ABSTRACT
BACKGROUND
Statins are prescribed to lower cholesterol, but also have anti-inflammatory properties. Some observational studies suggest that statins may reduce mortality from sepsis.
METHODS
Using a highly detailed administrative database, we conducted an observational cohort study of all patients aged ≥18 years who received a discharge diagnosis of pneumonia from 2003–2005 at 376 hospitals. Patients with contraindications to statins, and those unable to take oral medications or discharged within 2 days were excluded. We used multivariable logistic regression and propensity matching to compare mortality among patients who did and did not receive statins on hospital day 1 or 2.
RESULTS
Of the 121,254 patients who met the inclusion criteria, median age was 74; 56% were female and 70% were white; 19% received a statin on day 1 or 2. Compared to patients who did not receive statins, statin-treated patients were less likely to be admitted to intensive care (15.7% vs 18.1%, p < 0.001), require mechanical ventilation (6.9% vs. 9.3%, p < 0.001), or die in hospital (3.9% vs 5.7%, p < 0.001). After multivariable adjustment, including the propensity for statin treatment and severity at presentation, mortality was lower in statin-treated patients [OR for propensity-adjusted 0.86 (95% CI 0.79 to 0.93) OR for propensity-matched 0.90, (0.82 to 0.99)]. For patients admitted to intensive care the adjusted odds ratio for mortality with statins was 0.93 (95% CI 0.81 to 1.06), whereas outside intensive care it was 0.79 (95% CI 0.71 to 0.87).
CONCLUSIONS
Inpatient treatment with statins is associated with a modest reduction in pneumonia mortality outside of intensive care.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-011-1826-2) contains supplementary material, which is available to authorized users.
KEY WORDS: evidence-based medicine, hospital medicine, health services research
INTRODUCTION
Pneumonia is one of the most common causes of death in the US.1 Patients who die of pneumonia frequently succumb to respiratory distress syndrome or other complications of sepsis.2 Because these processes seem to be mediated by inflammatory cytokines, agents that could decrease inflammation might have a beneficial effect on mortality.3 HMG-CoA reductase inhibitors or “statins” have been shown to decrease inflammatory markers in acute coronary syndromes and also in patients with sepsis.4 These anti-inflammatory effects are thought to play a role in preventing acute cardiovascular syndromes, and one randomized trial of patients with bacterial infections found that treatment with simvastatin reduced levels of IL-6 and TNF-α at 72 h.5 Mouse models of sepsis demonstrate that statins reduce both inflammatory markers and mortality.6–9
Although randomized controlled trials are underway, a number of observational studies have demonstrated that patients taking statins in the ambulatory setting are less likely to die from bacteremia, pneumonia, and sepsis.10 Others investigators have suggested that a “healthy-user” bias11 may account for the benefits observed, because adjustment for previously unmeasured confounders eliminated the association between statins and mortality.12 Several other observational studies have found an association between statins administration during the hospitalization and improved survival.13–15 While these studies attempted to control for differences in treated and untreated patients, the studies were limited by the available data and residual confounding may fully or partially explain the observed association. Given the potential public health impact of this simple, low-cost intervention, we examined the association between statin therapy and mortality in a highly detailed administrative database, attempting to control for variables that may be associated with healthy or unhealthy patients. Because statins are believed to act primarily in patients with sepsis, we also examined subsets of patients to see whether the association of statins with outcomes occurred in patients at the highest risk of mortality (i.e., patients in intensive care and those treated with broad-spectrum antibiotics).
METHODS
Setting and Subjects
We conducted a retrospective cohort study of all patients hospitalized for pneumonia between 1 January 2003 and 31 December 2005 at 376 acute care facilities in the US that participated in Premier’s Perspective, a database developed for measuring quality and healthcare utilization. Participating hospitals represent all regions of the United States, and are primarily small to medium-sized non-teaching hospitals located mostly in urban areas. In contrast to standard hospital discharge files, which contain only basic demographic information about patients, International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes, and physician information, the Perspective database includes a date-stamped log of all billed items, including diagnostic tests, medications, and other treatments, for individual patients, allowing for a highly detailed assessment of the care delivered. The Institutional Review Board at Baystate Medical Center approved the study.
Patients were included if they were ≥18 years and had a principal diagnosis of pneumonia, or a principal diagnosis of respiratory failure or sepsis paired with a secondary diagnosis of pneumonia (ICD-9-CM codes 481, 482–482.83, 482.89–483, 483.8, 484.8–486). In accordance with coding guidelines,16 patients with a principal diagnosis of respiratory failure or sepsis were included to capture the full severity spectrum of pneumonia cases. We excluded patients with a length of stay <2 days; those transferred to or from another acute care facility, because we could not ascertain what treatments they received prior to admission or their vital status at discharge; those treated with neither an antibiotic nor an antiviral by hospital day 2, because this cast doubt on the diagnosis; and those with liver disease or myopathy, because these are contraindications to statin therapy. We also excluded patients who did not receive medications by the oral route on the first 2 hospital days because we were concerned that inability to take oral medications reflected severe illness, and because statins are not available in intravenous formulations. Failure to exclude such patients would bias the study toward showing a benefit of statins as a result of confounding by ability to take oral medications.
