Abstract
Background
Hospice programs improve the quality of life and quality of death for men dying of cancer. We sought to characterize hospice use by men dying of prostate cancer and to compare the use of high-intensity care between those who did or did not enroll in hospice.
Methods
We used linked Surveillance, Epidemiology, and End Results–Medicare data to identify a cohort of Medicare beneficiaries who died of prostate cancer between 1992 and 2005. We created 2 multivariable logistic regression models, one to identify factors associated with hospice use and one to determine the association of hospice use with the receipt of diagnostic and interventional procedures and physician visits at the end of life.
Results
Of 14 521 men dying of prostate cancer, 7646 (53%) used hospice for a median of 24 days. Multivariable modeling demonstrated that African American ethnicity (odds ratio [OR], 0.78; 95% confidence interval [CI], 0.68–0.88) and higher Charlson comorbidity index (OR, 0.49; 95% CI, 0.44–0.55) were associated with lower odds of hospice use, while having a partner (OR, 1.23; 95% CI, 1.14–1.32) and more recent year of death (OR, 1.12; 95% CI, 1.11–1.14) were associated with higher odds of hospice use. Men dying of prostate cancer who enrolled in hospice were less likely (OR, 0.82; 95% CI, 0.74–0.91) to receive high-intensity care, including intensive care unit admissions, inpatient stays, and multiple emergency department visits.
Conclusions
The proportion of individuals using hospice is increasing, but the timing of hospice referral remains poor. Those who enroll in hospice are less likely to receive high-intensity end-of-life care.
The hospice model of end-of-life care seeks to control pain, promote patient and family-centered care and autonomy, and address spiritual concerns as death approaches. Hospice programs improve both the quality of life and the quality of death for men dying of cancer.1–4 Since hospice care was added as a Medicare entitlement in 1983, the proportion of dying Americans who use hospice services has increased steadily.5–13 Known predictors of hospice use among individuals with cancer include type of insurance, partnership status, education level, income level, ethnicity, sex, age, and geographic location.5,7,8,10–12 The trajectory of prostate cancer differs significantly from other malignant conditions, however, with a disease arc that is typically longer and more variable. While this presents ample opportunities to offer hospice services to those with prostate cancer, it also adds the challenge of determining when the end of life is approaching, and predictors of hospice use among those with advanced prostate cancer are poorly understood.
Although more than one-third of Americans who die use hospice care, many choose aggressive, often futile treatments at the end of life.14,15 Approximately 30% of lifetime Medicare resources are expended in the last year of life, but hospice care may reduce these costs, especially for individuals dying of cancer.13,16–20 When patients do seek hospice care, their median hospice stay lasts only 26 days, in large measure because of late referrals by health care providers.6
Of the more than 28 000 American men who die of prostate cancer each year, only one-third enroll in hospice.5,7–10,21,22 We sought to characterize not only hospice use by men dying of prostate cancer but also differences in the care these men receive compared with men not enrolled in hospice. The primary purpose of our analysis was to identify patient characteristics associated with hospice use among men dying of prostate cancer. Our secondary aim was to compare the use of diagnostic and interventional procedures and physician visits between those who did or did not enroll in hospice. While hospice entry is generally associated with lower use of services, the types of studies and services that are and are not used when an individual enrolls in hospice are incompletely understood. Delineating use of medical care at the end of life, with and without hospice enrollment, can be important in developing policies to deliver high-quality, affordable care at the end of life. We hypothesized a priori that white ethnicity, higher household income, and more formal education would be associated with greater hospice use.10–12,22 We also posited that hospice use would be correlated with fewer intensive care unit (ICU) and inpatient admissions and with fewer interventional procedures in the last 6 months of life, especially those procedures involving placement of decompressive ureteral stents or nephrostomy tubes.
METHODS
DATA SOURCE
We used linked data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program and the Centers for Medicare and Medicaid Services (CMS) to identify our cohort. SEER is a population-based cancer registry representative of the US population that includes information regarding tumor characteristics, incidence, treatment, and mortality. More than 95% of Medicare-eligible individuals identified in SEER are successfully linked to Medicare claims, which contain comprehensive medical and surgical data.
STUDY POPULATION
We identified 14 521 men 66 years or older who died of prostate cancer between 1992 and 2005. We excluded subjects not enrolled in Parts A and B of Medicare for at least 12 months after prostate cancer diagnosis and those cases diagnosed at autopsy. We then searched inpatient claims in the Medicare Provider Analysis and Review file, based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes, and physician claims in the Carrier Claims file, based on American Medical Association Current Procedural Terminology (CPT) and ICD-9-CM codes for prostate cancer diagnosis and procedure codes (eTables 1–3; http://www.archinternmed.com). We categorized hospice enrollment by any unique claim in the SEER hospice file. While prostate cancer was the cause of death for all individuals included in our study, the admitting diagnosis for hospice enrollment was not ascertained.
