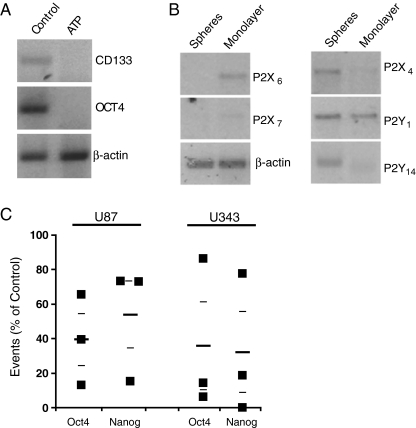
Cells treated with ATP presented less expression of the stem cell markers CD133 and Oct-4 as analyzed by RT-PCR (Fig. 4a), and the CD133, Oct-4, and Nanog staining analyzed by flow cytometry was lower in cells treated with ATP when compared to untreated cells (Fig. 4c and Supplementary Fig. 1b, c). Next, we wondered whether components of the purinergic system were differently expressed in glioma cells present in spheres or attached to the culture dish. As expected, different glioma cell lines present a heterogeneous expression of purinergic receptors (Supplementary Table 2). When comparing adherent versus spheres, expression of P2X6 and P2X7 was found increased in cells from the adherent population, whereas P2X4, P2Y1, and P2Y14 were more expressed in spheres (Fig. 4b). The other purinergic receptors expressed by U87 cells (Supplementary Table 2) were not differentially expressed between spheres and attached cells.
Fig. 4.
Purinergic system and tumor sphere formation: expression of CD133 and Oct-4 mRNA as analyzed by RT-PCR of cells treated with 100 μM ATP for 7 days (a). Expression of mRNA of purinergic receptors in spheres and adherent cells as analyzed by RT-PCR (representative of five independent experiments) (b). Proportion of Oct-4 and Nanog-positive cells in U87 and U343 lines grown on NSC medium and treated with ATP 100 μM for 7 days, when compared to control. Average ± SEM are shown as small lines (c)
Discussion
U87 glioma cells grown in the presence of serum form tumor spheres with characteristics that are similar to gliomas grown on selected growth factors with regard to growth of spheres and increased expression of markers of undifferentiation such as CD133, Nanog, and Oct-4. The percentage of cells positive for different stemness marker reported in glioma tumor spheres is quite broad, with primary cultures of brain tumors or cell lines presenting from 1% to about 50% of CD133 [17, 22–24]. Our analysis indicates that, despite variability among experiments, the proportion of cells positive for stemness markers are, with the exception of one experiment, always higher in the NSC medium when compared to the FBS medium. The number of cells needed to form a sphere was three times higher in the serum-containing medium when compared to U87 grown with FGF and EGF in the absence of serum, but consistent with the 0.15% CD133+ cells found in the parental U87 cell line grown under serum conditions [25]. The reduced amount of stemness positive cells may be a reflection of the reduction of CSCs due to the presence of serum. An additional indication that the tumor spheres observed in our culture conditions harbor bona fide CSCs is the observation that temozolomide, at a sub-toxic concentration of 5 μM, reduced the formation of spheres by 50% (data not shown), similar to the reduction found in primary glioblastoma tumors cultivated with defined factors [10].
Although the majority of the data with gliomas CSCs were obtained in defined serum-free medium, some glioma cell lines readily form clones in the serum-containing medium [23]. The maintenance of a CSC population in spheres, in spite of the presence of 5% serum, may be due to the establishment of a microenvironment that favors the maintenance and growth of CSCs. As already pointed out by Shen et al. [23], glioma cell lines grown on serum-containing medium for several years must be able to maintain a CSC population under this condition in order to remain tumorigenic, as is the case of U87. U87 cells grown on serum-containing medium are able to form tumors when injected into the brain of nude mice, indicating that under these conditions, the main feature of CSCs, which is the formation of tumor, is maintained [2, 26, 27].
Activation of P2Y receptors was shown to be mitogenic for mice neural progenitor cells, but had no effect on cell differentiation. Distinct expression of purinergic receptors in undifferentiated versus differentiated cells was observed in P19 embryonic carcinoma cells, a model of neural differentiation [16]. Several purinergic receptors changed their expression over differentiation, with P2X6 and P2X7 presenting a more clear-cut increase in expression. In our study, these receptors were also more expressed in the more differentiated population of glioma cells, i.e., the adherent cells when compared to spheres. The opposite was true for receptors more expressed in spheres, which were found to reduce its expression along differentiation of P19 cells, except for P2Y14 which was not analyzed in this study [16]. This suggests that there are similarities in the expression of purinergic receptors between neural stem cells and glioma CSCs and their more differentiated counterparts.
