Abstract
Purpose of review
Intracranial atherosclerotic disease (IAD) is likely the most common cause of stroke world-wide, and is associated with a very high risk of recurrence. It results in cerebral ischemia due to a variety of mechanisms, including artery-to-artery embolism, hemodynamic failure and occlusion of penetrating arteries. New imaging modalities focused on physiological consequences of IAD have become available and recent treatment trials have been completed.
Recent findings
We review the traditional imaging modalities, emphasizing the advantages and limitations of each method, and discuss novel physiological approaches that interrogate physiological process to indicate specific mechanisms of ischemia. These allow deeper understanding of the pathophysiological processes that underlie IAD-related ischemia. The key findings of recent therapeutic trails are reviewed, including the landmark randomized studies showing advantage of antiplatelet agents and risk factor modification, and a significant risk of complications with endovascular approaches.
Summary
Current evidence argues for aggressive medical management and suggests caution with interventional treatments. We propose that mechanistic information will further refine the risk assessment of patients with IAD to offer targeted therapy.
Keywords: stroke, intracranial atherosclerosis, stroke etiology, stroke therapy, intracranial stenting
Introduction
Intracranial atherosclerotic disease (IAD) is a common cause of stroke; its incidence and prevalence vary by ethnicity. IAD is more common in Asians, Hispanics, and those of African descent, compared to Caucasians. It causes approximately 10% of all strokes in the US. [1,2] From Asian studies, IAD accounts for 33–50% of all strokes in China, 47% in Thailand, 48% in Singapore, and 10–25% in Korea.[3] Given these numbers and the shift in world populations, IAD may well be the most common subtype of ischemic stroke worldwide.[3,4] Furthermore, population based studies of stroke-free adults with vascular risk factors have shown a prevalence of asymptomatic IAD of 8.6%.[5] This review focuses on key findings and recent advances both in diagnosis, with an emphasis on novel approaches to defining the underlying mechanisms of stroke in IAD, and in treatment, including medical and endovascular approaches.
Imaging of Intracranial Stenosis
Diagnosing IAD and defining the mechanism of cerebral ischemia is important, as it will lead to targeted and aggressive treatment to lower recurrent stroke risk. While anatomic diagnosis of arterial narrowing is made with reasonable accuracy, ascribing an etiology for the stenosis remains challenging as radiographic mimics of IAD such as partial embolus recanalization, dissection, vasculitis, and vasospasm are often encountered and may require repeat or multi-modal imaging to distinguish between them. Furthermore, determining whether the stenosis is symptomatic or asymptomatic may not be straightforward, as cardioembolism, extracranial large artery disease and small artery occlusion can co-exist with IAD; up to 19% of recurrent strokes in IAD may be due to another mechanism.[6]
Diagnosis of intracranial stenosis
Catheter-based digital subtraction angiography (DSA), the reference standard of neurovascular imaging, provides excellent visualization of vessel contour, anatomic localization, and assessment of the degree and length of stenosis, and of collateral circulation. Three-dimensional reconstruction provides even greater detail. The risk of peri-procedural neurologic injury, associated access site injury, and dye-related complications limit its use.[7,8]
Transcranial Doppler (TCD) correlates blood flow patterns and velocity changes with arterial diameter. Since direct anatomic visualization is unavailable, its performance depends greatly on technical expertise. Color-coded duplex can overcome this limitation but needs more study.[9] Given its performance characteristics in IAD excellent sensitivity, specificity, and negative predictive (NPV) but only modest positive predictive (36–75%) value (PPV),[10–12] TCD is useful to exclude significant IAD with high certainty but requires confirmatory testing when stenosis is suggested.
Magnetic resonance angiography (MRA) measures flow signal intensity on time-of-flight (TOF) MRA to visualize changes in the flow of blood. Motion artifact and flow directional changes from vertical to horizontal can render ambiguous or erroneous results.[13,14] Its inability to distinguish between high-grade stenosis and occlusion is another major disadvantage.[14] In addition, it has limited to no use in morbidly obese and claustrophobic patients and those with implanted metallic objects. Overall, studies suggest MRA has the similar performance characteristics to TCD in diagnosing IAD: high sensitivity, specificity, and NPV, but modest PPV (59–66%).[10,15] Given their modest PPV, TOF-MRA requires confirmatory diagnostic testing. Contrast-enhanced MRA provides better anatomic visualization, particularly in regions of changing blood flow directions; however, visualization of smaller arteries remains limited.[16,17] Quantitative MRA (QMRA), utilizing phase-contrast techniques, has shown promise to diagnosis hemodynamic failure in posterior circulation stenosis[18] and screen for stenosis of intracranial stents.[19,20] High-resolution MRI (HR-MRI) is capable of characterizing plaque morphology, and discriminating from other non-atherosclerotic etiologies.[21,22] Further study using these novel modalities may increase the diagnostic utility of MRA and provide valuable prognostic data.
Computed tomography angiography (CTA) provides better anatomic visualization compared to MRA,[15,23] and is superior in assessing high-grade stenosis. However, it can be degraded by local calcium or metallic artifacts. Compared to DSA, the high sensitivity (81–98%), specificity (97–99%), PPV (79–93%), and NPV (98–100%) of CTA make this an excellent, and perhaps preferred, non-invasive diagnostic test for intracranial stenosis.[9,15,24] Its disadvantages, besides radiation exposure, are contrast reactions and nephrotoxicity.
The choice of non-invasive imaging modality is guided by availability, cost, advantages and disadvantages (table 1) but other factors should be considered: CTA can better visualize high-grade stenoses than MRA since the latter tends to overestimate the degree of stenosis, while MRA is better at evaluating the petrous and cavernous carotid as bony artifacts affect CTA. The concordance of any two non-invasive tests results obviates the need for confirmatory DSA.
Table 1.
