Abstract
Pannexin1 is a prime candidate to represent an ATP release channel. The pannexin1 channel can be activated by extracellular ATP through purinergic receptors P2X7 or P2Y. Recent studies have shown that the Pannexin1 channel is inhibited by its own permeant ion, ATP, and also by P2X7 receptor agonists and antagonists. However, the dose dependence of this inhibition indicated that significant inhibition was prominent at ATP concentrations higher than required for activation of purinergic receptors, including P2X7 and P2Y2. The inhibitory effect of ATP is largely decreased when R75 in the first extracellular loop of Pannexin1 is mutated to alanine, indicating that ATP regulates this channel presumably through binding. To further investigate the structural property of the putative ATP binding site, we performed alanine-scanning mutagenesis of the extracellular loops of pannexin1. Mutations on W74, S237, S240, I247 and L266 in the extracellular loops 1 and 2 severely impaired the inhibitory effect of BzATP, indicating that they might be the essential amino acids in the putative binding site. Mutations on R75, S82, S93, L94, D241, S249, P259 and I267 moderately (≥50%) decreased BzATP sensitivity, suggesting their supporting roles in the binding. Mutations of other residues did not change the BzATP potency compared to wild-type except for some nonfunctional mutants. These data demonstrate that several amino acid residues on the extracellular loops of Pannexin1 mediate ATP sensitivity. Cells expressing mutant Panx1W74A exhibited an enhanced release of ATP, consistent with the removal of a negative feedback loop controlling ATP release.
Keywords: ATP release, Pannexin, Alanine scanning mutagenesis, Channel inhibition, BzATP, BBG
Introduction
Pannexins represent a recently discovered second family of gap junction proteins in vertebrates. However, instead of forming the intercellular channels of gap junctions as connexins do, pannexins operate as unpaired pannexons, allowing the flux of molecules from the cytoplasm to the extracellular space and vice versa [1]. It has been recognized that Pannexin1 (Panx1) exerts a key role in the release of ATP [2–5]. In this function Panx1 contributes to the propagation of intercellular calcium waves. Several observations link Panx1 to inflammation, including signaling for the release of the pro-inflammatory cytokine IL-1beta and entry of bacterial peptides [6–8]. Panx1 has also been shown to mediate the activation of the inflammasome and secondary cell death [9, 10]. Whether these phenomena are based exclusively on the ATP release function of Panx1 or involve additional or alternative mechanisms is presently not resolved.
The function of extracellular nucleotides as signaling molecules has been established for decades [11, 12]. It appears that ATP release occurs by more than one mechanism. In addition to the classical vesicular release of ATP most cells also involve a channel-mediated process as indicated by pharmacological data and by the uptake of extracellular tracer molecules correlated with ATP release. The identity of the nucleotide release channel has been the subject of some speculation. The characterization of the Panx1 channel indicates that this channel fulfills the criteria of the long-sought ATP conduit. Besides some basic properties that are expected for an ATP release channel involved in calcium wave propagation, such as high permeability to ATP [2], mechanosensitivity [2], activation by increased cytoplasmic calcium and activation by ATP through purinergic receptors [13], Panx1 also exhibits a series of additional properties consistent with an ATP release channel function.
The evidence for the ATP release function of Panx1 channels includes that Panx1 is expressed in cells that are documented to have channel-mediated ATP release, including erythrocytes, endothelial cells, astrocytes and airway epithelial cells [3–5, 14]. Notable in airway epithelial cells, the expression of Panx1 is restricted to the apical membrane of these cells, where the ATP release takes place [3]. Furthermore, ATP release or surrogate measures are correlated with Panx1 expression levels. In knockdown experiments with Panx1 siRNA/shRNA, the ATP release is largely attenuated [3, 14]. Also, the pharmacology of Panx1 channels matches that of ATP release. Almost all compounds that are reported to inhibit non-vesicular ATP release are effective on the Panx1 channel including connexin mimetic peptides and the inhibitor of the organic anion transporter probenecid [15, 16]. Finally, the Panx1 channel is controlled by ATP via a negative feedback loop [17].
Panx1 forms a large channel with a unitary conductance of up to 500 pS [2]. Prolonged opening of this kind of large pore could lead to rundown of transmembrane gradients and loss of cell constituents, causing cell death. Given that Panx1 can be activated by ATP through purinergic receptors, this channel has been proposed to be involved in the secondary cell death after traumatic brain and spinal cord injury as well as stroke [9, 10]. To prevent ATP-induced cell death under normal physiological conditions, the simplest and most common way is a negative feedback, which the Panx1 inhibition by ATP apparently represents.