Three different antagonists of purinergic P2Y and P2X receptors decreased the differentiation of P19 neural stem cells to NMDA-responsive cells suggesting that activation of these receptors is pro-differentiative for neural stem cells [16]. Here we observed that ATP, which can activate P2Y and P2X receptors, reduces the number and size of spheres, increases the number of cells needed to form a sphere, and decreases the number of CD133, Nanog and Oct-4 positive CSCs. This suggests that in CSCs, ATP also has a pro-differentiative role. Accordingly, proliferation during the course of the experiment (7 days) was also reduced by ATP. FGF is a growth factor fundamental for the maintenance and growth of glioma CSCs, and ATP may act by blocking the activation of the ERK by FGF, as was shown in astrocytes [28].
Due to the low degradation rates of extracellular nucleotides by gliomas [20], it is thought that they accumulate ATP at its border, which was already observed in melanoma tumors [29]. We have shown that ATP has a positive effect on tumor growth, mainly considering the drastic reduction in tumor growth with the injection of apyrase, an ATP and ADP scavenger enzyme which produced AMP [14]. On the other hand, degrading ATP with NTPDase2 (which produces mostly ADP), the tumor size was increased [15]. Therefore, it is difficult to isolate the effect of ATP on CSC differentiation from proliferation [11], death [13, 18], and angiogenesis or modulation of the immune system [15].
Several inhibitors of neurosphere proliferation were evaluated in a broad chemical screen, and several compounds presented inhibitory effects on the proliferation of cultures enriched for brain cancer stem cells [5]. These chemicals induced different neurosphere phenotypes, altering for example sphere number, size, and adhesion properties. Appropriate neurotransmission signaling seems to be required in neural stem cell maintenance, and chemical compounds that affect dopaminergic, cannabinoid, and purinergic receptors, among others, influenced the formation of tumor spheres [5]. Therapies that affect differentiation can also affect tumor malignity and should therefore be explored as treatment alternatives.
In summary, ATP induces several cellular responses, such as proliferation and differentiation, and here we show that ATP reduces the number of spheres in U87 cell line, as well as the amount of CD133+ cells. Co-expression of Oct-4 and Nanog has been linked to carcinogenesis and reduced survival prognosis, and overexpression of Oct-4/Nanog has been shown to enhance sphere formation and the percentage of CD133+ cells in lung adenocarcinoma [30]. Also, expression of those genes, among others, has been considered as a stemness signature in different grade gliomas [31]. We believe that the purinergic system is involved in the formation of cancer stem cells and therefore has to be considered in the search for alternative treatments for glioblastoma multiforme.
Electronic supplementary materials
Presence of CD133 marker in spheres versus monolayer and in control versus ATP treatments. Spheres and monolayer cells were stained with anti-CD133 antibody and analyzed by flow cytometry (a). CD133 analysis by flow cytometry of cells grown for 7 days in the presence or absence of ATP in soft agar plates (spheres) or adherent plates in the same conditions as used in the sphere formation assay with FBS medium (representative of three independent experiments) (b, c) (PDF 980kb)
Titration of ATP effect on sphere formation. Proportion of spheres formed in control and in ATP treatment at concentrations of 1, 10, and 100 μM, in U87 and U343 cells grown on NSC medium (a). Bars represent SEM (n = 4). t test values: *p < 0.05 and **p < 0.001. Representative figures of U87 cells treated with the indicated concentrations of ATP for 7 days (b). Bar size = 100 μm (PDF 2242kb)
Primers used in RT-PCR and annealing temperature (T) (DOC 48 kb)
mRNA expression of purinergic receptors in different gliomas (DOC 38 kb)
Acknowledgments
The present research was supported by grants from Brazilian funding agencies FAPERGS (Procoredes III 06/1376.0 and Pronex 10/0044-3) and CNPq (420036/2005-9). PL, ESV, DGF. and GL are recipient of CNPq fellowships.
Conflict of interest We declare that we have no conflict of interest.
