Abstract
Quality in laboratory has huge impact on diagnosis and patient management as 80–90% of all diagnosis is made on the basis of laboratory tests. Monitoring of quality indicators covering the critical areas of pre-analytical, analytical and post-analytical phases like sample misidentification, sample rejection, random and systemic errors, critical value reporting and TATs have a significant impact on performance of laboratory. This study was conducted in diagnostic laboratories receiving approximately 42,562 samples for clinical chemistry, hematology and serology. The list of quality indicators was developed for the steps of total testing process for which errors are frequent and improvements are possible. The trend was observed for all the QI before and after sensitisation of the staff over the period of 12 months. Incomplete test requisition form received in the lab was the most poor quality indicator observed (7.89%), followed by sample rejection rate (4.91%). Most significant improvement was found in pre- and post-analytical phase after sensitisation of staff but did not have much impact on analytical phase. Use of quality indicators to assess and monitor the quality system of the clinical laboratory services is extremely valuable tool in keeping the total testing process under control in a systematic and transparent way.
Keywords: Patient safety, Quality indicator, Pre-analytical phase, Analytical phase, Post-analytical phase, Total testing process
Introduction
More than 10 years back the Institute of Medicine (IOM) report on medical errors ‘To Err is Human’ published in 1999 started a movement focussing on patient safety [1]. The IOM estimated that up to 98,000 deaths per year in US were attributed to medical errors [2]. Medical errors can be traditionally clustered into 4 categories which include errors of diagnosis, errors of treatment, errors of prevention and miscellaneous category [3]. With approximately 60–70% of medical decisions related to diagnosis and treatment involving the laboratory, no other discipline is better positioned to the pivotal in the patient safety solution [4]. Hence, it is obvious that laboratory errors may have a major adverse impact on patient care. However, providing high quality effective laboratory services is not new to the laboratory profession. Laboratory medicine has been a pioneer in the field of patient safety with the College of American pathologist first calling the attention to the patient safety issue in 1946.
Laboratory error is defined as any defect from ordering tests to reporting results and appropriately interpreting and reacting on these. Laboratory errors have a reported frequency of 0.012–0.6% of all test results which in turn has huge impact on diagnosis and patient management as 80–90% of all diagnosis are made on the basis of laboratory tests [5]. First and foremost management strategy to prevent laboratory errors is the identification of those activities at greater risk. It is important to monitor the system and identify the critical areas so that human and economical resources are not wasted in dealing with mistakes that are unlikely to occur. The identification of vulnerable areas is achieved by implementation of error detecting systems specifically developed to target all three phases of total testing process, i.e., pre-analytical, analytical and post-analytical phases [6]. Efforts were made in last decade to implement quality indicators for laboratory tests which either focused on analytical performance or the achievement of a specific efficiency target like turnaround time (TATs) [7]. As every step in the process of patient care may carry a risk to patient safety, a systematic approach is required to mitigate the errors. Use of quality indicators to monitor every process of service delivery and implementation of quality system is the first step in this direction. It is also important to keep the total testing process under control in a systematic and transparent way as it promotes and encourages investigations when error occur. It also leads to the identification of strategies and procedures for improving it [8].
Quality Indicators
Quality indicator is the information, qualitative or quantitative, associated to an event (or process or result) put under observation, that is able to evaluate it’s changes during the time and to verify achievement of the defined quality goals, in order to take the correction decision and choices (Standard UNI 11097). The ISO 15189:2007, International standard for accreditation of medical laboratories requires the laboratory to identify and develop quality indicators and implement them for systematic monitoring and evaluation of the laboratory’s contribution to patient care, but no guidelines are available for their identification and development. ISO 15189:2007 suggests the areas to be monitored but does not define the method to be used for developing quality indicator. Hence, laboratories are using different ways to develop quality indicators as per requirements of certification/accreditation standards, to monitor and improve the quality and patient safety in their laboratory [8].