COVARIATES
We ascertained demographic and clinical information, including age at diagnosis, ethnicity, marital status, and tumor stage, using SEER variables. Subject income level represents the median income of his US census tract; education level represents that census tract’s percentage of non–high school graduates. We measured pre-existing comorbidity with the Charlson index23 from inpatient and physician claims. The Charlson comorbidity index assigns a range of comorbid conditions a score of 1 to 6 based on the risk of mortality from each condition, with the summed score predicting mortality. We identified primary treatment type for men receiving external beam radiation, radiation implants, or prostatectomy by searching the corresponding CPT codes (eTables 1–3). We identified cause of death from death certificates and included participants with prostate cancer as the immediate cause of death. We defined time from diagnosis to death as the interval from the SEER date of diagnosis to the SEER date of death.
DIAGNOSTIC AND TREATMENT PROCEDURES
We ascertained diagnostic and treatment procedures performed in the last 6 months of life by searching Medicare claims ICD-9-CM and Healthcare Common Procedure Coding System (HCPCS) codes for nephrostomy tube placement and cystoscopic or urethroscopic manipulations (eTables 1–3). Because this yielded few ureteral interventions, we reviewed and categorized all diagnostic and treatment procedures performed during the last 6 months of life (eTables 1–3). We focused on the most common procedures performed: laboratory studies, imaging, palliative radiation, physical therapy, prostate-specific antigen test, electrocardiography, Foley catheter placement, ureteral stent or nephrostomy, cystoscopy, cardiopulmonary resuscitation, chemotherapy, intravenous medication injection, and transportation. Radiation at the end of life for men dying of prostate cancer is palliative rather than curative, while the aim of chemotherapy is to extend life. Cystoscopy, Foley catheter placement, and ureteral stent or nephrostomy tube placement can be either palliative (if the individual is symptomatic) or not (if, for instance, the goal is to reverse azotemia).
PHYSICIAN VISITS
We identified all emergency department visits, outpatient office visits, inpatient hospital stays, and ICU admissions using the Medicare Provider Analysis and Review file, the National Claims History records, and the hospital variables derived from Medicare’s Healthcare Cost Report and Provider of Service survey.
STATISTICAL ANALYSIS
In our bivariate models, we used χ2 tests to evaluate associations between patient-level covariates and hospice use. We categorized timing of hospice referral as late if the subject was enrolled for fewer than 7 days before dying, and as early if he was enrolled for more than 180 days before dying.5,24 We compared utilization of health care services, stratified by hospice use, with independent samples t tests or analysis of variances.
We then created 2 multivariable logistic regression models. Examination of claims of men enrolled more than 180 days prior to death demonstrated marked heterogeneity in hospice use; these subjects were likely to join hospice, disenroll, and reenroll. They represented a heterogeneous cohort and were excluded from our models. The first model determined the independent association of subjects’ sociodemographic and clinical characteristics with hospice use. We controlled for age, ethnicity, comorbidity, treatment type, education, income, and partnership status based on bivariate significance. We created a separate multivariable model to determine the association of hospice use and receipt of high-intensity care in the last 6 months of life, controlling for age, ethnicity, comorbidity, treatment type, education, income, and partnership status. We defined high-intensity care as an inpatient hospital stay, ICU admission, more than 1 emergency department visit, cystoscopy, placement of a ureteral stent or nephrostomy tube, cardiopulmonary resuscitation, or administration of chemotherapy. For chemotherapy and palliative radiation, we considered courses of treatment.
Statistical testing was 2-sided and performed with SAS 9.1 software (SAS Institute Inc, Cary, North Carolina). We considered findings to be statistically significant if P < .05.
RESULTS
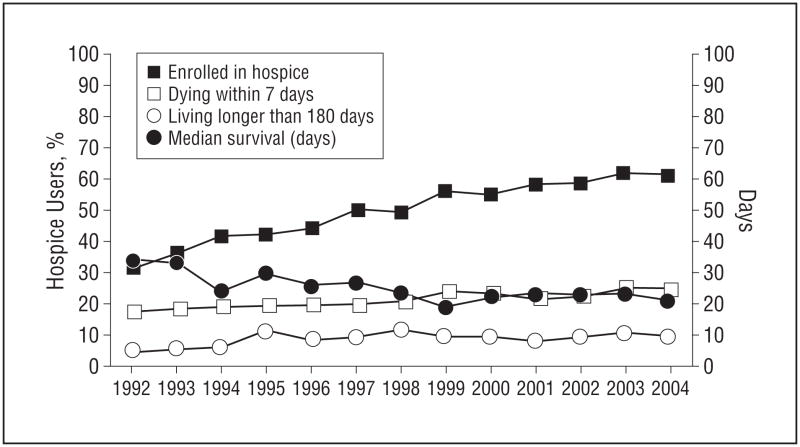
Of 14 521 men dying of prostate cancer, 7646 (53%) used hospice. Enrollment within 7 days of death was noted in 1699 (22% of hospice users), and enrollment more than 180 days prior to death was seen in 717 (9% of hospice users). Subjects who used hospice for 7 to 180 days were enrolled for a mean (SD) of 47 (41) days (median, 24 days) prior to death. The Figure displays yearly trends in hospice enrollment and length of hospice stay prior to death. While the proportion of subjects using hospice increased over time, so did the proportion of those who died within 7 days of hospice enrollment.
Figure.
Hospice enrollment and median hospice stays by year. “Days” in right vertical axis represent median survival.