Data Elements
For each patient, we extracted age, gender, race, and insurance status, principal diagnosis, comorbidities, and specialty of the attending physician. Pneumonias were classified into three categories: staphylococcal; non-staphylococcal bacterial; and other pneumonias, which included “not otherwise specified.” Comorbidities were identified from ICD-9-CM secondary diagnosis codes and Diagnosis Related Groups using Healthcare Cost and Utilization Project Comorbidity Software, version 3.1, based on the work of Elixhauser.17 We examined receipt of a large number of treatments on the first 2 days of hospitalization, including all statins, and other lipid-lowering medications, as well as angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs) because they might have anti-inflammatory effects. Then we assessed receipt of specific antibiotic classes as potential confounders. Finally, we assessed 21 additional non-pneumonia treatments given in the first 2 hospital days that might indicate poor health (e.g., antipsychotic medications, restraints, or a gastric feeding tube), and 25 additional medications (e.g., antidepressants) that have previously been shown to be associated with statin use.12 Hospitals were categorized by region (Northeast, South, Midwest, or West), bed size, setting (urban vs rural), and teaching status.
Statin Treatment and Outcome Variables
Exposure to statins was defined as at least one dose of any HMG-CoA reductase inhibitor on hospital day 1 or 2. Statins were further classified by dosage and type (lipophilic vs hydrophilic). Because statins are rarely initiated in the hospital for patients with pneumonia, we assumed that patients who received statins after day 2 were resuming outpatient statin therapy as their condition improved. Such late statin use might be considered a marker of good prognosis, and including these patients in the statin-treated group could bias our results. Consequently, patients whose statins were initiated after hospital day 2 were grouped with the non-statin patients. Our primary outcome was all-cause in-hospital mortality.
Statistical Analysis
Summary statistics were computed using frequencies and percents for categorical variables, and means, standard deviations, medians and interquartile ranges for continuous variables. Associations between early statin treatment and patient and hospital characteristics were assessed using chi-square tests for categorical variables and z-tests for continuous variables.
We developed a multivariable logistic regression model using a logit link function to evaluate the impact of early statin therapy on mortality while adjusting for all patient and hospital factors, the effects of other treatments and accounting for within-hospital correlation. Further, in order to reduce the threat of selection bias, we created a non-parsimonious propensity model in which receipt of statin therapy on day 1 or 2 was the outcome. The model included patient factors prior to admission (e.g., demographic variables and comorbidities); additional early non-pneumonia treatments that indicate poor general health, physician specialty, hospital factors (e.g., size and teaching status), and other medications associated with statin use; as well as a number of interaction terms. Additional factors associated with presenting severity of pneumonia, such as the need for mechanical ventilation on the first hospital day, and treatment with vancomycin, were not included in the propensity score, but were adjusted for separately. We did this in order to separate out the potential effect of statin use prior to admission, which would affect severity on presentation, from the effects of statins given in the hospital after adjusting for differences in initial severity.
Unadjusted, covariate-adjusted, propensity- and covariate-adjusted models were compared. In addition, patients who received statin therapy on day 1 or 2 were matched to patients with a similar propensity score yet who did not receive a statin, or in whom treatment was begun after day 2, using a greedy 5-to-1 digit matching algorithm.18 Conditional logistic models were developed using this propensity-matched subsample of patients, adjusted for covariables that remained unbalanced after propensity matching. For each model, adjusted odds ratios for mortality with associated 95% confidence intervals for statin treatment were calculated. In addition, to assess for heterogeneity of treatment effect associated with severity of illness, we examined the association between statin therapy and mortality in two pre-specified stratified analyses: first, we examined the association across quintiles of severity based on the risk of mortality, as predicted by a mortality model that excluded statin status; second, we compared the association among patients initially admitted and not admitted to intensive care. We also performed an exploratory analysis examining patients who received initial treatment with antibiotics against staphylococcal or pseudomonal pneumonia, infections associated with high mortality. Finally, we examined the relationship between statin type (lipophilic vs hydrophilic) and statin dose (≥40 mg vs <40 mg of simvastatin) and mortality.
All analyses were performed using SAS version 9.1 (SAS Institute Inc, Cary, NC).