Selection of Quality Indicators
The quality indicators are selected in such a way that they cover the critical activities of pre-, intra- and post-analytical phases which may have a significant impact on performance of laboratory [9]. The critical steps which can affect the quality of test results are patient and sample identification, specimen collection and transport, analytical quality, rapid transmission of laboratory results particularly critical test results and interpretative service and other tools for allowing a more accurate interpretation of laboratory data [10].The quality indicator selected should be suitable for vulnerable event under observation, capable of providing information that reflects the real situation, user friendly, easy to measure, provide the information for improving the performance, understandable, and encourage prompt and suitable corrective/preventive action.
Traditionally, patient safety initiatives in the laboratory have focussed on error review which is based on the assumption that if personnel follow policies and procedures, errors should not occur. To improve patient safety and quality within three phases of total testing process, i.e., pre-analytical, analytical and post-analytical, the laboratory must have a process to identify and investigate these errors. These evidences can help in assessing the laboratory performance accurately and its safety. Hence, the next step in this direction will be to design the indicators with the following objectives [8].
Evaluation of collected data based on evidence of compliance with specifications defined by the laboratory.
Steps being used in data collection are fair and precise.
Identification of events which are non compliance with the specification (errors/near misses).
The main objectives of the present study are to identify the indicators which are most crucial for recognising the errors in the laboratory and to assess the role of formal training of medical, nursing and laboratory personnel regarding patient preparation, sample collection and transport, quality assurance and reporting of results in addressing the errors occurring during TTP, thereby improving the performance of the laboratory over the period of 12 months, i.e., from January to December, 2010.
Materials and Methods
Study Design
This study was conducted in diagnostic laboratories in Institute of Human Behavior and Allied Sciences (IHBAS), Delhi, India, a tertiary care neuropsychiatric super speciality hospital during 1 year period from January 2010 to December, 2010 with final data submission by 7th January, 2011. Its diagnostic laboratories have defined policies and procedures regarding sample preparation (includes patient preparation, phlebotomy techniques, sample handling and transport), equipment procurement and maintenance, testing performed, their validation and quality assurance program followed. In addition to this, laboratory staff undergoes regular training pertaining to these policies and procedures, their implementation and documentation. To ensure the continuity of quality of care and continuous quality improvement, the laboratory prospectively reviews its own performance in all the processes during pre-analytic, analytic and post-analytic phases of total testing process through monitoring of indicators developed by the laboratory.
All the blood specimens received for routine clinical chemistry, haematology and serology were included in the present study. Samples other than blood, i.e., histopathology and microbiology (other than serology) samples were excluded from the study. All the tests were analysed using fully automated auto analysers-XL-300 from Transasia Pvt. Ltd. and Ion Selective Electrolyte Analyser from CL Micromed (For clinical chemistry) and SYSMEX (For haematology) from Transasia Pvt. Ltd.
Laboratory Characteristics
Clinical chemistry, haematology and serology laboratories participated in this study over the period of 12 months from January to December, 2010. During this period, 42,562 samples were received in these laboratories. Out of total samples, 40% were from outpatient clinics and 60% from inpatient wards.
Quality Indicators Measured
List of indicators was developed to help in improving the quality and reliability of test results, health providers and patient safety. The performance indicators selected were those for which errors were frequent and improvements was possible, monitoring the critical step of total testing process and measurement of which on long term basis was possible. In the present study, indicators measured the performance of each discipline of laboratory undertaken, namely, clinical chemistry, haematology and serology (Tables 1, 2). However, some performance indicators, such as specimen identification and rejection, test turn around time (TATs), urgent and critical value reporting and outliers in proficiency testing were applied to all the sections of clinical laboratory. This list also fulfils the requirement of clinical laboratory improvement amendments (CLIA) which states that a laboratory’s quality improvement program must monitor all the steps of the total testing process.
Table 1.
Critical quality indicators
| Indicator | Lab discipline | Phase of testing | Frequency of data collection |
|---|---|---|---|
| Lab personnel awareness | All | All Three phases | Yearly |
| Wrong identification | All | Pre-analytical | Monthly |
| Incomplete forms | All | Pre-analytical | Monthly |
| Sample rejection rate | All | Pre-analytical | Monthly |
| No. of accidents reported | All | All three phases | Monthly |
| Random errors | Biochemistry, Haematology | Analytical | Monthly |
| Systemic errors | Biochemistry, Haematology | Analytical | Monthly |
| Non-conformity with QC | Biochemistry, Haematology | Analytical | Monthly |
| Number of repeat testing (to confirm the results) | Biochemistry, Haematology | Analytical | Monthly |
| Urgent sample reporting | All | Post Analytical | Monthly |
| Critical value reporting | All | Post Analytical | Monthly |
| Turnaround time (TATs) | Biochemistry, Haematology | All three phases | Monthly |
Adapted from [9]
Table 2.