Comparison of commonly used diagnostic tests for intracranial stenosis
| Test | Advantages | Disadvantages |
|---|---|---|
| DSA | High resolution (microns) Measure stenosis precisely Measure antegrade and collateral flow Gold standard |
Invasive Radiation risks Contrast risks Availability Costs |
| TCD | Non-invasive Inexpensive Serial examination High specificity and NPV Emboli detection Vasomotor reactivity testing |
Low PPV Reliability Technical limits (i.e. absent windows) |
| MRA | Non-invasive High specificity and NPV Modest cost No radiation Widely available |
Motion artifact Magnet contraindications Overestimates stenosis Low PPV |
| CTA | High sensitivity, specificity, NPV Good PPV Modest cost Widely available |
Radiation risk Contrast risk and contraindications Calcium and metal artifacts |
Stroke mechanisms and imaging markers of stroke risk
The mechanisms of ischemia in IAD include impaired antegrade flow, artery-to-artery embolism, penetrator vessel occlusion, and impaired collateral flow and cerebrovascular reserve. It is likely that these mechanisms interact and co-exist in the same patient.[25–28] Non-invasive imaging can provide physiologic data on these mechanisms, which may be useful in calibrating stroke risk, developing mechanism-specific prevention and treatment strategies, and assisting in patient selection for endovascular therapies.
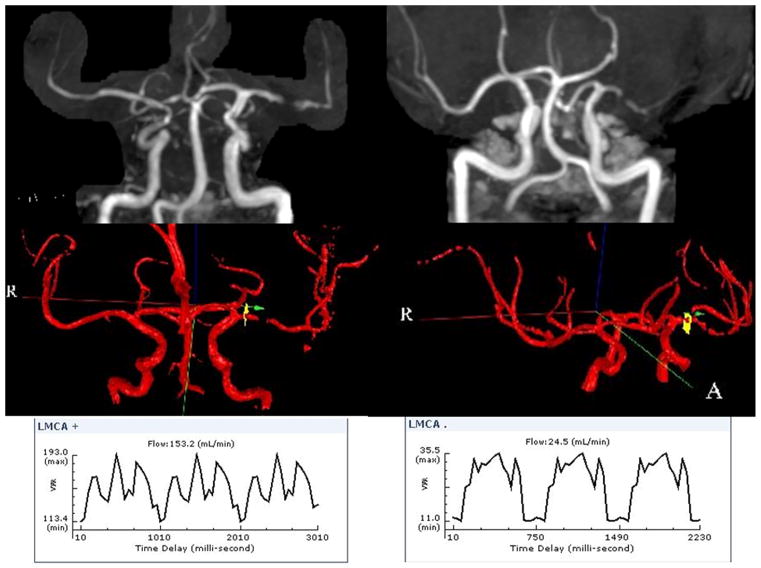
Anterograde blood flow at and distal to the site of stenosis can be quantified by QMRA; it exploits the phase shift in the signal of flowing blood, which is proportional to flow velocity, to quantify flow rate in medium and large vessels.[29] QMRA can quantify the physiologic significance of the anatomic degree of stenosis in IAD. For instance, at similar degrees of stenosis, patients may have different flow rates representing differential risk, as exemplified in the figure. In a study of 47 patients symptomatic vertebral or basilar 50–100% disease (72% with intracranial disease), low flow on QMRA was noted in one-third of patients; at 24 months, 97.5% of those with normal QMRA flow were event-free, compared to 81.5% with low flow.[18]
Figure. QMRA in two patients with similar stenosis by MRA but different volumetric flow rate.
On the right, the top panel shows a left middle cerebral artery flow gap. The QMRA, shown in the right middle and right lower panels, shows the area interrogated and a low volumetric flow rate of 24.5 mL/min. The left upper panel depicts a similar left middle cerebral artery stenosis, but the volumetric flow rate shown in the left lower panels is not decreased at 153.2 mL/min.
Serial monitoring of flow velocities by TCD detects progression of IAD. Progression was noted in upto one-third of patients with symptomatic middle cerebral artery (MCA) stenosis at 26-month follow-up and was associated with a near 3-fold increased risk of recurrence.[30] Others have noted progression of stenosis in 9–13% of patients at shorter time intervals even with aggressive medical therapy.[28,31] TCD monitoring can also detect embolic signals (ES) in approximately 33% of patients with symptomatic MCA stenosis in the acute phase.[32–34] MRI studies have confirmed that ES correlate with multiple strokes indicative of an embolic process, [35] and their presence is predictive of early recurrent ischemic events.[32]
Impaired cerebral hemodynamics is a well-established predictor in large artery stroke.[26,36,37] Cerebral perfusion pressure distal to a high-grade stenosis or occlusion depends on collateral sources of blood flow. The anterior and posterior communicating arteries provide most collateral flow in internal carotid and basilar artery stenosis (primary collaterals), while distal pial and leptomeningeal anastomoses (secondary collaterals) are important in MCA stenosis [26]. Collateral flow on DSA was found to be absent in 69% of patients with symptomatic IAD and an independent predictor of recurrent ipsilateral stroke.[38,39]
Autoregulatory vasodilation in response to a vasodilatory challenge, such as hypercapnia or acetazolamide, defines vasomotor reactivity (VMR), a measure of dynamic cerebrovascular reserve capacity.[36] Impaired VMR has been used to identify those patients who are at a high-risk for stroke in both intra- and extracranial large vessel disease.[37,40] TCD with CO2 challenge correlates with degree of stenosis in IAD.[41] Using acetazolamide Xenon-SPECT, impaired VMR has been reported in 14–33%, with annual risk of ipsilateral stroke 17.4–21.8% versus 0.6–2.4% in those with impaired and normal VMR.[42,43].
Perfusion imaging of the brain using MRI, CT, and positron emission tomography (PET) measures tissue-level perfusion distal to a stenosis; it may reflect the true net effect of antegrade and collateral flow. Although extensively studied in acute ischemic stroke,[44,45] little data exist in chronic IAD.[26] Ongoing studies may provide further evidence of the prognostic value of perfusion defects in IAD.
A high-risk profile of physiologic markers emerges by combining these modalities (table 2). Therefore, markers of antegrade and collateral flow, static tissue perfusion, dynamic cerebrovascular reserve, and plaque features of embolic potential and morphology, may refine risk stratification adding to clinical and anatomic (i.e. percent stenosis) predictors of stroke risk.
Table 2.