RESULTS
Of the 121,254 patients who met the inclusion criteria the median age was 74; 56% were female and 70% were white (Table 1). Eighty-five percent of patients had a primary diagnosis of pneumonia, 7% had sepsis, and 8% had respiratory failure. The most common pneumonia diagnosis was “not otherwise specified.” The most common comorbidities were chronic pulmonary disease, hypertension, and diabetes. Most patients were treated with either a third-generation cephalosporin or a quinolone. Eighteen percent of patients spent time in intensive care, 9% required mechanical ventilation, and 5.4% died in hospital; another 11.8% were readmitted to the same hospital within 30 days. Median length of stay was 6 days.
Table 1.
Patient Characteristics of 121,254 Adults with Pneumonia Who Did or Did Not Receive Statins in the First 2 Hospital Days
| No statin | Statin | p-value | |||||
|---|---|---|---|---|---|---|---|
| All | N = 97,969 | N = 23,285 | |||||
| N | (%) | N | (%) | N | (%) | ||
| Age (mean, SD) | 70.2 | 15.9 | 69.6 | 16.7 | 72.4 | 11.5 | <0.001 |
| Female | 67,975 | (56.1) | 56,146 | (57.3) | 11,829 | (50.8) | <0.001 |
| Race | <0.001 | ||||||
| White | 84,688 | (69.8) | 67,400 | (68.8) | 17,288 | (74.2) | |
| Black | 14,728 | (12.1) | 12,686 | (12.9) | 2,042 | (8.8) | |
| Hispanic | 4,454 | (3.7) | 3,731 | (3.8) | 723 | (3.1) | |
| Other | 17,384 | (14.3) | 14,152 | (14.4) | 3,232 | (13.9) | |
| Primary diagnosis | <0.001 | ||||||
| Community acquired pneumonia | 102,973 | (84.9) | 82,403 | (84.1) | 20,570 | (88.3) | |
| Septicemia | 8,935 | (7.4) | 7,722 | (7.9) | 1,213 | (5.2) | |
| Respiratory failure | 9,346 | (7.7) | 7,844 | (8.0) | 1,502 | (6.5) | |
| Pneumonia type | <0.001 | ||||||
| Influenza/not otherwise specified | 104,954 | (86.6) | 84,184 | (85.9) | 20,770 | (89.2) | |
| Bacterial | 11,862 | (9.8) | 10,022 | (10.2) | 1,840 | (7.9) | |
| Staphylococcal | 4,438 | (3.7) | 3,763 | (3.8) | 675 | (2.9) | |
| Indicators of general health status | |||||||
| Smoker | 17,177 | (14.2) | 14,507 | (14.8) | 2,670 | (11.5) | <0.001 |
| Admitted from skilled nursing facility | 1,900 | (1.6) | 1,683 | (1.7) | 217 | (0.9) | <0.001 |
| Thiamine* | 2,313 | (1.9) | 2,087 | (2.1) | 226 | (1.0) | <0.001 |
| Nutritional supplements* | 870 | (0.7) | 763 | (0.8) | 107 | (0.5) | <0.001 |
| Total parenteral nutrition* | 1,226 | (1.0) | 1,055 | (1.1) | 171 | (0.7) | <0.001 |
| Gastrostomy or jejunostomy-tube* | 864 | (0.7) | 776 | (0.8) | 88 | (0.4) | <0.001 |
| Indwelling urinary catheter* | 14,647 | (12.1) | 12,296 | (12.6) | 2,351 | (10.1) | <0.001 |
| Restraints* | 1,959 | (1.6) | 1,715 | (1.8) | 244 | (1.0) | <0.001 |
| Calcium supplements* | 5,270 | (4.3) | 4,147 | (4.2) | 1,123 | (4.8) | <0.001 |
| Indicators of pneumonia severity | |||||||
| Mechanical ventilation* | 6,583 | (5.4) | 5,713 | (5.8) | 870 | (3.7) | <0.001 |
| Antipseudomal penicillins | 11,634 | (9.6) | 9,863 | (10.1) | 1,771 | (7.6) | <0.001 |
| Vancomycin/linezolid | 17,226 | (14.2) | 14,621 | (14.9) | 2,605 | (11.2) | <0.001 |
| Attending specialty | <0.001 | ||||||
| Internal medicine | 67,194 | (55.4) | 54,158 | (55.3) | 13,036 | (56.0) | |
| General practice/family medicine | 25,328 | (20.9) | 20,629 | (21.1) | 4,699 | (20.2) | |
| Pulmonology | 8,475 | (7.0) | 6,956 | (7.1) | 1,519 | (6.5) | |
| Cardiology/nephrology/other | 20,257 | (16.7) | 16,226 | (16.6) | 4,031 | (17.3) | |
| Hospital characteristics | |||||||
| Teaching hospital | 38,945 | (32.1) | 31,110 | (31.8) | 7,835 | (33.6) | <0.001 |
| Region | <0.001 | ||||||
| South | 65,822 | (54.3) | 53,595 | (54.7) | 1,2227 | (52.5) | |
| Midwest | 23,411 | (19.3) | 18,611 | (19.0) | 4,800 | (20.6) | |
| West | 13,812 | (11.4) | 11,436 | (11.7) | 2,376 | (10.2) | |
| Northeast | 18,209 | (15.0) | 14,327 | (14.6) | 3,882 | (16.7) | |