Table 1 gives the bivariate associations between sociodemographic and clinical characteristics and hospice use. We included in our analysis those who used hospice for 7 to 180 days prior to death, which we had defined as appropriate hospice use. Sixteen percent of the men in our cohort were younger than 70 years, the majority were white, and most had localized or locoregional disease at diagnosis. The P value represents differences in hospice use by sociodemographic factors; age, ethnicity, partnership status, Charlson comorbidity index, stage, primary prostate cancer treatment, household income, education level, and time from diagnosis to death were associated with hospice use.
Table 1.
Characteristics of the Study Sample Stratified by Hospice Use
| Characteristic | Men, No. (%)
|
P Value | |||
|---|---|---|---|---|---|
| Total | Hospice | No Hospice | |||
| Age, y | |||||
| 65–69 | 2355 (16) | 911 (39) | 1071 (45) |
|
<.001 |
| 70–74 | 3785 (26) | 1422 (38) | 1723 (46) | ||
| 75–79 | 3787 (26) | 1395 (37) | 1728 (46) | ||
| 80–84 | 2756 (19) | 933 (34) | 1381 (50) | ||
| 85–89 | 1372 (9) | 435 (32) | 721 (53) | ||
| ≥90 | 466 (3) | 134 (29) | 251 (54) | ||
| Ethnicity | |||||
| White | 11 479 (79) | 4307 (38) | 5216 (45) |
|
<.001 |
| African American | 1787 (12) | 555 (31) | 958 (54) | ||
| Hispanic | 637 (4) | 214 (34) | 319 (50) | ||
| Other | 618 (4) | 154 (25) | 382 (62) | ||
| Partnership status | |||||
| Single | 1037 (7) | 316 (30) | 581 (56) |
|
<.001 |
| Partnered | 9559 (65) | 3603 (38) | 4239 (45) | ||
| Widowed/divorced | 2852 (20) | 882 (31) | 1511 (53) | ||
| Unknown | 1183 (8) | 210 (18) | 544 (46) | ||
| Charlson comorbidity index | |||||
| 0 | 6237 (43) | 2573 (41) | 2524 (40) |
|
<.001 |
| 1–2 | 5994 (41) | 2048 (34) | 3039 (51) | ||
| ≥3 | 2290 (16) | 609 (27) | 1312 (57) | ||
| Stage | |||||
| Localized/locoregional | 8223 (58) | 3016 (37) | 3761 (46) |
|
<.001 |
| Distant | 3782 (27) | 1403 (37) | 1780 (47) | ||
| Unstaged | 2183 (15) | 723 (33) | 1126 (52) | ||
| Primary prostate cancer treatment | |||||
| Radical prostatectomy | 905 (6) | 383 (7) | 376 (5) |
|
<.001 |
| Radiation therapy | 5587 (38) | 2163 (41) | 2490 (36) | ||
| Primary androgen deprivation | 8029 (55) | 2684 (51) | 4009 (58) | ||
| Median census tract household income | |||||
| <$38 500 | 4922 (35) | 1711 (35) | 2454 (50) |
|
<.001 |
| $38 500–$48 499 | 3409 (25) | 1261 (37) | 1583 (46) | ||
| $48 500–$62 000 | 3061 (22) | 1096 (36) | 1394 (46) | ||
| >$62 000 | 2487 (18) | 921 (37) | 1152 (46) | ||
| % Of census tract with <high school education | |||||
| <20 | 9208 (66) | 3508 (38) | 4113 (45) |
|
<.001 |
| 20–40 | 3907 (28) | 1263 (32) | 2022 (52) | ||
| >40 | 764 (6) | 218 (29) | 448 (59) | ||
| Time from diagnosis to death, mo | |||||
| 12–24 | 3691 (25) | 1229 (33) | 1947 (53) |
|
<.001 |
| 25–36 | 2565 (18) | 898 (35) | 1274 (50) | ||
| 37–48 | 1985 (14) | 689 (35) | 963 (49) | ||
| 49–60 | 1510 (10) | 559 (37) | 698 (46) | ||
| >60 | 4770 (33) | 1855 (39) | 1993 (42) | ||
Our multivariable model evaluating factors associated with hospice use demonstrated that white ethnicity, primary treatment type, income, lower Charlson comorbidity index, being partnered, and more recent year of death were associated with higher odds of hospice use (Table 2). We also identified significant regional variation, with lower hospice use in the Los Angeles registry compared with several other SEER registries. This variation remained significant after incorporating an interaction between subject ethnicity and SEER region.
Table 2.