Measurement of quality indicators
| Indicator | Numerator | Denominator | Measurement |
|---|---|---|---|
| Lab personnel awareness ratea | Sum of all lab personnel score | Sum of all maximal score | Mean lab personnel score |
| Wrong identification | No. of patients incorrectly identified | No. of patients’ sample received | % Improperly identified |
| Incomplete formsb | No. of incomplete forms received | Total No. of forms received | % Incomplete forms |
| Sample rejection ratec | No. of specimens rejected | Total no. of specimens received in the lab. | % Specimens rejected |
| No. of accidents reportedd | No. of accidentsreported3 | – | Total no. of accidents occurred |
| Random errors | No. of random errors reported | Total no. of specimens received in the lab. | % Random errors occurred |
| Systemic errors | No. of systemic errors reported | Total no. of specimens received in the lab. | % Systemic errors occurred |
| Non-conformity with QC (%) | No. of outliers observed | – | Total no. of failures |
| Number of repeat testinge | No. of tests repeated | Total no. of tests performed | % Repeat tests |
| Urgent sample reportingf | No. of times report not informed to caregiver stat | Total no. of samples received | % Caregivers not reached |
| Critical value reportingg | No. of times caregivers not reached | Total no. of tests performed | % Caregivers not reached |
| Turnaround time (TATs) | No. of times test reported beyond TATs | – | Total no. of outliers |
aLab personnel awareness in all the phases of total testing process was assessed
bIncomplete forms included forms not signed, writing not clear & inappropriate form received in the lab
cHaemolysed/clotted samples, sample quantity not sufficient & samples received in inappropriate vial were rejected
dAccidents reported in the lab & included in the study were needle stick injury, gloves not worn during handling the samples, spillage of samples in working area & waste disposal in wrong bag
eAll the tests repeated to confirm the results were included in this indicator
fSamples marked by the clinician as urgent were included in this parameter
gTest results found critical for patient care as per the critical values defined by the lab
Upon receiving the samples in the laboratories, quality indicators are documented in the lab after careful screening of the sample and accompanying test requisition form (TRF) by the laboratory technician to monitor pre-analytical phase. The prefixed criteria checked for this phase were completeness of TRFs (name, age, sex, registration no., OPD/ward/emergency, requesting physician’s name and signature, clinical/diagnostic information, date and time of sample collection), quality of sample (haemolysed/clotted/lipemia/quantity not sufficient/inappropriate vials) and sample identification (ID/registration no. verification between sample and TRF). The performance during analytical phase is monitored in terms of repeat testing and proficiency testing performance, whereas performance indicators monitored in post-analytical phase are critical and urgent sample reporting and (TATs) for reporting of results in clinical chemistry, haematology and serology. Repeat testing was done in two situations to confirm the test value by the lab personnel and on request of treating physician.
As part of continuous medical education, the laboratory technical, medical and nursing staff of the clinical departments are periodically oriented and sensitised to the quality assurance program and all the activities of laboratory services including patient preparation, filling of TRF, sample collection and reporting of results by group discussions and practical demonstrations in our laboratories. In the present study the total period of quality indicators monitoring was divided into two phases, i.e., Phase I in which QI were monitored before sensitisation of the medical, nursing and laboratory staff which extended from January to June, 2010 and Phase II in which QI were monitored after sensitisation and was from July to December, 2010. The trend was observed for all the QI before and after sensitisation of the staff over the period of 12 months and attempt had been made to find out the parameters which showed significant improvement after intervention.
Statistical Analysis
Paired Student-t and Z-proportion two sample tests applied as per requirement of data for all the parameters of quality indicators.