Independent studies on ATP-induced activation of the inflammasome and cell death have shown that Panx1 is the pore-forming unit of the P2X7 death complex [6, 9, 17]. In our previous study on ATP effects on Panx1 [18], we have observed that ligands of the P2X7 receptor are also effective inhibitors on Panx1 but with different affinities. This implies that the mechanism of autoinhibition of Panx1 by ATP may be through ligand binding similar to the P2X7 receptor. However, the concentrations of ligands required for inhibition of Panx1 channels are substantially higher than those affecting P2X7 receptors.
We suspected that the site of ATP action on Panx1 was extracellular because of the prevailing high intracellular ATP concentration and because application of extracellular ATP to the closed channel resulted in substantial attenuation of currents at first opening [18]. The most common ATP binding motif, the Walker loop [19], is absent from Panx1 protein, which has four transmembrane segments, two extracellular loops and cytoplasmic localization of the amino- and carboxy-terminal segments. Our previous mutagenesis data suggest that ATP works on Panx1 through ligand binding and R75 plays an important role in the binding because the inhibitory effect of ATP is largely decreased when R75 on the first extracellular loop of Panx1is mutated to alanine. A SCAM analysis of Panx1 [20] revealed that the permeation pathway of the Panx1 channel shares some similarity with the connexin26 channel [21] but is also distinct. The external portion of the channel pore is similar and is constructed by the amino acids from the first transmembrane segment and the first extracellular loop. Here, we tested whether residue R75 is located at the external portion of the Panx1 permeation pathway, which allows the accessibility of the ligands. To further characterize the ATP binding site, we used alanine-scanning mutagenesis on both extracellular loops of Panx1 to identify residues important for the inhibition of Panx1 channel activity by ATP.
Materials and methods
Materials Mouse pannexin1 was kindly provided by Dr. Rolf Dermietzel (University of Bochum). The plasmid containing mouse pannxin1 and its mutants in pCS2 was linearized with Not I. In vitro transcription was performed with SP6 polymerase, using the Message Machine kit (Ambion, Austin, TX). mRNAs were quantified by absorbance (260 nm), and the proportion of full-length transcripts was checked by agarose gel electrophoresis. The ATP, BzATP, BBG were purchased from SigmaAldrich. The thiol reagents MTSET, MTSEA and MTSES were purchased from Toronto Research Chemicals.
Mutagenesis The alanine mutants were engineered with QuickChange II site-directed mutagenesis kit (Stratagene) according to the manufacturers specifications. The purified mutant plasmids were sequenced by Genewiz.
Preparation of oocytes Preparation of oocytes and electrophysiological recording were performed as described [22]. All procedures were conducted in accordance with the Guiding Principles for Research Involving Animals and Human Beings of American Physiological Society. Ovaries of Xenopus were cut into small pieces for digestion in zero calcium OR2 containing 2.5 mg/ml collagenase (Worthington) for about 3 h. After wash in regular OR2, the mature, follicle cell devoid oocytes with even pigmentation were picked and saved for experimental use. In vitro transcribed mRNAs (∼40 nl) were injected into Xenopus oocytes. Cells were incubated in regular frog Ringer solution OR2 (82.5 mM NaCl, 2.5 mM KCl, 1 mM Na2HPO4, 1 mM MgCl2, 1 mM CaCl2 and 5 mM HEPES) with 10 mg/ml streptomycin.
Electrophysiology Whole cell membrane current of single oocytes was measured using a two-electrode voltage clamp and recorded with a chart recorder. Both voltage-measuring and current-passing microelectrodes were pulled with a vertical puller (Kopf) and filled with 3 M KCl. The recording chamber was perfused continuously with solution. Membrane conductance was determined using voltage pulses. Oocytes expressing pannexin1 were held at −50 mV, and pulses to +50 mV were applied to transiently open the channels.
ATP release assay ATP flux was determined by luminometry. Oocytes, two days after injection of pannexin1 and mutant messenger RNAs, were pretreated in OR2 solution with and without BBG for 10 min and stimulated by incubation in OR2 solution (negative control), KGlu solutions (positive control), KGlu solution with BBG, respectively, for 10 min. The supernatant was collected and assayed with luciferase/luciferin (Promega, Madison, USA). Human erythrocytes (G.D’s) were washed three times in Krebs solution by low speed centrifugation. Erythrocytes were suspended at 25% hematocrit and aliquots of 100 μl were used for experiments. Erythrocytes were preincubated in Krebs solution with or without the drugs (BBG or probenecid) for 5 min. A 10× excess of Krebs solution or 150 mM potassium gluconate (KGlu) buffered with 5 mM HEPES and containing the drugs, when applicable, was added to the erythrocytes. All experimental conditions were performed at room temperature. After a 10-min incubation period, ATP was determined in the supernatant of a 100×g spin with a luciferase assay.
