Abstract
Primary cancer of the liver (hepatocellular carcinoma, HCC) is one of the most common cancers worldwide; HBV is the major cause of HCC. A vaccine that protects against HBV infection was invented in 1969 and is now one of the most commonly used vaccines. National vaccination programs have dramatically reduced the prevalence of HBV infection and carriers, with a concomitant decrease in the incidence of HCC in the vaccine-impacted populations. HBV vaccine is the first widely used cancer prevention vaccine; a second that protects against papilloma virus and cancer of the cervix has recently been introduced.
Appropriate treatment of HBV carriers with antivirals can reduce the titers of HBV in their blood and thereby greatly reduce the risk of HCC and chronic liver disease. Further data are required to establish criterion for treatment to enable protocols for medical and prevention programs.
There are other viral caused cancers and an understanding of their pathogenesis is an important future direction for research to reduce the human burden of cancer.
Keywords: Hepatitis B Virus, HBV, Hepatocellular Carcinoma (HCC, HBV vaccine, Primary prevention, Secondary prevention, Prevention by delay, Treatment of HBV and HCC
2. INTRODUCTION
Primary cancer of the liver (hepatocellular carcinoma, HCC) is one of the most common cancers in the world. It is estimated to be the third most common cause of cancer death in males and the seventh most common in females. About 70-80 % of HCC is associated with HBV as an etiological agent. Hepatitis C virus accounts for a large part of the remainder. As for all cancers there are multiple factors that contribute to the risk of HCC. In addition to HBV and HCV infection these include chronic alcohol use, increased iron storage levels, gender (males are much more likely than females to develop HCC), aflatoxins, (carcinogenic products of fungi such as Aspregillis that can infest food stuffs), possibly arsenic in water and food, and others. The vaccine that prevents infection with HBV is now used widely and several studies show that it decreases the incidence of HCC as well as acute and chronic liver disease. This is the first cancer prevention vaccine, a second has recently been introduced, and it is highly likely that others will be found in the future. Cancer prevention is the most effective means to ease the world's burden of cancer and further research and application in the field should be encouraged and supported (1).
3. HEPATITIS B VIRUS EPIDEMIOLOGY
Infection with HBV is extremely common. Two billion people (about one third of the world's population) have been infected with the virus. In most cases the infected persons remain well and produce protective antibody against the surface antigen of HBV (anti-HBs) or they may produce antibodies against the core protein (anti-HBc) or other antibodies. Some develop acute hepatitis from which they ordinarily recover, while a few develop fulminant hepatitis that can lead to severe illness and death. A significant percentage – the amount varies from place to place – becomes chronic carriers of HBV. The prevalence's are 10 - 15% in eastern Asia and the Pacific Islands, 5-10% in Africa, and 10% or more in the Amazon basin. There are also high frequencies in eastern and southern Europe, among Australian aborigines, in Arctic Inuits, and others. The prevalence is low in Western Europe (although higher in southern and Eastern Europe), and in North America although there are higher frequencies in some groups in all populations. Altogether there are about 400 million carriers worldwide. This represents an enormous actual and potential disease load. In some studies, the lifetime risks for liver-related death in male HBV carriers is 40% ; about one million deaths a years are ascribed to HBV infection.
HBV is transmitted at high frequency from infected mothers to their children, venerealy, between young sibs, by needle injection, and by other means that facilitate the transfer of blood from an infected to an uninfected person.
4. PRIMARY CANCER OF THE LIVER AND HBV
It had been suspected for many years that chronic hepatitis was part of the etiological process that resulted in primary cancer of the liver. Pathologists studying the disease in Africa noted that most cases of HCC developed on a background of chronic liver disease and cirrhosis (2,3). However, it was not possible to test this hypothesis directly until we had introduced the test for HBsAg and the carrier state could be detected. In 1969 we hypothesized that HBV was a cause of HCC (4). This was soon confirmed in studies from many laboratories worldwide.