| Number of beds | <0.001 | ||||||
| 0–200 | 23,319 | (19.2) | 19,248 | (19.6) | 4,071 | (17.5) | |
| 201–500 | 63,280 | (52.2) | 51,078 | (52.1) | 12,202 | (52.4) | |
| >500 | 34,655 | (28.6) | 27,643 | (28.2) | 7,012 | (30.1) | |
| Co-morbid illnesses | |||||||
| Ischemic heart disease | 31,295 | (25.8) | 20,050 | (20.5) | 11,245 | (48.3) | <0.001 |
| Peripheral vascular disease | 7,440 | (6.1) | 5,172 | (5.3) | 2,268 | (9.7) | <0.001 |
| Hypertension | 56,301 | (46.4) | 42,849 | (43.7) | 13,452 | (57.8) | <0.001 |
| Diabetes | 33,394 | (27.5) | 23,467 | (24.0) | 9,927 | (42.6) | <0.001 |
| Renal failure | 9,429 | (7.8) | 7,045 | (7.2) | 2,384 | (10.2) | <0.001 |
| Chronic pulmonary disease | 59,766 | (49.3) | 47,473 | (48.5) | 12,293 | (52.8) | <0.001 |
| Deficiency anemias | 27,574 | (22.7) | 22,337 | (22.8) | 5,237 | (22.5) | 0.31 |
| Other neurological disorders | 14,496 | (12.0) | 12,376 | (12.6) | 2,120 | (9.1) | <0.001 |
| Hypothyroidism | 15,673 | (12.9) | 12,302 | (12.6) | 3,371 | (14.5) | <0.001 |
| Depression | 12,810 | (10.6) | 10,281 | (10.5) | 2,529 | (10.9) | 0.10 |
| Obesity | 7,613 | (6.3) | 5,714 | (5.8) | 1,899 | (8.2) | <0.001 |
| Solid tumor without metastasis | 14,608 | (12.0) | 11,716 | (12.0) | 2,892 | (12.4) | 0.05 |
| Other medications | |||||||
| Cephalosporin | 75,294 | (62.1) | 60,989 | (62.3) | 14,305 | (61.4) | 0.02 |
| Penicillin | 7,920 | (6.5) | 6,531 | (6.7) | 1,389 | (6.0) | <0.001 |
| Other antibiotics | 30,696 | (25.3) | 25,870 | (26.4) | 4,826 | (20.7) | <0.001 |
| Angiotensin-converting enzyme inhibitor | 25,023 | (20.6) | 17,354 | (17.7) | 7,669 | (32.9) | <0.001 |
| Angiotensin receptor blocker | 10,054 | (8.3) | 6,810 | (7.0) | 3,244 | (13.9) | <0.001 |
| Resin/fibrate/niacin/ezetimibe | 4,600 | (3.8) | 2,794 | (2.9) | 1,806 | (7.8) | <0.001 |
| Outcomes | |||||||
| Died in hospital | 6,518 | (5.4) | 5,617 | (5.7) | 901 | (3.9) | <0.001 |
| Readmitted within 30 days | 14,253 | (11.8) | 11,494 | (11.7) | 2,759 | (11.8) | 0.61 |
| Any mechanical ventilation | 10,545 | (8.7) | 8,939 | (9.1) | 1,606 | (6.9) | <0.001 |
| Admission to intensive care unit | 21,326 | (17.6) | 17,678 | (18.0) | 3,648 | (15.7) | <0.001 |
*If initiated on hospital day 1 or 2
Nineteen percent of patients received at least one dose of a statin during the first 2 days of hospitalization, most commonly simvastatin, atorvastatin, or pravastatin. An additional 2% of patients initiated a statin later in hospitalization. Statin users differed from non-users in many important ways. Statin users were older, and more likely to be male and white. They were much more likely to have ischemic heart disease, hypertension, and diabetes, but less likely to have treatments associated with frailty or poor health status, including Foley catheters and psychotropic medications. They were also more likely to receive ACE inhibitors, ARBs, and other cholesterol-lowering medications. Finally, they were less likely to be admitted with a principal diagnosis of respiratory failure or sepsis.
Unadjusted Analyses
Compared to patients who did not receive statins in the first 2 days, statin-treated patients were less likely to be admitted to the ICU (15.7% vs 18.1%, p < 0.001), to require mechanical ventilation (6.9% vs 9.3%, p < 0.001), or to die in-hospital (3.9% vs 5.7%, p < 0.001), but had similar rates of readmission. Patients treated with statins also had a shorter length of stay (6.9 days vs 7.5 days, p < 0.001) and lower hospital costs ($9,149 vs $10,144, p < 0.001). Among patients who received statins, those receiving lipophilic statins had lower mortality than patients receiving hydrophilic statins (1.7% vs 2.4%, p = 0.02). While not statistically significant, there appeared to be a dose-response curve, with higher doses associated with lower mortality (p for trend = 0.08).