Mechanisms and surrogate imaging markers of stroke risk in intracranial stenosis
| Mechanism | Marker (s) | Imaging modality |
|---|---|---|
| Decreased antegrade flow | VFR | QMRA |
| TICI flow grade | DSA | |
| Flow velocity | TCD | |
|
| ||
| Progression of stenosis | Changes in: | |
| VFR | QMRA | |
| TICI flow grade | DSA | |
| Flow velocity | TCD | |
|
| ||
| Poor collateral flow | Collateral flow grade | DSA |
| COW collaterals | DSA, TCD, QMRA | |
|
| ||
| Impaired vasomotor reactivity | Cerebrovascular reactivity | |
| Breath-holding index | TCD | |
| CO2 inhalation | TCD | |
| Acetazolamide | SPECT | |
|
| ||
| Poor tissue perfusion | CBF, CBV, transit time | MRP or CTP |
| Metabolic rate, oxygen extraction | PET | |
|
| ||
| Artery-to-artery embolism | Microembolic signals | TCD |
|
| ||
| Vulnerable plaque | Plaque enhancement/hemorrhage | HR-MRI |
Abbreviations: VFR-volumetric flow rate; TICI-thrombolysis in cerebral infarction; CO2-carbon dioxide; CBF-cerebral blood flow; CBV-cerebral blood volume; DSA-digital subtraction angiography; TCD-transcranial Doppler; MRA-magnetic resonance angiography; QMRA-quantitative magnetic resonance angiography; SPECT-spectral emission computerized tomography; PET-positron emission tomography; MRP-magnetic resonance perfusion; CTP-computerized tomography perfusion; HR-MRI-high-resolution magnetic resonance imaging
Management
After stroke or TIA, the 1-year risk of ischemic stroke, intracerebral hemorrhage, or vascular death is 12–15%, while the risk of recurrent stroke in the same territory is 11–12%.[46,47] The most important predictors of stroke recurrence are the degree of stenosis and time from event.[48] Symptomatic IAD with 70–99% stenosis has greater risk compared to those with 50–69% stenosis (hazard ratio [HR] 2.08). The first 2–3 weeks are also particularly perilous (HR 1.67 for symptoms ≤ 17 days). Other factors are less clearly associated with increased risk, although women may have a marginally increased risk. The type of event, stroke vs. TIA, is not an independent predictor, but when stratified with degree of stenosis, can be used to stratify risk; for example, those with a stroke and high-grade 70–99% stenosis have an eightfold risk of stroke compared with those with a TIA and moderate stenosis. In sum, these clinical data may identify a high-risk population eligible for more aggressive treatment interventions.
Antithrombotic therapy
Warfarin is not more efficacious and is associated with greater risk than aspirin in IAD. The seminal Warfarin-Aspirin Symptomatic Intracranial Disease trial (WASID)[47] compared aspirin 1200 mg/day vs. warfarin (target INR 2–3) in symptomatic IAD with 50–99% stenosis. There was no difference between the treatment arms; ischemic stroke, intracerebral hemorrhage, or vascular death occurred at a staggering 22% during 1.8 years of follow-up (annual rate of 16%). Warfarin was associated with a two-fold increase in death and almost a three-fold increase in major hemorrhages. There was no benefit of warfarin for those who had their initial event on antiplatelet therapy.[49] As most events occur early after the initial event, a brief course of anticoagulation was considered to decrease risk while avoiding the long-term risks of warfarin.[50] However, nadroparin for 10 days followed by aspirin was not superior to aspirin alone when started even within 2 days of symptoms.[51]
The combination of aspirin and clopidogrel was tested in the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial.[46] Patients with a non-disabling stroke or TIA due to IAD with 70–99% stenosis within 30 days of symptoms, a high-risk population,[48] were treated with the combination of aspirin 325 mg/day and clopidogrel 75 mg/day for 90 days followed by aspirin 325 mg/day and aggressive treatment of other vascular risk factors, and randomized to angioplasty and stenting or medical therapy alone. As detailed below, the study was stopped due to excess complications in the angioplasty and stenting arm. Patients with medical management alone had a 30-day rate of stroke or death of 5.8%, lower than the anticipated 10.7% rate from the WASID sub-analysis of similar patients. The 1-year risk of stroke or death at 30 days or recurrent ischemic stroke in the territory of the stenotic vessel beyond 30 days was estimated at 12.2%. These data also imply that the combination of aspirin and clopidogrel for a short period of time, combined with statins and antihypertensive agents, is effective and probably superior to aspirin alone and standard risk factor modification.
Other antiplatelet drug combinations have also been evaluated. A randomized study comparing cilostazol and clopidogrel vs. cilostazol and aspirin in patients ischemic stroke within 2 weeks of symptoms did not find a difference between the treatment arms in progression of stenosis (primary outcome) or recurrent stroke, new ischemic lesions on MRI and hemorrhagic complications (secondary outcomes).[31] Combination antiplatelet therapy was associated with a 6.1% risk of stroke, major hemorrhage and vascular death during a follow-up period of 7 months, similar to the observed rates in SAMMPRIS.
Risk factor modification
Patients with IAD have a high burden of vascular risk factors and their control is associated with a lower rate of recurrent events. In the WASID trial, a recurrent stroke was more likely in those with poor control of risk factors during follow up; systolic blood pressure (SBP) ≥140 mm Hg (HR 1.58), total cholesterol >200 mg/dL (HR 1.95), and no alcohol consumption (HR 1.76) were significant risk factors.[52] Initial concern that blood pressure reduction would cause hypoperfusion was dispelled in further analysis of the WASID study: a recurrent stroke in the territory of the stenotic artery occurred at similar rates for SBP categories of <120, 120–139, and 140–159 mm Hg, and a higher risk was noted for those with SBP ≥160 mm Hg during follow-up,[53] although this analysis did not involve the acute post-stroke period. The SAMMPRIS study managed vascular risk factors in an intense manner with goal SBP <140 mm Hg (<130 mm Hg in diabetics) and LDL < 70 mg/dL, protocol-driven control of other risk factors and institution of a lifestyle modification program. After 4 months, important reductions in SBP (11 mm Hg), LDL (20–25 mg/dL), and tobacco use (7–10%) and an increase in moderate or vigorous exercise (22–27%) were achieved, demonstrating the feasibility of significantly altering vascular risk factors with intense medical treatment. These interventions likely stabilize the atherosclerotic plaque and prevent further IAD progression.