There were subsequent convincing studies on the etiological role of HBV in the pathogenesis of HCC. Beasley and his colleagues conducted a prospective study of male workers in Taiwan some of whom were carriers of HBV and others were not (5). They were followed for about five years or longer; death and cases of HCC were recorded. The vast majority of HCC cases were in individuals who were HBV carriers at the beginning of the study. The annual incidence of HCC in the entire study population was 55/100,000 while in the carriers it was 351/100,000. They estimated that the relative risk of HCC for male HBV carriers was 223. Similar but lower risk estimates have been made in other studies.
Woodchucks (groundhogs, Marmota monax) may be infected with Woodchuck Hepatitis Virus (WHV) a virus that is very similar to HBV. (The DNA homology between HBV and WHV is more than 70%). Millman showed that the probability of HBV carriers developing the woodchuck equivalent of HCC was extremely high and increased with age (6). Tennant was able to breed woodchucks under laboratory conditions and he and his colleagues showed that woodchucks inoculated with WHV soon after birth and who became carriers of WHV had nearly a 100% probability of developing HCC (7). By analogy, these studies strongly support the hypothesis that HBV is an etiologic agent for a large proportion of HCC cases.
There have been extensive studies of the molecular biology of HBV and its host in order to understand how a virus can cause cancer. For example, the HBx gene inserted into transgenic mice causes cancer of the liver in these experimental animals and the relation of elements of the HBV genome to “cancer genes” has been the subject of many investigations (8, 9).
5. HBV VACCINE AND PREVENTION OF HCC
We discovered the hepatitis B virus in 1967 and invented the vaccine two years later using a novel method (10, 11, 12). Particles made up only of the surface antigen of HBV (HBsAg) are produced in large quantities in the peripheral blood of carriers by the S gene of HBV inserted into the genome of the infected human liver cells. These particles, that are not infectious or apparently pathogenic, are separated form the whole virus particles that are infectious and pathogenic, treated to kill any remaining living virus and preservatives and adjuvants are added. This vaccine was field tested by Szmuness and his colleagues in a high risk (for hepatitis B) population and the vaccine was found to be effective and safe (13, 14). Their study population included 1083 people (549 given the vaccine and 534 a placebo). The results were conclusive; the protection rate was over 90% and there was no evidence of toxicity. The FDA approved the blood derived vaccine within two years of the publication of the Szmuness paper. By the early 1980s, national vaccination programs were already in place and effective. Trials of vaccine usually require much larger numbers; for example the field trial for poliomyelitis vaccine required over a million children and the projected trials for the so far HIV vaccine have and will employ tens of thousands of individuals. A small percentage of HBV vaccine non-responders could be due to vaccine escape mutants. Vaccines containing pre-S epitopes have been formulated and tested to attempt to respond to this problem (15).
HBV vaccine is now produced by recombinant methods.
By 2004 about 80% of the nations in the World Health Organization had national vaccination programs. In many jurisdictions well over 90% of newborn children are vaccinated and programs for adolescents and adults are increasing. Within two decades of the introduction of the vaccine and less than a decade since its widespread use there have been striking decreases in the prevalence of the virus in the vaccinated populations. For example, in a regional study in China, the prevalence of HBV carriers dropped from 16% before vaccination to 1.4% afterwards. Similarly, the drop in Taiwan was from 9.8% to 1.3%, in Spain from 9.3% to 0.9%, and there were similar dramatic drops in many other locations. In some studies the prevalence has also decreased in the non-vaccinated cohorts although the reason for this is not clear.
Of more relevance to the topic of this paper is the effect of the vaccination program on the incidence of HCC (TABLE 1). In a national study in Taiwan, the average annual incidence in children 6 - 9 years fell from 0.52/100,000 before the vaccination program to 0.13/100,000 afterwards (16). In a similar study in Korea that included adults, the results were in the same direction (17). The cohort consisted of 370,285 males over 30 of whom 39,934 had been vaccinated. The risk ratio for HCC among those who were not vaccinated and did not have natural protective antibody was 18.1 compared to unvaccinated and initially uninfected individuals. It was 0.58 in the vaccinated group and 0.34 in those with naturally acquired immunity.