Multivariable Logistic Regression Model of Factors Associated With Hospice Use Among 14 521 Men Dying of Prostate Cancer
| Characteristic | OR (95% CI) |
|---|---|
| Age | 1.00 (0.99–1.00) |
| Ethnicity vs white | |
| Hispanic | 1.00 (0.83–1.19) |
| African American | 0.78 (0.68–0.88) |
| Other | 0.59 (0.48–0.73) |
| Charlson comorbidity index vs 0 | |
| 1 | 0.68 (0.62–0.74) |
| 2 | 0.63 (0.57–0.70) |
| ≥3 | 0.49 (0.44–0.55) |
| Primary treatment, vs primary androgen deprivation | |
| Radiation prostatectomy | 1.28 (1.10–1.50) |
| Radiation therapy | 1.37 (1.26–1.49) |
| % Of census tract with >high school education vs ≤high school education | 1.15 (1.03–1.28) |
| Median census tract household income vs | |
| <$38 500 | |
| $38 500–$48 499 | 1.12 (1.01–1.24) |
| $48 500–$62 000 | 1.21 (1.07–1.37) |
| >$62 000 | 1.19 (1.03–1.37) |
| With partner vs without partner | 1.23 (1.14–1.32) |
| Distant stage at diagnosis vs localized/locoregional | 1.13 (1.04–1.23) |
| Year of death vs 1992 | 1.12 (1.11–1.14) |
| SEER region vs Los Angeles | |
| San Francisco | 1.22 (1.03–1.45) |
| Connecticut | 1.05 (0.91–1.22) |
| Detroit | 2.50 (2.17–2.87) |
| Hawaii | 1.34 (1.01–1.77) |
| Iowa | 2.01 (1.72–2.35) |
| New Mexico | 1.55 (1.27–1.88) |
| Seattle | 1.17 (1.00–1.38) |
| Utah | 1.48 (1.23–1.78) |
| Atlanta | 1.69 (1.39–2.05) |
| San Jose | 1.33 (1.07–1.65) |
| Rural Georgia | 1.78 (0.99–3.22) |
| Kentucky | 1.65 (1.19–2.29) |
| Louisiana | 1.53 (1.08–2.16) |
| New Jersey | 1.17 (0.91–1.52) |
Abbreviations: CI, confidence interval; OR, odds ratio; SEER, Surveillance, Epidemiology, and End Results.
Table 3 displays procedures and physician visits during the last 6 months of life. In our bivariate analysis, subjects not enrolled in hospice had more imaging studies, electrocardiograms, and cardiopulmonary resuscitations performed but received less palliative radiation. They made more emergency department visits and had more inpatient hospital stays and ICU admissions but made a comparable number of outpatient office visits.
Table 3.
Mean Number of Procedures and Physician Visits Among Men Dying of Prostate Cancer in the Last 6 Months of Life
| Characteristic | Total (N=13 804) | No Hospice (n=6875) | Hospicea (n=6929) | P Value |
|---|---|---|---|---|
| Procedures in last 6 mo of life | ||||
| Laboratory studies, excluding PSA | 22.42 | 23.32 | 22.16 | .007 |
| Imaging | 8.35 | 9.40 | 7.51 | <.001 |
| Palliative radiation | 2.18 | 2.01 | 2.53 | <.001 |
| Physical therapy/rehabilitation | 2.34 | 2.42 | 2.41 | .89 |
| PSA | 1.52 | 1.48 | 1.60 | .001 |
| ECG | 1.52 | 1.76 | 1.25 | <.001 |
| Foley catheter placement | 0.26 | 0.27 | 0.28 | .46 |
| Ureteral stent or nephrostomy | 0.50 | 0.52 | 0.48 | .23 |
| Cystoscopy | 0.19 | 0.19 | 0.17 | .04 |
| Cardiopulmonary resuscitation | 0.12 | 0.20 | 0.06 | <.001 |
| Chemotherapy | 1.78 | 1.82 | 1.75 | .44 |
| Intravenous medication injection | 7.66 | 7.82 | 7.54 | .33 |
| Other | 6.01 | 6.91 | 5.27 | <.001 |
| Physician visits in last 6 mo of life | ||||
| Emergency department visit | 1.53 | 1.69 | 1.44 | .001 |
| Outpatient office visit | 8.23 | 8.29 | 8.55 | .07 |
| Inpatient hospital admission | 1.18 | 1.36 | 0.99 | <.001 |
| Intensive care unit admission | 0.17 | 0.23 | 0.10 | <.001 |
Abbreviations: ECG, electrocardiography; PSA, prostate-specific antigen.
Time from hospice enrollment to death < 180 d.
Table 4 gives the adjusted odds of receiving high-intensity care for subjects enrolled in hospice compared with those not enrolled. Given that referral to hospice occurred less than a month before death in the median subject, we examined the odds of high-intensity care for hospice-enrolled subjects over the final 1, 2, 3, and 6 months of life. Men not enrolled in hospice were more likely to receive high-intensity care, including ICU admissions, inpatient stays, and emergency department visits. These disparities were amplified as death approached. Examining the last 30 days of life for our cohort, those enrolled in hospice had a 40% to 77% lower odds of use of high-intensity services.
Table 4.
Adjusted Odds of Receiving High-Intensity Care for Subjects Enrolled in Hospice Compared With Those Not Enrolleda
| Type of High-Intensity Care | OR (95% CI)
|
|||
|---|---|---|---|---|
| Last 180 d | Last 90 d | Last 60 d | Last 30 d | |
| Any high-intensity care | 0.82 (0.74–0.91) | 0.52 (0.48–0.57) | 0.41 (0.38–0.45) | 0.31 (0.29–0.33) |
| ICU admission | 0.51 (0.46–0.56) | 0.40 (0.35–0.45) | 0.34 (0.29–0.38) | 0.23 (0.20–0.28) |
| Inpatient admission | 0.58 (0.53–0.63) | 0.44 (0.41–0.48) | 0.38 (0.35–0.41) | 0.31 (0.29–0.34) |
| >1 Emergency department visit | 0.89 (0.83–0.96) | 0.71 (0.66–0.78) | 0.59 (0.54–0.65) | 0.45 (0.40–0.51) |
| Stent or nephrostomy | 0.99 (0.87–1.10) | 0.84 (0.74–0.95) | 0.75 (0.66–0.86) | 0.54 (0.46–0.64) |
| Cystoscopy | 1.04 (0.94–1.15) | 0.87 (0.76–0.99) | 0.78 (0.67–0.91) | 0.59 (0.48–0.74) |
| Chemotherapy | 1.16 (1.08–1.26) | 0.94 (0.86–1.03) | 0.76 (0.69–0.84) | 0.54 (0.47–0.62) |
| Cardiopulmonary resuscitation | 0.35 (0.31–0.41) | 0.30 (0.26–0.35) | 0.27 (0.23–0.32) | 0.23 (0.19–0.27) |
Abbreviations: CI, confidence interval; ICU, intensive care unit; OR, odds ratio.
