P2X receptors are ligand-gated ion channels that induce the flux of cations across the membrane when activated by ATP, resulting in membrane depolarization and Ca2+ entry [1–4]. In mammals, P2X receptors have been shown to play a critical role in regulating physiological processes in a wide range of cell types such as neurons, glial cells, muscle, endothelial and epithelial cells, osteoclasts, and hematopoietic cells [1, 3]. The cloning of the first P2X receptor P2X1 in 1994 revealed an unusual structure of P2X receptors containing two transmembrane segments [5, 6], which is distinct from other ionotropic receptors such as glutamate receptors and acetylcholine receptors [3]. P2X receptors appear to be present in all vertebrate and many invertebrate genomes but are absent in some invertebrates such as Caenorhabditis elegans and Drosophila melanogaster [4, 7, 8]. It should be noted that C. elegans and D. melanogaster are also known to lack two other Ca2+-permeable channels—the voltage-gated CatSper Ca2+ channels at the plasma membrane [9] and the two-pore channels underlying NAADP-sensitive Ca2+ release from acidic Ca2+ stores [10].
ATP is widely utilized as an energy source in many organisms, and so, ATP could serve as an evolutionarily conserved means to mediate cell–cell communications or intracellular signaling through P2X receptors. The emergence of purinergic signaling by P2X receptors is believed to have occurred in early evolution of eukaryotes [4, 8], which is supported by the identification and characterization of P2X receptor homologs in non-metazoan organisms such as the dictyostelid social amoeba Dictyostelium discoideum [11], the green alga Ostreococcus tauri [12], and the choanoflagellate Monosiga brevicollis, one of the closet unicellular relatives of animals [12, 13]. Interestingly, even though a P2X receptor homolog is characterized in D. discoideum [11], P2X receptor homologs have not been detected in known genomes of fungal species [4, 8]. It is generally believed that P2X receptors had been lost in fungi [4, 8].
We have recently demonstrated in the choanoflagellate M. brevicollis the presence of extensive Ca2+ signaling and amplification pathways, most of which are previously believed to be animal specific [13]. To further explore the origin of the Ca2+ signaling machinery, we have more recently reported the examination of several genomes at the Origins of Multicellularity Database [14, 15], the Broad Institute and the NCBI genomic databases including the genomes of three basal fungi Allomyces macrogynus, Spizellomyces punctatus, and Batrachochytrium dendrobatidis [16].
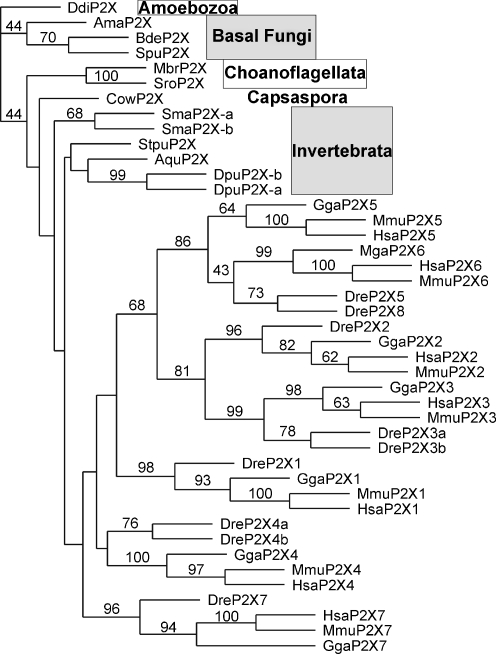
Surprisingly, our analysis revealed the presence of P2X receptor homologs in the three basal fungi (AmaP2X, SpuP2X, and BdeP2X) (Fig. 1; Electronic supplementary material). These three fungal P2X receptor homologs display strong sequence similarity with animal P2X receptors, primarily in the two transmembrane segments, for example, the YxxxK motif conserved in vertebrate P2X receptors (Fig. 2). Fungal P2X receptor homologs also show sequence divergence, possibly reflecting lineage-specific adaptation [16]. For instance, as shown in Fig. 2, basal fungal P2X receptor homologs contain a Glu residue in the second transmembrane segment [16], which is conserved as an Asp residue in vertebrate P2X receptors [11].
Fig. 1.
Phylogenetic analysis of P2X receptor homologs in select animal species and unicellular relatives of animals and three basal fungi. The phylogenetic tree was constructed by the maximum likelihood approach, with the P2X receptor in D. discoideum (DdiP2X) [11] as an outgroup. Bootstrap values of more than 40 are shown at the nodes. Abbreviations for species: Ama A. macrogynus, Aqu A. queenslandica, Bdi B. dendrobatidis, Cow C. owczarzaki, Ddi D. discoideum, Dpu Daphnia pulex, Dre Danio rerio, Gga Gallus gallus, Hsa Homo sapiens, Mbr M. brevicollis, Mga Meleagris gallopavo, Mmu Mus musculus, Sma Schistosoma mansoni, Spu S. punctatus, Stpu Strongylocentrotus purpuratus, Sro Salpingoeca rosetta
Fig. 2.
Sequence alignment of regions surrounding the second transmembrane segments of select P2X receptor sequences. The horizontal black bar indicates the position of TMS2. Functionally important regions and residues, a Lys residue (K308 equivalent in rat P2X2 receptor), an Asp residue (conserved in the TMS2 of most P2X receptors), and the conserved YxxxK motif, are indicated by a filled circle, an asterisk symbol, and a gray bar, respectively. For species abbreviations, see Fig. 1 caption
The identification of P2X receptor homologs in the basal fungi demonstrates that the loss of P2X receptors in fungi had occurred in recent fungal lineages. Interestingly, selective evolutionary loss of P2X receptors might have also occurred independently in the nematode and arthropod lineages, based on recent characterization of a P2X receptor from the tardigrade Hypsibius dujardini and the identification of P2X receptor-like sequences from the nematode Xiphinema index and the chelicerate Boophilus microplus [17].