TABLE 1.
THE INCIDENCE OF HEPATOCELLULAR CARCINOMA PER 100,000 POPULATION BEFORE AND AFTER HBV VACCINATION
| LOCATION | BEFORE | AFTER | NOTES |
|---|---|---|---|
| Taiwan | 0.70 | 0.36 | Ages 6-14 |
| " | 0.52 | 0.13 | Ages 6-9 |
| Korea | 18.1 | 1)Vaccinated 0.58 2)“natural” anti-HBs 0.34 |
Cohort 370,285 m. 30+. 35,934, vaccinated |
These studies demonstrate a major reduction in a deadly cancer following the institution of HBV vaccination. If these findings continue to be supported, then HBV vaccine is the first “Cancer Prevention Vaccine”. In 2007, twenty five years after the first cancer prevention vaccine a second, which protects against certain strains of papilloma virus, was introduced (18). Based on field trials and models it has been predicted that the papilloma vaccine will significantly decreases the risk of cancer of the cervix, a very common disease particularly in developing countries, and other cancers (19).
6. TREATMENT OF HBV CARRIERS TO PREVENT HCC AND CHRONIC LIVER DISEASE
The apparent success of the vaccination program in decreasing the probability of HCC makes it likely that, in time, the incidence of HCC due to HBV will decrease significantly as more and more of the population becomes immune. But, there are still 400 million carriers in the world and there will be many carriers in the population for years to come.
Early in our research on HBV we recognized that there is a very long delay between infection and the perceived recognition of illness. Many carriers – in some places, most – are infected about the time of birth, but disease, if it occurs, may not happen until much later. We introduced the concept of “Prevention (or treatment) by delay” for diseases associated with HBV (1, 11).
HBV is a patient virus; it remains in its carrier host for three, four or even five or more decades without causing any perceptible trouble. A goal of “therapy” would be to delay the onset of symptoms in the carrier so that the asymptomatic period would prevail still longer and the host could live out his or her expected life span and die of some other disease. Meanwhile, in populations protected by the vaccine, the incidence and prevalence of HBV should gradually decrease or even conceivably, disappear.
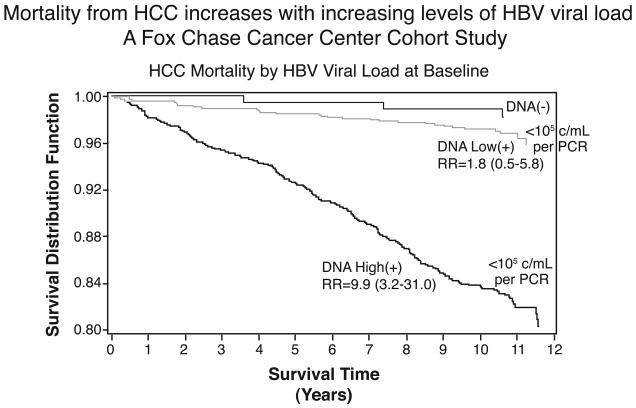
There is now considerable evidence that prevention by delay is not only feasible but is happening. Treatment of carriers with antivirals for HBV can greatly reduce the risk of HCC, chronic liver disease (CLD), cirrhosis, and terminal liver failure. London, Evans and others at Fox Chase Cancer Center along with their colleagues in Senegal, West Africa, and in Haimen City, China studied HBV carriers in respect to the development of HCC. Carrier rates for HBV are high in both locations but the risk for HCC among carriers is much lower in Senegal than in China. They concluded from the analysis of their prospective study that viral load is associated with increased mortality from HCC and CLD in people infected with HBV (FIGURE 1). They also found that increased HBV titers at the start of the prospective study greatly increased the probability of developing HCC (20).
Figure 1.
