Abstract
Objectives
Chronic opioid therapy for chronic noncancer pain has increased dramatically in recent years. Research on associated risks has typically focused on opioid abuse and dependence, and opioid misuse or aberrant drug use behaviors, but these risks have been defined from the providers’ perspective. The aim of this article was to develop a psychometrically sound method for assessing difficulties patients attribute to chronic opioid therapy.
Methods
A cross-sectional, observational study of patients prescribed opioids for chronic noncancer pain was conducted in a large integrated service delivery network in Washington State. Data were obtained from a phone interview and electronic health records including pharmacy data. Exploratory and confirmatory factor analyses were conducted using a split sample design.
Results
The interview response rate was 56.5% and a total of 1144 patients were included in analyses. A 2 factor solution was obtained and replicated with excellent fit statistics. Two correlated factors were identified—opioid control concerns and psychosocial problems— with 50% of the sample reporting difficulties with prescribed opioids: 24% reported elevated psychosocial problems and 36% reported elevated concerns about controlling their use of prescribed opioids.
Discussion
The Prescribed Opioid Difficulties Scale identifies common difficulties that patients ascribe to chronic opioid therapy. This scale may provide both an entry point and a framework for a patient-centered clinical dialog about the pros and cons of use of opioid medicines for managing chronic pain.
Keywords: prescription opioids, chronic pain, functioning, psychosocial, measurement
Chronic opioid therapy (COT) for chronic noncancer pain has increased in recent years.1,2 Rates of COT are higher among those with mental health and substance abuse problems.3,4 Rates of problems with prescription type opioids, including nonmedical use, addiction, and fatal overdose have also increased in recent years.5–7
Research concerning problems with COT has typically focused on opioid abuse and dependence, opioid misuse, or aberrant drug use behaviors, but prior research has several limitations. Diagnostic and Statistical Manual of Mental Disorder-IV (DSM–IV) abuse and dependence diagnoses8 for prescribed opioids have been shown to have poor correspondence with clinical assessments of problematic use.9,10 Recent research has shown that among chronic pain patients on opioids, the traditional DSM IV diagnostic categories of dependence and abuse are distinct from many of the misuse problems that patients report.11,12 The most appropriate approach seems to be that of the American Society of Addiction Medicine, which characterizes addiction in the context of pain treatment with opioids as including any of the following 3 elements: (1) adverse consequences, (2) loss of control, or (3) preoccupation with obtaining opioids.13 However, earlier research with pain patients on COT indicates a range of salient problems beyond addiction.12 In general, potential difficulties with COT from the patient’s perspective have not been studied. New perspectives and approaches are needed.
Opioid misuse and aberrant drug use behaviors include such phenomena as borrowing medications, using nonprescribed drugs, or requesting early refills.14–16 However, these types of behaviors can be interpreted as a consequence of misuse or pseudoaddiction. Furthermore, they are defined as problems from the perspective of the prescribing clinician, rather than from the perspective of the patient. Focus on these behaviors may contribute to an adversarial relationship between physicians and patients receiving COT. Programs that have used evidence of misuse or aberrant behavior to discontinue COT have led to substantial proportions of patients being lost to follow-up, with questionable clinical outcomes.15,17 In addition, research on aberrant drug using behaviors may not capture the full range of problems that patients experience with prescribed opioids.
Psychosocial problems are commonly observed among chronic pain patients on COT,18–20 but prior research has not assessed the extent to which patients themselves attribute these problems to their use of opioid pain medicines. Understanding the problems and concerns that patients specifically attribute to their use of prescribed opioid pain medicines is one important step toward clarifying the extent and nature of difficulties that patients receiving COT experience. This is particularly important given the dramatic increase in prevalent long-term opioid use for chronic, noncancer pain nationally, and an increase from 2.4% to 4.7% of all adults from 1997 to 2005 in the health plan in which this research was carried out.2 Another analysis from the same study indicates that although long-term users represent a minority (5.6%) of the total proportion of individual episodes of opioid use, they represent a majority (80.6%) of the total morphine equivalents dispensed.1
This article develops psychometrically sound methods for assessing difficulties with COT as perceived from the patients’ perspective, focusing on problems and concerns that chronic pain patients attribute to their use of prescribed opioid medications. This study refines the measurement of opioid-related difficulties based on the patients’ perspective. On the basis of earlier studies11,12 and clinical experience, we expected to find 2 domains of difficulties: psychosocial problems attributed to opioids and concerns about control of opioid medicines.
MATERIALS AND METHODS
Setting and Participants
Data used for these analyses were obtained from a survey of adults, aged 21 to 80 years, who were current recipients of COT for chronic noncancer pain. This survey was conducted as a component of CONSORT-CONsortium to Study Opioid Risks and Trends among Group Health (GH) patients.1 GH provides comprehensive care on a prepaid basis to approximately 500,000 persons in Washington State who are demographically representative of the general population.
Inclusion/Exclusion
Eligibility criteria included filling at least 10 opioid prescriptions and receiving at least 120 days supply in 1-year period before the sample selection date, with at least 90 days between the first and last opioid dispensing in that year. These criteria for long-term opioid use have been shown to predict a high probability of sustained opioid use 1 year later.1 Use of opioids was ascertained from GH’s electronic pharmacy database. Additional eligibility criteria were continuous enrollment in GH for at least 1 year before sampling and enrollment at a Western Washington Group Health clinic. To narrow the population to chronic, noncancer pain patients, patients were excluded who had received a cancer diagnosis in the Western Washington cancer registry (with the exception of nonmelanoma skin cancer) or who had 2 or more cancer diagnoses in the automated visit records in the year before sampling. For the analyses in this article, only those respondents who reported having used opioid medications in the 2 weeks before the interview were included, excluding only a small number of respondents (47 of 1191, 4%). These patients were excluded because several items in the scale being developed measured issues related to opioid use in the prior 2 weeks.