Results
A putative ATP binding site
At the resting membrane potential, Panx1 channels are closed but can be activated by various mechanisms including voltage steps to positive potentials or by increasing the extracellular potassium ion concentration [2, 10, 19]. We have observed previously that Panx1 mediated currents as well as uptake of extracellular tracer molecules and ATP release from cells can be inhibited by ATP and other ligands of the P2X7 receptor, irrespective of whether the ligands are acting as agonist or antagonist at the receptor [18]. Following the lead from theses previous studies that ATP and analogues may act by binding to the extracellular aspect of the Panx1 channel we further analyzed the role of extracellular amino acids in Panx1 by an alanine-scanning analysis. Replacement of autochthonous amino acids by alanine residues is typically well tolerated unless the replaced amino acid exerts a specific role in protein folding or function.
Alanine-scanning mutagenesis of mPanx1 and responses of mutants to ATP/BzATP
As shown in Fig. 1, ATP (500 μM) and more effectively BzATP (100 μM) inhibited currents in Xenopus oocytes expressing wild-type Panx1 while this inhibition was attenuated in Panx1 R75A as reported earlier [18]. Mutation of neighboring amino acids had an even more pronounced effect (Panx1 W74A) or did not impair the ATP/BzATP effect (Q76A, S73A). Alanine substitution of F72 failed to form the typical voltage-gated Panx1 channel. To identify other residues on Panx1 important for ATP binding, a complete alanine-scanning mutagenesis analysis of the extracellular amino acids of Panx1 was performed. Several online softwares, i.e., TMpred (http://www.ch.embnet.org/software/TMPRED_form.html) and TMHMM (http://www.cbs.dtu.dk/services/TMHMM) were used to predict the transmembrane segments and consequently the position of the extracellular loops of mouse Panx1. The derived first extracellular loop extends from I58 to K107 and the second extracellular loop is from S237 to G270. All these residues were systematically substituted by alanine one at a time and the endogenous alanines were replaced by cysteine, another well tolerated replacement amino acid.
Fig. 1.
Inhibition of membrane currents by ATP or BzATP in oocytes expressing wtPanx1 (top) or a series of alanine mutants of Panx1. Membrane currents were induced by −50 to +50 mV voltage steps in voltage-clamped Xenopus oocytes. The currents were reversibly attenuated by ATP and BzATP for wtPanx1 and the mutants Panx1S73A and Panx1Q76A. Panx1 W74A was not affected by these compounds, while Panx1R75A was slightly inhibited by 100 μM BzATP but not by 500 μM ATP
Of the 81 mutants we engineered, 24 mutants did not exhibit any channel activity when expressed in oocytes (Figs. 2 and 3). Presently, it is unclear whether the failure to form functional channels by these mutants is due to impaired channel structure or due to misfolding and failure to insert into the plasma membrane. Except for these null mutants, alanine mutations on the remaining 57 residues were well tolerated, and channel activities were similar to wt, i.e. gating by membrane voltage and blockage by carbenoxolone (CBX).
Fig. 2.
Quantitative analysis of inhibition of membrane currents in oocytes expressing wtPanx1 and alanine mutants covering the first (a) and second (b) extracellular loop sequences of Panx1. All data are normalized to the % inhibition observed by 100 μM BzATP for wtPanx1 currents. For three positions (A86, A87, A268) no mutants were obtained. Means ± SD, n = 3–4
Fig. 3.
Summary of alanine scan analysis of Panx1 current inhibition by BzATP
For most experiments we used BzATP (100 μM) instead of ATP because of its higher stability [23] and because both compounds have the similar effect on Panx1, including current inhibition and dye-uptake inhibition, implying these two compounds may share a similar binding site on Panx1. Furthermore, BzATP has a stronger effect on Panx1 than ATP, which increases the sensitivity of mutant screening. Moreover, we tested the effects of both ATP and BzATP on some of the mutants and observed similar consequences of the mutation on the effects of both drugs, confirming that BzATP is a faithful alternative for the ATP binding site studies.
