Abstract
OBJECTIVES
We conducted a systematic literature review of smoking cessation interventions for patients with histories of depressive disorders or current significant depressive symptoms. We examined the comparative effectiveness of smoking cessation strategies on abstinence rates, differential effects of cessation strategies by depression status (i.e., history positive vs. current depression), and differential effects by gender.
DATA SOURCES
Peer-reviewed literature in MEDLINE, Embase, PsycINFO, and Cochrane Library.
Study eligibility criteria, participants, and interventions
Randomized controlled trials or secondary analysis of RCT data comparing two or more smoking cessation interventions or intervention to control, and reporting cessation outcomes in adults with depression.
STUDY APPRAISAL AND SYNTHESIS METHODS
Two trained researchers screened articles for inclusion. When possible, we estimated pooled risk ratios with 95% confidence intervals by using a random effects model with the Mantel–Haenszel method. We synthesized other studies qualitatively. We classified each intervention as antidepressants, nicotine replacement therapy (NRT), brief smoking cessation counseling, smoking cessation behavioral counseling, or behavioral mood management.
RESULTS
We identified 16 unique RCTs, of which, only three trials recruited participants with current depression. Meta-analysis demonstrated a small, positive effect of adding behavioral mood management (RR = 1.41, 95% CI 1.01–1.96). All included antidepressant trials showed small, positive effects, but risk ratio summary was not significant (RR = 1.31, 95% CI 0.73–2.34). Three NRT trials demonstrated small, positive effects on smoking cessation rates. We found insufficient evidence to examine gender and depression status moderator effects.
LIMITATIONS
Few RCTs exist that test smoking cessation interventions among adults with depression. To make meaningful comparisons, we created broad intervention categories that contained heterogeneity.
CONCLUSIONS AND IMPLICATIONS OF KEY FINDINGS
Few trials enrolled smokers with current depression. Most of data identified were from subgroup analyses of patients history-positive for depression. However, several promising interventions exist. Healthcare providers should consider encouraging their patients with significant depressive symptoms or depression histories to seek smoking cessation services that include NRT and behavioral mood management.
Key Words: smoking cessation, depression, tobacco use, systematic review, meta-analysis
BACKGROUND
Tobacco smoking is the single greatest preventable cause of disease in the United States.1,2 Patients with depression are about twice as likely to be smokers than are individuals who are not depressed.3–5 Smokers with depression are more likely to relapse from a quit attempt, have higher nicotine dependence, suffer negative mood symptoms from withdrawal, and suffer greater smoking-related morbidity and mortality than the general population of smokers.6–12
For the general population, effective smoking cessation interventions include nicotine replacement therapy (NRT),13 antidepressants bupropion and nortriptyline,14 nicotine receptor partial agonists varenicline,15 and smoking cessation counseling.16–18 Pharmacological and behavioral strategies increase the likelihood of successful quits attempt by 1.5 to 2 times compared to placebo or usual care. However, gender and depression status (current vs. history positive) may impact smoking cessation intervention effectiveness. When trying to quit smoking, women may have more difficulty with withdrawal symptoms compared to their male counterparts and, consequently, experience higher rates of smoking relapse to alleviate withdrawal symptoms.19 Depression status may influence patients’ ability to engage in smoking cessation.6,7,9 Some evidence supports that smokers with current depression are less likely to succeed with smoking cessation compared to history positive patients.20
Despite the complex relationship between tobacco use and depression, smokers with depression need effective smoking cessation services.21,22 No systematic reviews have synthesized smoking cessation strategies for patients with depression. We conducted a systematic literature review and meta-analyses to address the following questions.
Among patients with histories of depressive disorders or current significant depressive symptoms:
What is the comparative effectiveness of smoking cessation strategies on smoking abstinence rates?
Are there differential effects of smoking cessation strategies by depression status (i.e., history positive vs. current depression)?
Are there differential effects of smoking cessation strategies by gender?
This paper summarizes three of five original key questions addressed in a technical report commissioned by the Department of Veteran Affairs .23
METHODS
Data Sources and Study Selection
We searched English-language publications in MEDLINE, Embase, PsycINFO, and Cochrane Library from database inception through March 2010 and supplemented electronic searching by examining bibliographies of included studies. Major search terms included “smoking cessation,” “depression,” and “depressive disorder”; we added filters for randomized control trials. Two trained researchers independently screened titles and abstracts. To be included, studies had to (1) be randomized controlled trials (RCT), (2) compare two or more smoking cessation interventions or compare intervention to control, and (3) report smoking abstinence outcomes in adults with depression. (Table 1)
Table 1.
Summary of Inclusion and Exclusion Criteria
| Study characteristic | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Study design | RCTs or a secondary data analysis from RCTs of smoking cessation interventions | Non-English language publication, cross-sectional studies |
| Population | Adults age 18 and over with a history of a depressive disorder (including current depression) or current significant depressive symptoms* | Pregnant women, adolescents, postpartum depression, depressive symptoms secondary to another primary condition (e.g., substance abuse, schizophrenia) |
| Interventions | Any patient-level smoking cessation strategies (e.g., self-help, quit lines, physician or brief advice, behavioral counseling, pharmacologic therapies) alone or in combination with other strategies | Policy-level interventions (e.g., smoking bans), mass media campaigns |
| Comparators | Active comparators or control (e.g., usual care or placebo) | None |
| Setting | Outpatient (e.g., mental health clinics, primary care) or delivered through remote communication technologies (e.g., telephone, Web) | Hospital-based (inpatient) interventions |
| Outcome | Smoking abstinence reported at ≥ 3 months postrandomization | Relapse prevention† |
*History of depression was defined as having a lifetime diagnosis of depression. Current depression was defined as having an ongoing episode of a depressive disorder or current significant symptoms. Both history and current depression were assessed using validated diagnostic instrument (e.g., DSM-IV, PRIME-MD). We define significant depressive symptoms as meeting a designated threshold on a validated assessment instrument (e.g., CES-D, BDI)†Intervention strategies that reduce the likelihood of recent quitters returning to smoking
Abbreviations: BDI = Beck Depression Inventory; CES-D = Center for Epidemiologic Studies-Depression Scale; DSM-IV =Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; PRIME-MD = Primary Care Evaluation of Mental Disorders; RCT = randomized controlled trial
Data Extraction and Quality Assessment
Trained researchers abstracted data from published reports into evidence tables; second reviewers overread tables. We abstracted information about study eligibility, sample size, followup duration, intervention characteristics, adverse effects, and smoking abstinence rates. We defined smoking abstinence as (1) point prevalence abstinence (in past 7 days) or (2) extended abstinence (since quit date). We included one effect size per study and categorized them as short-term (3<6 months) or long-term (≥6 months) confirmed by self-report, biochemical validation, or both. Two investigators independently rated risk of bias using criteria described in the Agency for Healthcare Research and Quality Methods Guide for Effectiveness and Comparative Effectiveness Reviews24 and assigned summary quality scores of good, fair, or poor. We adjudicated disagreements about abstracted data elements or study quality by consensus between two independent investigators or by obtaining a third reviewer’s opinion when consensus could not be reached.
