Abstract
HIV infection and antiretroviral therapy (ART) are now established independent risk factors for osteoporosis. With a spate of recent studies reporting significant elevations in fracture prevalence in HIV patients, and a rapidly aging demographic, defining the mechanisms underlying HIV/ART-induced skeletal decline has become imperative. The recent emergence of the field of “osteoimmunology” has provided a conceptual framework to explain how the immune and skeletal systems interact. Furthermore, it is becoming clear that inflammatory states leading to perturbations in the immuno-skeletal interface, a convergence of common cells and cytokine mediators that regulate both immune and skeletal systems, conspire to imbalance bone turnover and induce osteoporosis. In this review we examine the role of inflammation in the bone loss associated with diverse inflammatory conditions and new concepts into how the underlying mechanisms by which inflammation and immune dysregulation impact bone turnover may be pertinent to the mechanisms involved in HIV/ART-induced bone loss.
Keywords: HIV, AIDS, osteoporosis, inflammation, immune-skeletal interface
Introduction
Evidence continues to emerge for the existence of an inexplicable convergence within the immune and skeletal systems, the result of a centralization of common cell types and cytokine mediators that may be described as the “immuno-skeletal interface” [1]. The clinical implications of the immuno-skeletal interface are underscored by the changes in bone turnover and loss of bone mineral density (BMD) commonly associated with conditions such as rheumatoid arthritis [2], periodontal infection [3], inflammatory bowel disease [4], type I and II diabetes [5–7], systemic lupus erythematosus [5], and sickle cell disease [8]. All of these pathologic conditions are intimately associated with immune dysregulation and chronic inflammation. Interestingly, animal models, backed up by limited clinical data, now support the contention that postmenopausal osteoporosis, the archetypal bone disease of women, is itself the result of immune dysregulation associated with a persistent low-grade inflammatory state [9, 10]. Given the well-documented correlation between inflammation and bone disease and the importance of the immuno-skeletal interface in the regulation of basal and pathological bone turnover, we recently proposed that disruption of the immune system may in part underlie the skeletal abnormalities ubiquitously documented in the setting of HIV infection [11••], a condition associated with severe immune deficiency and a persistent state of immune activation and chronic inflammation [12, 13]. This review examines the depth of the integration between the immune and the skeletal systems and emerging concepts pertinent to immune and inflammatory changes that may underlie skeletal deterioration in the setting of HIV-1 infection and antiretroviral therapy (ART).
The Skeletal Profile in the Setting of HIV Infection and ART
The Direct Effects of HIV Infection on the Skeleton
As a result of a series of observational cohort studies conducted over the past decade we have come to appreciate the extent of skeletal abnormalities associated with HIV infection [14–16]. In fact, it is now clearly established that HIV infection is an independent risk factor for osteopenia and osteoporosis [17, 18]. Current data suggest that two of every three HIV-seropositive individuals naïve to ART exhibit osteopenia and have a 3.7 higher odds of developing osteoporosis [14]. Teasing out the relative contribution of direct viral effects on the skeleton vis-à-vis the contribution of other traditional osteoporosis risk factors such as drug abuse, smoking and alcohol consumption, associated with patient lifestyle, and AIDS-related pathologies such as muscle wasting, kidney disease, vitamin D deficiency, and hypogonadism that abound in this patient population, has been challenging. As a consequence, many believe that the etiology of bone decline in the setting of HIV infection is likely multifactorial.
Indirect Effects of Antiretroviral Therapy on the Skeleton
Another perplexing paradox is that unlike its effect on many other HIV-related pathologies, ART exacerbates, rather than ameliorates bone loss [19, 20]. Interestingly, the skeletal effects of ART, though varied in magnitude, appear universal regardless of regimen [21•] and are typically documented within the first 1–2 years of therapy [20, 22–24]. Clinical studies routinely find an average loss in BMD of 2%–6% at the femur, lumbar spine, or hip [20, 25, 26], all common fracture prone anatomical sites in the body. Contextually, bone loss of this magnitude is not inconsequential, and approaches that sustained in women during the first 2–5 years following menopause, a time when rapid bone deterioration is in effect [20, 27]. This loss of BMD further compounds the bone loss already sustained in the majority of patients as a result of chronic HIV infection.