To quantify the effect of the mutations, BzATP responses, were recorded from all of the functional mutant channels and normalized to the effect on wt Panx1. Reversible inhibitions were still displayed in most of the mutants (45 of 57) with no significant change compared to wt. A modest 40% to 60% decrease in BzATP efficacy was seen for seven mutants, R75A, L93A, L94A, D241A, S249A, P259A and I267A; inhibition of Panx1 currents by BzATP was essentially eliminated in mutants W74A, S237A, S240A, I247A and L266A (Figs. 2 and 3).
Effect of charge on ATP inhibition of Panx1 currents
ATP binding sites on proteins characteristically include the positively charged amino acids arginine and lysine. The two extracellular loops of Panx1 contain 7 positively charged amino acids. The contribution of 2 of them could not be assessed because mutation to alanine resulted in loss of channel function. Mutation of 4 remaining positively charged amino acids to alanine did not affect the inhibitory action of ATP on the membrane currents as compared to wild-type Panx1. However, as shown previously [18] channels formed by Panx1 R75A or Panx1 R75C exhibited a significantly attenuated response of the currents to ATP or its more potent analogue BzATP. Replacement of the arginine in position 75 by glutamate abolished the ATP effect while the replacement of the arginine with lysine remained without consequence [18]. To test whether the effect of ATP or BzATP could be restored by other positively charged moieties we utilized charged methanethiosulfonate (MTS) thiol reagents in combination with the cysteine replacement mutant of R75.
We recently found that the carboxy-terminal cysteine in wt Panx1 is reactive to thiol reagents, indicating that this cysteine is in the permeation pathway of Panx1 [20]. In order to test the accessibility of R75 without the interference of the endogenous reactive cysteine, a double mutant, R75C/C426S, was constructed and tested for the effect of the thiol reagents MTSET (+), MTSEA (+), MTSES (−), and MBB (−).
As shown in Fig. 4, the thiol-reagent MTSET by itself attenuated the current of R75C/C426S suggesting that the thiol-reagent reacted with the engineered cysteine at 75 thereby presumably blocking the permeation pathway of Panx1. Consequently, R75 is accessible from the extracellular side and it is localized in or close to the pore of Panx1. Application of BzATP resulted in further inhibition of the currents when the positively charged MTSET was used for thiol reaction (Fig. 4). A similar but less pronounced effect was observed after reaction with MTSEA, which is neutral at higher pH (pKa 8.5) but positively charged at pH 7.5. In contrast, after reaction of Panx1 R75C/C426S with the negatively charged MTSES or MBB, no inhibition of currents was observed after application of BzATP (Fig. 4).
Fig. 4.
Effect of BzATP on membrane currents in oocytes expressing Panx1-R75C/C426S after reaction with thiol reagents. A pulse protocol as described in Fig. 1 was applied. The thiol reagents MTSET and MTSEA (positively charged), and MTSES and MBB (negatively charged) were applied at 1 mM or 100 μM (MBB) concentrations and BzATP (50 μM) was added after currents reached a steady state. In c and d the MTS solutions were applied before the beginning of the traces. In the quantitative analysis (e), the effect of BzATP on the channel not reacted with the thiol reagent was set to 1 and the data obtained after thiol reaction were normalized to it. Means ± SD, n = 4
Regulation of ATP release is impaired by alanine mutation
The effect of ATP and analogues on Panx1 channels is not limited to inhibition of ionic currents. Brilliant Blue G (BBG), for example, also inhibited ATP release from oocytes expressing Panx1 exogenously [18] or from erythrocytes (Fig. 5). Because Panx1 is an ATP release channel, the inhibition of ATP on Panx1 provides a negative feedback regulation. We tested whether this feedback was impaired by alanine mutations. We examined the BBG effect on ATP release from oocytes expressing W74A, 102A and wt respectively. Panx1 W74A currents were not affected by 1 μM BBG, while currents in oocytes expressing wtPanx1 or Panx1 P102A (not shown) were highly attenuated. ATP release was attenuated by 1 μM BBG in wt and P102A, but not in W74A, consistent with the current measurements (Fig. 6).
Fig. 5.
Effect of BBG on ATP release from human erythrocytes. ATP release from erythrocytes was stimulated with potassium gluconate (150 mM) solution. 1 μM BBG was as effective in inhibiting the potassium induced ATP release as the Panx1 inhibitor probenecid (prob). Means ± SD, n = 4
Fig. 6.