Animals and fungi diverged from a common unicellular ancestor of Opisthokonta about one billion years ago [14, 18]. Animal P2X receptors function at the plasma membrane by binding to extracellular ATP [3]. In contrast, P2X receptors in the dictyostelid social amoeba D. discoideum [11] and the green alga O. tauri [12] are thought to play a role in intracellular purinergic signaling, which suggests ancestral P2X receptors emerged to serve functional roles on intracellular organelle membranes. Therefore, functional characterization of these fungal P2X receptor homologs will reveal whether P2X receptor-mediated signaling at the plasma membrane predates the divergence of animals and fungi or it had occurred specifically in the lineage leading to animals.
We also detected the presence of P2X receptor homologs in the amoeboid holozoan Capsaspora owczarzaki (CowP2X), a unicellular relative branching close to animals and choanoflagellates. In addition, a P2X receptor homolog is also identified in the sea sponge Amphimedon queenslandica (AquP2X) (Fig. 1; Electronic supplementary material).
In conclusion, the identification of new P2X receptor homologs in basal fungi and primitive species in the animal lineage [16] sheds novel evolutionary and mechanistic insights into the purinergic signaling and provides new molecular tools to explore P2X receptor signaling in fungal biology.
Electronic supplementary material
(PDF 163 kb)
Acknowledgments
I thank the Broad Institute and the investigators of “the Origins of Multicellularity Sequencing Project, Broad Institute of Harvard and MIT” (http://www.broadinstitute.org/) for making data publicly available. I also thank Yanhong Zhang for excellent technical assistance and for critical reading of the manuscript.
References
- 1.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 2.Erb L, Liao Z, Seye CI, Weisman GA. P2 receptors: intracellular signaling. Pflugers Arch. 2006;452:552–562. doi: 10.1007/s00424-006-0069-2. [DOI] [PubMed] [Google Scholar]
- 3.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- 4.Fountain SJ, Burnstock G. An evolutionary history of P2X receptors. Purinergic Signal. 2009;5:269–272. doi: 10.1007/s11302-008-9127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brake AJ, Wagenbach MJ, Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994;371:519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- 6.Valera S, Hussy N, Evans RJ, Adami N, North RA, Surprenant A, Buell G. A new class of ligand-gated ion channel defined by P2x receptor for extracellular ATP. Nature. 1994;371:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- 7.Agboh KC, Webb TE, Evans RJ, Ennion SJ. Functional characterization of a P2X receptor from Schistosoma mansoni. J Biol Chem. 2004;279:41650–41657. doi: 10.1074/jbc.M408203200. [DOI] [PubMed] [Google Scholar]
- 8.Burnstock G, Verkhratsky A. Evolutionary origins of the purinergic signalling system. Acta Physiol (Oxf) 2009;195:415–447. doi: 10.1111/j.1748-1716.2009.01957.x. [DOI] [PubMed] [Google Scholar]
- 9.Cai X, Clapham DE. Evolutionary genomics reveals lineage-specific gene loss and rapid evolution of a sperm-specific ion channel complex: CatSpers and CatSperβ. PLoS ONE. 2008;3:e3569. doi: 10.1371/journal.pone.0003569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brailoiu E, Churamani D, Cai X, Schrlau MG, Brailoiu GC, Gao X, Hooper R, Boulware MJ, Dun NJ, Marchant JS, et al. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J Cell Biol. 2009;186:201–209. doi: 10.1083/jcb.200904073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fountain SJ, Parkinson K, Young MT, Cao L, Thompson CR, North RA. An intracellular P2X receptor required for osmoregulation in Dictyostelium discoideum. Nature. 2007;448:200–203. doi: 10.1038/nature05926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fountain SJ, Cao L, Young MT, North RA. Permeation properties of a P2X receptor in the green algae Ostreococcus tauri. J Biol Chem. 2008;283:15122–15126. doi: 10.1074/jbc.M801512200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai X. Unicellular Ca2+ signaling ‘toolkit’ at the origin of Metazoa. Mol Biol Evol. 2008;25:1357–1361. doi: 10.1093/molbev/msn077. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz-Trillo I, Burger G, Holland PW, King N, Lang BF, Roger AJ, Gray MW. The origins of multicellularity: a multi-taxon genome initiative. Trends Genet. 2007;23:113–118. doi: 10.1016/j.tig.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Sebe-Pedros A, Roger AJ, Lang FB, King N, Ruiz-Trillo I. Ancient origin of the integrin-mediated adhesion and signaling machinery. Proc Natl Acad Sci U S A. 2010;107:10142–10147. doi: 10.1073/pnas.1002257107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai X, Clapham DE (2011) Ancestral Ca2+ signaling machinery in early animal and fungal evolution. Mol Biol Evol. doi:10.1093/molbev/msr149 [DOI] [PMC free article] [PubMed]
- 17.Bavan S, Straub VA, Blaxter ML, Ennion SJ. A P2X receptor from the tardigrade species Hypsibius dujardini with fast kinetics and sensitivity to zinc and copper. BMC Evol Biol. 2009;9:17. doi: 10.1186/1471-2148-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rokas A. The origins of multicellularity and the early history of the genetic toolkit for animal development. Annu Rev Genet. 2008;42:235–251. doi: 10.1146/annurev.genet.42.110807.091513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 163 kb)