Sampling
To obtain adequate numbers of respondents with higher doses of opioids, potentially eligible patients were stratified into 3 equally sized groups based on the average daily opioid dose dispensed over the past year: persons receiving less than 50 mg morphine equivalent dose (MED) per day; 50 to less than 100mg MED per day; and those receiving 100mg MED per day or more.
Telephone Survey Procedures
Patient interviews were conducted between June and November 2008. A letter explaining the study was sent to all potentially eligible patients, with a 2-dollar bill included as a pre-incentive. GH’s Survey Research Program’s interviewers then called potential participants. The interviewers, highly experienced in research interview techniques, used a Computer-assisted Telephone Interview. Potential participants were told the telephone interview would take 25 to 30 minutes and were asked for permission for study staff to access their electronic healthcare data from the time they enrolled in GH until 3 years after the interview. Before starting the interview, staff asked respondents for their birth month and year to confirm their identity. All interview questions were fully structured and research interviewers received training on the survey questions and format. Interviewers were required to successfully complete a test interview with supervisors before they were allowed to begin interviewing participants. Calls with participants were randomly audited by survey research supervisors to ensure fidelity to interview protocols (participants were informed that calls could be monitored for quality assurance purposes). Remuneration of $20 was mailed to patients who completed the interview. The GH Institutional Review Board approved all study procedures including the use of oral informed consent.
Assessment of Problems and Concerns
To determine whether psychosocial difficulties were specifically attributed to prescribed opioids by the patients interviewed, questions were developed that asked respondents about problems that they perceived were related to their use of opioids. Items were included based on a previous interview study (n=778) with identical inclusion criteria, that asked a broader array of questions regarding problems and concerns related to opioids including the full Prescription Drug Use Questionnaire (PDUQ)13 and DSMIV for opioid abuse and dependence.11 For this earlier study, interviewers, who used a Computer-assisted Telephone Interview, documented respondents’ comments about specific items and the instrument as a whole. All of these comments were reviewed by one of the authors (C.J.B.) and informed development of interview questions for this study. In addition, the coverage of items included in this study was reviewed by 2 of the co-authors who have extensive clinical experience with chronic pain patients on opioids, a psychiatrist who works in a pain clinic at an academic medical center (M.D.S.) and an internist/addiction specialist who works in an adult medicine clinic in a large public hospital (J.O.M.). We also drew on the research literature, considering the specific items used in other studies from content and statistical perspectives.
Item frequencies were documented with 5 or 3-point Likert scales. Items and response scales are detailed in Appendix A. Several items pertaining to patients’ concerns about opioid use were based upon items from the PDUQ. The response scale for the PDUQ-derived items was changed from binary to a 5-point Likert scale to increase the sensitivity of the items. The time frame for the questions was made more specific and uniform with a focus on recent problems and concerns related to opioid use from the patient’s perspective. Most of the psychosocial problem questions were analogous to cognitive symptoms or side effects and the goal was to relate these recent problems to recent opioid use. A short time frame was used (2 wk) for these items to ensure accurate recall. Items addressing control concerns were about events or concerns that occur with lower frequency, thus a longer time frame was used (1 y) to ensure adequate yield and to identify clinically important events that may occur less frequently. Item response prevalence from similar items in a similar study population informed the time frames used for these questions.12 Items 1 through 8 address psychosocial problems attributed by patients to opioid use, and items 9 through 15 address concerns about control of opioids from the patients’ perspective (Appendix A). An item about the helpfulness of opioids (number 16) was included as a patient-centered measure of opioid benefit, but was not scored as part of the scale.
Item and Scale Scoring
To assign equal weight to each item, the maximum score for each item was 4 and the minimum score was 0. On the basis of results of exploratory factor analyses (EFA) in the derivation sample, some Likert-type items were recoded as binary for scoring and interpretation. Specifically, items assessing the level of agreement with a statement were recoded as binary and were scored as 4 for responses of “agree” and “strongly agree” and 0 otherwise. Two items (questions 5 and 6) which used a 5-point scale to document frequency of events from a range of “never” to “always or almost every day” were assigned scores from 0 to 4. The same 0 through 4 scoring was used for an item (question 8) which measured how bothersome side effects were from “not at all” to “extremely.” Question 7 which used a 3-level frequency scale, never, “once or twice,” and “3 or more times” was scored 0, 2, and 4, respectively. Total scores were created by summing the scores for all items. Recoding items in this manner allows for simpler scoring and yielded a better fit in the derivation sample. This scoring is also easier to interpret because 11 of the 15 items are scored based on whether the respondent agreed with the statement or not. We report psychometric analyses for both the original and the recoded scoring methods for comparison.
Statistical Analyses
Factor analyses were conducted on the original scoring of the items and on recoded items. Respondents were randomly divided into 2 samples: a derivation sample (N=573) and validation sample (N=571). With the derivation sample, EFA were conducted. Factor analytic procedures were selected according to the distributional characteristics of the data. This consisted of factor analyzing responses to the items using the robust weighted least squares parameter estimation procedure for categorical data (Muthén B, du Toit SHC, Spisic D, 1997, unpublished manuscript), which allows for missing data to be taken into account by the usual approach of maximum likelihood and the missing at random assumption,21,22 and with an oblique, geomin rotation.23,24 Missing responses accounted for less than 1% of the data: 0.49% with the derivation sample and 0.45% with the validation sample.
To compare the dimensionality of the factor pattern derived from the original scoring and the recoded scored items, coefficients of congruence25,26 between the exploratory factor loadings were calculated. The values of these coefficients were assessed according to the guidelines used by MacCallum et al27 (0.98 to 1.00=excellent, 0.92 to 0.98=good, 0.82 to 0.92=borderline, 0.68 to 0.82=poor, and below 0.68=terrible).