Data Synthesis
When study designs and outcomes reported were similar, we estimated pooled risk ratios with 95% confidence intervals using random effects models with Mantel–Haenszel methods. We qualitatively synthesized other study. We grouped trials by primary intervention comparison into the following categories: antidepressants, NRT, brief smoking cessation counseling, smoking cessation behavioral counseling, or behavioral mood management.
We defined brief smoking cessation counseling as similar to advice given during physician visits and behavioral counseling as multisession therapy using behavioral strategies, such as those common in cognitive behavioral therapy (CBT), to influence tobacco use. Behavioral mood management was defined as counseling intended to influence negative mood and improve depression symptomatology beyond standard smoking cessation counseling.
There were sufficient studies to perform meta-analyses for two comparisons: 1) antidepressants plus cotreatments versus placebo plus cotreatments; and 2) behavioral mood management plus cotreatments versus cotreatments only. We defined cotreatments as any type of smoking cessation intervention strategy (e.g. NRT, behavioral counseling). For behavioral mood management comparison, we conducted subgroup analysis of studies using NRT alone or in combination with antidepressants. Two studies25,26 used factorial designs; we treated these comparisons as separate studies in analyses.
We evaluated heterogeneity visually and with the Cochran Q statistic27 using threshold p-value of less than 0.1028 and I2 statistic.29 We considered I2 statistic thresholds of 0% to 40%, 30% to 60%, 50% to 90%, and 75% to 100% to represent between-study heterogeneity as important, moderate, substantial, or considerable, respectively.30 We planned a priori to conduct subgroup analyses by depression status and gender; there were insufficient studies to conduct these analyses. We used Review Manager 5.0 to conduct analysis (Cochrane Collaboration, Oxford).
RESULTS
Literature Search and Study Characteristics
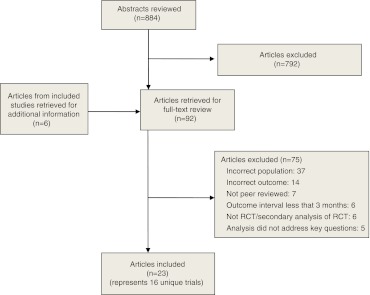
We identified 884 titles and performed full-text reviews on 92 articles. We retrieved six additional papers for supplemental information on included studies. Of these 98 papers, we excluded 75. The 23 included reports encompassed 16 unique trials. (Fig. 1)
Figure 1.
Study flow diagram.
All studies were conducted in the U.S. with English-speaking participants except Munoz (1997) which was conducted with Spanish speakers.31 Ten studies were of good quality and only six trials used depression status as an inclusion criterion.32–37(Table 2) Data from the remaining ten studies included in this review were from subgroup analysis of trials that recruited general populations of smokers; many of these trials cited current depression as an exclusion criteria. Thus, depressed participants included in this review from these ten studies were history positive for MDD or exceeded a screening threshold for significant depressive symptoms but were not recruited based on depression status.
Table 2.
Characteristics of Trials Included in this Systematic Review of Smoking Cessation Interventions for Patients with Depression
| Study, year | Sample size‡ (% depressed by study condition) | Study quality* | Intervention Arm | Comparator | Pharmacotherapy Dosing† | Proportion Achieving Smoking Abstinence |
|---|---|---|---|---|---|---|
| Nicotine Repayment Therapy Studies | ||||||
| Hall, 199626‡ | 201 (23% NRT gum, 20% placebo gum, 20% mood management, and 24% health education arms MDD history positive) | Fair | NRT gum + 5 group sessions of CBT mood management + 5 group sessions of smoking cessation counseling or 10 session health education | Placebo gum + 5 group sessions of CBT mood management + 5 group sessions of smoking cessation counseling or 10 sessions health education | NRT: 2 mg gum starting at session 3 (quit date) for 8 weeks at 1 piece per hour for at least 12 hours/day during first 3 weeks; then use gum as needed weeks 4-8 | NRT gum: 22% |
| Placebo gum: 33% | ||||||
| Mood management: 33% | ||||||
| Health education: 22% (at 52 weeks) | ||||||
| Hall, 200633 | 322‡ (100% with current depression) | Good | NRT patch (or bupropion if failed NRT) + staged motivational feedback + 6 sessions of individual CBT smoking cessation counseling | Brief contact with self-help guide + list of referrals to smoking cessation programs and stop smoking guide | NRT: 21 mg patch for 6 weeks, then 14 mg week 7–8, and 7 mg weeks 9–10 | Drugs + feedback + counseling: 18.4% |
| Bupropion: Dose not reported | ||||||
| Brief contact: 13.2% (at 18 months) | ||||||
| Kinnunen, 199643 | 269‡ (33% NRT gum and 36% counseling arms met criteria for depression) | Good | NRT gum + one-time brief individual behavioral counseling | Placebo gum + one-time brief individual behavioral counseling | NRT: 2 or 4 mg gum taken as needed with target usage of 9–15 pieces/day for 3 months | NRT + counseling:29.5% |
| Counseling: 12.5% (at 3 months) | ||||||
| Kinnunen, 200844 | 608‡ (31% NRT gum and 34% counseling arms met criteria for depression) | Good | NRT gum + 9 brief in-person individual counseling sessions | Placebo gum + 9 brief in-person individual counseling sessions | NRT: 2 or 4 mg gum with target use of 9–15 pieces/day for 2 months | NRT + counseling: 15% |
| Counseling: 5.7% (at 12 months) | ||||||
| Antidepressant Studies | ||||||