Controlling for age, ethnicity, comorbidity, treatment type, education, income, and partnership status (referent; total, N=13 804; no hospice, n=6875; hospice, n=6929). High-intensity care included an inpatient hospital stay, ICU admission, more than 1 emergency department visit, cystoscopy, placement of a ureteral stent or nephrostomy tube, cardiopulmonary resuscitation, or administration of chemotherapy.
COMMENT
We characterized predictors of hospice use and aggressive end-of-life care in a population-based cohort of men dying of prostate cancer. Our study has several important findings. First, we found that over half of men dying of prostate cancer enrolled in hospice. The proportion of individuals using hospice increased over time, but the timing of hospice referral remained poor, with almost one-third of subjects enrolling either within 7 days of death or more than 180 days before dying. By 2004, when 61% of men dying of prostate cancer enrolled in hospice, 25% of those enrolled died within 7 days of referral, and 10% lived longer than 180 days. Almost all regions in the United States have access to hospice services, with the exception of a small number of rural areas.11 Hospice resources employ a multidisciplinary team of physicians, nurses, social workers, home health aids, speech therapists, volunteers, occupational counselors, physical therapists, nutritionists, and spiritual advisors who offer illness-related medical care, respite care, medical supplies, and family bereavement support for up to 12 months after an individual dies.25 Hospice stays shorter than 7 days are too brief to maximize the benefit of enrollment, and individuals making shorter stays receive fewer services and benefit less from the input of the full interdisciplinary team.5,24,26,27 Late hospice referral and low hospice enrollment indicate low-quality end-of-life care.28 Family members who perceive referrals as late report more concerns with the quality of care, lower overall satisfaction, and greater unmet needs.25,29
At the other end of the spectrum, the Medicare hospice benefit requires that a primary care physician and a hospice medical director certify than an individual’s expected prognosis does not exceed 180 days when he or she is enrolled in hospice.13,30 Despite these guidelines, physicians routinely overestimate their patients’ survival, with prognostic accuracy declining the longer the clinician has known the patient.31 The use rate we report builds on previous population-based studies of men dying of prostate cancer, in which use rates ranged from 18% to 43%, with a temporal trend toward increased use.5,7–10,21,22
While overall rates of hospice use by all patients with cancer, including those with prostate cancer, have improved over time, the timing of hospice referral has not changed significantly over the past 25 years.5,13 Our findings highlight both the successes of increased hospice use, to a zenith of 62% of men dying of prostate cancer in 2003, as well as avenues for improvement in the timing of hospice referral.
Second, we found that in the last 6 months of life, men not enrolled in hospice underwent more imaging studies and received more high-intensity care, which comprised inpatient hospital stays, ICU admissions, multiple emergency department visits, chemotherapy, cystoscopy, and ureteral stent or nephrostomy tube placement. Our hypothesis that stent or nephrostomy tube placement would represent the most frequent end-of-life intervention in men dying of prostate cancer was incorrect. Men enrolled in hospice were at least as likely as those not enrolled to make outpatient visits and to receive palliative radiation, suggesting that hospice enrollment did not prevent individuals from receiving primary or palliative care. Medicare expenditures for imaging services have risen approximately 17% per year in the last decade, from $3.6 billion in 2000 to $7.6 billion in 2006.32,33 No data endorse a connection between increased use of imaging studies and patient outcomes, especially at the end of life.34 Although the mean number of imaging studies performed on all men in our analysis was high overall, it was substantially higher in those not enrolled in hospice.
Likewise, the significant burden of high-intensity procedures performed in the last 6 months of life, particularly in men not enrolled in hospice, raises concern over what Emanuel35 has termed cost without benefit. While our population-based analysis did not allow us to characterize the specific reason for each individual emergency department visit or hospital admission, a high number of emergency department visits and hospital admissions, including ICU stays, indicates poor-quality end-of-life care, according to the quality of care indicators proposed by Earle et al.28 Data from the National Hospice Study and other analyses suggest that hospice enrollment improves symptom management, quality of death, and quality of life at the end of life.3,4 While prospectively collected data are lacking, observational data suggest that hospice enrollment significantly reduces the cost of caring for individuals at the end of life.5,7,13,16,18–20 If hospice enrollment is associated with lower cost and higher quality of life at the end of life, and if, as our analysis shows, hospice enrollment portends the performance of fewer costly high-intensity interventions, hospice use may minimize resources spent with limited benefit. Approximately one-third of the total Medicare budget is expended on individuals at the end of life.13 The United States currently spends approximately $2.2 trillion per year on Medicare and Medicaid, accounting for 16% of the gross domestic product, and, as the current Director of the Office of Management and Budget pointed out in 2007, “The long-term fiscal balance of the United States will be determined primarily by the future rate of growth of health care costs.”36,37(p1885) The Obama Administration’s fiscal year 2010 budget aims at reducing hospital admission rates as one way of controlling cost without sacrificing quality.38,39 Our findings suggest that increasing indicated hospice enrollment may aid in achieving this goal specifically. It also may provide other avenues for controlling cost and maximizing quality of life, by minimizing expensive interventions that improve neither the quality of care nor the quality of life at the end of life. Reducing the proportion of health care resources expended at the end of life might liberate resources that could then be used in other areas of need.