Glossary
- CSC
Cancer stem cell
- Oct-4
Octamer-4
- GLAST
Glutamate–aspartate transporter
- CAMKII
Calcium-calmodulin-dependent kinase II
- NMDA
N-methyl-d-aspartate
- FGF
Fibroblast growth factor
- EGF
Epidermal growth factor
References
- 1.Demuth T, Berens ME. Molecular mechanisms of glioma cell migration and invasion. J Neurooncol. 2004;70(2):217–228. doi: 10.1007/s11060-004-2751-6. [DOI] [PubMed] [Google Scholar]
- 2.Flores DG, Ledur PF, Abujamra AL, Brunetto AL, Schwartsmann G, Lenz G, Roesler R. Cancer stem cells and the biology of brain tumors. Curr Stem Cell Res Ther. 2009;4(4):306–313. doi: 10.2174/157488809789649214. [DOI] [PubMed] [Google Scholar]
- 3.Lenz G. Transient oncogenes. Med Hypotheses. 2010;75(6):660–662. doi: 10.1016/j.mehy.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Vlashi E, Kim K, Lagadec C, Donna LD, McDonald JT, Eghbali M, Sayre JW, Stefani E, McBride W, Pajonk F. In vivo imaging, tracking, and targeting of cancer stem cells. J Natl Cancer Inst. 2009;101(5):350–359. doi: 10.1093/jnci/djn509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diamandis P, Wildenhain J, Clarke ID, Sacher AG, Graham J, Bellows DS, Ling EK, Ward RJ, Jamieson LG, Tyers M, Dirks PB. Chemical genetics reveals a complex functional ground state of neural stem cells. Nat Chem Biol. 2007;3(5):268–273. doi: 10.1038/nchembio873. [DOI] [PubMed] [Google Scholar]
- 6.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, Joshua B, Kaplan MJ, Wapnir I, Dirbas FM, Somlo G, Garberoglio C, Paz B, Shen J, Lau SK, Quake SR, Brown JM, Weissman IL, Clarke MF. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458(7239):780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 8.Yu SC, Ping YF, Yi L, Zhou ZH, Chen JH, Yao XH, Gao L, Wang JM, Bian XW. Isolation and characterization of cancer stem cells from a human glioblastoma cell line u87. Cancer Lett. 2008;265(1):124–134. doi: 10.1016/j.canlet.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Qiang L, Yang Y, Ma YJ, Chen FH, Zhang LB, Liu W, Qi Q, Lu N, Tao L, Wang XT, You QD, Guo QL. Isolation and characterization of cancer stem like cells in human glioblastoma cell lines. Cancer Lett. 2009;279(1):13–21. doi: 10.1016/j.canlet.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Beier D, Rohrl S, Pillai DR, Schwarz S, Kunz-Schughart LA, Leukel P, Proescholdt M, Brawanski A, Bogdahn U, Trampe-Kieslich A, Giebel B, Wischhusen J, Reifenberger G, Hau P, Beier CP. Temozolomide preferentially depletes cancer stem cells in glioblastoma. Cancer Res. 2008;68(14):5706–5715. doi: 10.1158/0008-5472.CAN-07-6878. [DOI] [PubMed] [Google Scholar]
- 11.Morrone FB, Jacques-Silva MC, Horn AP, Bernardi A, Schwartsmann G, Rodnight R, Lenz G. Extracellular nucleotides and nucleosides induce proliferation and increase nucleoside transport in human glioma cell lines. J Neurooncol. 2003;64(3):211–218. doi: 10.1023/A:1025699932270. [DOI] [PubMed] [Google Scholar]
- 12.Wei W, Ryu JK, Choi HB, McLarnon JG. Expression and function of the p2x(7) receptor in rat c6 glioma cells. Cancer Lett. 2008;260(1–2):79–87. doi: 10.1016/j.canlet.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 13.Morrone FB, Horn AP, Stella J, Spiller F, Sarkis JJ, Salbego CG, Lenz G, Battastini AM. Increased resistance of glioma cell lines to extracellular ATP cytotoxicity. J Neurooncol. 2005;71(2):135–140. doi: 10.1007/s11060-004-1383-1. [DOI] [PubMed] [Google Scholar]
- 14.Morrone FB, Oliveira DL, Gamermann P, Stella J, Wofchuk S, Wink MR, Meurer L, Edelweiss MI, Lenz G, Battastini AM. In vivo glioblastoma growth is reduced by apyrase activity in a rat glioma model. BMC Cancer. 2006;6:226. doi: 10.1186/1471-2407-6-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braganhol E, Morrone FB, Bernardi A, Huppes D, Meurer L, Edelweiss MI, Lenz G, Wink MR, Robson SC, Battastini AM. Selective NTPDase2 expression modulates in vivo rat glioma growth. Cancer Sci. 2009;100(8):1434–1442. doi: 10.1111/j.1349-7006.2009.01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Resende RR, Majumder P, Gomes KN, Britto LR, Ulrich H. P19 embryonal carcinoma cells as in vitro model for studying purinergic receptor expression and modulation of N-methyl-d-aspartate-glutamate and acetylcholine receptors during neuronal differentiation. Neuroscience. 2007;146(3):1169–1181. doi: 10.1016/j.neuroscience.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 17.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–5828. [PubMed] [Google Scholar]