The Health Hazards of Combined HIV/ART-Related Skeletal Deterioration
Because fragility fractures are typically a rare event in younger populations, especially in men, evidence for higher fracture incidence in the comparatively young HIV/AIDS population has until very recently been mostly anecdotal and contentious. However, a series of recent clinical studies have now unambiguously demonstrated a dramatic rise in fracture prevalence with HIV infection. Fracture rates twofold to fourfold higher than the general population have consistently been documented over a wide age range. In the landmark study by Triant et al. [28••] involving 8525 HIV-infected and 2,208,792 non–HIV-infected control patients, an increase in fracture prevalence among HIV-infected patients of up to fourfold was observed in both men and women over a wide age range. Importantly, while fracture rates in HIV-seronegative men have been historically low until advanced age because of intrinsically higher BMD and increased bone size, HIV-infected men in this cohort demonstrated a dramatic rise in fracture prevalence at relatively young ages. Similarly, fracture rates for the HIV/AIDS population were twofold to fourfold higher in the HIV Outpatient Study (HOPS) cohort, a study involving 5826 HIV-infected patients [29••]. In the Veteran Administration (VA) cohort comprising 39,375 HIV-infected patients, fracture rates were 24%–32% higher [30]. A smaller Canadian study with 138 HIV-infected women and 402 controls reported a close to twofold higher fracture rate with HIV infection [31]. These important epidemiological data underscore the clinical and public health implication of fragility bone disease in the HIV/AIDS population and highlight the urgency of uncovering the underlying mechanisms of HIV-induced and ART-induced bone loss.
Bone Modeling and the Homeostatic Bone Remodeling Processes
Full appreciation of the depth of the immuno-skeletal interface and its impact on bone health requires an understanding of the structure and regulation of the skeleton and the roles of the key cells involved in skeletal remodeling. Eighty-five percent of adult bone mass is achieved by 18 years of age in girls and by 20 years of age in boys [32]. By early adulthood (25–35 years of age), skeletal formation is essentially complete. At this time “bone remodeling,” a process of bone renewal, begins and involves the clearance of damaged bone and bone with diminished load-bearing properties by osteoclasts, and the resynthesis of new bone by osteoblasts. In early adulthood, this process of bone breakdown and reformation ultimately leads to a period of relative stability in BMD because the amount of old bone removed is balanced by the amount of new bone formed with no net gain or loss of BMD, a state of “homeostatic” balance. However, this state of skeletal stability is relatively transient, and by the forth decade of life, the rate of bone resorption typically exceeds that of formation, resulting in a gradual but persistent decline in BMD. While in men this process of age-related skeletal decline is relatively linear, in women bone loss is markedly accentuated for approximately 5–10 years following menopause [33]. This accelerated bone loss associated with menopause is compounded by the fact that the bones in women are typically smaller and less dense than those in men, resulting in bone fracture incidence that is in excess of twofold higher in women than men [33]. Nonetheless, both sexes are at risk and 50% of women and 20% of men over the age of 50 will sustain a fracture in their remaining lifetimes as a consequence of osteoporosis [32].
The Depth of the Immuno-Skeletal Interface
The process of homeostatic bone remodeling is complex and susceptible to changes in many physiologic processes and biochemical systems in the body, and as such, can be impacted by diverse pathologic conditions. Over the last decade we have begun to appreciate the degree of the influence of immune cells on bone metabolism [5, 34, 35].
Although our understanding of the interactions between the immune system and the bone building machinery is still rudimentary, it is well recognized that mesenchymal stem cells (MSCs) give rise to bone marrow stromal cells, a population containing the precursor of bone-forming osteoblasts. MSCs are known to exert an important regulatory influence on immune cell function [36] while osteoblasts themselves regulate the hematopoietic stem cell niche from whence all other immune and blood cells are derived [37]. In addition, cytokine mediators of immune origin such as tumor necrosis factor alpha (TNFα) are potent inhibitors of bone formation [38, 39] and may drive down bone formation under inflammatory states, widening the gap between formation and resorption and exacerbating bone loss. By contrast, activated T cells also secrete factors with the capacity to stimulate the differentiation of bone marrow stromal cells into the osteoblast phenotype [40]. Recently we identified a novel T-cell–secreted cytokine termed secreted osteoclastogenic factor of activated T cells (SOFAT) that not only promotes osteoclast formation but is capable of eliciting production of interlukin-6 (IL-6) from osteoblasts [41•]. Finally, osteoblasts may feedback on T cells by secretion of interlukin-7 (IL-7) [42], a master regulator of T-cell development and function [43].