Third, several sociodemographic characteristics of our patients were associated with hospice use. In our multivariable model, white ethnicity, lower Charlson comorbidity index, higher socioeconomic status, and partnered relationship status were associated with increased hospice use. Retrospective analyses of population-based data reveal conflicting findings about sociodemographic variation in hospice use. Virnig et al12 found no sociodemographic predictors of hospice use, but Lackan et al7 noted variations in age, marital status, insurance type, cancer type, education level, income level, race, and sex. The analysis by Lackan et al7 demonstrated decreasing variation over time for subgroups defined by type of insurance, marital status, urban residence, and income, but increasing variation over time as a function of age and cancer type. Other analyses suggest that men who have never been married are less likely to enroll in hospice.8 We were surprised to find an inverse relationship between Charlson comorbidity index and hospice referral. It is possible that men who were sicker with multiple illnesses had more difficulty coping in a home setting with hospice and therefore did not get referred; this would be of interest for further study.
Several authors have expressed concern over misuse of hospice to manage acute complications in the last few days before death, as opposed to more appropriate use earlier in the end of life arc.7,9,15 That individuals with more comorbidity were less likely enroll in hospice suggests that they were not enrolled with the goal of palliating pain as death approached, although nuances in referral patterns stratified by comorbidity remains an intriguing area for future study. Inappropriate variation in use of medical treatments is considered poor-quality care,40 and identifying subgroups that use hospice resources less frequently may help target interventions aimed at increasing appropriate hospice use.
Our findings are limited by several methodological considerations. First, because our sample was restricted to Medicare enrollees older than 65 years, our findings may not apply to younger individuals. However, SEER-Medicare data are representative of the US population and allow valuable temporal analyses that are not feasible in many other data sets. Similarly, although many men are diagnosed as having prostate cancer at younger ages, the majority of men dying of prostate cancer are of Medicare age.21 Second, our analysis missed all hospice costs paid out of pocket, although this accounts for less than 20% of total hospice expenditures.13 The lost opportunity cost as well as the actual out of pocket cost was not accounted for in our analysis. Third, we could not incorporate patient preference for end-of-life care into our analysis. Prospective studies to examine patient preferences for hospice care at the end of life would be invaluable. Fourth, use of death certificates to establish prostate cancer as the cause of death risks attribution bias, although death certificates for men dying of prostate cancer have been shown to be accurate. We captured men dying of prostate cancer, not men with prostate cancer, but could not ascertain the acute reason leading to their final hospital admission or death.41 Fifth, by using SEER-Medicare claims data we did not capture all individuals dying of prostate cancer, but SEER-Medicare claims have been shown to be representative of trends for the overall population. Sixth, our model did not examine the temporal relationship between hospice use and high-intensity care, and our results reflect associations, not causation. Elucidating the aforementioned temporal relationships would be important in improving our understanding of end-of-life care for men with prostate cancer. Although we did not prove causality, we believe that the marked differences in the care received by men who did and did not enroll in hospice are important to note and should be further examined. Seventh, during the study period, standards of care regarding chemotherapy for advanced prostate cancer changed. Specifically, the benefit of docetaxel for those with good performance status was not fully appreciated until late in our study period.
We found that the proportion of men dying of prostate cancer who use hospice resources has increased over time, although the percentage of those enrolling very close to the end of life remains too high. Those who enroll in hospice are less likely to undergo imaging procedures and to receive high-intensity medical care at the end of life. Increasing appropriate hospice use may improve the quality of death for men at the end of life while rationalizing health care expenditures during this high-cost period.
Acknowledgments
Funding/Support: The Urologic Diseases in America Project is sponsored by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), part of the National Institutes of Health.
Footnotes
Author Contributions: Study concept and design: Bergman, Saigal, Lorenz, Hanley, Miller, and Litwin. Acquisition of data: Bergman, Saigal, Hanley, and Litwin. Analysis and interpretation of data: Bergman, Saigal, Lorenz, Hanley, Gore, and Litwin. Drafting of the manuscript: Bergman and Miller. Critical revision of the manuscript for important intellectual content: Bergman, Saigal, Lorenz, Hanley, Miller, Gore, and Litwin. Statistical analysis: Bergman, Saigal, Hanley, Gore, and Litwin. Obtained funding: Bergman, Saigal, and Litwin. Administrative, technical, and material support: Saigal, Lorenz, Hanley, and Litwin. Study supervision: Lorenz, Hanley, Miller, and Litwin.