Revascularization strategies
Extracranial to intracranial (EC/IC) bypass surgery has not been successful; in the EC/IC bypass surgery, patients with severe middle cerebral artery stenosis had worse outcomes with surgery.[54] Endovascular approaches with angioplasty alone, angioplasty and stenting with balloon mounted stents (BMS) and self-expanding stents (SES) have been tested in IAD. Intracranial endovascular treatment is challenging as the intracranial vessels are accessed through a tortuous path, they are not embedded within tissue but free floating in CSF, have a thinner media, and have small penetrators arising at right angles. These characteristics underlie the complications encountered in intracranial procedures including peri-procedural stroke, vessel rupture, hyperperfusion syndrome, stent thrombosis and restenosis.
Angioplasty alone has been evaluated in a number of case series reporting wide ranges of periprocedural complications. Larger series where angioplasty was performed electively documented relatively low complication rates,[55] but lesion recoil, restenosis and dissection are potential complications.[56] Indirect comparison of angioplasty and stenting case series suggest that angioplasty alone may be inferior to stenting,[57] but this intervention has not been evaluated in a randomized manner with blinded adjudication.
Balloon mounted stents offer the advantage of a one-step procedure and less residual stenosis, but the rigid delivery system makes it more difficult to navigate through tortuous anatomy. That BMS can be deployed was indicated by the SSYLVIA study,[58] a non-randomized study of vertebral stenosis treated with a BMS, but a 6.6% peri-procedural stroke rate plus a 7.3% subsequent risk of stroke was similar to the risk found with medical management in the WASID study.[47] A number of subsequent single center series employing a variety of stents analyzed in systematic reviews show a peri-procedural risk of stroke of 7.7%,[59] similar to large single center series,[60,61] although variability in peri-procedural complications and unblinded outcome ascertainments may bias results. Available data suggest that when the index event is related to occlusion of a perforating artery, the risk of peri-procedural stroke is higher.[62] There is little data on drug eluting stents (DES) in IAD, but DES do not overcome the challenges posed by other BMS, and may increase the risk of thrombogenicity.[63] Furthermore, DES did not show an advantage on restenosis in a small case series.[64]
Self-expanding stents require a two-step procedure (subtotal angioplasty stent deployment), but offer easier delivery through tortuous vessels, and were thought to have less peri-procedural complications. In the US, the Wingspan stent and Gateway balloon are approved by the FDA for IAD with >50% stenosis and recurrent events on medical therapy. Three registries using this system showed a 4.4–7.5% peri-procedural risk of stroke and death, and a 7.1–31.2% risk of restenosis.[65–69] However, the SAMMPRIS study, which employed the Wingspan/Gateway system, was stopped due to excess complications in the stented arm, with a 30-day risk of stroke or death of 14.7%, higher than the anticipated rate from the registries. The peri-procedural complications included ischemic strokes (70%) and intracranial hemorrhages (30%), many likely related to reperfusion. After 30 days, the risk of recurrent stroke was similar for both medical and stenting arm, and therefore a beneficial late effect of stenting is unlikely.[46] Long term follow-up in two Wingspan registries confirm that post-procedural beyond 30 days events continue to occur at a rate of 4–10%, with few events beyond one year, and associated with restenosis and antiplatelet cessation.[70,71]
There has been no head-to-head comparison of BMS and SES, but systematic reviews of case series have not shown significant differences in complications and equivocal benefit of BMS on restenosis rates.[59,60]
Conclusions
Intracranial atherosclerosis is associated with very high risk of recurrence, which can be modified by aggressive medical management (table 3). The role of stenting in the management of IAD is still unclear; the surprising high rate of complications in the SAMMPRIS study has given pause to recommending stenting for IAD. The current American Heart Association guidelines stating that endovascular approaches with angioplasty or stenting may be considered for symptomatic IAD >70% that failed medical therapy (class 2b recommendation, level of evidence C)[72] may need to be revised. Stenting, if used at all, should only be performed by experienced operators[73] in the setting of recurrent cerebral ischemia due to high-grade stenosis despite maximal medical therapy. It remains to be seen whether stenting will play a more prominent role in the future treatment of IAD as hardware, training, and experience improve. It is likely that advances in neuroimaging may refine risk stratification. There is evidence that poor collateral flow, impaired vasomotor reactivity, and presence of microembolic signals predict risk. Future studies integrating pathophysiological mechanisms into risk stratification may identify specific high-risk groups and time windows to offer targeted therapies that may modify the course of this condition.
Table 3.
Treatment recommendations for recently symptomatic IAD
|
Key Points.
IAD may well be the most common world-wide cause of ischemic stroke and is associated with various mechanisms of cerebral ischemia.
Current diagnostic methods assess the degree of stenosis but novel physiological markers provide mechanistic information that may refine risk stratification and lead to more targeted interventions.
Recent trials have shown that antiplatelet therapy and aggressive risk factor modification reduce the risk of stroke recurrence.
The role of endovascular treatment of IAD is still unclear given the high risk of complications.
Footnotes
Disclosures and Conflict of Interest:
Drs. Romano and Prabhakaran are co-principal investigators of the Mechanisms of Stroke in Intracranial Stenosis Study (MOSIS) (NINDS 1R01NS069938-01). They are also the site principal investigators of the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial (NINDS U01 NS058728-01A1) at their respective institutions.
Contributor Information
Shyam Prabhakaran, Email: Shyam_Prabhakaran@rush.edu, Department of Neurological Sciences, Head, Cerebrovascular Disease & Neurocritical Care, Rush University Medical Center, 1725 W. Harrison St. Suite 1121, Chicago, IL 60612, Tel: 312-563-2518 Fax: 312-563-2206.
Jose G. Romano, Email: jromano@med.miami.edu, Cerebrovascular Division, University of Miami, Miller School of Medicine, 1120 NW 14th St. Suite 1357, Miami FL 33136, Tel: 305-243-2336, Fax: 305-243-7081.