Lack of inhibition by BzATP and BBG of membrane currents and ATP release from oocytes expressing Panx1-W74A. BzATP and BBG had no detectable effect on currents carried by Panx1-W74A (a) but attenuated currents through wtPanx1 channels (b). ATP release from oocytes expressing wt Panx1, Panx1W74A or Panx1P102A was stimulated by superfusion with potassium gluconate solution. BBG (1 μM) inhibited ATP release through wt Panx1 and Panx1P102A but not through Panx1W74A (c). Data were normalized to stimulated release by wt Panx1. Means ± SD, n = 5
Notably, ATP release in the absence of drugs from oocytes expressing W74A mutant and stimulated with KGlu for 10 min was significantly (up to tenfold) higher than that from wt expressing oocytes: 39,471 ± 4823 (n = 6) versus 4,574 ± 684 (n = 6) luminescence units, respectively (see also Fig. 7a and b). This large increase in ATP release occurred despite only slightly increased current amplitudes (less than twofold over wt Panx1). This phenomenon can be explained by the loss of negative feedback control of ATP release through mutated Panx1 channels. With longer incubation periods the difference in ATP release between oocytes expressing wt Panx1 and the W74A mutant diminished probably due to exhaustion of the ATP supply (Fig. 7a and b). As another test of the negative feedback hypothesis, we analyzed volume-dependent ATP release from wt and W74A mutant. For wt Panx1, the amount of ATP released from oocytes in a 100 μl extracellular volume was much smaller than the ones in 1 ml extracellular space, suggesting a concentration dependent inhibition of ATP release by extracellular ATP. The W74A mutant, which is likely to have removed a potential ATP binding site, released a significantly larger amount of ATP with no difference between small and large extracellular volumes (Fig. 7b and c). Similar results were observed in the other three Panx1 mutants, S237A, S240A and L266A, that do not exhibit BzATP mediated inhibition of currents (Fig. 7d).
Fig. 7.
Loss of negative feedback resulting in increased ATP release. Time course of ATP release from oocytes expressing wt Panx1 (a) or Panx1W74A (b) stimulated by potassium gluconate is shown. c A plot of the amount of ATP released from oocytes through wt Panx1 or Panx1W74A into a small (100 μl) or a large (1 ml) fluid volume after 10 min of incubation. d An equivalent plot to c for mutants S237A, S240A and L266A. In c and d data were normalized to the value obtained for the small volumes. Means ± SD, n = 5
It is likely, that the ATP concentrations measured in the bulk solution are proportional to but do not reflect the actual concentrations at the mouth of the Panx1 channel because of unstirred layer effects. The oocytes used in these experiments had the follicle cells removed but were not devitellinized. The glycoprotein meshwork forming the circa 5 μm thick vitelline layer on the oocyte surface can be expected to trap molecules and reduce the exchange with the bulk solution. This effect is aggravated in the absence of perfusion, which was not applied in the experiments.
Discussion
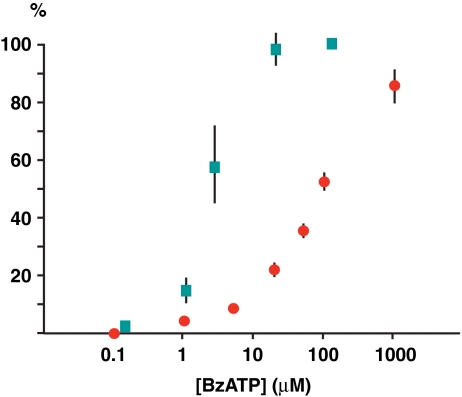
As shown previously, extracellular application of ATP has an inhibitory effect on Panx1 currents [18, 24]. Since Panx1 is a serious candidate to represent the prime ATP release channel, it seems counterintuitive that ATP would inhibit Panx1. However, because Panx1 forms a large channel with poor selectivity, excessive loss of ATP and other cellular constituents through open Panx1 channels would be deleterious to cells. This could be prevented by a negative feedback regulation of the channel. The inhibitory effect of ATP on Panx1 channel would represent the simplest and most direct form of negative feedback control of channel activity. Moreover, Panx1 can be activated by extracellular ATP through purinergic receptors, the probable mechanism underlying ATP-induced ATP release. This represents a positive feedback loop that would keep the Panx1 channel in an open configuration unless there was a deactivation mechanism to keep this channel in check and thereby maintaining cell integrity. This dual regulation of Panx1 channel activity is realized by different ATP concentrations required for the activation and inhibition of Panx1. The concentration of BzATP required to inhibit Panx1 channels is almost two orders of magnitude higher than that required to activate Panx1 through P2X7R (Fig. 8). This property allows for the coexistence of a positive feedback loop and a negative feedback loop. The former is operative at low ATP concentrations, while the latter kicks in as the extracellular ATP concentration builds up.