The mortality from HCC increases with increasing levels of HBV viral load measured at the start of the study. From the Fox Chase Cancer Center Cohort Study (Reference 20).
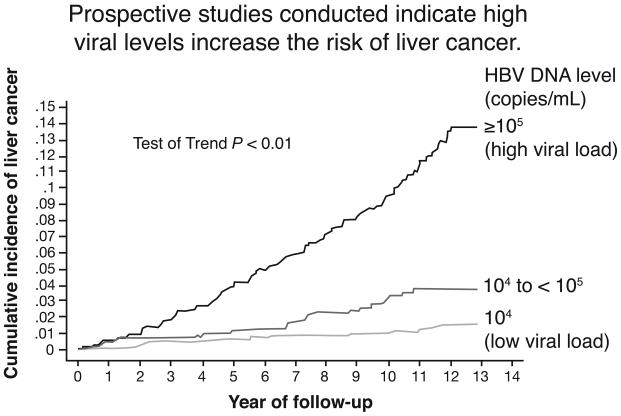
In the REVEAL study from Taiwan (FIGURE 2), Chen and colleagues showed that the cumulative number of cases of HCC increased significantly if the HBV DNA viral load was equal to or exceeded 105 copies/mL compared to carriers whose load was less than 104 copies/mL. The number of cases was intermediate for intermediate viral loads (21). Investigators in Taiwan reported that HBV titer is the major risk factor for the development of cirrhosis and chronic liver disease (22).
Figure 2.
The R.E.V.E.A.L. HBV study, Taiwan. Individuals with a low HBV viral load (<104 /mL) have a much lower risk for HCC than those with a high titer (> or =105). Patients with an intermediate titer have an intermediate risk (Reference 21).
In the past few years interventional studies, that is, treatment of HBV with antivirals, have been accomplished. In an early study overall survival and survival without clinical complications were significantly longer in patients who were seronegative for HBeAg after therapy with interferon than in those who remained seropositive (23). In a regression analysis, clearance of HBeAg was the strongest predictor of survival.
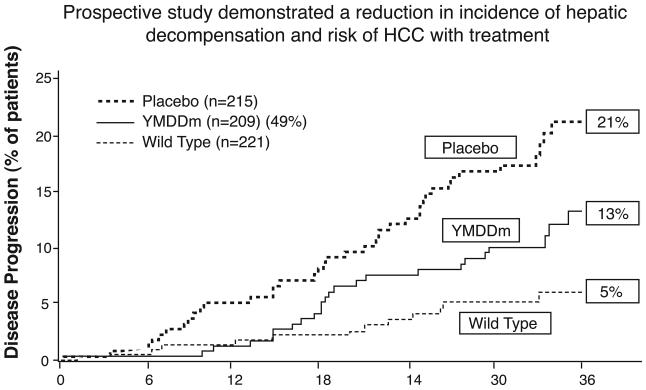
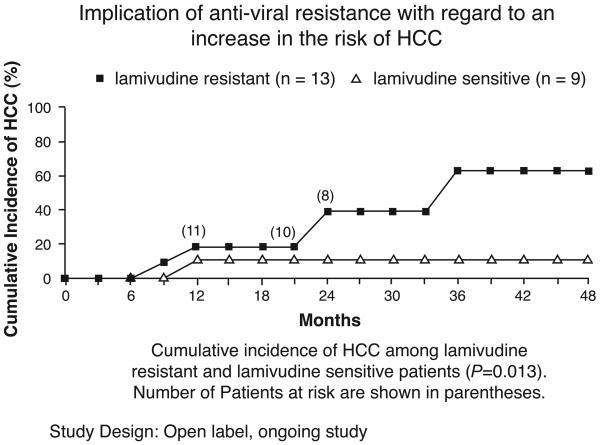
In a long-term study, antiviral treatment significantly decreased the risk of cancer and liver decompensation but the effectiveness of treatment was decreased by the development of resistance to the antiviral (FIGURE 3) (24). Over the course of three years only 5% of treated patients who were not resistant to the antiviral showed disease progression to HCC and hepatic decomposition, while 21% of those treated with a placebo did so. Treated patients who carried the mutated sequence (YMDD) associated with antiviral resistance had an intermediate level of disease progression (13%). Another study showed that the cumulative incidence of HCC was 60% in resistant patients but 10% in those not resistant to the antiviral (FIGURE 4) (25). The discovery and use of additional antivirals with lower resistance frequencies could further delay the progression of the liver disease and the onset of liver disease.