On the basis of the results of the EFA, the responses to the items from the validation sample were used in a confirmatory factor analysis (CFA), using the same weighted least squares parameter estimation procedure, allowing the latent factors to be correlated. All factor analyses were conducted with the MPLUS software.28 The number of factors to extract with the EFA was determined by considering 3 criteria: comparison of the obtained Eigen values with those derived by parallel analysis and the SAS program provided by O’Connor (2000), the interpretability of the factors derived, and the values of indices of model fit.29–31 CFA was based on the exploratory results and its adequacy assessed by similar indices of model fit. These indices included the comparative fit index (CFI),32 the Tucker-Lewis incremental fit index (TLI),33 the root mean square error of approximation (RMSEA),34 the standardized root mean square residual (SRMR),35 and the weighted root mean square residual (WRMR).36 The magnitudes of the fit indices were evaluated on recommendations given by Hu and Bentler,37 Yu and Muthén,38 and Yu,39 and consideration of the non-normality of the data (>0.95 for the CFI and TLI, <0.06 for the RMSEA, <0.07 for SRMR, and <0.95 for WRMR).
RESULTS
Sample Characteristics
A total of 1191 interviews were conducted among the 2109 who were approached and met eligibility criteria per electronic records, for a response rate of 56.5%. Of the respondents, 47 had not used opioids in the past 2 weeks and were excluded from analyses because they were skipped out of questions pertaining to opioid use in the prior 2 weeks, leaving a study population of 1144. Tables 1 to 3 present the demographics, pain problem, and opioid use characteristics of the derivation and validation samples. Samples did not differ significantly (P<0.05) on the variables of interest, except for a slight difference in ethnicity. The proportions of White, African-American, and those of other ethnicities were 82%, 4%, and 13% for the derivation sample compared with 85%, 6%, and 9% for the validation sample (χ2=7.07, P=0.03). Almost all (98%) reported 2 or more pain problems in the prior 6 months with back and leg pain the most common types. The average number of days in pain in the prior 6 months was 165 and the average pain intensity was 5.9 out of 10. Opioids were taken on average 13 of the prior 14 days.
TABLE 1.
Demographic Characteristics
| Total Sample (N=1144) (%) | |
|---|---|
| Age (y) | |
| 21–44 | 194 (17.0) |
| 45–64 | 719 (62.9) |
| 65+ | 231 (20.2) |
| Sex (female) | 700 (61.2) |
| Ethnicity* | |
| African-American | 57 (5.1) |
| White | 942 (83.9) |
| Other | 124 (11.0) |
| Marital status | |
| Married/cohabitating | 762 (66.7) |
| Never married | 130 (11.4) |
| Widowed/separated/divorced | 251 (22.0) |
| Education | |
| 12+years | 1070 (93.5) |
| Employment | |
| Full time | 382 (33.5) |
| Part time | 57 (5.0) |
| School or vocational training | 3 (0.3) |
| Retired | 290 (25.4) |
| Homemaker | 30 (2.6) |
| Unemployed, laid off, or looking for work | 24 (2.1) |
| Permanently disabled or unable to work due to health | 292 (25.6) |
| Temporarily unable to work for health reasons | 64 (5.6) |
| Body mass index (mean and SD) | 30.9 (8.2) |
χ2=7.07 (P=0.03) for the difference between randomly drawn derivation and validation samples.
TABLE 3.
Opioid Use Characteristics
| Total Sample (N=1144) (%) | |
|---|---|
| Most frequently used opioids in the past 3 mo | |
| Long-acting morphine | 283 (24.7) |
| Hydrocodone | 231 (20.2) |
| Oxycodone | 193 (16.9) |
| Long-acting oxycodone | 184 (16.1) |
| Methadone | 114 (10.0) |
| Days took opioids in past 2 weeks (mean and SD) | 13.1 (2.7) |
| Times per day in past 2 weeks | |
| 1 | 137 (12.1) |
| 2 | 355 (31.3) |
| 3 | 384 (33.8) |
| 4 | 163 (14.4) |
| 5+ | 90 (7.9) |
| Usual pain before opioid use (mean and SD)* | 6.5 (2.0) |
| Usual pain after opioid use (mean and SD)* | 3.9 (2.0) |
| Median percent change in pain after opioid use | −40 |
| Helpfulness of opioids in pain relief | |
| Not at all | 10 (0.9) |
| A little | 77 (6.7) |
| Moderate | 357 (31.2) |
| Very | 440 (38.5) |
| Extremely | 259 (22.7) |
Rated on a 11-point Likert scale, 0=no pain to 10=pain as bad as could be.
For individual items, patients infrequently attributed specific psychosocial problems to opioids. For instance, the proportion reporting they agreed or strongly agreed that opioids “caused me to lose interest in my usual activities” was 10% and that “side effects… interfered with your work, family, or social responsibilities” was 9% (Table 4). A larger proportion reported they were worried about control of opioid pain medicines, including that they “might be dependent on or addicted” (31%) and that they wanted to “stop using…or cut down” (38%). An item included in the scale (number 16), but which was not scored asks “how helpful have you found opiate pain medicines in relieving your pain?” to which 61% responded very or extremely.
TABLE 4.