Results
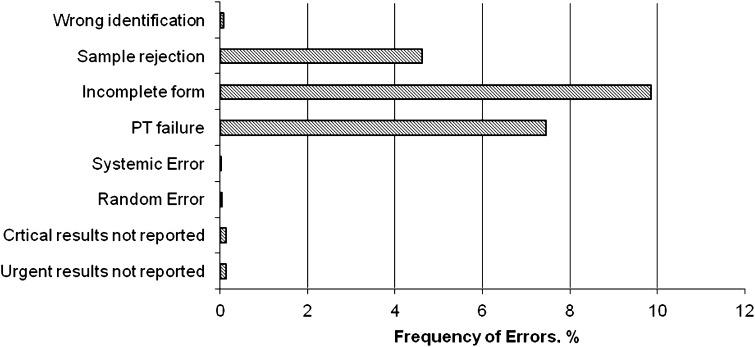
During 12 months period from January 2010 to December 2010, 42,562 samples were received for clinical chemistry, haematology and serology testing. Table 3 shows the performance indicator monitored during pre-analytical, analytical and post-analytical phase over 1 year period. During pre-analytical phase, incomplete TRF received in the lab was the most poor quality indicator observed (7.89%) which was followed by sample rejection rate (4.91%) (Fig. 1). The most common cause of sample rejection was inadequate quantity of sample received (2.75%) followed by haemolysis (0.74%). Other quality indicators assessed were wrong identification (0.05%), number of accidents reported in the lab (3 episodes) and lab personnel awareness (Tables 3, 4). During assessment of lab personnel’s awareness, it was observed the awareness of staff was below satisfactory (20.80 ± 2.29) and they needed encouragement with regards to implementation of the lab’s policies and procedures more stringently. Quality indicators covering the analytical phase were random error, systemic error, non conformity to quality control (proficiency testing) and repeat testing. During 1 year period, non conformity to QC was observed 14 times whereas there were 18 and 8 instances of random and systemic errors respectively. These errors were detected on regular monitoring of Levy Jenings chart for each test parameter. Most commonly random errors were occurring due reagent contamination, pipetting error and calibration failures. Systemic errors were mostly observed due to problems arising from tubing and probes or reduced sensitivity of filters/lamp of auto analysers. Repeat testing of sample to reconfirm the results was done on regular basis, both in case of doubt at laboratory level but also on request of physicians and was taken as a positive indicator for monitoring the reliability of reports. During our study, it was noted that approximately 22% tests were retested over 1 year period. Retesting was performed on the same samples. All the results of retesting were under predefined coefficient of variable (CV %) of the lab. As shown in Table 3, in only 0.29% of cases, reports marked urgent and 0.11% critical values could not be communicated to the concerned physician. In our lab we do not have LIS system and only manual reporting is done. However, the lab has defined the TATs for all clinical chemistry, haematology and serology which is 4–6 h. In our study we observed 11 instances when the test could not be dispatched in stipulated time excluding the stat and critical samples reporting.
Table 3.
Prevalence of quality indicators during 1 year period (2010)
| S. No | Parameters | (n = 42562) Frequency (%) |
|---|---|---|
| Pre-analytical phase | ||
| 1. | Wrong identification | 23 (0.05) |
| 2. | Incomplete forms | 3360 (7.89) |
| 3. | Sample rejection | 2056 (4.91) |
| Haemolysed/clotted sample | 316 (0.74) | |
| Inappropriate vial | 10 (0.023) | |
| Insufficient quantity | 1162 (2.75) | |
| 4. | No. of accidents reported | |
| Analytical phase | ||
| 5. | Random errors | 18 (0.04) |
| 6. | Systemic errors | 08 (0.02) |
| 7. | PT failure (non-conformity with QC) | 14 |
| 8. | Number of repeat testing | 9753 (22.91) |
| Post-analytical phase | ||
| 9. | Urgent sample reporting | 125 (0.29) |
| 10. | Critical value reporting | 46 (0.11) |
| 11. | Turnaround time (TATs) | 11 (0.025) |
Fig. 1.
The frequency of errors for critical steps of total testing process
Table 4.