In contrast to the cross-talk between osteoblasts and immune cells, our understanding of the immuno-skeletal interface in the regulation of osteoclastic bone resorption is relatively well developed. First, it has long been recognized that osteoclasts, the bone resorbing cells, are of myeloid origin and derived from precursors that circulate within the macrophage and monocyte lineage. More recently, was the recognition that these precursors may be distinguished from other monocytic cells by the expression on their membrane surface of receptor activator of NF-κB (RANK). The ligand for RANK (RANK ligand [RANKL]) is recognized to have important immunological functions including the integration and regulation of T-cell growth and dendritic cell functions [44]. However, in the context of bone biology RANKL is now generally considered to be the key final effector of osteoclast differentiation and activity [45]. The association of RANKL with RANK on osteoclast precursors induces their differentiation into pre-osteoclasts which ultimately fuse together into the mature multinucleated giant cells, osteoclasts, responsible for bone resorption.
A delicate control network involving osteoprotegerin (OPG), a TNF receptor superfamily member and RANKL decoy receptor, prevents RANKL from binding to RANK, moderating RANKL-induced osteoclast formation and activity [45–47].
B Cells: A Major Source of OPG in response to T-Cell Costimulation
B cells are an essential component of the adaptive immune system and of humoral immunity. Although B cells also present antigens to T cells, a principal function of B cells is to make antibodies against soluble antigens. B cells, however, are also central to basal skeletal regulation. Although it was initially thought that basal osteoclastogenesis is controlled primarily by RANKL and OPG production by cells of the osteoblast lineage [48], studies in the human system identified B cells as a prominent additional source of OPG, and responsive to T-cell costimulatory ligands in vitro [49]. More recently, in vivo data from our group have validated B-lineage OPG production as critical to the regulation of basal osteoclastogenesis and the maintenance of basal bone mass in the murine system [50]. Interestingly, as originally suggested by in vitro studies using human B cells [49], association of CD40L on activated T cells with its cognate receptor CD40 on B cells potently enhances B-cell OPG secretion in mice in vivo. The importance of this interaction was further reflected by the documentation of diminished OPG production and severe osteoclastic bone loss in murine models of B-cell, T-cell, CD40, and CD40L deficiency [50]. The importance of CD40L in the regulation of basal bone mass is further underscored by evidence of a high rate of osteopenia and fractures in humans with the inherited immune deficiency disorder X-linked hyper-IgM syndrome, a genetic disease caused by mutations in the CD40L gene [51].
T Cells and B Cells: A Significant Source of RANKL Under Inflammatory Conditions
Besides the influence of T cells and B cells on the basal production of bone-sparing OPG, under inflammatory conditions activated T cells [9, 52–54] and B cells [11••, 54] become a significant additional source of RANKL. In fact, activated lymphocytes have been implicated as key protagonists of the bone loss associated with pathologies as diverse as postmenopausal osteoporosis [10, 55, 56], rheumatoid arthritis [2], and in alveolar bone loss associated with periodontal infection [3, 54]. We have also recently reported the identification of SOFAT, a novel cytokine secreted by activated T cells that has the capacity to promote osteoclast formation in a RANKL-independent fashion that may further directly drive up osteoclastic bone resorption during inflammation, as well as indirectly promote osteoclastogenesis by stimulating RANKL production from osteoblasts through induction of IL-6 [41•].
In the presence of permissive concentrations of the survival factor macrophage colony-stimulating factor (M-CSF), RANKL (and SOFAT) are final unique effectors of osteoclast formation. However, it is recognized that many other inflammatory cytokines play a significant role in driving bone loss. Prominent among these are IL-1, IL-6, IL-7, TNFα, IFNγ, M-CSF, VEGF, IGF-1, and IL-17 [9, 57]. These inflammatory cytokines are able to upregulate osteoclastogenesis through one or more distinct mechanisms including stimulating RANKL production by lymphocytes and/or osteoblast-lineage cells, upregulating the RANKL receptor RANK on osteoclast precursors, or in the case of TNFα by amplifying the activity of RANKL at the level of signal transduction [9, 56]. By contrast, cytokines such as IL-4 and IFN-γ may mediate inhibitory actions on osteoclastogenesis. While IFNγ potently upregulates antigen presentation and drives up T-cell activation and TNFα and RANKL production [58, 59], it paradoxically has direct inhibitory effects on osteoclast differentiation by impeding RANK signal transduction [60]. Interestingly, in the context of HIV infection, high levels of suppressor of cytokine signaling-1 (SOCS-1) may modify IFNγ responses by impeding its signal transduction [61]. The actions of common inflammatory cytokines on osteoclastogenesis are summarized in Table 1.