Percent Response by Item
| Strongly Disagree (%) | Disagree (%) | Neutral (%) | Agree (%) | Strongly Agree (%) | Missing (%) | |
|---|---|---|---|---|---|---|
| Problems scale | ||||||
| 1. Opiate medicines have caused me to lose interest in my usual activities | 38.2 | 45.0 | 7.1 | 6.7 | 2.5 | 0.4 |
| 2. Opiate medicines have caused me to have trouble concentrating or remembering | 33.6 | 43.0 | 7.4 | 10.8 | 3.8 | 1.4 |
| 3. Opiate medicines have caused me to feel slowed down, sluggish, or sedated | 31.0 | 42.8 | 9.3 | 14.5 | 2.1 | 0.3 |
| 4. Opiate pain medications have caused me to feel depressed, down, or anxious | 35.6 | 47.9 | 8.7 | 5.8 | 1.4 | 0.6 |
| Never | Rarely | Sometimes | Often | Always/Almost Every Day | Missing | |
| 5. How often have side effects of opiate medicine interfered with your work, family, or social responsibilities? | 56.1 | 25.2 | 10.8 | 3.9 | 3.9 | 0.1 |
| 6. How often did opiate medicine make it hard for you to think clearly? | 65.2 | 19.1 | 10.1 | 2.8 | 1.8 | 1.1 |
| Never | Once or Twice | Three or More | Missing | |||
| 7. In the past year, about how many times did opiate medicines make you sleepy or less alert when you were driving, operating machinery, or doing something else where you needed to be alert? | 72.6 | 17.7 | 8.7 | — | — | 1.0 |
| Not at All Bothersome | A Little Bothersome | Moderately Bothersome | Very Bothersome | Extremely Bothersome | Missing | |
| 8. Considering the side effects of opiate medicines you experienced in the past month, how bothersome were these side effects? | 48.0 | 24.1 | 16.4 | 7.1 | 4.4 | 0.0 |
| Concerns scale | Strongly Disagree | Disagree | Neutral | Agree | Strongly Agree | Missing |
| 9. I have been preoccupied with or thought constantly about use of opiate pain medicines | 43.8 | 43.3 | 6.1 | 4.7 | 1.8 | 0.3 |
| 10. In the past year, I have felt that I could not control how much or how often I used opiate medicine | 57.3 | 31.4 | 6.5 | 3.7 | 0.9 | 0.4 |
| 11. I have needed to use a higher dose of opiate pain medicine to get the same effect | 32.1 | 30.9 | 8.8 | 20.8 | 6.6 | 0.8 |
| 12. I have worried that I might be dependent on or addicted to opiate pain medicines | 29.8 | 28.4 | 10.8 | 22.6 | 7.8 | 0.5 |
| 13. I have wanted to stop using opiate pain medicines or to cut down on the amount of opiate medicines that I use | 18.4 | 25.8 | 15.0 | 28.8 | 11.4 | 0.8 |
| 14. In the past year, opiate medicines have caused me to have problems with family, friends or coworkers | 58.1 | 33.7 | 3.1 | 3.8 | 1.3 | 0.0 |
| 15. Family or friends have thought that I may be dependent on or addicted to opiate pain medicines | 43.6 | 34.1 | 6.3 | 11.5 | 3.6 | 0.9 |
| Asked, but not scored | Not at All | A Little | Moderately | Very | Extremely | Missing |
| 16. Over the past month, how helpful have you found opiate pain medicines in relieving your pain | 0.9 | 6.7 | 31.2 | 38.5 | 22.7 | 0.1 |
Factor Analyses
The results of the EFA with the derivation sample indicated a 2-factor solution best fit the data (Table 5). The indices of fit were marginal with the original scoring of the data, especially the CFI=0.913 and the RMSEA=0.095. However, with recoded items, excellent indices of fit were obtained: CFI=0.995, TLI=0.997, RMSEA=0.018, and SRMR=0.052. Coefficients of congruence between the factor structures obtained with the original and recoded items were high, 0.982 for the factor or subscale designated as Problems and 0.957 for the factor or subscale defined as Concerns, indicating excellent and good similarity of the underlying dimensions, respectively. With the recoded data, item 4 had a salient loading on both factors (0.500 and 0.363), where a salient loading is defined as being 0.316 (approximately 10% of the variance) or higher. This may be because of the effect that when recoded there is sparseness in the value; that is only 8.0% would be coded as one and the remaining 92.0% of the responses as 0. Item 6 had large loadings, 0.92 for the original scoring and exceeding 1 (1.01) for the recoded items. Such high values are appropriate, but perhaps indicate a high degree of multi-colinearity with this item and other items defining this factor.40
TABLE 5.
Results of Exploratory and Confirmatory Factor Analyses (Factor Loadings)
| Scoring Item | Exploratory
|
Confirmatory
|
||||||
|---|---|---|---|---|---|---|---|---|
| (Derivation Sample, N=573)
|
(Validation Sample, N=571)
|
|||||||
| Original
|
Recoded
|
Original
|
Recoded
|
|||||
| Problems | Concerns | Problems | Concerns | Problems | Concerns | Problems | Concerns | |
| 1 | 0.455 | 0.329 | 0.694 | 0.046 | 0.789 | 0.786 | ||
| 2 | 0.696 | 0.173 | 0.892 | 30.049 | 0.839 | 0.872 | ||
| 3 | 0.733 | 0.096 | 0.816 | 0.011 | 0.772 | 0.718 | ||
| 4 | 0.564 | 0.310 | 0.500 | 0.363 | 0.842 | 0.833 | ||
| 5 | 0.759 | 0.007 | 0.741 | 0.074 | 0.672 | 0.749 | ||
| 6 | 0.920 | 30.110 | 1.013 | 30.192 | 0.759 | 0.836 | ||
| 7 | 0.651 | 30.011 | 0.604 | 0.093 | 0.576 | 0.614 | ||
| 8 | 0.507 | 30.016 | 0.464 | 0.176 | 0.490 | 0.617 | ||
| 9 | 0.055 | 0.610 | 0.044 | 0.626 | 0.711 | 0.637 | ||
| 10 | 30.140 | 0.733 | 30.036 | 0.642 | 0.681 | 0.688 | ||
| 11 | 0.018 | 0.566 | 0.012 | 0.491 | 0.589 | 0.535 | ||
| 12 | 0.007 | 0.699 | 30.068 | 0.831 | 0.699 | 0.773 | ||
| 13 | 0.192 | 0.325 | 0.148 | 0.420 | 0.458 | 0.604 | ||
| 14 | 0.093 | 0.631 | 0.281 | 0.479 | 0.808 | 0.783 | ||
| 15 | 30.023 | 0.791 | 0.045 | 0.779 | 0.777 | 0.812 | ||
| Reliability | 0.85 | 0.75 | 0.83 | 0.63 | 0.85 | 0.79 | 0.81 | 0.65 |
| Interfactor correlations | ||||||||
| 0.579 | 0.627 | 0.701 | 0.589 | |||||
| Indices of model fit | ||||||||
| CFI | 0.913 | 0.995 | 0.905 | 0.982 | ||||
| TLI | 0.962 | 0.997 | 0.958 | 0.987 | ||||
| RMSEA | 0.095 | 0.018 | 0.106 | 0.032 | ||||
| SRMR | 0.054 | 0.052 | — | — | ||||
| WRMR | — | — | 1.505 | 0.865 | ||||
Values in bold for the exploratory factor analysis indicate a salient loading >0.316.