We observed inconsistent mortality effects for other cholesterol-lowering therapies: fibrates and ezetimibe were associated with lower mortality (3.0% and 2.9%, vs 5.6%, p < 0.001 for both), whereas resins were associated with higher mortality (9.2% vs 5.6%, p < 0.001). Receipt of niacin was not associated with mortality.
Results of Multivariable Analyses
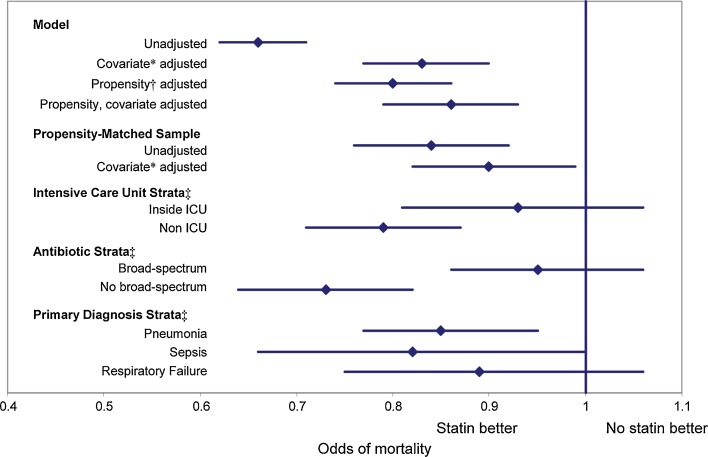
In the unadjusted analysis, patients treated with statins had an odds ratio for mortality of 0.66 (95% CI 0.62 to 0.71) compared to untreated patients (Fig. 1). Adjusting for demographics, comorbidities, and initial treatments associated with mortality increased the odds ratio to 0.85 (95% CI 0.79 to 0.90). In contrast, after adjustment, no other cholesterol-lowering therapy was associated with mortality (Table 2).
Figure 1.
Relative odds of mortality associated with statin use in the first 2 hospital days. *Adjusted for: age, gender, smoking, congestive heart failure, lymphoma, metastatic cancer, other neurologic disorders, obesity, pulmonary circulation disease, renal failure, solid tumor without metasasis, valvular disease, weight loss, depression, hypertension, psychoses, primary diagnosis, pneumonia type, initial antibiotic(s) received, early treatment (day 1 or 2) with angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, Foley catheter, restraints, nutritional supplements, total parenteral nutrition, gastrostomy or jejunostomy tube, thiamine, calcium or mechanical ventilation. †Variables in propensity model: All variables above, plus admission from skilled nursing facility, insurance type, marital status, race/ethnicity, hospital geographic region, number of beds, teaching hospital, and setting (urban/rural), attending specialty, acquired immune sufficiency syndrome, alcohol abuse, deficiency anemia, collagen vascular disease, chronic blood loss anemia, chronic pulmonary disease, diabetes, drug abuse, hypothyroidism, ischemic heart disease, paralysis, peripheral vascular disease, peptic ulcer disease and bleeding, aspirin, bisphosphonates, clopidogrel, folic acid, glucosamine, multivitamin, vitamin B2, B5, B6, C, D or E, dementia medications, collagenase, prealbumin, psychotropic drugs, silvadene, antidepressants, beta blockers, calcium channel blocker, diuretics, ezetimibe, fibrates, amiodorone, inhaled bronchodialators, inhaled corticosteroids, insulin, immunosuppressants, niacin, nitroglycerin, non-steroidal anti-inflammatory drugs, proton pump inhibitors, resins, steroids, thyroid replacement therapy, and warfarin. ‡All stratified analyses adjusted for propensity score and co-variates.
Table 2.
Odds of Mortality Associated with Other Cholesterol Medication
| Medication | OR | 95% CI | p-value |
|---|---|---|---|
| Ezetimibe | |||
| Crude | 0.51 | 0.36–0.72 | 0.0001 |
| Adjusted* | 0.82 | 0.57–1.19 | 0.29 |
| Fibrates | |||
| Crude | 0.54 | 0.41–0.70 | <0.0001 |
| Adjusted* | 0.79 | 0.60–1.05 | 0.10 |
| Resins | |||
| Crude | 1.79 | 1.45–2.21 | <0.0001 |
| Adjusted* | 0.97 | 0.77–1.22 | 0.78 |
| Niacin | |||
| Crude | 0.90 | 0.65–1.26 | 0.55 |
| Adjusted* | 1.36 | 0.96–1.94 | 0.09 |
*Adjusted for covariates + propensity quintile
Propensity matching was largely effective at balancing covariates among the two groups of patients (Online Appendix). In this propensity-matched cohort, patients treated with statins were still slightly more likely to be male and to have received non-statin lipid-lowering therapy. Because markers of pneumonia severity were not included in the propensity score, they remained unbalanced. Patients treated with statins had much less severe illness on presentation, as evidenced by the fact they were less likely to have sepsis or respiratory failure, to receive broad-spectrum antibiotics, to need initial mechanical ventilation, or to be admitted initially to the intensive care unit. In the propensity-matched cohort, statins continued to be associated with lower mortality [OR 0.84 (95% CI 0.76 to 0.92)]. After adjustment for unbalanced covariates, including those representing severity of presenting illness, the association was attenuated, but still statistically significant [OR 0.90, (95% CI 0.82 to 0.99)].