Table 1.
Effect of inflammatory cytokines on bone cells
| Inflammatory cytokine |
Abbreviation | Bone effect |
|---|---|---|
| Interferon-gamma | IFNγ | A major product of activated T cells, IFNγ has been found to promote inflammatory bone loss during estrogen deficiency by upregulating the activity of antigen-presenting cells (especially macrophages) and leading to further T-cell activation and enhanced osteoclastogenic cytokine production [59]. Conversely, IFNγ has been reported to mediate direct inhibitory activity on osteoclast differentiation by inducing degradation of the RANK adapter protein, TRAF6, which is required for RANKL signal transduction [60]. |
| Interleukin | IL-1 | IL-1 is a potent inflammatory cytokine long associated with bone loss in postmenopausal osteoporosis [80] and animal models of estrogen deprivation [81]. IL-1 promotes RANKL production by osteoblasts [82] and is a potent amplifier of TNF-induced RANKL production by bone marrow stromal cells [83]. |
| Interleukin | IL-6 | IL-6, a potent inflammatory cytokine, has long been associated with estrogen deprivation [84] and inflammatory states leading to bone loss. IL-6 is a major product of osteoblasts and their progenitors [85] and appears to be potently osteoclastogenic; however, the exact role of IL-6 and its mechanism of action in bone turnover remains unclear. IL-6 is reported to stimulate the proliferation of osteoclast progenitors [86] and RANKL expression by synovial cells [87], but not osteoblasts [82]. Osteoclastogenic effects of IL-6 in models of rheumatoid arthritis may also stem from indirect generalized proinflammatory effects on the immune system [88]. |
| Interleukin-7 | IL-7 | IL-7 is a master regulator of T-cell production and function [43] and a potent upstream osteoclastogenic cytokine as it promotes RANKL production by T cells and is a central player in the bone loss associated with estrogen deprivation in mice by stimulating bone resorption and suppressing bone formation [9, 42, 79, 89, 90]. |
| Macrophage colony-stimulating factor | M-CSF | M-CSF is a key survival factor for cells of the monocyte/macrophage lineage including osteoclasts and their precursors and although required for osteoclast formation, M-CSF alone is incapable of stimulating osteoclast differentiation in the absence of RANKL [91, 92]. M-CSF may further aid osteoclastogenesis by upregulating expression of the RANKL receptor RANK on osteoclast precursors, expanding the osteoclast precursor pool [11••, 93]. |
| Osteoprotegerin | OPG | OPG is a decoy receptor for RANKL and moderates RANKL activity by binding to it and preventing RANKL from binding to, and initiating signal transduction from, its receptor RANK. The ratio of RANKL to OPG is a key determinant of osteoclast formation and activity [45–47, 91, 92]. |
| Receptor activator of NF-κB ligand | RANKL | RANKL, also known as osteoprotegerin ligand (OPGL), tumor necrosis factor–related activation-induced cytokine (TRANCE), and osteoclast differentiation factor (ODF), is a TNF receptor superfamily member that enhances T-cell growth and dendritic cell function [44]. RANKL is also considered to be the key osteoclastogenic cytokine and the final effector of osteoclast formation and activity [45, 91, 92, 94]. |
| Secreted osteoclastogenic factor of activated T cells | SOFAT | A recently identified novel cytokine secreted by activated T cells that directly promotes osteoclastogenesis in a RANKL-independent manner, and indirectly by inducing IL-6 production by osteoblasts [41•]. |
| Transforming growth factor-β | TGF-β | TGF-β is an important multifaceted regulator of bone metabolism. TGF-β possesses anti-inflammatory properties that suppress T-cell activation, thus reducing production of osteoclastogenic cytokines, and may limit bone loss associated with estrogen deficiency [95]. TGF-β also directly suppresses osteoclastogenesis by initiating apoptosis of osteoclasts [96, 97] and may further antagonize osteoclast formation by inducing OPG production by osteoblasts [98, 99]. Paradoxically, in vitro TGF-β can promote RANKL-induced osteoclastogenesis [100]. TGF-β is also an early osteoblast differentiation commitment factor [101] and promotes migration of osteoblast progenitors to sites of bone resorption [102]. |