Figure 3.
In a prospective therapeutic study, treated HBV patients who developed resistance to lamivudine (YMDD mutation) had a higher percentage of progression to hepatic decompensation and HCC than treated patients who did not have the mutation. Placebo controls had a much higher percentage of progression than either (Reference 24).
Figure 4.
Treated HBV patients who developed lamivudine resistance had a higher incidence of HCC than patients who did not develop resistance (Reference 25).
7. DISCUSSION
It appears that treatment that lowers the titer of HBV DNA can considerably decrease the risk of the dire long term effects of HBV infection, chronic liver disease, liver failure, and cancer of the liver. It may not be necessary to completely eliminate the virus. That implies that a moderately effective antiviral or a very effective one in low dose that decreases the probability of detrimental side effects, would be satisfactory for long term treatment. This dosage level could be adjusted to achieve significant reduction of disease risk and minimize drug side effects. An essential characteristic of a good antiviral; would be a low incidence of resistance to therapy along with effective antiviral action. Combination therapy is effective and the future invention of drugs that act on different features of pathogenesis could further improve outcome (26, 27). The Hepatitis B Foundation maintains a current list of available medications for chronic HBV infection (28).
The treatment by delay approach is an unusual form of cancer therapy. Most forms of cancer treatment are based on the elimination of cancer cells by the use of surgery, radiation, and/or chemotherapy. This is often accomplished with the concomitant loss of normal cells to the great discomfort of the patient. Antiviral treatment is directed to decreasing viral replication and other approaches to diminish the viral pathogenic effects. The destruction of the patient's non-infected cells could be minimal with subsequent benefit to the patient.
In many medical communities current practice is to treat only carriers with symptoms, biopsy evidence of chronic disease and other indicators of established disease. However, in some communities (e.g., Taiwan, Germany) criterion for starting treatment are less stringent including treatment of patients with sustained elevations of ALT above normal, and/or HBV DNA titers greater than 104 copies/mL. There could be a re-evaluation of the upper limits of normal if the ALT elevation criterion is used. Recently, in a study of adolescent boys in a correctional institution, a significantly lower upper limit of normal ALT was recommended (29). The decision to extend the number of carriers treated will depend on the accumulation of more data and ongoing analysis of the evidence-based criterion.
If treatment of more carriers is instituted there will be several important consequences. Screening of high risk and other populations will need to be increased to identify those who should be treated. It would also help to identify those who come in contact with carriers and require vaccination. Treatment of potentially infectious but asymptomatic carriers will enhance the vaccination program as it will decrease the pool of carriers who could infect others. This would speed the eventual control of HBV.
An unfortunate outcome of the identification of carriers of HBV is that, in some situations, it has led to their stigmatization (30, 31). Carriers without any evidence of disease and who in normal social interactions were not infectious have been forced to leave their jobs, been excluded from professional school and have had difficulties with relationships. It remains a serious issue particularly in places where there is inadequate knowledge of HBV. Appropriate treatment of carriers would decrease their infectivity and thereby greatly decrease the risk of transmission. This would be advantageous to the carrier and to those he or she contacts, and may also relieve the public anxiety and stigmatization of carriers. The resolution of this problem would be a major benefit of the treatment of carriers including those who are asymptomatic.