We also explored whether the observed mortality might differ across risk strata as determined by our mortality prediction model, ICU admission, or antibiotic treatment regimen (Fig. 1). For the quintile with lowest predicted mortality, the model failed to converge. For the remaining quintiles, from lowest to highest risk, the odds ratios for mortality associated with statin treatment were 0.71 (95% CI 0.53 to 0.96), 0.77 (95% CI 0.62 to 0.95), 0.93 (95% CI 0.79 to 1.10), and 0.87 (95% CI 0.78 to 0.97). Results were also consistent across quintiles of propensity. However, the adjusted odds ratios for the association varied by location in the hospital. For patients admitted directly to intensive care the adjusted odds ratio for mortality was 0.93 (95% CI 0.81 to 1.06), whereas outside intensive care it was 0.79 (95% CI 0.71 to 0.87). Similarly, for patients treated initially with antibiotics directed at Staphylococcus aureas or Pseudomonas (vancomycin, antipseudomonal penicillins, or fourth-generation cephalosporins), the odds of mortality with statin use was 0.95 (95% CI 0.86 to 1.06), whereas for those not receiving these antibiotics it was 0.73 (95% CI 0.64 to 0.82).
DISCUSSION
In this large observational cohort study, we found that statin use in the hospital was associated with a small reduction in hospital mortality from pneumonia. However, patients taking statins had much less severe disease at presentation, even in a sample matched on the propensity for treatment. Once we adjusted for the severity of pneumonia at the time of admission, this association was attenuated but still significant. Additionally, among the sickest patients—those admitted to intensive care units and those treated with antibiotics against Staphylococcus or Pseudomonas, there was no association between receipt of statin in the hospital and mortality.
Our study adds to the ongoing debate about a possible role for statins in the treatment of severe pneumonia, specifically, whether the extraordinary benefits of statins seen in many observational studies are real or the result of unmeasured biases. Earlier studies raised the possibility that statins could dramatically reduce pneumonia mortality—perhaps by 50% or more.19–21 Several later studies challenged these findings as confounded by a “healthy-user” effect, in which patients in general good health are the ones most likely to receive statins.12,22,23 In one prospective analysis, adjustment for functional status, which is not usually available in retrospective studies, negated any benefit of statin therapy.12
Few studies have assessed the effects of statins given in the hospital, and none has looked specifically at patients with pneumonia. Donino et al. studied 2,036 patients hospitalized for suspected infection and found that statin receipt was associated with a 73% reduction in mortality.13 The study relied on pharmacy records to identify which patients received statins during their hospital stay, and outcomes were adjusted for initial pneumonia severity based on ED chart review using the MEDS score. In contrast to our study, they found that statin-treated patients had more severe presentation, with an average MEDS score of 6.0 vs. 3.0 for the no-statin group. Dobesh et al., in a retrospective study of 188 patients with severe sepsis, found in-hospital statin use to be associated with a 58% reduction in mortality.14 There was no difference in severity of presentation as measured by APACHE II scores between statin users and non-users, but the benefit of statins was observed only in patients with APACHE II scores of >24 (32% mortality among statin users vs 58% in non-users). Finally, Kruger et al., studied 438 patients admitted with acute bacteremia and found that patients who had taken statins had a 61% reduction in mortality.15 Patients who took statins at home had slightly less severe presentation as measured by septic shock (8% vs 18%) and APACHE II scores (14.7 vs 16.6), but neither of these reached statistical significance. Entry criteria for these last two studies may have attenuated any differences in severity at presentation.