| Tumor necrosis factor-alpha | TNFα | TNFα is a unique osteoclastogenic cytokine by virtue of its capacity to synergize with RANKL to amplify its osteoclastogenic and resorptive activity [56, 103–106]. TNFα can also promote RANKL production by osteoblasts [82, 83]. Furthermore, TNFα is a potent inhibitor of osteoblast differentiation and activity [39] and in vivo bone formation leading to reduced peak BMD in mice [38]. TNFα is a key protagonist of bone loss associated with estrogen deficiency [9]. |
Together these observations have led to the emergence of the field of “osteoimmunology” and have given rise to the notion that overexpression of inflammatory cytokines by cells of the immune system underlies the high rate of skeletal abnormalities associated with inflammatory and autoimmune disorders [5] and in postmenopausal osteoporosis, a condition that exhibits significant characteristics of an inflammatory state [9].
HIV-1 Infection and Inflammation
HIV infection is another disease that has now been shown to be associated with high rates of osteopenia and osteoporosis [1, 18]. A hallmark of HIV infection is a continuous stimulation of the immune system contributing to loss of CD4+ T cells as a consequence of activation-induced cell death. This process is further exacerbated by poor T-cell restoration due to reduced thymic function associated with HIV infection [12]. Although near total depletion of CD4+ T cells is a hallmark of HIV infection, there is extensive damage to the entire immune system affecting cellular, humoral, and innate immune response. This leads to severe B-cell and T-cell (both CD4+ and CD8+) exhaustion [62, 63] resulting in a dysfunctional memory B-cell compartment, and predisposing patients to AIDS-related secondary diseases and opportunistic infections [64, 65••]. Loss of CD4+ T cells is accompanied by increased immune activation affecting all major cell populations of the immune system [65••].
As previously stated, T cells are critical for B-cell function, and consequently HIV infection causes numerous direct and indirect (via T cells) perturbations in the B-cell population. B-cell numbers are significantly diminished along with a concomitant increase in the frequency of immature/transitional B cells that are associated with CD4+ T-cell lymphopenia [65••]. Lymph nodes (LNs) represent the principal site where antigen-specific memory T-cell and B-cell responses are primed and differentiated into memory and effector cells. During chronic HIV infection substantial structural changes to LNs occur, leading to fibrotic LNs. These changes are only partly reversed by ART [66] in part due to diminished space for B-cell engraftment. Furthermore, while memory B cells (CD27+) in the peripheral blood of healthy individuals comprise both B220+ and B220− subsets, HIV-infected individuals show a significant reduction in CD27+ B220− populations [65••, 67]. The cause of these changes in B-cell number and in memory subpopulations is poorly understood but cytokine imbalance, decreased T-cell function, and direct exposure to virus and viral antigens all play significant roles [67].
In addition to the direct disruption of the immune cells described above, chronic immune activation is a recognized feature of HIV infection and a strong predictor of disease progression [68•]. This is thought to result in part from HIV-induced gastrointestinal mucosal damage leading to systemic translocation of bioactive microbial cell wall products including lipopolysaccharide (LPS) that are capable of activating both the innate and adaptive immune systems [69]. LPS is well established to stimulate osteoclast production by promoting osteoblast production of RANKL, IL-1, and TNFα [70].
Taken together, these data indicate that the pathophysiologic changes associated with HIV infection could affect bone metabolism at multiple levels including the direct disruption of B-cell and T-cell functions, and induction of chronic activation of the innate and adaptive immune response via translocation of gut microbial cell wall products.