Lab personnel awareness rate
| Frequency | Maximal score (n = 30) | Total score (by the lab personnel) | Mean ± SD |
|---|---|---|---|
| January (before initiating the study) | 750 | 624 | 20.80 ± 2.29 |
| July (in between the study) | 750 | 685 | 22.83 ± 2.00* |
*P < 0.001
During first 6 months of present study, in process of vigorous monitoring of quality indicators, lab personnel were encouraged to improve their phlebotomy technique along with more stringent checking of TRFs, quality of samples received, running of internal and external quality control materials and reporting of urgent and critical samples. In addition to this, lab personnel, medical and nursing staff were given more formal training on the phlebotomy techniques and patient preparation. Table 5 shows the effect of such intervention on quality indicators. Z proportion independent test was applied and it was found that the frequency (proportion) of change after intervention for all the Quality Indicators was statistically highly significant (P < 0.001). There was marked improvement in quality indicators of pre and post analytical phase especially wrong identification which dropped from 0.11 to 0.01%, inappropriate TRFs received in the lab decreased from 13.32 to just 2.7%. Also, there was substantial improvement in urgent sample reporting from 0.54 to 0.06%. There was almost 100% critical value reporting with just one incidence of non reporting in last 6 months. Also, the staff showed significant improvement in their awareness regarding TTP. However, there was not much improvement noted for indicators like non conformity with QC, random and systemic errors and TATs.
Table 5.
Effect of intervention over the prevalence of quality indicators
| S. No | Parameters | Pre-intervention (January–June, 2010) | Post-intervention (July–December, 2010) |
|---|---|---|---|
| Frequency (%) (n = 20,810) | Frequency (%) (n = 21,752) | ||
| Pre-analytical phase | |||
| 1. | Wrong identification | 21 (0.11) | 2 (0.01) |
| 2. | Incomplete forms | 2772 (13.32) | 588 (2.7) |
| 3. | Sample rejection rate | 1174 (5.64) | 882 (4.05) |
| 4. | No. of accidents reported | 03 | 00 |
| Analytical phase | |||
| 5. | Random errors | 10 (0.05) | 08 (0.04) |
| 6. | Systemic errors | 04 (0.02) | 03 (0.02) |
| 7. | PT failure (non-conformity with QC) | 08 | 06 |
| 8. | Number of repeat testing | 5564 (26.73) | 4189 (19.25) |
| Post-analytical phase | |||
| 9. | Urgent sample reporting | 113 (0.54) | 12 (0.06) |
| 10. | Critical value reporting | 45 (0.21) | 01 (0.004) |
| 11. | Turnaround time (TATs) | 04 (0.02) | 07 (0.03) |
Discussion
A single error in any step during TTP from sample collection, transport, analysis of sample to the reporting of test results invalidates the quality. To ensure up to date performance and service, the process of identification and correction of error risk should be integrated in the quality system of the laboratory. The implementation of quality indicators in the laboratory is essential not only to detect the error but also to formulate quality improvement strategies. The efficiency of the use of quality indicators is demonstrated by the improvement found in performance.
In the present study we tried to monitor number of indicators pertaining to the activities covering all the phases of TTP to find out the areas most prone to error in the laboratory thereby reducing the patient safety. Also, attempts have been made to take measures in the lab in terms of sensitisation of lab personnel, medical and nursing staff and assess its effect on prevalence of quality indicators. To achieve this goal we assessed the frequency of rejection due to haemolysis/clotting of sample, inappropriateness of vial or insufficient quantity of sample received. Over the period of 12 months from January to December, 2010, 4.91% samples were rejected due to these causes, which was much higher than the rejection rate reported by Dale et al. [11] (0.3%), Stark et al. [12] (0.74%) and Chawla et al. [13] (1.54%). In present study insufficient quantity of sample was the most common cause of sample rejection as 2.3% samples were rejected due to it as compared to 0.74% due to haemolysis, whereas Ricos et al. [14] and Chawla et al. [13] reported haemolysis as main cause of sample rejection. It may be due to the fact that in our set up most of the patients are chronic or staying for longer duration and they also have poor nutritional status. Hence repeated sampling leads to collapse of veins which makes it difficult to take appropriate amount of sample. To avoid this, we held sensitisation session on phlebotomy techniques. This led to reduction in sample rejection due to QNS from 5.64 to 4.05%. We also encourage the nursing staff to avoid repeat sampling for additional tests during patients’ stay by storing the samples for 48 h at 2–40 C for those parameters which are stable during this period. In additional to this use of evacuated tubes for sample collection has been initiated. This led to further reduction in sample rejection due to QNS. The sensitisation of staff regarding patient preparation, filling of TRF and appropriate labelling of samples has led to significant improvement in pre-analytical errors from 19.07 to 6.76%. Though there was a remarkable decrease in errors in pre-analytical phase, sample rejection did not show significant improvement, indicating that we need to further improve the skills of our staff by holding practical sessions. Till now focus was on improvement in lab personnel’s knowledge and awareness which did not meet much success in improvement of quality of sample collected. Hence, practical training has been planned for all the steps of sample collection to improve the same.