Financial Disclosure: None reported.
Online-Only Material: eTables 1, 2, and 3 are available at http://www.archinternmed.com.
Group Information: For more information on the Urologic Diseases in America Project, see http://www.udaonline.net.
References
- 1.Steinhauser KE, Christakis NA, Clipp EC, McNeilly M, McIntyre L, Tulsky JA. Factors considered important at the end of life by patients, family, physicians, and other care providers. JAMA. 2000;284(19):2476–2482. doi: 10.1001/jama.284.19.2476. [DOI] [PubMed] [Google Scholar]
- 2.Morrison RS, Meier DE. Clinical practice: palliative care. N Engl J Med. 2004;350 (25):2582–2590. doi: 10.1056/NEJMcp035232. [DOI] [PubMed] [Google Scholar]
- 3.Wallston KA, Burger C, Smith RA, Baugher RJ. Comparing the quality of death for hospice and non-hospice cancer patients. Med Care. 1988;26(2):177–182. doi: 10.1097/00005650-198802000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Dy SM, Shugarman LR, Lorenz KA, Mularski RA, Lynn J RAND-Southern California Evidence-Based Practice Center. A systematic review of satisfaction with care at the end of life. J Am Geriatr Soc. 2008;56(1):124–129. doi: 10.1111/j.1532-5415.2007.01507.x. [DOI] [PubMed] [Google Scholar]
- 5.McCarthy EP, Burns RB, Ngo-Metzger Q, Davis RB, Phillips RS. Hospice use among Medicare managed care and fee-for-service patients dying with cancer. JAMA. 2003;289(17):2238–2245. doi: 10.1001/jama.289.17.2238. [DOI] [PubMed] [Google Scholar]
- 6.Lamont EB, Christakis NA. Physician factors in the timing of cancer patient referral to hospice palliative care. Cancer. 2002;94(10):2733–2737. doi: 10.1002/cncr.10530. [DOI] [PubMed] [Google Scholar]
- 7.Lackan NA, Ostir GV, Freeman JL, Mahnken JD, Goodwin JS. Decreasing variation in the use of hospice among older adults with breast, colorectal, lung, and prostate cancer. Med Care. 2004;42(2):116–122. doi: 10.1097/01.mlr.0000108765.86294.1b. [DOI] [PubMed] [Google Scholar]
- 8.Lackan NA, Ostir GV, Kuo YF, Freeman JL. The association of marital status and hospice use in the USA. Palliat Med. 2005;19(2):160–162. doi: 10.1191/0269216305pm981oa. [DOI] [PubMed] [Google Scholar]
- 9.Locher JL, Kilgore ML, Morrisey MA, Ritchie CS. Patterns and predictors of home health and hospice use by older adults with cancer. J Am Geriatr Soc. 2006;54(8):1206–1211. doi: 10.1111/j.1532-5415.2006.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ngo-Metzger Q, McCarthy EP, Burns RB, Davis RB, Li FP, Phillips RS. Older Asian Americans and Pacific Islanders dying of cancer use hospice less frequently than older white patients. Am J Med. 2003;115(1):47–53. doi: 10.1016/s0002-9343(03)00258-4. [DOI] [PubMed] [Google Scholar]
- 11.Virnig BA, Kind S, McBean M, Fisher E. Geographic variation in hospice use prior to death. J Am Geriatr Soc. 2000;48(9):1117–1125. doi: 10.1111/j.1532-5415.2000.tb04789.x. [DOI] [PubMed] [Google Scholar]
- 12.Virnig BA, Morgan RO, Persily NA, DeVito CA. Racial and income differences in use of the hospice benefit between the Medicare managed care and Medicare fee-for-service. J Palliat Med. 1999;2(1):23–31. doi: 10.1089/jpm.1999.2.23. [DOI] [PubMed] [Google Scholar]
- 13.Hogan C, Lunney J, Gabel J, Lynn J. Medicare beneficiaries’ costs of care in the last year of life. Health Aff (Millwood) 2001;20(4):188–195. doi: 10.1377/hlthaff.20.4.188. [DOI] [PubMed] [Google Scholar]
- 14.National Hospice and Palliative Care Organization. 2006 National Summary of Hospice Care: Statistics and Trends From the 2006 National Data Set and the 2006 NHPCO Membership Survey. Alexandria, VA: National Hospice and Palliative Care Organization; Nov, 2007. p. 4. [Google Scholar]
- 15.Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, Weeks JC. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22 (2):315–321. doi: 10.1200/JCO.2004.08.136. [DOI] [PubMed] [Google Scholar]
- 16.Taylor DH, Jr, Ostermann J, Van Houtven CH, Tulsky JA, Steinhauser K. What length of hospice use maximizes reduction in medical expenditures near death in the US Medicare program? Soc Sci Med. 2007;65(7):1466–1478. doi: 10.1016/j.socscimed.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 17.Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100(9):630–641. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 18.Emanuel EJ. Cost savings at the end of life: what do the data show? JAMA. 1996;275(24):1907–1914. [PubMed] [Google Scholar]