Early in the HBV and cancer research we encouraged the search for other vaccine preventable cancers (32). We noted (33)
The associations of HBV and WHV with this carcinoma suggest that there may be other, similar virus-cancer relations that could be dealt with by primary-prevention strategies. We believe that searching for such viruses and cancers in animals and human beings is an important … direction for cancer research.
In 2007, the second cancer prevention vaccine was introduced (18). A vaccine against certain strains of papilloma virus is effective in preventing infection with these viruses and cancer of the cervix. It may also be protective against other cancers. It has taken nearly forty years since the invention of the HBV vaccine and about 25 years since its widespread use, for the introduction of the second vaccine, but there is every reason to hope that additional cancer prevention vaccines will be developed soon.
There are a number of candidates - EBV and Burkitt's lymphoma and nasopharyngeal cancer; HHV-8 and Kaposi's sarcoma; HCV and hepatocellular carcinoma; HTLV–1 and Adult T cell leukemia/lymphoma; HTLV – 2 and hairy cell leukemia (34). Other possibilities include Adenoma virus and mesothelioma. An interesting association, not yet fully explored, is between HBV and cancer of the pancreas. A population based cohort study found that the presence of HBsAg along with HBeAg was an independent risk factor for cancer of the pancreas with a seven-fold increased risk (35). A recent study has confirmed the association (36). If confirmed, this implies that HBV may have an etiological role in the pathogenesis of pancreatic cancer, and/or that there is a similar virus that is part of its etiological process.
8. PERSPECTIVE
Prevention is a more effective means for disease control than treatment after the onset of disease symptoms. However, it may be less dramatic; if all goes well, nothing happens, and the putative patient is unaware of benefit. Great advances in cancer control have resulted from prevention; the campaign for cessation of smoking, colonoscopy for cancer of the colon, cervical smears for cancer of the cervix, early detection of cancer of the breast and other cancers, probably some forms of dietary control, and others. Prevention of viral caused cancers by vaccination for HBV and papilloma viruses are now accepted as important preventive methods and their effects will be increasingly apparent in the next few years. The value of treatment of those already infected with antivirals is already apparent for HBV. The increasing understanding of the molecular biology of hosts and viruses will aid in the process of drug discovery. The use of antivirals should also decrease the current difficulties of stigmatization of HBV carriers
These wholesome outcomes with HBV and pappiloma virus should, and I believe will, increase research on cancers caused by infectious agents and, in time, the deployment of vaccines and other methods for infection control.
9. ACKNOWLEDGEMENTS
The project described was supported by Grant Number P30 CA006927 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the NIH.
10. REFERENCES
- 1.Blumberg BS, London WT. Hepatitis B virus and the prevention of primary hepatocellular carcinoma. Editorial. N. Engl. J. Med. 1981;304:782–784. doi: 10.1056/NEJM198103263041312. [DOI] [PubMed] [Google Scholar]
- 2.Payet M, Camaine R, Rene P. Le cancer primitive du foie, etude critique a propos de 240 cas. Rev. Intern. Hepatol. 1956;4:1–20. [PubMed] [Google Scholar]