References
- 1.Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke. 1995;26:14–20. doi: 10.1161/01.str.26.1.14. [DOI] [PubMed] [Google Scholar]
- 2.Suri MF, Johnston SC. Epidemiology of intracranial stenosis. J Neuroimaging. 2009;19 (Suppl 1):11S–16S. doi: 10.1111/j.1552-6569.2009.00415.x. [DOI] [PubMed] [Google Scholar]
- 3.Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke. 2006;1:158–159. doi: 10.1111/j.1747-4949.2006.00045.x. [DOI] [PubMed] [Google Scholar]
- 4.Gorelick PB, Wong KS, Bae H-J, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. 2008;39:2396–2399. doi: 10.1161/STROKEAHA.107.505776. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Cancio E, Dorado L, Millan L, Alzamora M, Reverte S, Sunol A, Masuet A, Galan A, Davalos A, Arenilla J. Final Results Of The Barcelona-ASymptomatic Intracranial Atherosclerosis (ASIA) Study: Prevalence And Risk Factors. Stroke. 2011;42:e87–e88. doi: 10.1016/j.atherosclerosis.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Famakin BM, Chimowitz MI, Lynn MJ, Stern BJ, George MG. Causes and severity of ischemic stroke in patients with symptomatic intracranial arterial stenosis. Stroke. 2009;40:1999–2003. doi: 10.1161/STROKEAHA.108.546150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cloft HJ, Joseph GJ, Dion JE. Risk of cerebral angiography in patients with subarachnoid hemorrhage, cerebral aneurysm, and arteriovenous malformation: a meta-analysis. Stroke. 1999;30:317–320. doi: 10.1161/01.str.30.2.317. [DOI] [PubMed] [Google Scholar]
- 8.Cloft HJ, Lynn MJ, Feldmann E, Chimowitz M. Risk of cerebral angiography in patients with symptomatic intracranial atherosclerotic stenosis. Cerebrovasc Dis. 2011;31:588–591. doi: 10.1159/000324951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roubec M, Kuliha M, Jonszta T, Prochazka V, Fadrna T, Filip M, Kanovsky P, Langova K, Herzig R, Skoloudik D. Detection of intracranial arterial stenosis using transcranial color-coded duplex sonography, computed tomographic angiography, and digital subtraction angiography. J Ultrasound Med. 2011;30:1069–1075. doi: 10.7863/jum.2011.30.8.1069. [DOI] [PubMed] [Google Scholar]
- 10.Feldmann E, Wilterdink JL, Kosinski A, Lynn M, Chimowitz MI, Sarafin J, Smith HH, Nichols F, Rogg J, Cloft HJ, et al. The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) trial. Neurology. 2007;68:2099–2106. doi: 10.1212/01.wnl.0000261488.05906.c1. [DOI] [PubMed] [Google Scholar]
- 11.Martinelli O, Benedetti-Valentini F. Trancranial Doppler: value in clinical practice. Int Angiol. 2009;28:249–253. [PubMed] [Google Scholar]
- 12*.Zhao L, Barlinn K, Sharma VK, Tsivgoulis G, Cava LF, Vasdekis SN, Teoh HL, Triantafyllou N, Chan BP, Sharma A, et al. Velocity Criteria for Intracranial Stenosis Revisited: An International Multicenter Study of Transcranial Doppler and Digital Subtraction Angiography. Stroke. 2011;42:1–6. doi: 10.1161/STROKEAHA.111.621235. This study of 102 patients using standard protocol for TCD compared to DSA confirmed the accuracy of threshold velocities from SONIA used in diagnosis of IAD ≥ 50% stenosis and also developed novel criteria for diagnosis of ≥70% stenosis. [DOI] [PubMed] [Google Scholar]
- 13.Choi CG, Lee DH, Lee JH, Pyun HW, Kang DW, Kwon SU, Kim JK, Kim SJ, Suh DC. Detection of intracranial atherosclerotic steno-occlusive disease with 3D time-of-flight magnetic resonance angiography with sensitivity encoding at 3T. AJNR Am J Neuroradiol. 2007;28:439–446. [PMC free article] [PubMed] [Google Scholar]
- 14.Sadikin C, Teng MM, Chen TY, Luo CB, Chang FC, Lirng JF, Sun YC. The current role of 1.5T non-contrast 3D time-of-flight magnetic resonance angiography to detect intracranial steno-occlusive disease. J Formos Med Assoc. 2007;106:691–699. doi: 10.1016/S0929-6646(08)60030-3. [DOI] [PubMed] [Google Scholar]
- 15.Bash S, Villablanca JP, Jahan R, Duckwiler G, Tillis M, Kidwell C, Saver J, Sayre J. Intracranial vascular stenosis and occlusive disease: evaluation with CT angiography, MR angiography, and digital subtraction angiography. AJNR Am J Neuroradiol. 2005;26:1012–1021. [PMC free article] [PubMed] [Google Scholar]
- 16.Willinek WA, von Falkenhausen M, Born M, Gieseke J, Holler T, Klockgether T, Textor HJ, Schild HH, Urbach H. Noninvasive detection of steno-occlusive disease of the supra-aortic arteries with three-dimensional contrast-enhanced magnetic resonance angiography: a prospective, intra-individual comparative analysis with digital subtraction angiography. Stroke. 2005;36:38–43. doi: 10.1161/01.STR.0000149616.41312.00. [DOI] [PubMed] [Google Scholar]
- 17.Wright VL, Olan W, Dick B, Yu H, Alberts-Grill N, Latour LL, Baird AE. Assessment of CE-MRA for the rapid detection of supra-aortic vascular disease. Neurology. 2005;65:27–32. doi: 10.1212/01.wnl.0000167606.81882.68. [DOI] [PubMed] [Google Scholar]
- 18.Amin-Hanjani S, Du X, Zhao M, Walsh K, Malisch TW, Charbel FT. Use of quantitative magnetic resonance angiography to stratify stroke risk in symptomatic vertebrobasilar disease. Stroke. 2005;36:1140–1145. doi: 10.1161/01.STR.0000166195.63276.7c. [DOI] [PubMed] [Google Scholar]