CFI indicates comparative fit index; RMSEA, root mean square error of approximation; SRMR, standardized root mean square residual; TLI, Tucker-Lewis incremental fit index; WRMR, weighted root mean square residual.
The CFAs with the validation sample indicated that the same 2 factors were easily replicated and produced similar indices of model fit compared with the exploratory results (Table 5). With the original scoring, the indices were CFI=0.905, TLI=0.958, RMSEA=0.106, and WRMR =1.505; whereas the solution with the recoded items again revealed excellent indices of model fit: CFI=0.982, TLI=0.987, RMSEA=0.032, and WRMR=0.865.
Internal consistency reliability was estimated with Cronbach’s α coefficient.41 The internal consistency of the Problems subscale exceeded 0.80 for the original and recoded versions in both the derivation and validation samples (Table 5). The internal consistency of the Concerns subscale exceeded 0.75 for the original version for the derivation and validation sample, but the recoded version had internal consistency that was lower than 0.70. Alpha coefficients for the total scale, combining both factors, were similar for both samples: 0.87 and 0.82 with the derivation sample for the original and recoded items, respectively; and 0.87 and 0.81 with the validation sample for the original and recoded items, respectively.
Distribution of Scale Scores
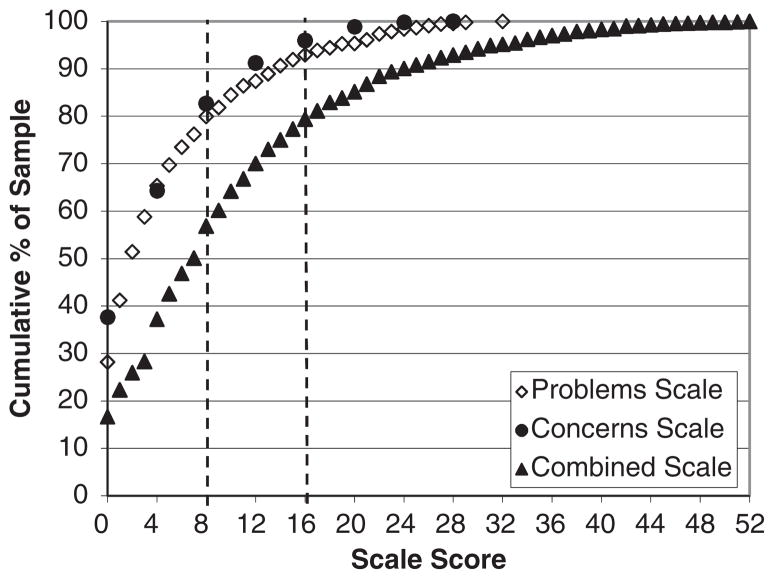
The distribution of scores for the Problems and Concerns subscales and the combined scale score are presented in Table 6 and Figure 1. A cutpoint of 8 was selected as a medium score level and 16 as a high score. These scores generally correspond to endorsing 2 and 4 items, respectively. These cutpoints were used for the subscales and the combined scale. For the Problems subscale, 15.7% had a medium score and 8.0% a high score. On the Concerns subscale, 26.9% of patients scored in the medium range and 8.7% in the high range. Combining the 2 scales, the proportion with a medium score was 27.2% and 22.7% were in the high range.
TABLE 6.
Distribution of Scores on Problems, Concerns, and Combined Scales
| Score | Problems Scale (%) | Concerns Scale (%) | Combined Scale (%) |
|---|---|---|---|
| <8 | 76.2 | 64.3 | 50.1 |
| 8–15 | 15.7 | 26.9 | 27.2 |
| ≥16 | 8.0 | 8.7 | 22.7 |
FIGURE 1.
Distribution of scores on Problems, Concerns, and Combined Scales.
DISCUSSION
In this study, we assessed patients’ difficulties with COT and found 2 types of difficulties: opioid control concerns and psychosocial problems. The patient-centered approach of the 15-item instrument that we propose is unique in both its perspective and potential uses. By eliciting patients’ own experiences with opioids, rather than focusing on aberrant behaviors which can create a pejorative or adversarial perspective, we believe the instrument can provide value in the clinical setting by informing and guiding dialog between provider and patient. Scale scores and cutpoints were presented as a way to summarize the frequency of different levels of problems and concerns. The intent of these scales is to identify patient problems and concerns attributed to the use of opioids, not as a screening scale to identify problem patients. The recommended cutpoints are offered for circumstances in which it is useful to report grouped data, not as thresholds for identifying patients with abnormal results. Several instruments to identify opioid misuse or aberrant behaviors have been developed; however, reviews indicate that no instrument or conceptual approach has been shown to work best.42–44
The Problem subscale asks the patient whether, from their perspective, opioids are related to problems with mood and cognition, providing the patient and clinician an entry point for discussion of psychological symptoms as well as potential negative impacts of opioids on well-being. The questions about patients’ function within the scale directly address whether opioids are impairing one’s ability to function, an issue that initially may be of secondary import in physicians’ or patients’ minds compared with pain control. Impaired functioning as a direct consequence of opioid medicines rather than the pain conditions for which they were prescribed is a critical issue that may not be routinely addressed by physicians in deciding whether to authorize refills of opioids for chronic noncancer pain. The time frame assessed for most of the Problems subscale items was 2 weeks. The endorsement rates for these items were relatively low, the most commonly endorsed item, opiate medicines caused me to feel “sluggish or sedated,” was endorsed by 17% of patients. It is possible that this 2-week time frame resulted in lower estimates of opioid-related problems than would be observed with a longer reporting window.