| Covey, 200235 | 134‡ (100% MDD history positive) | Good | Sertraline+ 9 individual in-person smoking cessation behavioral counseling sessions augmented with supportive approach to manage negative affect associated with quitting smoking | Placebo + 9 individual in-person smoking cessation behavioral counseling sessions augmented with supportive approach to manage negative affect associated with quitting smoking | Sertraline: 50 mg/day for week 1, then 100 mg/day for week 2; increased to 150 mg/day for week 3, and increased again to 200 mg/day for week 4-9 | Sertraline + counseling: 11.8% |
| Counseling: 16.7% (at 6 months) | ||||||
| Evins, 200834 | 199‡ (100% MDD history positive) | Good | Bupropion + 13 group CBT smoking cessation counseling + NRT patch | Placebo + 13 group CBT smoking cessation counseling + NRT patch | Bupropion: 150 mg/day for first 3 days, then 150 mg twice daily | Bupropion + counseling + NRT: 36% |
| NRT: 21 mg patches for weeks 2–6, then 14 mg patches for weeks 7–8, decreased to 7 mg patches weeks 9 -10 | Counseling + NRT: 31% (at 13 weeks) | |||||
| Hayford, 199940 | 615‡ (18% 100 mg, 12% 150 mg, 13% 300 mg and 18% placebo arms MDD history positive) | Good | Bupropion + 11 brief in-person individual counseling sessions | Placebo + 11 brief in-person individual counseling sessions | Bupropion: (3 doses tested) | |
| - 50 mg twice daily for 7 weeks | 100 mg: 14% | |||||
| - 150 mg/day for 7 weeks | 150 mg: 26% | |||||
| - 150 mg daily for 3 days and then 150 mg twice daily for 7 weeks | 300 mg: 20% | |||||
| Placebo: 7% (at 12 months) | ||||||
| Hall, 199825§ | 199‡ (33% mood management/ortriptyline, 31% nortriptyline/health education,33% mood management only, and 33% health education only arms MDD history positive) | Good | Nortriptyline + 5 group sessions of CBT mood management + 5 group sessions of smoking cessation counseling or 10 session health education | Placebo + 5 group sessions of CBT mood management + 5 group sessions of smoking cessation counseling or 10 session health education | Nortriptyline: 25 mg/day for 3 days; increased to 50 mg/day for 4 days; dose increased to 75 mg/day if therapeutic level not attained; increased to 100 mg/day if necessary at week 6. Modal dose was 100 mg/day and participants | Mood management + nortriptyline: 24% |
| Nortriptyline + health education: 20% | ||||||
| Mood management only: 29% | ||||||
| Health education only: 13% (at 64 weeks) | ||||||
| Saules, 200441 | 150‡ (23% 20 mg, 22% 40 mg, and 16% NRT/counseling arms were MDD history positive) | Fair | Fluoxetine + 6 group CBT smoking cessation counseling + NRT patch | Placebo + 6 group CBT smoking cessation counseling + NRT patch | Fluoxetine: 20 mg or 40 mg of fluoxetine started 4 weeks before quit date, then continued for another 10 weeks | 20 mg fluoxetine + counseling + NRT: 54.4% |
| NRT: 15 mg patch starting at quit date, then 6 week on 15 mg, then 2 weeks on 10 mg, and 2 weeks on 5 mg patch | 40 mg fluoxetine + counseling + NRT: 54.4% | |||||
| Counseling + NRT:37.5% (at 15 weeks) | ||||||
| Behavioral Mood Management Studies | ||||||
| Brown, 200136 | 179‡(100% MDD history positive) | Good | 8 group sessions of mood management and smoking cessation CBT | 8 group sessions of smoking cessation CBT | N/A | Mood management: 32.5% |
| Smoking cessation counseling: 24.7% (at 12 months) | ||||||
| Duffy, 200638 | 184‡ (38% intervention and 31% comparator arms depressed smokers) | Good | 9 to 11 session of combined smoking, depression, alcohol abuse telephone CBT + bupropion monotherapy OR combination bupropion + NRT patch or gum (if failed bupropion monotherapy in the past) OR NRT patch/gum + paroxetine (if failed bupropion in he past for depression) | One-time behavioral counseling and referral to appropriate services for substance use/abuse and/or depression | Bupropion: 150 mg twice daily | Counseling + drugs: 51% (at 6 months) |
| Paroxetine: Dose not reported | One-time counseling: 17% (13 weeks) | |||||
| NRT: Dose not reported | ||||||
| Hall, 199439 | 149‡ (37% intervention and 24% comparator arms MDD history positive) | Fair | 5 group sessions of CBT mood management + 5 group sessions of smoking cessation counseling + NRT gum | 5 group sessions of smoking cessation counseling + NRT gum | NRT: 2 mg gum taken as needed for 8 weeks | Mood management + NRT: 34% |
| Counseling + NRT: 24% (at 52 weeks) | ||||||
| Hall, 199626‡ | 201‡ (23% NRT gum, 20% placebo gum, 20% mood management, and 24% health education arms MDD history positive) | Fair | NRT gum + 5 group sessions of CBT mood management + 5 group sessions of smoking cessation counseling or 10 session health education | Placebo gum + 5 group sessions of CBT mood management + 5 group sessions of smoking cessation counseling or 10 sessions health education | NRT: 2 mg gum starting at session 3 (quit date) for 8 weeks at 1 piece per hour for at least 12 hours/day during first 3 weeks; then use gum as needed weeks 4-8 | Mood management: 33% |
| Health education: 22% | ||||||
| NRT gum: 22% | ||||||
| Placebo gum: 33% (at 52 weeks) | ||||||
| Hall, 199825§ | 199‡ (33% mood management/nortriptyline, 31% nortriptyline/health education, 33% mood management only, and 33% health education only arms MDD history positive) | Good | Nortriptyline + 5 group sessions of CBT mood management + 5 group sessions of smoking cessation counseling or 10 session health education | Placebo + 5 group sessions of CBT mood management + 5 group sessions of smoking cessation counseling or 10 session health education | Nortriptyline: 25 mg/day for 3 days; increased to 50 mg/day for 4 days; dose increased to 75 mg/day if therapeutic level not attained; increased to 100 mg/day if necessary at week 6. Modal dose was 100 mg/day and participants | Mood management + nortriptyline: 24% |