- 19.Amin-Hanjani S, Alaraj A, Calderon-Arnulphi M, Aletich VA, Thulborn KR, Charbel FT. Detection of intracranial in-stent restenosis using quantitative magnetic resonance angiography. Stroke. 2011;41:2534–2538. doi: 10.1161/STROKEAHA.110.594739. [DOI] [PubMed] [Google Scholar]
- 20.Prabhakaran S, Warrior L, Wells KR, Jhaveri MD, Chen M, Lopes DK. The utility of quantitative magnetic resonance angiography in the assessment of intracranial in-stent stenosis. Stroke. 2009;40:991–993. doi: 10.1161/STROKEAHA.108.522391. [DOI] [PubMed] [Google Scholar]
- 21.Degnan AJ, Gallagher G, Teng Z, Lu J, Liu Q, Gillard JH. MR Angiography and Imaging for the Evaluation of Middle Cerebral Artery Atherosclerotic Disease. AJNR Am J Neuroradiol. 2011 Sep 22; doi: 10.3174/ajnr.A2697. (e-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turan TN, Bonilha L, Morgan PS, Adams RJ, Chimowitz MI. Intraplaque hemorrhage in symptomatic intracranial atherosclerotic disease. J Neuroimaging. 2011;21:159–161. doi: 10.1111/j.1552-6569.2009.00442.x. [DOI] [PubMed] [Google Scholar]
- 23.Katz DA, Marks MP, Napel SA, Bracci PM, Roberts SL. Circle of Willis: evaluation with spiral CT angiography, MR angiography, and conventional angiography. Radiology. 1995;195:445–449. doi: 10.1148/radiology.195.2.7724764. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen-Huynh MN, Wintermark M, English J, Lam J, Vittinghoff E, Smith WS, Johnston SC. How accurate is CT angiography in evaluating intracranial atherosclerotic disease? Stroke. 2008;39:1184–1188. doi: 10.1161/STROKEAHA.107.502906. [DOI] [PubMed] [Google Scholar]
- 25.Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. 1998;55:1475–1482. doi: 10.1001/archneur.55.11.1475. [DOI] [PubMed] [Google Scholar]
- 26.Derdeyn CP, Powers WJ, Grubb RL. Hemodynamic effects of middle cerebral artery stenosis and occlusion. AJNR Am J Neuroradiol. 1998;19:1463–1469. [PMC free article] [PubMed] [Google Scholar]
- 27.Wong KS, Gao S, Chan YL, Hansberg T, Lam WWM, Droste DW, Kay R, Ringelstein EB. Mechanisms of acute cerebral infarctions in patients with middle cerebral artery stenosis: a diffusion-weighted imaging and microemboli monitoring study. Ann Neurol. 2002;52:74–81. doi: 10.1002/ana.10250. [DOI] [PubMed] [Google Scholar]
- 28.Wong KS, Li H, Lam WWM, Chan YL, Kay R. Progression of middle cerebral artery occlusive disease and its relationship with further vascular events after stroke. Stroke. 2002;33:532–536. doi: 10.1161/hs0202.102602. [DOI] [PubMed] [Google Scholar]
- 29.Zhao M, Charbel FT, Alperin N, Loth F, Clark ME. Improved phase-contrast flow quantification by three-dimensional vessel localization. Magn Reson Imaging. 2000;18:697–706. doi: 10.1016/s0730-725x(00)00157-0. [DOI] [PubMed] [Google Scholar]
- 30.Arenillas JF, Molina CA, Montaner J, Abilleira S, Gonzalez-Sanchez MA, Alvarez-Sabin J. Progression and clinical recurrence of symptomatic middle cerebral artery stenosis: a long-term follow-up transcranial Doppler ultrasound study. Stroke. 2001;32:2898–2904. doi: 10.1161/hs1201.099652. [DOI] [PubMed] [Google Scholar]
- 31*.Kwon SU, Hong KS, Kang DW, Park JM, Lee JH, Cho YJ, Yu KH, Koo JS, Wong KS, Lee SH, et al. Efficacy and safety of combination antiplatelet therapies in patients with symptomatic intracranial atherosclerotic stenosis. Stroke. 2011;42:2883–2890. doi: 10.1161/STROKEAHA.110.609370. This study comparing two combinations of antiplatelet agents in IAD further supports the SAMMPRIS findings that a short course of combination antiplatelet therapy is safe. [DOI] [PubMed] [Google Scholar]
- 32.Gao S, Wong KS, Hansberg T, Lam WWM, Droste DW, Ringelstein EB. Microembolic signal predicts recurrent cerebral ischemic events in acute stroke patients with middle cerebral artery stenosis. Stroke. 2004;35:2832–2836. doi: 10.1161/01.STR.0000147035.31297.b6. [DOI] [PubMed] [Google Scholar]
- 33.Markus HS, Droste DW, Brown MM. Detection of asymptomatic cerebral embolic signals with Doppler ultrasound. Lancet. 1994;343:1011–1012. doi: 10.1016/s0140-6736(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 34.Segura T, Serena J, Castellanos M, Teruel J, Vilar C, Davalos A. Embolism in acute middle cerebral artery stenosis. Neurology. 2001;56:497–501. doi: 10.1212/wnl.56.4.497. [DOI] [PubMed] [Google Scholar]
- 35.Wong KS, Gao S, Chan YL, Hansberg T, Lam WW, Droste DW, Kay R, Ringelstein EB. Mechanisms of acute cerebral infarctions in patients with middle cerebral artery stenosis: a diffusion-weighted imaging and microemboli monitoring study. Ann Neurol. 2002;52:74–81. doi: 10.1002/ana.10250. [DOI] [PubMed] [Google Scholar]
- 36.Derdeyn CP, Grubb RL, Powers WJ. Cerebral hemodynamic impairment: methods of measurement and association with stroke risk. Neurology. 1999;53:251–259. doi: 10.1212/wnl.53.2.251. [DOI] [PubMed] [Google Scholar]
- 37.Silvestrini M, Vernieri F, Pasqualetti P, Matteis M, Passarelli F, Troisi E, Caltagirone C. Impaired cerebral vasoreactivity and risk of stroke in patients with asymptomatic carotid artery stenosis. JAMA. 2000;283:2122–2127. doi: 10.1001/jama.283.16.2122. [DOI] [PubMed] [Google Scholar]