Fig. 8.
Difference in concentrations of BzATP required to activate (green squares) and to inhibit (red dots) Panx1 channels. Activation of Panx1 channels by BzATP was obtained by co-expression of Panx1 and P2X7R in oocytes. Original data from [9] and [17], n = 3–5
The binding pocket for ATP is well characterized for a number of ATP-regulated proteins. The Walker motif, for example is found in myosin, ATP synthase, various kinases and the CFTR chloride channel [25, 26].
However, not every ATP-regulated protein contains a conventional ATP binding motif, including the P2X receptors. Recent crystallographic data indicate the ATP binding pocket in P2X receptors is formed by the positively charged amino acids lysine and arginine and two phenylalanines, confirming earlier mutagenesis data [27–29]. Because the pharmacological properties of P2X7R and Panx1 channel inhibition are similar [18, 30] one might expect a similar ATP binding site. However, sequence alignment between these proteins did not reveal a common motif in their extracellular segments.
Here, a series of amino acids have been identified mediating the inhibitory effect of ATP on Panx1 currents. With the mutational approach taken, it cannot be discriminated whether the attenuation of the ATP effect on Panx1 currents is due to impaired binding of ATP to the channel, due to misfolding of the binding pocket, or due to changes in gating of the channel as a consequence of the alanine substitution. Common to all ATP binding sites is the presence of basic amino acids. The replacement of one of the seven basic amino acids in the extracellular loops of Panx1 (R75) indeed abolished the effect of ATP on Panx1 currents. Replacement of two other basic amino acids (K248 and K256) by alanine resulted in loss of channel activity precluding an evaluation of their potential contribution to the ATP effect.
Replacement of Panx1R75 with a cysteine had the same consequence as the alanine replacement. However, the reaction of the substituting cysteine with a positively charged thiol reagent in part restored the BzATP induced channel inhibition, stressing the importance of a positive charge in this position. Taken together, these observations are consistent with an ATP binding site in Panx1. This site likely includes R75 and some of the amino acids in the positions where alanine replacement attenuated or abolished the inhibitory effect on Panx1 channel currents.
Typically, ATP binding sites involve more than one positive charge. Because the contribution of two positions with positively charged amino acids could not be evaluated, it is not clear whether R75 is the sole contributor to the putative ATP binding site in Panx1 channels. Even so, it is possible that the binding site is formed from interaction of residues between adjacent subunits which is a common phenomenon for ligand-binding proteins, such as P2X receptor [28], nicotinic acetylcholine receptors [31] and γ-aminobutyric acid A receptor [32].
Covalent modification by MBB of the cysteine replacing R75 resulted in an irreversible block of conductance. This observation suggests that the pore of the Panx1 channel extends further into the first extracellular loop than previously reported [20]. If so, the ATP binding site is located close to or within the extracellular vestibulum of the channel and the inhibitory effect of ATP and related substances on channel conductance could be by steric obstruction of the permeation pathway.
Aside from the basic amino acids, other residues on the extracellular portion of Panx1 were also engineered into alanine mutants one at a time and it was inferred that a residue was involved directly or indirectly in ATP binding if the inhibitory effect of BzATP on the mutant was statistically significantly smaller than the effect on the wild-type channel. Of the 84 mutants tested, 24 did not yield detectable channel activity. For three positions (86, 87, 268) no mutants were obtained. In the remaining 57 residues, alanine substitution (a) had no effect on BzATP inhibitory potency, indicating these residues are not essential for binding; (b) had moderate reduction in BzATP potency (R75A, S93A, L94A, D241A, S249A, P259A, I267A); (c) had significantly decreased BzATP potency (W74A, S237A, S240A, I247A, L266A).
Several lines of evidence indicate that one of the physiological roles of Panx1 is that of an ATP release channel in various cell types. The present study provides further evidence for this function. Removal of the inhibitory effect of ATP on Panx1 channel currents by alanine replacement led to an increased ATP release from oocytes. The ATP release in oocytes expressing any of the four mutant Pannexins tested (Panx1 W74A, S237A, S240A and L266A) was five times higher than that observed in oocytes expressing wt Panx1. The amount of ATP released from wt Panx1 expressing oocytes was also boosted, albeit to a lesser extent, by incubating the cells in a large volume. Apparently, the dilution of ATP in the larger volume lessens the channel inhibition by the released ATP. Consequently, it can be expected that the negative feedback inhibition of ATP release through Panx1 channels will be minimal in cells like erythrocytes in the blood stream. On the other hand, ATP released into narrow extracellular spaces as in the nervous system would be subject to the feedback inhibition. Thus activation of Panx1 channels by ATP through purinergic receptors would not necessarily result in cell death, a phenomenon nevertheless observed upon massive and prolonged stimulation and blockable by the Panx1 channel inhibitor probenecid [10, 16].