| Mood management only: 29% | ||||||
| Nortriptyline + health education: 20% | ||||||
| Health education only: 13% (at 64 weeks) | ||||||
| MacPherson, 201037 | 68‡ (100% with elevated depressive symptoms) | Good | 8 group sessions of smoking cessation CBT that included behavioral activation therapy + NRT patch | 8 group sessions of smoking cessation CBT + NRT patch | NRT: 21 mg for 4 weeks, followed by 14 mg for 2 weeks, and 7 mg for 2 weeks | Behavioral activation counseling + NRT: 14.3% |
| Counseling + NRT: 0% (at 26 weeks) | ||||||
| Other Intervention Strategies | ||||||
| Covey, 199942 | 80‡ (42% intervention and 53% comparator arms MDD history positive) | Fair | Naltrexone + 6 individual in-person behavioral counseling sessions | Placebo + 6 individual in-person behavioral counseling sessions | Naltrexone : 25 mg/day for 3–5 days prior to quit, then increased to 50 mg/day on quit date, and increased again to 75 mg/day if tolerated for 1 month | Naltrexone + counseling: 28.6 % |
| Counseling: 9.1% (at 6 months) | ||||||
| Munoz, 199731 | 136‡ (76% immediate and 78% delayed arms were MDD history positive or current MDD) | Fair | Mailed smoking cessation guide + mood management guide (immediate group) | Mailed smoking cessation guide + mood management guide at 3 months delayed (delayed group) | N/A | Immediate group: 17.9% (Current MDD) |
| 38.5% (MDD history positive) | ||||||
| Delayed group: 8% (Current MDD) | ||||||
| 7.4% (MDD history positive) (at 6 months) | ||||||
| Vickers, 200932 | 60‡ (100% with current depression) | Fair | 10 in-person individual exercise counseling sessions that include brief smoking cessation counseling + NRT patch | 10 in-person individual health education sessions that include brief smoking cessation counseling + NRT patch | NRT: 21 mg patch started on quit date, continued for 6 weeks | Exercise counseling + NRT: 6.3% |
| Health education + NRT: 6.7% (at 24 weeks) | ||||||
*Study quality was assessed via key quality criteria described in Agency for Healthcare Research and Quality’s General Methods Guide, adapted for this specific topic.24 We abstracted data on adequacy of randomization and allocation concealment, comparability of groups at baseline, blinding, completeness of followup and differential loss to followup, whether incomplete data were addressed appropriately, and validity of outcome measures. Using these data elements, we assigned a summary quality score of Good, Fair, or Poor to individual RCTs
†Studies reported variable levels of detail on dosage schedules for pharmacotherapy.
‡Study used a 2X2 factorial design to compare pharmacological and behavioral interventions; results were collapsed across arms (NRT gum vs. placebo and mood management vs. health education).
§ Study used a 2X2 factorial design to compare pharmacological and behavioral interventions; results of each condition are reported.
CBT = cognitive behavioral therapy; MDD = major depressive disorder; mg = milligram; N/A = not applicable; NRT = nicotine replacement therapy
All but Munoz (1997) and Brown (2001) tested treatments consisting of counseling and pharmacotherapy.31,36 Of the studies that included counseling, the most common therapy was in-person CBT. One included study, Duffy (2006), conducted behavioral counseling via telephone.38 Six studies included behavioral mood management.25,26,36–39 Behavioral mood management ranged from one-time mood management counseling to intensive multisession CBT.
Of the studies that included antidepressants, four used bupropion,33,34,38,40 and three tested sertraline, fluoxetine, or nortriptyline.25,35,41 Of studies that included antidepressants, three used NRT patches as a cotreatment,34,38,41 and one used NRT patches as first-line therapy before offering bupropion.33 One study tested behavioral counseling plus naltrexone.42 No varenicline studies met eligibility criteria.
Key Questions
What is the comparative effectiveness of smoking cessation strategies on smoking abstinence rates?
Nicotine Replacement Therapy Evidence Synthesis
We identified four studies that compared adding NRT to other cotreatments versus active comparators. Outcomes and cotreatments were too heterogeneous to conduct meta-analysis. Pharmacotherapy dosing information and cessation rates are detailed in Table 2.
Kinnunen and colleagues (1996) compared adding 2 or 4 mg nicotine gum to one-time brief counseling.43 In subgroup analysis of participants classified as depressed via the Center for Epidemiologic Studies Depression Scale (CESD-D) (n = 93), smokers receiving active gum were more likely to quit smoking than those receiving placebo gum at 3 months post–quit date. In another trial, Kinnunen and colleagues (2008) reported long-term effects of adding 2 or 4 mg nicotine gum to 9 brief counseling sessions.44 Among participants with depression measured via the CES-D (n = 196), smokers receiving nicotine gum were more likely to remain abstinent at 12 month post–quit date than smokers receiving placebo.
In a 2X2 factorial design, Hall and colleagues (1996) compared 2 mg nicotine gum versus placebo gum with 10 sessions of group CBT smoking cessation counseling versus 10 sessions of health education.26 For MDD history-positive participants as measured by the Diagnostic Interview Schedule (n = 88), 22% receiving nicotine gum were abstinent compared to 33% receiving placebo gum at 52 weeks (p-value NR).