- 38.Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Cloft HJ, Chimowitz MI. Collateral circulation in symptomatic intracranial atherosclerosis. J Cereb Blood Flow Metab. 2011;31:1293–1301. doi: 10.1038/jcbfm.2010.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39**.Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Turan TN, Cloft HJ, Chimowitz MI. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol. 2011;69:963–974. doi: 10.1002/ana.22354. In the WASID cohort, this study implicates the prominent role of collateral flow in predicting ipsilateral recurrent stroke, independent of standard clinical and anatomic predictors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uzunca I, Asil T, Balci K, Utku U. Evaluation of vasomotor reactivity by transcranial Doppler sonography in patients with acute stroke who have symptomatic intracranial and extracranial stenosis. J Ultrasound Med. 2007;26:179–185. doi: 10.7863/jum.2007.26.2.179. [DOI] [PubMed] [Google Scholar]
- 41.Lee JY, Lee YS. Vasomotor reactivity in middle cerebral artery stenosis. J Neurol Sci. 2011;301:35–37. doi: 10.1016/j.jns.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 42.Ogasawara K, Ogawa A, Yoshimoto T. Cerebrovascular reactivity to acetazolamide and outcome in patients with symptomatic internal carotid or middle cerebral artery occlusion: a xenon-133 single-photon emission computed tomography study. Stroke. 2002;33:1857–1862. doi: 10.1161/01.str.0000019511.81583.a8. [DOI] [PubMed] [Google Scholar]
- 43.Kuroda S, Houkin K, Kamiyama H, Mitsumori K, Iwasaki Y, Abe H. Long-term prognosis of medically treated patients with internal carotid or middle cerebral artery occlusion: can acetazolamide test predict it? Stroke. 2001;32:2110–2116. doi: 10.1161/hs0901.095692. [DOI] [PubMed] [Google Scholar]
- 44.Kidwell C, Alger JR, Saver J. Beyond mismatch: evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke. 2003;34:2729–2735. doi: 10.1161/01.STR.0000097608.38779.CC. [DOI] [PubMed] [Google Scholar]
- 45.Konstas AA, Wintermark M, Lev MH. CT perfusion imaging in acute stroke. Neuroimaging Clin N Am. 2011;21:215–238. ix. doi: 10.1016/j.nic.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 46**.Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, Janis LS, Lutsep HL, Barnwell SL, Waters MF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993–991003. doi: 10.1056/NEJMoa1105335. This randomized controlled study of medical treatment vs. stenting showed that aggressive medical treatment is effective at reducing early stroke recurrence, and that stenting with self –expanding stents is associated with a high risk of complications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, Levine SR, Chaturvedi S, Kasner SE, Benesch CG, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–1316. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 48.Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, Levine SR, Chaturvedi S, Benesch CG, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006;113:555–563. doi: 10.1161/CIRCULATIONAHA.105.578229. [DOI] [PubMed] [Google Scholar]
- 49.Turan TN, Maidan L, Cotsonis G, Lynn MJ, Romano JG, Levine SR, Chimowitz MI. Failure of antithrombotic therapy and risk of stroke in patients with symptomatic intracranial stenosis. Stroke. 2009;40:505–509. doi: 10.1161/STROKEAHA.108.528281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koroshetz WJ. Warfarin, aspirin, and intracranial vascular disease. N Engl J Med. 2005;352:1368–1370. doi: 10.1056/NEJMe058022. [DOI] [PubMed] [Google Scholar]
- 51.Wong KS, Chen C, Ng PW, Tsoi TH, Li HL, Fong WC, Yeung J, Wong CK, Yip KK, Gao H, et al. Low-molecular-weight heparin compared with aspirin for the treatment of acute ischaemic stroke in Asian patients with large artery occlusive disease: a randomised study. Lancet Neurol. 2007;6:407–413. doi: 10.1016/S1474-4422(07)70079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chaturvedi S, Turan TN, Lynn MJ, Kasner SE, Romano J, Cotsonis G, Frankel M, Chimowitz MI. Risk factor status and vascular events in patients with symptomatic intracranial stenosis. Neurology. 2007;69:2063–2068. doi: 10.1212/01.wnl.0000279338.18776.26. [DOI] [PubMed] [Google Scholar]
- 53.Turan TN, Cotsonis G, Lynn MJ, Chaturvedi S, Chimowitz M. Relationship between blood pressure and stroke recurrence in patients with intracranial arterial stenosis. Circulation. 2007;115:2969–2975. doi: 10.1161/CIRCULATIONAHA.106.622464. [DOI] [PubMed] [Google Scholar]
- 54.Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. The EC/IC Bypass Study Group. N Engl J Med. 1985;313:1191–1200. doi: 10.1056/NEJM198511073131904. [DOI] [PubMed] [Google Scholar]
- 55.Marks MP, Wojak JC, Al-Ali F, Jayaraman M, Marcellus ML, Connors JJ, Do HM. Angioplasty for symptomatic intracranial stenosis: clinical outcome. Stroke. 2006;37:1016–1020. doi: 10.1161/01.STR.0000206142.03677.c2. [DOI] [PubMed] [Google Scholar]
- 56.Derdeyn CP, Chimowitz MI. Angioplasty and stenting for atherosclerotic intracranial stenosis: rationale for a randomized clinical trial. Neuroimaging Clin N Am. 2007;17:355–363. doi: 10.1016/j.nic.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siddiq F, Memon MZ, Vazquez G, Safdar A, Qureshi AI. Comparison between primary angioplasty and stent placement for symptomatic intracranial atherosclerotic disease: meta-analysis of case series. Neurosurgery. 2009;65:1024–1033. doi: 10.1227/01.NEU.0000360138.54474.52. [DOI] [PubMed] [Google Scholar]