Upon request, we refer to Chekeni et al. [33], who showed that Panx1 in addition to be capable of releasing ATP, in certain cells under certain conditions can be cleaved by caspase 3 to yield a carboxyterminus depleted Panx1. The authors concluded that the irreversible Panx1 truncation is a prerequisite for Panx1 channel opening and ensuing ATP release. This conclusion is not supported by the findings in several laboratories, that Panx1 channels can be opened and closed in rapid succession in various cell types in a voltage dependent fashion, by mechanical stress, by ATP through purinergic receptors, by glutamate through NMDA receptors and in a low oxygen environment. Furthermore, erythrocytes, astrocytes, macrophages and airway epithelial cells exhibit repetitive Panx1-mediated ATP release without ensuing cell death [2–6, 13, 19, 34–37]. Last but not least, there is direct experimental evidence that Panx1 channels can be opened with the carboxyterminus attached. The terminal cysteine in Panx1 is reactive to thiol reagents and the reaction requires an open channel, proving that at least in oocytes the complete Panx1 protein can form open channels [20]. However, the caspase cleavage of Panx1 could very well play a role late in the signaling cascade, when it commits cells to death. This would be consistent with the long time required (>1 h) for cleavage to become prominent [33].
Acknowledgments
We thank Dr. Peter Larsson for reading an early version of the manuscript. This work was supported by NIH grant GM 48610.
Glossary
- Panx1
Pannexin1
- BzATP
2′(3′)-O-(4-Benzoylbenzoyl)adenosine-5′-triphosphate
- BBG
Brilliant Blue G
- MTSET
[2-(trimethylammonium)ethyl] methanethiosulfonate bromide
- MTSEA
2-aminoethyl methanethiosulfonate hydrobromide
- MTSES
Sodium 2-sulfonatoethyl methanethiosulfonate
- MBB
Maleimidobutyryl-biocytin
- CBX
Carbenoxolone
References
- 1.Dahl G, Locovei S. Pannexin: to gap or not to gap, is that a question? IUBMB Life. 2006;58:409–419. doi: 10.1080/15216540600794526. [DOI] [PubMed] [Google Scholar]
- 2.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M. Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol. 2009;41:525–534. doi: 10.1165/rcmb.2008-0367OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte "hemichannels". J Neurosci. 2009;29:7092–7097. doi: 10.1523/JNEUROSCI.6062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci U S A. 2006;103:7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelegrin P, Surprenant A (2006). Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X(7) receptor. Embo J [DOI] [PMC free article] [PubMed]
- 7.Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Nunez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Marina-Garcia N, Franchi L, Kim YG, Miller D, McDonald C, Boons GJ, Nunez G. Pannexin-1-mediated intracellular delivery of muramyl dipeptide induces caspase-1 activation via cryopyrin/NLRP3 independently of Nod2. J Immunol. 2008;180:4050–4057. doi: 10.4049/jimmunol.180.6.4050. [DOI] [PubMed] [Google Scholar]
- 9.Locovei S, Scemes E, Qiu F, Spray DC, Dahl G. Pannexin1 is part of the pore forming unit of the P2X7 receptor death complex. FEBS Lett. 2007;581:483–488. doi: 10.1016/j.febslet.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverman WR, Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, Keane RW, Dahl G. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem. 2009;284:18143–18151. doi: 10.1074/jbc.M109.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drury AN, Szent-Gyorgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929;68:213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnstock G. Neural nomenclature. Nature. 1971;229:282–283. doi: 10.1038/229282d0. [DOI] [PubMed] [Google Scholar]
- 13.Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Scemes E, Suadicani SO, Dahl G, Spray DC. Connexin and pannexin mediated cell-cell communication. Neuron Glia Biol. 2007;3:199–208. doi: 10.1017/S1740925X08000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Ma M, Locovei S, Keane RW, Dahl G. Modulation of membrane channel currents by gap junction protein mimetic peptides: size matters. Am J Physiol Cell Physiol. 2007;293:C1112–C1119. doi: 10.1152/ajpcell.00097.2007. [DOI] [PubMed] [Google Scholar]