Post-analytical phase was another area where substantial improvement was noted. In this phase, critical value reporting, urgent sample reporting and TATs were monitored. On scrutiny of urgent sample and critical value sample reporting, poor awareness among nursing and lab staff has been observed. As reluctant lab staff was to communicate these reports, the nursing staff was also not aware of their role in this regard. It was further complicated by difficulty in reaching treating physicians telephonically. Critical value reporting is an important aspect of post-analytical phase of the clinical laboratory testing process. It is defined as values that represent situations that could be life threatening without treatment. Ineffectiveness of critical values notification or the failure to provide notification within the target time might prove to be life threatening in certain cases [15].The literature reports frequency of critical value reporting from 1 in 2,000 [16] to 14 per 1,000 [13], where as we reported 0.21% which improved to 0.004% after intervention. Hence, the sensitisation of staff improved the reporting of such cases by almost 100% suggesting that such regular attempts play an active role in improving the reporting system in the lab. There were only 4 episodes of withholding of reports in first 6 months (January–June, 2010) and 7 episodes after intervention. Most of these episodes of delayed reporting were due to defect occurring in the instrument.
In analytical phase 14 instances of non conformity to QC were observed, which could be due to instability or inappropriate storage of reconstituted QC material in the lab, contamination or instability of reagents or calibration drift. Chawla et al. [13] has reported 0.1/1,000 of non conformity to QC whereas Jesus et al. [17] have reported a cut off of 0.8% for external control exceeding the target range. In our study, we reported 5.07% which was much higher as compared to the literature. To improve random and systemic errors our lab has developed the check list to ensure that the maintenance of all these variables pertaining to reagents/kits, pipettes and instruments are performed and checked regularly and more stringently. Sensitisation of staff for the same was done as part of intervention. The staff was given a questionnaire in the initial part of the study followed by reassessment using the same questionnaire after their formal training. They showed highly significant improvement not only in their knowledge as their mean score improved from 20.80 ± 2.29 to 22.83 ± 2.00 (P = 0.001), but also in their readiness to implement the policies and procedures laid down in the laboratory to ensure patient safety and good laboratory practices.
In the present study, most significant improvement after sensitisation of medical, nursing and laboratory staff was observed in pre-analytical and post-analytical phase but did not have much impact on analytical phase. This signifies that improvement during analytical phase can only be brought about by more frequent and stringent hands on practical training of laboratory staff along with the continuous monitoring as well as assessment of their performance. Another important finding was that regular monitoring of quality indicators acted as an informal awareness program for the lab staff which not only improved their awareness but also their performance. This led to improved work culture of the labs. Hence, such systemic approach in continuous quality improvement introduced in the total testing process can contribute to the patient safety.
Conclusion
Traditionally, patient safety initiatives in the laboratory have focussed on the ‘person approach’ which believes that human operator is responsible for error through carelessness, fatigue/overload or inattention. It involves error review which is based on the assumption that if personnel follow policies and procedures, errors should not occur. However, now this approach is getting replaced by the ‘systems approach’ which implies that errors arise due to faulty systems rather than careless or inattentive staff. One such systemic approach, use of quality indicators to assess and monitor the quality system of the clinical laboratory services is extremely valuable tool in keeping the total testing process under control in a systematic and transparent way leading to improvement of work place culture.