- 19.Pyenson B, Connor S, Fitch K, Kinzbrunner B. Medicare cost in matched hospice and non-hospice cohorts. J Pain Symptom Manage. 2004;28(3):200–210. doi: 10.1016/j.jpainsymman.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Campbell DE, Lynn J, Louis TA, Shugarman LR. Medicare program expenditures associated with hospice use. Ann Intern Med. 2004;140(4):269–277. doi: 10.7326/0003-4819-140-4-200402170-00009. [DOI] [PubMed] [Google Scholar]
- 21.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 22.Lackan NA, Ostir GV, Freeman JL, Kuo YF, Zhang DD, Goodwin JS. Hospice use by Hispanic and non-Hispanic white cancer decedents. Health Serv Res. 2004;39(4 pt 1):969–983. doi: 10.1111/j.1475-6773.2004.00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Miller SC, Kinzbrunner B, Pettit P, Williams JR. How does the timing of hospice referral influence hospice care in the last days of life? J Am Geriatr Soc. 2003;51(6):798–806. doi: 10.1046/j.1365-2389.2003.51253.x. [DOI] [PubMed] [Google Scholar]
- 25.Teno JM, Clarridge BR, Casey V, et al. Family perspectives on end-of-life care at the last place of care. JAMA. 2004;291(1):88–93. doi: 10.1001/jama.291.1.88. [DOI] [PubMed] [Google Scholar]
- 26.Rickerson E, Harrold J, Kapo J, Carroll JT, Casarett D. Timing of hospice referral and families’ perceptions of services: are earlier hospice referrals better? J Am Geriatr Soc. 2005;53(5):819–823. doi: 10.1111/j.1532-5415.2005.53259.x. [DOI] [PubMed] [Google Scholar]
- 27.Ngo-Metzger Q, Phillips RS, McCarthy EP. Ethnic disparities in hospice use among Asian-American and Pacific Islander patients dying with cancer. J Am Geriatr Soc. 2008;56(1):139–144. doi: 10.1111/j.1532-5415.2007.01510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Earle CC, Park ER, Lai B, Weeks JC, Ayanian JZ, Block S. Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J Clin Oncol. 2003;21(6):1133–1138. doi: 10.1200/JCO.2003.03.059. [DOI] [PubMed] [Google Scholar]
- 29.Teno JM, Shu JE, Casarett D, Spence C, Rhodes R, Connor S. Timing of referral to hospice and quality of care: length of stay and bereaved family members’ perceptions of the timing of hospice referral. J Pain Symptom Manage. 2007;34(2):120–125. doi: 10.1016/j.jpainsymman.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Christakis NA, Escarce JJ. Survival of Medicare patients after enrollment in hospice programs. N Engl J Med. 1996;335(3):172–178. doi: 10.1056/NEJM199607183350306. [DOI] [PubMed] [Google Scholar]
- 31.Christakis NA, Lamont EB. Extent and determinants of error in physicians’ prognoses in terminally ill patients: prospective cohort study. West J Med. 2000;172(5):310–313. doi: 10.1136/ewjm.172.5.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.US Government Accountability Office. Report to Congressional Requesters: Medicare Part B Imaging Services: Rapid Spending Growth and Shift to Physician Offices Indicate Need for CMS to Consider Additional Management Practices. Washington, DC: US Government Accountability Office; Jun, 2008. Publication GAO-08-452. [Google Scholar]
- 33.Iglehart JK. Health insurers and medical-imaging policy—a work in progress. N Engl J Med. 2009;360(10):1030–1037. doi: 10.1056/NEJMhpr0808703. [DOI] [PubMed] [Google Scholar]
- 34.Demmerle C, Glaudemans J. Diagnostic Imaging: Spending Trends and the Increasing Use of Appropriateness Criteria and Accreditation. Washington, DC: Avalere; 2008. [Google Scholar]
- 35.Emanuel EJ. Health Care, Guaranteed. New York, NY: Public Affairs; 2008. [Google Scholar]
- 36.US Department of Health and Human Services. National Health Expenditure Projections. Centers for Medicare and Medicaid Services; 2008–2018. [Accessed March 16, 2009]. Web site. http://www.cms.gov/NationalHealthExpendData/03_NationalHealthAccountsProjected.asp. [Google Scholar]
- 37.Orszag PR, Ellis P. Addressing rising health care costs—a view from the Congressional Budget Office. N Engl J Med. 2007;357(19):1885–1887. doi: 10.1056/NEJMp078191. [DOI] [PubMed] [Google Scholar]
- 38.Executive Office of the President of the United States, Office of Management and Budget. [Accessed March 7, 2009];A New Era of Responsibility: Renewing America’s Promise. http://www.gpoaccess.gov/usbudget/fy10/pdf/fy10-newera.pdf.
- 39.Iglehart JK. Budgeting for change—Obama’s down payment on health care reform. N Engl J Med. 2009;360(14):1381–1383. doi: 10.1056/NEJMp0901927. [DOI] [PubMed] [Google Scholar]
- 40.Wennberg JE. Practice variation: implications for our health care system. Manag Care. 2004;13(9 suppl):3–7. [PubMed] [Google Scholar]
- 41.Penson DF, Albertsen PC, Nelson PS, Barry M, Stanford JL. Determining cause of death in prostate cancer: are death certificates valid? J Natl Cancer Inst. 2001;93(23):1822–1823. doi: 10.1093/jnci/93.23.1822. [DOI] [PubMed] [Google Scholar]