Some of the items in the Concerns subscale have been previously shown to be distinct from DSM-IV opioid abuse and dependence.11 It is difficult to know whether these concerns represent insight into uncontrolled opioid use with negative social consequences or simply a fear of loss of control based on concerns expressed by family or society. The psychometric analyses presented here cannot answer this question. It is, however, a question that patients and their physicians may wish to explore as part of a discussion of the risks and benefits of COT.
Addiction is a commonly voiced concern in the setting of COT, and the incidence of iatrogenic opioid addiction continues to be debated. We believe the Problems and Concerns subscales provide a more collaborative framework for discussing problems with prescribed opioids. They identify common negative consequences of opioids recognized by chronic pain patients treated with opioids. Related research based on the same dataset examined the relationship of the Problems and Concerns subscales with measures of psychosocial dysfunction and aberrant drug use behaviors. 45 These findings indicate that patients who attribute problems to opioid pain medicines are significantly more likely to have psychosocial problems, including depression and anxiety, and that patients who express concerns about control of opioid pain medicines are more likely to manifest aberrant drug use behaviors. However, the Problems and Concerns subscales are distinct from primary mental disorders or addiction, rather, they reflect the patient’s views of difficulties they are experiencing with opioid medications. Although it is important to assess patient perspectives on problems they attribute to opioids, it is also essential for clinicians to independently evaluate whether patients using opioids are experiencing problems indicative of depression or addiction.
This study examined long-term opioid therapy among chronic noncancer pain patients in a large integrated healthcare plan, most of whom were insured through employment or Medicare. This is a population that might be considered to be stable and therefore less likely to exhibit aberrant behaviors or other problems related to opioids. However, half of the patients in this sample reported 2 or more problems or concerns that they attributed to the use of prescribed opioid medications. The likely impact of inclusion criteria that required long-term use and the exclusion of those with no opioid use in the prior 2 weeks may have been to exclude patients who discontinued opioid use because of opioid-related problems or concerns about becoming addicted to opioids. Despite this sample restriction, about half of the patients in this study reported problems attributed to the use of prescribed opioid medicines. Reasons for discontinuation of opioids are important area of investigation, although it is beyond the scope of this study.
Limitations of this study include a cross-sectional, observational design, and the use of automated pharmacy data to document opioid prescriptions. However, the days supply and number of days used in the past 14 was high, and earlier research indicates enrollees report obtaining more than 90% of their prescriptions from GHC.46 Content validity was not formally determined for the survey questions in this interview study. It is possible that other important issues to patients about their pain and opioid use were not addressed. Items were developed on the basis of an extensive review of the literature, previous experience with a large, interview study with the same population, and clinical experience of several authors. Construct validity of the Prescribed Opioid Difficulties Scale, the correspondence with related concepts, is addressed in a related paper summarized above.45 We relied on self-report for the attribution of mood and cognition problems to opioids. It is important, and difficult, to try to determine what factors might be causing problems that patients attribute to their use of opioids. The survey questions address the patients’ perceptions; the degree to which they correspond to the actual relationship between opioids and reported problems could not be determined. Regardless, addressing the patients’ perception of these issues through a dialog with the clinician seems worthwhile. These results are limited to chronic pain patients on COT. It would not be appropriate to generalize results to those with using opioids for acute pain conditions, those with chronic pain who discontinued opioid therapy after brief trials, or those with cancer-related pain.
Although a significant proportion of patients attributed problems and concerns to their opioid medications, most also identified opioids as very or extremely helpful for pain relief. These data cannot determine the reasons for this discrepancy or how patients and providers can best balance the risks and benefits of COT, although it highlights the need for further research exploring these phenomena. Future research should also explore the extent of patient-perceived problems and concerns attributed to the use of prescribed opioids in different populations and settings. It would be valuable to study the potential utility of this instrument in informing patient evaluation, prescribing decisions, and subsequent health outcomes.
This research on COT focused on the patients’ perspective and linked perceived difficulties directly to opioids. The result was 2 subscales, Problems and Concerns, which identify previously unaddressed issues with opioids that are relatively common among patients on COT. These subscales may provide both an entry point and a framework for a critical dialogue about the pros and cons of opioid medicines for chronic pain.
TABLE 2.
Pain-related Characteristics
| Total Sample (N=1144) (%) | |
|---|---|
| Most bothersome pain problem in the past 3 mo | |
| Back pain | 332 (29.6) |
| Widespread/multiple | 285 (25.4) |
| Leg | 220 (19.6) |
| Reporting 2+ pain problems in the past 6 mo | 1121 (98.0) |
| Most common pain conditions for which used opiates in past 3 mo | |
| Back | 686 (60.0) |
| Leg | 499 (43.6) |
| Neck | 224 (19.6) |
| Shoulder | 200 (17.5) |
| Hip | 183 (16.0) |
| Took opioids for multiple pains in last 3 mo | 730 (63.8) |
| Number of days in pain in past 6 mo (mean and SD) | 164.7 (40.7) |
| Pain intensity (mean and SD) | |
| Now | 5.0 (2.4) |
| Worst | 8.7 (1.5) |
| Average | 5.9 (1.9) |
| Pain-related disability days in the past 3 months (mean and SD)* | 41.1 (37.0) |
| Average daily dose dispensed year prior (mg/d) | |
| 0–49 | 387 (33.8) |
| 50–99 | 385 (33.7) |
| 100+ | 372 (32.5) |
Rated on a 11-point Likert scale, 0=no pain to 10=pain as bad as could be.
Acknowledgments
Supported by a grant from the National Institute on Drug Abuse Bethesda, Maryland, to Michael Von Korff (DA022557).
APPENDIX A
Problems Scale Items
*1. (In the past 2 wk) Opiate medicines have caused me to lose interest in my usual activities.
Strongly disagree (0) Disagree (1) Neutral (2) Agree (3) Strongly agree (4).
*2. (In the past 2 wk) Opiate medicines have caused me to have trouble concentrating or remembering.
Strongly disagree (0) Disagree (1) Neutral (2) Agree (3) Strongly agree (4).
*3. (In the past 2 wk) Opiate medicines have caused me to feel slowed down, sluggish, or sedated.