- 16.Silverman W, Locovei S, Dahl G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol. 2008;295:C761–C767. doi: 10.1152/ajpcell.00227.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qu, Y. et al. (2011). Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J Immunol [DOI] [PubMed]
- 18.Qiu F, Dahl G. A permeant regulating its permeation pore: inhibition of pannexin 1 channels by ATP. Am J Physiol Cell Physiol. 2009;296:C250–C255. doi: 10.1152/ajpcell.00433.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A. 2003;100:13644–13649. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Dahl G. SCAM analysis of Panx1 suggests a peculiar pore structure. J Gen Physiol. 2010;136:515–527. doi: 10.1085/jgp.201010440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maeda S, Nakagawa S, Suga M, Yamashita E, Oshima A, Fujiyoshi Y, Tsukihara T. Structure of the connexin 26 gap junction channel at 3.5 Å resolution. Nature. 2009;458:597–602. doi: 10.1038/nature07869. [DOI] [PubMed] [Google Scholar]
- 22.Dahl G, Pfahnl A. Mutagenesis to study channel structure. Methods Mol Biol. 2001;154:251–268. doi: 10.1385/1-59259-043-8:251. [DOI] [PubMed] [Google Scholar]
- 23.Williams N, Coleman PS. Exploring the adenine nucleotide binding sites on mitochondrial F1-ATPase with a new photoaffinity probe, 3′-O-(4-benzoyl)benzoyl adenosine 5′-triphosphate. J Biol Chem. 1982;257:2834–2841. [PubMed] [Google Scholar]
- 24.Ma W, Hui H, Pelegrin P, Surprenant A. Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J Pharmacol Exp Ther. 2009;328:409–418. doi: 10.1124/jpet.108.146365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker JE, Saraste M, Runswick MJ, Gay NJ. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang TC, Sheppard DN. Gating of the CFTR Cl- channel by ATP-driven nucleotide-binding domain dimerisation. J Physiol. 2009;587:2151–2161. doi: 10.1113/jphysiol.2009.171595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts JA, Evans RJ. ATP binding at human P2X1 receptors. Contribution of aromatic and basic amino acids revealed using mutagenesis and partial agonists. J Biol Chem. 2004;279:9043–9055. doi: 10.1074/jbc.M308964200. [DOI] [PubMed] [Google Scholar]
- 28.Ennion S, Hagan S, Evans RJ. The role of positively charged amino acids in ATP recognition by human P2X1 receptors. J Biol Chem. 2000;275:35656. doi: 10.1074/jbc.M003637200. [DOI] [PubMed] [Google Scholar]
- 29.Kawate T, Michel JC, Birdsong WT, Gouaux E. Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature. 2009;460:592–598. doi: 10.1038/nature08198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- 31.Czajkowski C, Karlin A. Structure of the nicotinic receptor acetylcholine-binding site. Identification of acidic residues in the delta subunit within 0.9 nm of the 5 alpha subunit-binding. J Biol Chem. 1995;270:3160–3164. doi: 10.1074/jbc.270.7.3160. [DOI] [PubMed] [Google Scholar]
- 32.Smith GB, Olsen RW. Functional domains of GABAA receptors. Trends Pharmacol Sci. 1995;16:162–168. doi: 10.1016/S0165-6147(00)89009-4. [DOI] [PubMed] [Google Scholar]
- 33.Chekeni FB, et al. Pannexin 1 channels mediate 'find-me' signal release and membrane permeability during apoptosis. Nature. 2010;467:863–867. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson RJ, Jackson MF, Olah ME, Rungta RL, Hines DJ, Beazely MA, MacDonald JF, MacVicar BA. Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science. 2008;322:1555–1559. doi: 10.1126/science.1165209. [DOI] [PubMed] [Google Scholar]
- 35.Iglesias R, Locovei S, Roque A, Alberto AP, Dahl G, Spray DC, Scemes E. P2X7 receptor-Pannexin1 complex: pharmacology and signaling. Am J Physiol Cell Physiol. 2008;295:C752–C760. doi: 10.1152/ajpcell.00228.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sridharan M, Adderley SP, Bowles EA, Egan TM, Stephenson AH, Ellsworth ML, Sprague RS. Pannexin 1 is the conduit for low oxygen tension-induced ATP release from human erythrocytes. Am J Physiol Heart Circ Physiol. 2010;299:H1146–H1152. doi: 10.1152/ajpheart.00301.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seminario-Vidal, L. et al. (2011). RHO signaling regulates pannexin 1-mediated ATP release from airway epithelia. J Biol Chem [DOI] [PMC free article] [PubMed]