Hall and colleagues (2006) offered NRT patches plus 6 sessions of individual staged-care CBT behavioral counseling and computerized motivational feedback to participants with current diagnoses of depression based on the Primary Care Evaluation of Mental Disorders (PRIME-MD). Counseling sessions lasted 30 minutes and took place over 8 weeks. If patients did not quit smoking with NRT or relapsed during treatment, patients could request bupropion. Brief contact with provision of self-help guide and smoking cessation referral served as the control condition. Smoking status was confirmed at 3, 6, 12, and 18 months postrandomization by expired carbon monoxide at ≤10 ppm. Staged-care counseling condition plus NRT outperformed brief contact control over time (OR=4.55, 95% CI 1.04-19.93).
Antidepressants Evidence Synthesis
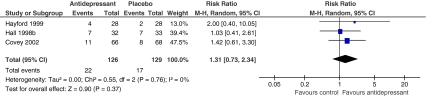
Five trials reported results of adding antidepressants to cotreatments for smokers with depression.25,34,35,40,41 Hayford (1999), Hall (1998), and Covey (2002) provided 6-month or greater outcomes data and were included in meta-analysis.25,35,40 These three studies compared either sertraline, nortriptyline, or buproprion plus behavioral counseling to placebo plus behavioral counseling. (Table 2 includes dosing information.) Participants receiving antidepressants plus behavioral counseling were not more likely to be abstinent compared to participants receiving placebo plus behavioral counseling at 6-month postrandomization (RR = 1.31, 95% CI 0.73-2.34, Cochran Q = 0.55, p = 0.76, I2 =0%). (Fig. 2)
Figure 2.
Risk of smoking abstinence at least 6 months after start of antidepressant therapy + behavioral counseling compared with placebo + behavioral counseling. *Events = number of participants who achieved smoking abstinence.
Two additional studies compared antidepressants plus cotreatments of behavioral counseling and NRT. These studies reported short-term outcomes and were not included in the meta-analysis.34,41 Evins and colleagues (2008) added 12 weeks of bupropion to 8 weeks of transdermal NRT and 13 sessions of group CBT smoking cessation counseling for patients with lifetime MDD histories (n = 199).34 Participants in the bupropion arm were no more likely to achieve smoking abstinence at end of treatment. Saules and colleagues (2004) added 14 weeks of either 20 or 40 mg fluoxetine to 10 weeks of transdermal NRT and 6 weeks of group CBT smoking cessation counseling,.41 Among participants who were MDD history positive (n = 30), Saules found no significant differences in abstinence rates when fluoxetine was added to NRT and intensive behavioral counseling.
Behavioral Mood Management Evidence Synthesis
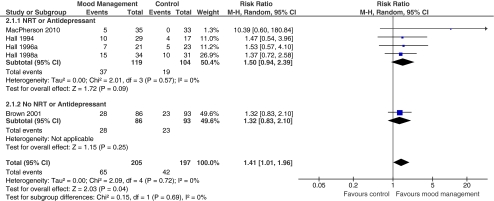
Six trials reported results of adding behavioral mood management to other types of behavioral counseling. Other cotreatments given to all participants include NRT,26,37–39 nortriptyline,25 or NRT plus bupropion or paroxetine.38 Five of these studies, involving 402 smokers with depression, provided sufficient data to meta-analyze the added effect of mood management therapy to other smoking cessation cotreatments.25,26,36,37,39
All studies included in the meta-analysis were in the expected direction, favoring behavioral mood management (RR = 1.41, 95% CI 1.01-1.96, Cochran Q=2.09, p = 0.71, I2 =0%). (Fig. 3) Subgroup analysis suggests smoking cessation may be more likely when behavioral mood management was added to NRT or antidepressants cotreatments in addition to counseling; however, this contrast was not statistically significant.
Figure 3.
Risk of smoking abstinence at least 6 months after start of behavioral mood management + cotreatment compared to active control. *Events = number of participants who achieved smoking abstinence.
One additional study tested behavioral mood management but could not be included in the meta-analysis. Duffy and colleagues (2006) tested a telephone-delivered smoking, depression, and alcohol abuse CBT intervention for head-and-neck cancer survivors.38 Depressed smokers were offered NRT and bupropion or paroxetine. One-time behavioral counseling and referral to followup services served as comparator condition. Participants in the CBT intervention who were smokers and depressed at baseline (n = 64) had greater smoking cessation rates at 6 months from end of treatment compared the control arm.
Other Intervention Strategies Evidence Synthesis
We identified three other types of smoking cessation strategies, each with only one RCT. Munoz and colleagues (1997) tested the efficacy of self-administered mood management intervention plus smoking cessation guide compared to smoking cessation guide alone delivered through the mail for Spanish-speaking smokers.31 Addition of mood management content improved cessation rates at 6 months postrandomization for MDD history positive participants but not for smokers with current MDD.
Covey and colleagues (1999) tested behavioral counseling plus naltrexone.42 Of the 36 participants with a history of MDD, six month quit rates favored use of naltrexone in combination with counseling over counseling plus placebo.
Vickers and colleagues (2009) conducted a randomized pilot to test feasibility of behavioral counseling to promote exercise as a smoking cessation intervention for depressed female smokers.32 Results demonstrated no effect for using exercise counseling as a smoking cessation intervention.
Are there differential effects of smoking cessation strategies by depression status (i.e., depression positive vs. current depression)?
Only two studies provided sufficient information to report differential effectiveness of smoking cessation intervention strategies by depression status. For both reports, researchers conducted subgroup analysis only; no treatment by depression interaction effects were directly tested. Evins and colleagues (2008) recruited 199 smokers who had a lifetime MDD diagnosis and randomized them to 12 weeks of bupropion versus placebo. Both groups also received 8 weeks of transdermal NRT and 13 sessions of group CBT smoking cessation counseling.34 Addition of bupropion did not significantly improve smoking cessation rates for either patients history positive for MDD or those with current depression. In Munoz (1997), mailed mood management content improved cessation rates over mailed smoking cessation guide at 6 months postrandomization for participants MDD history positive but not for smokers with current MDD.