- 58.SSYLVIA SI. Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA): study results. Stroke. 2004;35:1388–1392. doi: 10.1161/01.STR.0000128708.86762.d6. [DOI] [PubMed] [Google Scholar]
- 59.Groschel K, Schnaudigel S, Pilgram SM, Wasser K, Kastrup A. A systematic review on outcome after stenting for intracranial atherosclerosis. Stroke. 2009;40:340–347. doi: 10.1161/STROKEAHA.108.532713. [DOI] [PubMed] [Google Scholar]
- 60.Jiang W-J, Cheng-Ching E, Abou-Chebl A, Zaidat OO, Jovin TG, Kalia J, Hussain MS, Lin R, Malik AM, Hui F, et al. Multi-Center Analysis of Stenting in Symptomatic Intracranial Atherosclerosis. Neurosurgery. 2011 doi: 10.1227/NEU.0b013e31822d274d. [DOI] [PubMed] [Google Scholar]
- 61.Suh DC, Kim JK, Choi JW, Choi BS, Pyun HW, Choi YJ, Kim MH, Yang HR, Ha HI, Kim SJ, et al. Intracranial stenting of severe symptomatic intracranial stenosis: results of 100 consecutive patients. AJNR Am J Neuroradiol. 2008;29:781–785. doi: 10.3174/ajnr.A0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang WJ, Srivastava T, Gao F, Du B, Dong KH, Xu XT. Perforator stroke after elective stenting of symptomatic intracranial stenosis. Neurology. 2006;66:1868–1872. doi: 10.1212/01.wnl.0000219744.06992.bb. [DOI] [PubMed] [Google Scholar]
- 63.Fiorella D, Woo HH. Emerging endovascular therapies for symptomatic intracranial atherosclerotic disease. Stroke. 2007;38:2391–2396. doi: 10.1161/STROKEAHA.107.482752. [DOI] [PubMed] [Google Scholar]
- 64.Fields JD, Petersen BD, Lutsep HL, Nesbit GM, Liu KC, Dogan A, Lee DS, Clark WM, Barnwell SL. Drug eluting stents for symptomatic intracranial and vertebral artery stenosis. Interv Neuroradiol. 2011;17:241–247. doi: 10.1177/159101991101700217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bose A, Hartmann M, Henkes H, Liu HM, Teng MMH, Szikora I, Berlis A, Reul J, Yu SCH, Forsting M, et al. A novel, self-expanding, nitinol stent in medically refractory intracranial atherosclerotic stenoses: the Wingspan study. Stroke. 2007;38:1531–1537. doi: 10.1161/STROKEAHA.106.477711. [DOI] [PubMed] [Google Scholar]
- 66.Fiorella D, Levy EI, Turk AS, Albuquerque FC, Niemann DB, Aagaard-Kienitz B, Hanel RA, Woo H, Rasmussen PA, Hopkins LN, et al. US multicenter experience with the wingspan stent system for the treatment of intracranial atheromatous disease: periprocedural results. Stroke. 2007;38:881–887. doi: 10.1161/01.STR.0000257963.65728.e8. [DOI] [PubMed] [Google Scholar]
- 67.Fiorella DJ, Turk AS, Levy EI, Pride GL, Woo HH, Albuquerque FC, Welch BG, Niemann DB, Aagaard-Kienitz B, Rasmussen PA, et al. U.S. Wingspan Registry: 12-month follow-up results. Stroke. 2011;42:1976–1981. doi: 10.1161/STROKEAHA.111.613877. [DOI] [PubMed] [Google Scholar]
- 68.Levy EI, Turk AS, Albuquerque FC, Niemann DB, Aagaard-Kienitz B, Pride L, Purdy P, Welch B, Woo H, Rasmussen PA, et al. Wingspan in-stent restenosis and thrombosis: incidence, clinical presentation, and management. Neurosurgery. 2007;61:644–650. doi: 10.1227/01.NEU.0000290914.24976.83. [DOI] [PubMed] [Google Scholar]
- 69.Zaidat OO, Klucznik R, Alexander MJ, Chaloupka J, Lutsep H, Barnwell S, Mawad M, Lane B, Lynn MJ, Chimowitz M. The NIH registry on use of the Wingspan stent for symptomatic 70–99% intracranial arterial stenosis. Neurology. 2008;70:1518–1524. doi: 10.1212/01.wnl.0000306308.08229.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiang WJ, Yu W, Du B, Gao F, Cui LY. Outcome of patients with >70% symptomatic intracranial stenosis after wingspan stenting. Stroke. 2011;42:1971–1975. doi: 10.1161/STROKEAHA.110.595926. [DOI] [PubMed] [Google Scholar]
- 71.Fiorella DJ, Turk AS, Levy EI, Pride GL, Jr, Woo HH, Albuquerque FC, Welch BG, Niemann DB, Aagaard-Kienitz B, Rasmussen PA, Hopkins LN, Masaryk TJ, McDougall CG. U.S. Wingspan Registry: 12-month follow-up results. Stroke. 2011;42:1976–1981. doi: 10.1161/STROKEAHA.111.613877. [DOI] [PubMed] [Google Scholar]
- 72.Meyers PM, Schumacher HC, Higashida RT, Barnwell SL, Creager MA, Gupta R, McDougall CG, Pandey DK, Sacks D, Wechsler LR. Indications for the performance of intracranial endovascular neurointerventional procedures: a scientific statement from the American Heart Association Council on Cardiovascular Radiology and Intervention, Stroke Council, Council on Cardiovascular Surgery and Anesthesia, Interdisciplinary Council on Peripheral Vascular Disease, and Interdisciplinary Council on Quality of Care and Outcomes Research. Circulation. 2009;119:2235–2249. doi: 10.1161/CIRCULATIONAHA.109.192217. [DOI] [PubMed] [Google Scholar]
- 73.Nahab F, Lynn MJ, Kasner SE, Alexander MJ, Klucznik R, Zaidat OO, Chaloupka J, Lutsep H, Barnwell S, Mawad M, et al. Risk factors associated with major cerebrovascular complications after intracranial stenting. Neurology. 2009;72:2014–2019. doi: 10.1212/01.wnl.0b013e3181a1863c. [DOI] [PMC free article] [PubMed] [Google Scholar]