References
- 1.Kohn IT, Corrigan JM, Donaldson MS, editors. To err is human: building a safer health system. Washington: National Academy Press; 1999. [PubMed] [Google Scholar]
- 2.Brennan TA, Gawande A, Thomas E, Studdert D. Accidental deaths, saved lives and improved quality. N Engl J Med. 2005;353:1405–1409. doi: 10.1056/NEJMsb051157. [DOI] [PubMed] [Google Scholar]
- 3.Lippi G. Governance of preanalytical variability: travelling the right path to the bright side of the moon? Clin Chim Acta. 2009;404:32–36. doi: 10.1016/j.cca.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 4.American clinical laboratory association (ACLA). The value of clinical laboratory services. Web accessed February 4, 2009 at http://www.Clinical-labs.org/issues/value/index.shtml.
- 5.O’Kane M. The reporting, classification and grading of quality failures in the medical laboratory. Clin Chim Acta. 2009;404:28–31. doi: 10.1016/j.cca.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 6.McCay l, Lemer C, Wu AW. Laboratory safety and the WHO world alliance for patient safety. Clin Chim Acta. 2009;404:6–11. doi: 10.1016/j.cca.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Phlebani M. Quality specifications: self pleasure for clinical laboratories or added value for patient management? Clin Chem Lab Med. 2007;45:462–466. doi: 10.1515/CCLM.2007.094. [DOI] [PubMed] [Google Scholar]
- 8.Sciacovelli L, Plebani M. The IFCC working group on laboratory errors and patient safety. Clin Chim Acta. 2009;404:79–85. doi: 10.1016/j.cca.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 9.Howanitz PJ. Errors in laboratory medicine: practical lessons to improve patient safety. Arch Path Lab Med. 2005;129:1252–1261. doi: 10.5858/2005-129-1252-EILMPL. [DOI] [PubMed] [Google Scholar]
- 10.Phlebani M. Exploring the iceberg of errors in laboratory medicine. Clin Chim Acta. 2009;404:16–23. doi: 10.1016/j.cca.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Dale JC, Novis DA. Outpatient phlebotomy success and reasons for specimen rejection: a Q-probes study. Arch Pathol Lab Med. 2002;126:416–419. doi: 10.5858/2002-126-0416-OPSARF. [DOI] [PubMed] [Google Scholar]
- 12.Stark S, Jones BA, Chapman D, Well K, Krajenta R, Meier FA, Zarbo RJ. Clinical laboratory specimen rejection: association with the site of patient care and patients’ characteristics. Arch Pathol Lab Med. 1997;121:19–26. doi: 10.5858/2007-131-588-CLSRWT. [DOI] [PubMed] [Google Scholar]
- 13.Chawla R, Goswami B, Singh B, Chawla A, Gupta VK, Mallika V. Evaluating laboratory performance with quality indicators. Labmedicine. 2000;41(5):297–300. [Google Scholar]
- 14.Ricos C, Garcia-Victoria M, la Fuente B. Quality indicators and specifications for the extra analytical phases in clinical laboratory management. Clin Chem Lab Med. 2004;42:578–582. doi: 10.1515/CCLM.2004.100. [DOI] [PubMed] [Google Scholar]
- 15.Dighe AS, Jones JB, Parham S, Lewandrowski KBS. Survey of critical value reporting and reduction of false-positive critical value results. Arch Pathol Lab Med. 2008;132:1666–1671. doi: 10.5858/2008-132-1666-SOCVRA. [DOI] [PubMed] [Google Scholar]
- 16.Howanitz PJ, Steindel SJ, Heard NV. Laboratory critical values policies and procedures: a college of American pathologists’ Q-probe study in 623 institutions. Arch Pathol Lab Med. 2002;126:663–669. doi: 10.5858/2002-126-0663-LCVPAP. [DOI] [PubMed] [Google Scholar]
- 17.Kirchner MJ, Funes VA, Adzet CB, Clar MV, Escuer MI, Girona JM, et al. Quality indicators and specifications for key processes in clinical laboratories: a preliminary experience. Clin Chem Lab Med. 2007;45:672–677. doi: 10.1515/CCLM.2007.122. [DOI] [PubMed] [Google Scholar]