Are there differential effects of smoking cessation strategies by gender?
Only one included study reported treatment by gender interaction among study participants with depression.42 In Covey (1999), women history-positive for MDD (n = 26) experienced higher quit rates when randomized to receive naltrexone in combination with 6 sessions of individual behavioral counseling compared to women with depression receiving placebo at 6 months (22.2% versus 0%; p = 0.04). MDD history positive men (n = 10) did not experience significantly higher quit rates with naltrexone.
DISCUSSION
We identified 16 trials; only Hall (2006), MacPherson (2010), and Vickers (2009) recruited participants with current depression.32,33,37 Most patients included in this review were history-positive for depression; findings best apply to this population. We found sparse data on differential effects of gender and depression status on smoking cessation intervention efficacy for depressed patients. Findings, however, suggest some promising smoking cessation strategies for patients with depression.
Smokers with depression are more likely to have increased levels of negative mood precessation and postcessation.45–48 Also, negative mood is associated with greater smoking relapse rates.49,50 Behavioral mood management may serve to moderate negative mood associated with quit attempts.49 Thus, smokers with depression may achieve more success if smoking cessation interventions are augmented with behavioral mood management. Our meta-analysis results support this hypothesis; we found a small, positive effect of behavioral mood management that is comparable to effects of behavioral counseling in non-depressed populations.17,18
Included antidepressant trials showed small, positive effects on smoking cessation, but summary estimate was not statistically significant. Our results should be interpreted with caution. Sample sizes were small and numbers achieving cessation few, which limits precision of effect estimates and ability to detect statistically significant differences. We included five trials, which had considerable variability in antidepressant type. Only bupropion and nortriptyline have proven efficacy as smoking cessation pharmacotherapies.14 Selective serotonin reuptake inhibitors (e.g., sertraline, fluoxetine) used in other included studies show little smoking cessation benefit.14
Offering NRT to smokers with depression appears to have a small, positive effect on smoking cessation rates. Cessation rates ranged from 14% to 22% in included studies that reported outcomes of 12 months or longer.26,33,44 These cessation rates are higher than the 3 to 5% of smokers who successfully maintain quit attempts a year later without treatment aids51 and are comparable to NRT quit rates in the general population of smokers.13 Yet, long-term cessation rates across studies were lower for patients with current depressive symptoms33 than for those who were MDD history positive26 (14% versus 22%, respectively). Smokers with current depressive symptoms may have greater difficulty quitting due to intense nicotine withdrawal or worsening of depressive symptoms during a quit attempt.20 Smokers with current depressive symptoms may need additional support to achieve smoking abstinence.
Limitations
Our review has some limitations. Foremost, few RCTs exist that test smoking cessation interventions among smokers with depression. Thus, we created broad intervention categories in order to make meaningful comparisons. Within each category, there is considerable heterogeneity, which may influence estimates of effectiveness. Few trials recruited smokers with current depression; therefore, many reports based classifications of depression on self-reported screening criteria (e.g., CES-D) for significant depressive symptoms. Self-report scales may be measures of general emotional distress or negative affect rather than specific depressive symptoms. In primary care settings, positive depression screen has positive predictive value of ≤ 50% for MDD.52 Thus, our review contains heterogeneity among participants classified as depressed. We planned a proiri to stratify analysis by depression type, but there were too few trials in any intervention category to follow this approach. Also chronicity of depression and other important variations in depressive disorders may influence outcomes.3 Our review is unable to address these issues. In many instances, we examined subgroup data for this review. By doing so, we introduce the possibility of false-negative studies because studies may not be powered to detect clinically important treatment effects in subgroups. Meta-analysis helps to address this limitation, but with relatively few studies of small sample sizes, our analyses may remain underpowered.
Future Research
Future trials should be designed to test smoking cessation treatments for smokers with current depression. Within the trials we identified, we found little research on key moderators that may influence treatment effectiveness. Moderator analysis will facilitate subgroup identification, which may lead to better treatment matching.3 Also smokers with psychiatric comorbidities may benefit from combined pharmacotherapy and behavioral counseling with longer therapeutic approaches (i.e., exceeding 8–12 weeks) to reduce likelihood of dropout and depression relapse. 16,53–55 Future research should be designed to optimize dose, duration, sequencing, and frequency of both behavioral counseling and pharmacotherapies.
Conclusions
Patients with depression can stop smoking and should be offered evidence-based smoking cessation treatments available to other smokers. While this review provides evidence synthesis about smoking cessation strategies for patients with depression, extant evidence is insufficiently robust to make clear treatment recommendations. However, it is likely that patients with depression need strategies that target both depressive symptoms and smoking. Based on the available evidence, healthcare providers should consider encouraging their patients with depression who smoke to seek smoking cessation services that include both NRT and behavioral mood management.
Acknowledgments
We would like to thank Liz Wing for her expert editing skills and Megan Von Isenburg for her assistance in optimizing literature search strategies. We would like to thank the expert panel and reviewers of the original evidence synthesis report; their comments significantly improved this research. This study was funded with support from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development Evidence-based Synthesis Project [Project Number: ESP 09–010]. At the time of these analyses, Dr. Gierisch was funded by an AHRQ NRSA postdoctoral traineeship at Duke University Medical Center [Grant No. T-32-HS000079]. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Conflict of Interest
None disclosed.