- 18.Tamajusuku AS, Villodre ES, Paulus R, Coutinho-Silva R, Battasstini AM, Wink MR, Lenz G. Characterization of ATP-induced cell death in the GL261 mouse glioma. J Cell Biochem. 2010;109(5):983–991. doi: 10.1002/jcb.22478. [DOI] [PubMed] [Google Scholar]
- 19.Du Z, Jia D, Liu S, Wang F, Li G, Zhang Y, Cao X, Ling EA, Hao A. Oct4 is expressed in human gliomas and promotes colony formation in glioma cells. Glia. 2009;57(7):724–733. doi: 10.1002/glia.20800. [DOI] [PubMed] [Google Scholar]
- 20.Wink MR, Lenz G, Braganhol E, Tamajusuku AS, Schwartsmann G, Sarkis JJ, Battastini AM. Altered extracellular ATP, ADP and AMP catabolism in glioma cell lines. Cancer Lett. 2003;198(2):211–218. doi: 10.1016/S0304-3835(03)00308-2. [DOI] [PubMed] [Google Scholar]
- 21.Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006;58(1):58–86. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- 22.Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci USA. 2004;101(3):781–786. doi: 10.1073/pnas.0307618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen G, Shen F, Shi Z, Liu W, Hu W, Zheng X, Wen L, Yang X. Identification of cancer stem-like cells in the C6 glioma cell line and the limitation of current identification methods. In Vitro Cell Dev Biol Anim. 2008;44(7):280–289. doi: 10.1007/s11626-008-9115-z. [DOI] [PubMed] [Google Scholar]
- 24.Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66(16):7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 25.Annabi B, Laflamme C, Sina A, Lachambre MP, Beliveau R. A MT1-MMP/NF-kappaB signaling axis as a checkpoint controller of Cox-2 expression in CD133+ U87 glioblastoma cells. J Neuroinflammation. 2009;6:8. doi: 10.1186/1742-2094-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saidi A, Hagedorn M, Allain N, Verpelli C, Sala C, Bello L, Bikfalvi A, Javerzat S. Combined targeting of interleukin-6 and vascular endothelial growth factor potently inhibits glioma growth and invasiveness. Int J Cancer. 2009;125(5):1054–1064. doi: 10.1002/ijc.24380. [DOI] [PubMed] [Google Scholar]
- 27.Ouafik L, Sauze S, Boudouresque F, Chinot O, Delfino C, Fina F, Vuaroqueaux V, Dussert C, Palmari J, Dufour H, Grisoli F, Casellas P, Brunner N, Martin PM. Neutralization of adrenomedullin inhibits the growth of human glioblastoma cell lines in vitro and suppresses tumor xenograft growth in vivo. Am J Pathol. 2002;160(4):1279–1292. doi: 10.1016/S0002-9440(10)62555-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lenz G, Goncalves D, Luo Z, Avruch J, Rodnight R, Neary JT. Extracellular ATP stimulates an inhibitory pathway towards growth factor-induced cRaf-1 and MEKK activation in astrocyte cultures. J Neurochem. 2001;77(4):1001–1009. doi: 10.1046/j.1471-4159.2001.00299.x. [DOI] [PubMed] [Google Scholar]
- 29.Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Virgilio F. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS One. 2008;3(7):e2599. doi: 10.1371/journal.pone.0002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiou SH, Wang ML, Chou YT, Chen CJ, Hong CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS, Wu CW. Coexpression of Oct4 and Nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial–mesenchymal transdifferentiation. Cancer Res. 2010;70(24):10433–10444. doi: 10.1158/0008-5472.CAN-10-2638. [DOI] [PubMed] [Google Scholar]
- 31.Clement V, Sanchez P, Tribolet N, Radovanovic I, Ruiz I, Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17(2):165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Presence of CD133 marker in spheres versus monolayer and in control versus ATP treatments. Spheres and monolayer cells were stained with anti-CD133 antibody and analyzed by flow cytometry (a). CD133 analysis by flow cytometry of cells grown for 7 days in the presence or absence of ATP in soft agar plates (spheres) or adherent plates in the same conditions as used in the sphere formation assay with FBS medium (representative of three independent experiments) (b, c) (PDF 980kb)
Titration of ATP effect on sphere formation. Proportion of spheres formed in control and in ATP treatment at concentrations of 1, 10, and 100 μM, in U87 and U343 cells grown on NSC medium (a). Bars represent SEM (n = 4). t test values: *p < 0.05 and **p < 0.001. Representative figures of U87 cells treated with the indicated concentrations of ATP for 7 days (b). Bar size = 100 μm (PDF 2242kb)
Primers used in RT-PCR and annealing temperature (T) (DOC 48 kb)
mRNA expression of purinergic receptors in different gliomas (DOC 38 kb)