Two biases inherent in all three studies might account for the large and consistent benefit associated with inpatient statin use. First, because statins are only available orally and at the time were not considered crucial medications in sepsis, they were probably withheld from the sickest patients (a form of confounding by indication). Conversely, a statin initiated late in the hospital stay might be a marker that a patient was improving. Including the former patients in the no-statin group and the latter in the statin group would bias the outcome in favor of patients receiving statins. Indeed, in the Kruger study, patients who took statins at home and had them discontinued on admission had the highest mortality of any group (>60%). At least one retrospective study of myocardial infarction patients also found that discontinuing statins was associated with increased mortality, but it appears to be subject to the same sorts of bias.24 In addition, in all three studies, patients who did not survive long enough to receive a statin were included in the no statin group, thus introducing an important immortal time bias25 for statin recipients. Again, in the Kruger study, most patients who died did so on the first hospital day, confirming that failure to include these patients in the statin group may account for some or all of the observed benefit. We avoided confounding by indication by excluding patients who were unable to take oral medication. We avoided immortal time bias by limiting our analysis to patients with a length of stay of 2 days or longer, and only including patients in the statin group if they received a statin beginning on hospital day 1 or 2. Consequently, we observed a much smaller mortality benefit associated with in-hospital statin use.
Our study has a number of limitations. While we attempted to adjust for baseline differences in patient characteristics and disease severity, this was an observational study, not a randomized trial, and the associations we observed might still be the results of residual confounding. Increasingly detailed adjustment appeared to decrease the observed association between statin use and outcomes; further adjustment might completely negate any association. Second, because statins are not usually initiated during a hospitalization for pneumonia, we do not know what effect statins given prior to admission might have had on outcomes. We attempted to assess this through our propensity-matched analysis. The finding that statin-treated patients appeared to have less severe pneumonia at the time of admission, even after adjusting for demographic characteristics, co-morbidities, and other treatments associated with chronic disease, implies that chronic statin use may protect against severe pneumonia, or that patients who take statins are healthier than those who do not. Statin-treated patients were not only less likely to die, but they were less frequently admitted to an ICU, and were less likely to require mechanical ventilation or to be treated with antibiotics for Staphylococcus or Pseudomonas. Alternatively, statin users with mild disease might have been more likely to have their medication continued in hospital. Without knowledge of pre-hospital treatment it is impossible to differentiate between these two possibilities. However, after adjusting for measures of disease severity, including the ability to take medication by mouth, statin users still had a small survival advantage, implying an additional protective benefit of statins associated with their continuation during hospitalization.
One surprising finding was that patients in the intensive care unit and those who received antibiotics to cover Staphylococcus aureus and Pseudomonas aeruginosa did not appear to benefit from statin therapy. On the basis of previous observational trials, statins have been suggested as a potentially promising therapy for both sepsis and pneumonia, and as an inexpensive therapy in case of a serious influenza pandemic,26 and a number of randomized trials in sepsis are now underway. Our results suggest these trials are unlikely to show benefit. Statins may have some benefit in lower risk pneumonia patients, but randomized trials will be difficult to conduct in patients with a predicted 30-day mortality of less than 10%, if the benefit is as small as we observed. For now it seems prudent to continue statins in-hospital for those patients already taking them, but not to initiate statin use for infections until randomized trials have been conducted.
Electronic supplementary material
Online Appendix: Patient characteristics in the propensity-matched sample (n=44,495) (DOC 130 kb)
Funding
The authors are indebted to an anonymous physician for partially funding this study with personal funds. The donor had no other role in the study
Prior presentations
These data have not been presented previously.
The authors are also indebted to Dr. David Fedson for help in defining the study question, obtaining funding, and background research.
Conflict of Interest
None disclosed.
References
- 1.Minino AM, Xu J, Kochanek KD, Tejada-Vera B. Death in the United States. NCHS Data Brief. 2007;2009:1–8. [PubMed] [Google Scholar]
- 2.Marrie TJ. Pneumococcal pneumonia: epidemiology and clinical features. Semin Respir Infect. 1999;14:227–236. [PubMed] [Google Scholar]
- 3.Marshall JC. Sepsis: current status, future prospects. Curr Opin Crit Care. 2004;10:250–264. doi: 10.1097/01.ccx.0000134877.60312.f3. [DOI] [PubMed] [Google Scholar]