Footnotes
Systematic review registration number: Department of Veterans Affairs Evidence-based Synthesis Project-ESP 09–010
REFERENCES
- 1.Danaei G, Ding EL, Mozaffarian D, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6:e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 3.Ziedonis D, Hitsman B, Beckham JC, et al. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine and Tobacco Research. 2008;10:1691–715. doi: 10.1080/14622200802443569. [DOI] [PubMed] [Google Scholar]
- 4.Hall SM, Prochaska JJ. Treatment of smokers with co-occurring disorders: emphasis on integration in mental health and addiction treatment settings. Annu Rev Clin Psychol. 2009;5:409–31. doi: 10.1146/annurev.clinpsy.032408.153614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. JAMA. 2000;284:2606–10. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 6.Kenney BA, Holahan CJ, Holahan CK, Brennan PL, Schutte KK, Moos RH. Depressive symptoms, drinking problems, and smoking cessation in older smokers. Addict Behav. 2009;34:548–53. doi: 10.1016/j.addbeh.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cinciripini PM, Wetter DW, Fouladi RT, et al. The effects of depressed mood on smoking cessation: mediation by postcessation self-efficacy. J Consult Clin Psychol. 2003;71:292–301. doi: 10.1037/0022-006X.71.2.292. [DOI] [PubMed] [Google Scholar]
- 8.Glassman AH, Covey LS, Stetner F, Rivelli S. Smoking cessation and the course of major depression: a follow-up study. Lancet. 2001;357:1929–32. doi: 10.1016/S0140-6736(00)05064-9. [DOI] [PubMed] [Google Scholar]
- 9.Niaura R, Britt DM, Shadel WG, Goldstein M, Abrams D, Brown R. Symptoms of depression and survival experience among three samples of smokers trying to quit. Psychol Addict Behav. 2001;15:13–7. doi: 10.1037/0893-164X.15.1.13. [DOI] [PubMed] [Google Scholar]
- 10.Pomerleau CS, Zucker AN, Stewart AJ. Characterizing concerns about post-cessation weight gain: Results from a national survey of women smokers. Nicotine and Tobacco Research. 2001;3:51–60. doi: 10.1080/14622200020032105. [DOI] [PubMed] [Google Scholar]
- 11.Tsoh JY, Humfleet GL, Munoz RF, Reus VI, Hartz DT, Hall SM. Development of major depression after treatment for smoking cessation. A J Psychiatry. 2000;157:368–74. doi: 10.1176/appi.ajp.157.3.368. [DOI] [PubMed] [Google Scholar]
- 12.Pratt LA, Brody DJ. Depression and smoking in the U.S. household population aged 20 and over, 2005–2008. NCHS Data Brief. 2010:1–8. [PubMed]
- 13.Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2008. [DOI] [PubMed]
- 14.Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. SOURCE Cochrane Database Syst Rev. 2007 [DOI] [PubMed]
- 15.Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2008. [DOI] [PubMed]
- 16.Stead LF, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database Syst Rev. 2006. [DOI] [PubMed]
- 17.Stead LF, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst Rev. 2005. [DOI] [PubMed]
- 18.Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev. 2005. [DOI] [PubMed]
- 19.Weinberger AH, Maciejewski PK, McKee SA, Reutenauer EL, Mazure CM. Gender differences in associations between lifetime alcohol, depression, panic disorder, and posttraumatic stress disorder and tobacco withdrawal. Am J Addict. 2009;18:140–7. doi: 10.1080/10550490802544888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas AL, Munoz RF, Humfleet GL, Reus VI, Hall SM. Influences of mood, depression history, and treatment modality on outcomes in smoking cessation. J Consult Clin Psychol. 2004;72:563–70. doi: 10.1037/0022-006X.72.4.563. [DOI] [PubMed] [Google Scholar]
- 21.Hitsman B, Moss TG, Montoya ID, George TP. Treatment of tobacco dependence in mental health and addictive disorders. Can J Psychiatry. 2009;54:368–78. doi: 10.1177/070674370905400604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall SM. Nicotine interventions with comorbid populations. Am J Prev Med. 2007;33:S406–13. doi: 10.1016/j.amepre.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Gierisch JM, Bastian LA, Calhoun PS, McDuffie JR, Williams JW. Comparative effectiveness of smoking cessation treatments for patients with depression: A systematic review and meta-analysis of the evidence. VA-ESP Project #09-010; 2010 [PubMed]
- 24.Agency for Healthcare Research and Quality. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality. Available at: http://www.effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?pageaction=displayproduct&productid=318. Accessed September 27, 2011.
- 25.Hall SM, Reus VI, Muñoz RF, et al. Nortriptyline and cognitive-behavioral therapy in the treatment of cigarette smoking. Arch Gen Psychiatr. 1998;8:683–90. doi: 10.1001/archpsyc.55.8.683. [DOI] [PubMed] [Google Scholar]
- 26.Hall SM, Muñoz RF, Reus VI, et al. Mood management and nicotine gum in smoking treatment: a therapeutic contact and placebo-controlled study. J Consult Clin Psychol. 1996;5:1003–9. doi: 10.1037//0022-006x.64.5.1003. [DOI] [PubMed] [Google Scholar]
- 27.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–29. doi: 10.2307/3001666. [DOI] [Google Scholar]
- 28.Fleiss JL. Analysis of data from multiclinic trials. Control Clin Trials. 1986;7:267–75. doi: 10.1016/0197-2456(86)90034-6. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 30.Higgins JP, Greenberg S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane-handbook.org. Accessed September 27, 2011
- 31.Munoz RF, Marin BV, Posner SF, Perez-Stable EJ. Mood management mail intervention increases abstinence rates for Spanish-speaking Latino smokers. Am J Community Psychol. 1997;25:325–43. doi: 10.1023/A:1024676626955. [DOI] [PubMed] [Google Scholar]