- 3.Steiner E, Davies JN. Cirrhosis and primary liver carcinoma in Uganda Africans. Br J Cancer. 1957 Dec;11:523–34. doi: 10.1038/bjc.1957.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith JB, Blumberg BS. Viral hepatitis, post-necrotic cirrhosis and hepatocellular carcinoma. Lancet. 1969;2:953. doi: 10.1016/s0140-6736(69)90604-7. [DOI] [PubMed] [Google Scholar]
- 5.Beasley RP. Hepatitis B virus—the major etiology of the hepatocellular carcinoma. Cancer. 1988;1942:61. doi: 10.1002/1097-0142(19880515)61:10<1942::aid-cncr2820611003>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 6.Blumberg BS, Millman I, Venkateswaran PS, Thyagarajan SP. Hepatitis B virus and hepatocellular carcinoma--treatment of HBV carriers with Phyllanthus amarus. In: Nieburgs HE, editor. Cancer Detection and Prevention. CRC Press Inc.; Boca Raton FL: 1989. pp. 195–201. Boca Raton, FL. [PubMed] [Google Scholar]
- 7.Popper H, Roth L, Purcell RH, Tennant BC, Gerin JL. Hepatocarcinogenicity of the woodchuck hepatitis virus. Proc Natl Acad Sci USA. 1987;84:866. doi: 10.1073/pnas.84.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim C-Y, Koike K, Saito I, Miyamura T, Jay G. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature. 1991;351:317. doi: 10.1038/351317a0. [DOI] [PubMed] [Google Scholar]
- 9.Ueda H, Ullrich SJ, Gangemi JD, Kappel CA, Ngo L, Feitelson MA, Jay G. Functional inactivation but not structural mutation of p53 causes liver cancer. Nature Genetics. 1995;9:41–47. doi: 10.1038/ng0195-41. [DOI] [PubMed] [Google Scholar]
- 10.Blumberg BS. Australia antigen and the biology of hepatitis B. Science. 1977;197:17–25. doi: 10.1126/science.325649. [DOI] [PubMed] [Google Scholar]
- 11.Blumberg BS, Millman I. Vaccine Against Viral Hepatitis and Process. US Patent #3,636,191, Filed Oct. 8, 1969 Issued Jan. 18, 1972. [Google Scholar]
- 12.Blumberg BS, Hepatitis B. The Hunt for a Killer Virus. Princeton University Press; Princeton NJ: 2002. Princeton NJ. [Google Scholar]
- 13.Szmuness W, Stevens CE, Harley EJ, Zang EA, Oleszko WR, William DC, Sadovsky R, Morrison JM, Kellner A. Hepatitis B vaccine: demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States. N Engl J Med. 1980 Oct 9;303:833–841. doi: 10.1056/NEJM198010093031501. [DOI] [PubMed] [Google Scholar]
- 14.Szmuness W, Stevens CE, Zang EA, Harley EJ, Kellner A. A controlled clinical trial of the efficacy of the hepatitis B vaccine (heptavax B); a final report. Hepatology. 1981;1:377–385. doi: 10.1002/hep.1840010502. [DOI] [PubMed] [Google Scholar]
- 15.Tsuda F, Machida A, Mishiro S, Nakamura T. A synthetic peptide vaccine involving the product of the pre-S(2) region of hepatitis B virus DNA: protective efficacy in chimpanzees. PNAS. 1986 December 1;83(23):9174–9178. doi: 10.1073/pnas.83.23.9174. Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. N Engl J Med. 1997;336:1855–1859. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 17.Lee M-S, Kim D-H, Kim H, Lee H-S, Kim C-Y, Park T-S, Yoo K-Y, Park B-J, Ahn Y-O. Hepatitis B vaccination and reduced risk of primary liver cancer among male adults: a cohort study in Korea. Int. J. Epidemiol. 1998;27:316–319. doi: 10.1093/ije/27.2.316. [DOI] [PubMed] [Google Scholar]
- 18.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP). Quadrivalent Human Papilloma virus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007 Mar 23;56(RR-2):1–24. [PubMed] [Google Scholar]
- 19.Elbasha E, Dasbach EJ, Insinga RP. Model for assessing human papilloma virus vaccination. Emerg Infect Dis. 2007;13:29–41. doi: 10.3201/eid1301.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang B, Kruger WD, Chen G, Shen F, Lin WY, Mboup S, London WT, Evans AA. Hepatitis B viremia is associated with increased risk of hepatocellular carcinoma in chronic carriers. J Med Virol. 2004;72:35–40. doi: 10.1002/jmv.10559. [DOI] [PubMed] [Google Scholar]