- 4.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov. 2005;4:977–987. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 5.Novack V, Eisinger M, Frenkel A, et al. The effects of statin therapy on inflammatory cytokines in patients with bacterial infections: a randomized double-blind placebo controlled clinical trial. Intensive Care Med. 2009;35:1255–1260. doi: 10.1007/s00134-009-1429-0. [DOI] [PubMed] [Google Scholar]
- 6.Ando H, Takamura T, Ota T, Nagai Y, Kobayashi K. Cerivastatin improves survival of mice with lipopolysaccharide-induced sepsis. J Pharmacol Exp Ther. 2000;294:1043–1046. [PubMed] [Google Scholar]
- 7.Merx MW, Liehn EA, Graf J, et al. Statin treatment after onset of sepsis in a murine model improves survival HMG-CoA reductase inhibitor simvastatin profoundly improves survival in a murine model of sepsis. Circulation. 2005;112:117–124. doi: 10.1161/CIRCULATIONAHA.104.502195. [DOI] [PubMed] [Google Scholar]
- 8.Merx MW, Liehn EA, Janssens U, et al. HMG-CoA reductase inhibitor simvastatin profoundly improves survival in a murine model of sepsis. Circulation. 2004;109:2560–2565. doi: 10.1161/01.CIR.0000129774.09737.5B. [DOI] [PubMed] [Google Scholar]
- 9.Yasuda H, Yuen PS, Hu X, Zhou H, Star RA. Simvastatin improves sepsis-induced mortality and acute kidney injury via renal vascular effects. Kidney Int. 2006;69:1535–1542. doi: 10.1038/sj.ki.5000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tleyjeh IM, Kashour T, Hakim FA, et al. Statins for the prevention and treatment of infections: a systematic review and meta-analysis. Arch Intern Med. 2009;169:1658–1667. doi: 10.1001/archinternmed.2009.286. [DOI] [PubMed] [Google Scholar]
- 11.Brookhart MA, Patrick AR, Dormuth C, et al. Adherence to lipid-lowering therapy and the use of preventive health services: an investigation of the healthy user effect. Am J Epidemiol. 2007;166:348–354. doi: 10.1093/aje/kwm070. [DOI] [PubMed] [Google Scholar]
- 12.Majumdar SR, McAlister FA, Eurich DT, Padwal RS, Marrie TJ. Statins and outcomes in patients admitted to hospital with community acquired pneumonia: population based prospective cohort study. BMJ. 2006;333:999. doi: 10.1136/bmj.38992.565972.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnino MW, Cocchi MN, Howell M, et al. Statin therapy is associated with decreased mortality in patients with infection. Acad Emerg Med. 2009;16:230–234. doi: 10.1111/j.1553-2712.2009.00350.x. [DOI] [PubMed] [Google Scholar]
- 14.Dobesh PP, Klepser DG, McGuire TR, Morgan CW, Olsen KM. Reduction in mortality associated with statin therapy in patients with severe sepsis. Pharmacotherapy. 2009;29:621–630. doi: 10.1592/phco.29.6.621. [DOI] [PubMed] [Google Scholar]
- 15.Kruger P, Fitzsimmons K, Cook D, Jones M, Nimmo G. Statin therapy is associated with fewer deaths in patients with bacteraemia. Intensive Care Med. 2006;32:75–79. doi: 10.1007/s00134-005-2859-y. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Medicare and Medicaid Services. ICD-10-CM Official Guidelines for Coding and Reporting. 2010; available at http://www.cms.gov/ICD10/Downloads/7_Guidelines10cm2010.pdf. Accessed on February 12, 2011.
- 17.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Parsons L. Reducing Bias in a Propensity Score Matched-Pair Sample Using Greedy Matching Techniques. Cary, NC: SAS Institute; 2001. [Google Scholar]
- 19.Mortensen EM, Restrepo MI, Anzueto A, Pugh J. The effect of prior statin use on 30-day mortality for patients hospitalized with community-acquired pneumonia. Respir Res. 2005;6:82. doi: 10.1186/1465-9921-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlienger RG, Fedson DS, Jick SS, Jick H, Meier CR. Statins and the risk of pneumonia: a population-based, nested case-control study. Pharmacotherapy. 2007;27:325–332. doi: 10.1592/phco.27.3.325. [DOI] [PubMed] [Google Scholar]
- 21.Thomsen RW, Riis A, Kornum JB, Christensen S, Johnsen SP, Sorensen HT. Preadmission use of statins and outcomes after hospitalization with pneumonia: population-based cohort study of 29,900 patients. Arch Intern Med. 2008;168:2081–2077. doi: 10.1001/archinte.168.19.2081. [DOI] [PubMed] [Google Scholar]
- 22.Dublin S, Jackson ML, Nelson JC, Weiss NS, Larson EB, Jackson LA. Statin use and risk of community acquired pneumonia in older people: population based case-control study. BMJ 2009;338:b2137. doi:10.1136/bmj.b2137. [DOI] [PMC free article] [PubMed]
- 23.Kwong JC, Li P, Redelmeier DA. Influenza morbidity and mortality in elderly patients receiving statins: a cohort study. PLoS One. 2009;4:e8087. doi: 10.1371/journal.pone.0008087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fonarow GC, Wright RS, Spencer FA, et al. Effect of statin use within the first 24 hours of admission for acute myocardial infarction on early morbidity and mortality. Am J Cardiol. 2005;96:611–616. doi: 10.1016/j.amjcard.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 25.Levesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. Bmj. 2010;340:b5087. doi: 10.1136/bmj.b5087. [DOI] [PubMed] [Google Scholar]
- 26.Fedson DS. Pandemic influenza: a potential role for statins in treatment and prophylaxis. Clin Infect Dis. 2006;43:199–205. doi: 10.1086/505116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Appendix: Patient characteristics in the propensity-matched sample (n=44,495) (DOC 130 kb)