- 32.Vickers KS, Patten CA, Lewis BA, et al. Feasibility of an exercise counseling intervention for depressed women smokers. Nicotine & tobacco research. 2009;8:985–95. doi: 10.1093/ntr/ntp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall SM, Tsoh JY, Prochaska JJ, et al. Treatment for cigarette smoking among depressed mental health outpatients: a randomized clinical trial. Am J Publ Health. 2006;10:1808–14. doi: 10.2105/AJPH.2005.080382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evins AE, Culhane MA, Alpert JE, et al. A controlled trial of bupropion added to nicotine patch and behavioral therapy for smoking cessation in adults with unipolar depressive disorders. J Clin Psychopharmacol. 2008;6:660–6. doi: 10.1097/JCP.0b013e31818ad7d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Covey LS, Glassman AH, Stetner F, Rivelli S, Stage K. A randomized trial of sertraline as a cessation aid for smokers with a history of major depression. The American Journal of Psychiatry. 2002;10:1731–7. doi: 10.1176/appi.ajp.159.10.1731. [DOI] [PubMed] [Google Scholar]
- 36.Brown RA, Kahler CW, Niaura R, et al. Cognitive-behavioral treatment for depression in smoking cessation. J Consult Clin Psychol. 2001;69:471–80. doi: 10.1037/0022-006X.69.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacPherson L, Tull MT, Matusiewicz AK, et al. Randomized controlled trial of behavioral activation smoking cessation treatment for smokers with elevated depressive symptoms. J Consult Clin Psychol. 2010;78:55–61. doi: 10.1037/a0017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duffy SA, Ronis DL, Valenstein M, et al. A tailored smoking, alcohol, and depression intervention for head and neck cancer patients. Cancer Epidemiol Biomarkers Prev. 2006;15:2203–8. doi: 10.1158/1055-9965.EPI-05-0880. [DOI] [PubMed] [Google Scholar]
- 39.Hall SM, Munoz RF, Reus VI. Cognitive-behavioral intervention increases abstinence rates for depressive-history smokers. J Consult Clin Psychol. 1994;62:141–6. doi: 10.1037/0022-006X.62.1.141. [DOI] [PubMed] [Google Scholar]
- 40.Hayford KE, Patten CA, Rummans TA, et al. Efficacy of bupropion for smoking cessation in smokers with a former history of major depression or alcoholism. Br J Psychiatr. 1999;174:173–8. doi: 10.1192/bjp.174.2.173. [DOI] [PubMed] [Google Scholar]
- 41.Saules KK, Schuh LM, Arfken CL, Reed K, Kilbey MM, Schuster CR. Double-blind placebo-controlled trial of fluoxetine in smoking cessation treatment including nicotine patch and cognitive-behavioral group therapy. Am J Addict. 2004;13:438–46. doi: 10.1080/10550490490512762. [DOI] [PubMed] [Google Scholar]
- 42.Covey LS, Glassman AH, Stetner F. Naltrexone effects on short-term and long-term smoking cessation. J Addict Dis. 1999;1:31–40. doi: 10.1300/J069v18n01_04. [DOI] [PubMed] [Google Scholar]
- 43.Kinnunen T, Doherty K, Militello FS, Garvey AJ. Depression and smoking cessation: characteristics of depressed smokers and effects of nicotine replacement. J Consult Clin Psychol. 1996;4:791–8. doi: 10.1037//0022-006x.64.4.791. [DOI] [PubMed] [Google Scholar]
- 44.Kinnunen T, Korhonen T, Garvey AJ. Role of nicotine gum and pretreatment depressive symptoms in smoking cessation: twelve-month results of a randomized placebo controlled trial. Int J Psychiatr Med. 2008;3:373–89. doi: 10.2190/PM.38.3.k. [DOI] [PubMed] [Google Scholar]
- 45.Berlin I, Covey LS. Pre-cessation depressive mood predicts failure to quit smoking: the role of coping and personality traits. Addiction. 2006;101:1814–21. doi: 10.1111/j.1360-0443.2006.01616.x. [DOI] [PubMed] [Google Scholar]
- 46.Haaga DA, Thorndike FP, Friedman-Wheeler DG, Pearlman MY, Wernicke RA. Cognitive coping skills and depression vulnerability among cigarette smokers. Addict Behav. 2004;29:1109–22. doi: 10.1016/j.addbeh.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 47.Kahler CW, Brown RA, Ramsey SE, et al. Negative mood, depressive symptoms, and major depression after smoking cessation treatment in smokers with a history of major depressive disorder. J Abnorm Psychol. 2002;111:670–5. doi: 10.1037/0021-843X.111.4.670. [DOI] [PubMed] [Google Scholar]
- 48.Kahler CW, Brown RA, Strong DR, Lloyd-Richardson EE, Niaura R. History of major depressive disorder among smokers in cessation treatment: associations with dysfunctional attitudes and coping. Addict Behav. 2003;28:1033–47. doi: 10.1016/S0306-4603(02)00234-4. [DOI] [PubMed] [Google Scholar]
- 49.Carmody TP. Affect regulation, nicotine addiction, and smoking cessation. J Psychoactive Drugs. 1992;24:111–22. doi: 10.1080/02791072.1992.10471632. [DOI] [PubMed] [Google Scholar]
- 50.Hall SM, Munoz RF, Reus VI, Sees KL. Nicotine, negative affect, and depression. J Consult Clin Psychol. 1993;61:761–7. doi: 10.1037/0022-006X.61.5.761. [DOI] [PubMed] [Google Scholar]
- 51.Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 52.Williams JW., Jr Pignone M, Ramirez G, Perez Stellato C. Identifying depression in primary care: a literature synthesis of case-finding instruments. Gen Hosp Psychiatry. 2002;24:225–37. doi: 10.1016/S0163-8343(02)00195-0. [DOI] [PubMed] [Google Scholar]
- 53.An LC, Zhu SH, Nelson DB, et al. Benefits of telephone care over primary care for smoking cessation: a randomized trial. Arch Intern Med. 2006;166:536–42. doi: 10.1001/archinte.166.5.536. [DOI] [PubMed] [Google Scholar]
- 54.Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Quick Reference Guide for Clinicians. Rockville, MD: US Department of Health and Human Services. Public Health Service. April 2009. Available at: http://www.ahrq.gov/clinic/tobacco/tobaqrg.pdf. Accessed September 27, 2011.
- 55.Gelenberg AJ, Leon J, Evins AE, Parks JJ, Rigotti NA. Smoking cessation in patients with psychiatric disorders. Primary Care Companion to the Journal of Clinical Psychiatry. 2008;10:52–8. doi: 10.4088/PCC.v10n0109. [DOI] [PMC free article] [PubMed] [Google Scholar]