Strongly disagree (0) Disagree (1) Neutral (2) Agree (3) Strongly agree (4).
*4. (In the past 2 wk) Opiate pain medications have caused me to feel depressed, down, or anxious.
Strongly disagree (0) Disagree (1) Neutral (2) Agree (3) Strongly agree (4).
5. (In the past 2 wk) How often have side effects of opiate medicine interfered with your work, family, or social responsibilities?
Never (0) Rarely (1) Sometimes (2) Often (3) Always or almost every day (4).
6. (In the past 2 wk) How often did opiate medicine make it hard for you to think clearly?
Never (0) Rarely (1) Sometimes (2) Often (3) Always or almost every day (4).
7. In the past year, about how many times did opiate medicines make you sleepy or less alert when you were driving, operating machinery, or doing something else where you needed to be alert?
Never (0) Once or twice (2) Three or more times(4).
8. Considering the side effects of opiate medicines you experienced in the past month, how bothersome were these side effects?
Not at all bothersome (0) A little bothersome (1) moderately bothersome (2) very bothersome (3) Extremely bothersome (4).
*Items marked with an asterisk are scored with agree/strongly agree assigned a 4 and all other responses assigned a 0 for the recoded version of the scale.
Concerns Scale Items
*9. (In the past 2 wk) I have been preoccupied with or thought constantly about use of opiate pain medicines.
Strongly disagree (0) Disagree (1) Neutral (2) Agree (3) Strongly agree (4).
*10. In the past year, I have felt that I could not control how much or how often I used opiate medicine.
Strongly disagree (0) Disagree (1) Neutral (2) Agree (3) Strongly agree (4).
*11. (In the past year) I have needed to use a higher dose of opiate pain medicine to get the same effect.
Strongly disagree (0) Disagree (1) Neutral (2) Agree (3) Strongly Agree (4).
*12. [In the past year] I have worried that I might be dependent on or addicted to opiate pain medicines.
Strongly disagree (0) Disagree (1) Neutral (2) Agree (3) Strongly agree (4).
*13. (In the past year) I have wanted to stop using opiate pain medicines or to cut down on the amount of opiate medicines that I use.
Strongly disagree (0) Disagree (1) Neutral (2) Agree (3) Strongly agree (4).
*14. In the past year, opiate medicines have caused me to have problems with family, friends, or coworkers.
Strongly disagree (0) Disagree (1) Neutral (2) Agree (3) Strongly agree (4).
*15. (In the past year) Family or friends have thought that I may be dependent on or addicted to opiate pain medicines.
Strongly Disagree (0) Disagree (1) Neutral (2) Agree (3) Strongly Agree (4).
Asked but Not Scored
16. Over the past month, how helpful have you found opiate pain medicines in relieving your pain.
Not at all helpful (0) A little helpful (1) Moderately helpful (2) Very helpful (3) Extremely helpful (4).
*Items marked with an asterisk are scored with agree/strongly agree assigned a 4 and all other responses assigned a 0 for the recoded version of the instrument.
Footnotes
Disclosure of interests: Dr. Von Korff has a grant pending from Johnson & Johnson. Dr. Sullivan has received grant support from Wyeth, Lilly, Aetna, Johnson & Johnson, and Ortho McNeil and has been a consultant for Eli Lilly.
References
- 1.Von Korff M, Saunders K, Ray GT, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24:521–527. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boudreau D, Von Korff M, Rutter CM, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Safe. 2009;18:1166–1175. doi: 10.1002/pds.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan MD, Edlund MJ, Fan MY, et al. Trends in use of opioids for non-cancer pain conditions 2000–2005 in commercial and Medicaid insurance plans: the TROUP study. Pain. 2008;138:440–449. doi: 10.1016/j.pain.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weisner CM, Campbell CI, Ray GT, et al. Trends in prescribed opioid therapy for non-cancer pain for individuals with prior substance use disorders. Pain. 2009;145:287–293. doi: 10.1016/j.pain.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613–2620. doi: 10.1001/jama.2008.802. [DOI] [PubMed] [Google Scholar]
- 6.Paulozzi LJ, Xi Y. Recent changes in drug poisoning mortality in the United States by urban-rural status and by drug type. Pharmacoepidemiol Drug Safe. 2008;17:997–1005. doi: 10.1002/pds.1626. [DOI] [PubMed] [Google Scholar]
- 7.McCabe SE, Cranford JA, West BT. Trends in prescription drug abuse and dependence, co-occurrence with other substance use disorders, and treatment utilization: results from two national surveys. Addict Behav. 2008;33:1297–1305. doi: 10.1016/j.addbeh.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Psychiatric Institute. Diagnostic and Statistical Manual of Mental Disorder. 4. Washington DC: American Psychiatric Institute; 1994. [Google Scholar]
- 9.Compton W, Volkow N. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81:103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Sees KL, Clark HW. Opioid use in the treatment of chronic pain: assessment of addiction. J Pain Symptom Manage. 1993;8:257–264. doi: 10.1016/0885-3924(93)90154-n. [DOI] [PubMed] [Google Scholar]
- 11.Banta-Green CJ, Merrill JO, Doyle SR, et al. Measurement of opioid problems among chronic pain patients in a general medical population. Drug Alcohol Depend. 2009;104:43–49. doi: 10.1016/j.drugalcdep.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banta-Green CJ, Merrill JO, Doyle SR, et al. Opioid use behaviors, mental health and pain—development of a typology of chronic pain patients. Drug Alcohol Depend. 2009;104:34–42. doi: 10.1016/j.drugalcdep.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and “problematic” substance use: evaluation of a pilot assessment tool. J Pain Symptom Manage. 1998;16:355–363. doi: 10.1016/s0885-3924(98)00110-9. [DOI] [PubMed] [Google Scholar]