- 21.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. REVEAL-HBV Study Group.Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 22.Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-In HBV (the REVEAL-HBV) Study Group. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006:678–86. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 23.Niederau C, MD, Heintges T, MD, Lange S, MD, Goldmann G, MD, Niederau CM, MD, Mohr L, MD, Häussinger D., MD Long-Term Follow-Up of HBeAg-Positive Patients Treated with Interferon Alfa for Chronic Hepatitis B. N Engl J Med. 1996;334:1422–1427. doi: 10.1056/NEJM199605303342202. [DOI] [PubMed] [Google Scholar]
- 24.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Cirrhosis Asian Lamivudine Multicentre Study Group. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 25.Andreone P, Gramenzi A, Cursaro C, Biselli M, Cammà C, Trevisani F, Bernardi M. High risk of hepatocellular carcinoma in anti-HBe positive liver cirrhosis patients developing lamivudine resistance. J Viral Hepat. 2004;11:439–442. doi: 10.1111/j.1365-2893.2004.00564.x. [DOI] [PubMed] [Google Scholar]
- 26.Peters GM, Hann HW, Martin P, Heathcote EJ, Buggisch P, Rubin R, Bourliere M, Kowdley K, Trepo C, Gray DF, Sullivan M, Kleber K, Ebrahimi R, Xiong S, Brosgart CL. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology. 2004;126:91–101. doi: 10.1053/j.gastro.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 27.Marcellin P, Lau GKK, Bonino F, Farci P, Hadziyannis S, Jin R, Lu Z-M, Piratvisuth T, Germanidis G, Yurdaydin C, Diago M, Gurel S, Lai M-Y, Button P, Pluck N. Peginterferon Alfa-2a Alone, Lamivudine Alone, and the Two in Combination in Patients with HBeAg-Negative Chronic Hepatitis B for the Peginterferon Alfa-2a HBeAg-Negative Chronic Hepatitis B Study Group. New Eng. J. Med. 2004;351:1206–17. doi: 10.1056/NEJMoa040431. [DOI] [PubMed] [Google Scholar]
- 28.Web site of the Hepatitis B Foundation ( www.hepb.org)
- 29.Van der Poorten D, Kenny DT, Butler T, George J. Liver disease in adolescents: a cohort study of high-risk individuals. Hepatology. 2007;46:1750–1758. doi: 10.1002/hep.21918. [DOI] [PubMed] [Google Scholar]
- 30.Blumberg BS. Bioethical questions related to hepatitis B antigen. Am. J. Clin. Pathol. 1976;65:848–853. [PubMed] [Google Scholar]
- 31.Blumberg BS, Fox RC. The Daedalus effect: Changes in ethical questions relating to hepatitis B virus. Ann. Intern. Med. 1985;102:390–394. doi: 10.7326/0003-4819-102-3-390. [DOI] [PubMed] [Google Scholar]
- 32.Blumberg BS, Larouze B, London WT, Werner B, Hesser JE, Millman I, Saimot G, Payet M. The relation of infection with the hepatitis B agent to primary hepatic carcinoma. Am. J. Pathol. 1975;81:669–682. [PMC free article] [PubMed] [Google Scholar]
- 33.Blumberg BS, London WT. Hepatitis B virus and the prevention of primary hepatocellular carcinoma. Editorial. N. Engl. J. Med. 1981;304:782–784. doi: 10.1056/NEJM198103263041312. [DOI] [PubMed] [Google Scholar]
- 34.DaVita VT, Hellman S, Rosenberg SA. Cancer: Principles and Practice of Oncology. 7th edition. Lippincott Williams and Wilson; Philadelphia: 2005. Philadelphia. 2005. [Google Scholar]
- 35.Nunez M. Hepatitis B Virus May Be a Risk Factor for Other Cancers Besides Hepatocellular Carcinoma. Digestive Disease Week. 2005 Abstracts. [Google Scholar]
- 36.Manal MH, Li D, Adel SE-D, Wolf RA, Bondy ML, Davila M, Abbruzzese JL. Association between Hepatitis B virus and pancreatic cancer. J. Clin. Oncol. 2008;26:4557–4562. doi: 10.1200/JCO.2008.17.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]