- 14.Morasco BJ, Dobscha SK. Prescription medication misuse and substance use disorder in VA primary care patients with chronic pain. Gen Hosp Psychiatry. 2008;30:93–99. doi: 10.1016/j.genhosppsych.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Chabal C, Erjavec MK, Jacobson L, et al. Prescription opiate abuse in chronic pain patients: clinical criteria, incidence, and predictors. Clin J Pain. 1997;13:150–155. doi: 10.1097/00002508-199706000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Fleming MF, Davis J, Passik SD, et al. Reported lifetime aberrant drug-taking behaviors are predictive of current substance use and mental health problems in primary care patients. Pain Med. 2008;9:1098–1106. doi: 10.1111/j.1526-4637.2008.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chelminski PR, Ives TJ, Felix KM, et al. A primary care, multi-disciplinary disease management program for opioid-treated patients with chronic non-cancer pain and a high burden of psychiatric comorbidity. BMC Health Serv Res. 2005;5:3. doi: 10.1186/1472-6963-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reid MC, Engles-Horton LL, Weber MB, et al. Use of opioid medications for chronic noncancer pain syndromes in primary care. J Gen Intern Med. 2002;17:173–179. doi: 10.1046/j.1525-1497.2002.10435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleming MF, Balousek SL, Klessig CL, et al. Substance use disorders in a primary care samples receiving daily opioid therapy. J Pain. 2007;8:573–582. doi: 10.1016/j.jpain.2007.02.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullivan MD, Edlund MJ, Zhang L, et al. Association between mental health disorders, problem drug use, and regular prescription opioid use. Arch Intern Med. 2006;23:2087–2093. doi: 10.1001/archinte.166.19.2087. [DOI] [PubMed] [Google Scholar]
- 21.Rubin DB. Inference and missing data. Biometrika. 1976;63:581–592. [Google Scholar]
- 22.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley and Sons, Inc; 1987. [Google Scholar]
- 23.Yates A. Multivariate Exploratory Data Analysis: A Perspective on Exploratory Factor Analysis. Albany: State University of New York Press; 1988. [Google Scholar]
- 24.Browne MW. An overview of analytic rotation in exploratory factor analysis. Multivariate Behav Res. 2001;36:111–150. [Google Scholar]
- 25.Tucker LR. Personnel Research Section Report No. 984. Washington, DC: Department of the Army; 1951. A Method of Synthesis of Factor Analysis Studies. [Google Scholar]
- 26.Wrigley CS, Neuhaus JO. The matching of two sets of factors. Am Psychol. 1955;10:418–419. [Google Scholar]
- 27.MacCallum RC, Widaman KF, Zhang S, et al. Sample size in factor analysis. Psychol Methods. 1999;4:84–99. [Google Scholar]
- 28.Muthén LK, Muthén BO. Mplus User’s Guide. Los Angeles, CA: Muthén & Muthén; 1998–2008. [Google Scholar]
- 29.Hayton JC, Allen DG, Scarpello V. Factor retention decisions in exploratory factor analysis: a tutorial on parallel analysis. Organ Res Methods. 2004;7:191–205. [Google Scholar]
- 30.Horn JL. A rationale and test for the number of factors in factor analysis. Psychometrika. 1965;30:179–185. doi: 10.1007/BF02289447. [DOI] [PubMed] [Google Scholar]
- 31.O’Connor BP. SPSS and SAS programs for determining the number of components using parallel analysis and Velicer’s MAP test. Behav Res Methods Instrum Comput. 2000;32:396–402. doi: 10.3758/bf03200807. [DOI] [PubMed] [Google Scholar]
- 32.Bentler PM. Comparative fit indices in structural models. Psychol Bull. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- 33.Tucker LR, Lewis C. A reliability coefficient for maximum likelihood factor analysis. Psychometrika. 1973;38:1–10. [Google Scholar]
- 34.Steiger JH, Lind JM. Statistically based tests for the number of common factors. Paper presented at the annual meeting of the Psychometric Society; Iowa City, IA. 1980. [Google Scholar]
- 35.Bentler PM. EQS Structural Equations Program Manual. Encino, CA: Multivariate Software Inc; 1995. [Google Scholar]
- 36.Muthén K, Muthén BO. Mplus User’s Guide. Los Angeles, CA: Muthén & Muthén; 1998–2001. [Google Scholar]
- 37.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Model. 1999;6:1–55. [Google Scholar]
- 38.Yu C, Muthén BO. Evaluation of Model Fit Indices for Latent Variable Models with Categorical and Continuous Outcomes. Paper presented at the annual conference of the American Educational Research Association; New Orleans. 2002. [Google Scholar]
- 39.Yu C. Evaluating Cutoff Criteria of Model Fit Indices for Latent Variable Models With Binary and Continuous Outcomes. Los Angeles: Dissertation, University of California; 2002. [Google Scholar]
- 40.Jöreskog KG. How large can a standardized coefficient be? 1999 [see http://www.ssicentral.com/lisrel/techdocs/HowLargeCanaStandardizedCoefficientbe.pdf]
- 41.Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. [Google Scholar]
- 42.Turk DC, Swanson KS, Gatchel RJ. Predicting opioid misuse by chronic pain patients: a systematic review and literature synthesis. Clin J Pain. 2008;24:497–508. doi: 10.1097/AJP.0b013e31816b1070. [DOI] [PubMed] [Google Scholar]
- 43.Passik S, Kirsh KL, Casper D. Addiction-related assessment tools and pain management: instruments for screening, treatment planning and monitoring compliance. Pain Med. 2008;9(suppl 2):S145–S166. [Google Scholar]
- 44.Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic noncancer pain: prediction and identification of aberrant drugrelated behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:131–146. doi: 10.1016/j.jpain.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Sullivan MD, Von Korff M, Banta-Green C, et al. Problems and concerns of patients receiving chronic opioid therapy for chronic non-cancer pain. Pain. 2010;149:345–353. doi: 10.1016/j.pain.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boudreau DM, Doescher MP, Jackson JE, et al. Impact of healthcare delivery system on where HMO-enrolled seniors purchase medications. Ann Pharmacother. 2004;38:1317–1318. doi: 10.1345/aph.1D569. [DOI] [PubMed] [Google Scholar]