Evidence of HIV Disruption of the Immuno-Skeletal Interface
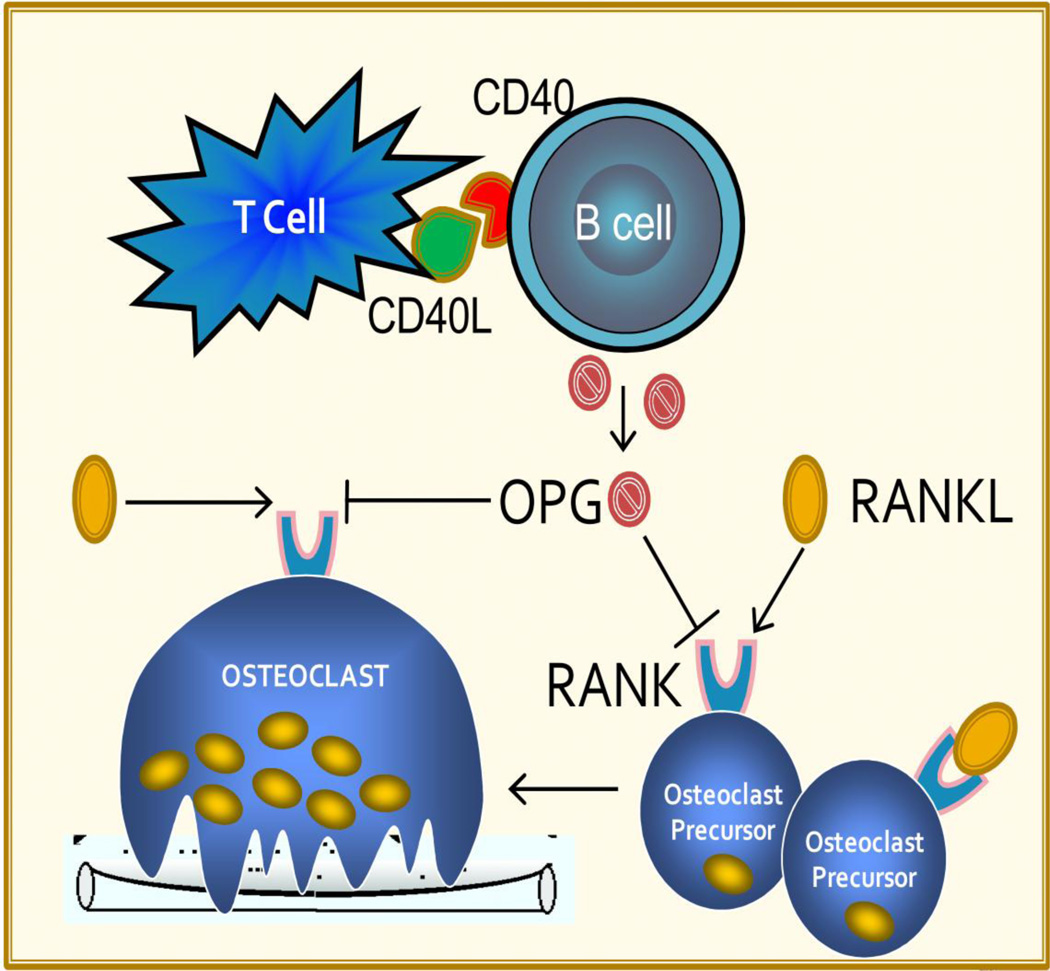
In an attempt to better understand the pathophysiology of HIV-induced bone loss, our group recently evaluated the impact of HIV infection on the immuno-skeletal interface using the HIV-1 transgenic rat model. In this murine model, there is constitutive expression of HIV-1 viral proteins as a consequence of a replication defective HIV-1 viral genome integrated into the rat DNA [71]. This animal has been demonstrated to recapitulate many of the immunologic and clinical abnormalities seen with human HIV/AIDS [71]. Similar to the skeletal changes observed in human HIV infection, HIV-1 transgenic rats underwent severe loss of BMD and extensive disruption of bone architecture and structure. Histologic and serum biochemical markers were consistent with markedly increased osteoclast number and in vivo bone resorption. Mechanistically, osteoclastogenesis was associated with altered B-cell function leading to a significant decline in production of bone-sparing OPG, an increased expression of the osteoclastogenic cytokine RANKL, and compounded by a dramatic increase in the number of osteoclast precursors [11••]. These intriguing observations provided some of the first evidence of a direct disruptive impact of HIV on the immuno-skeletal interface. This model of HIV-induced bone loss is outlined diagrammatically in Figure 1. Studies to ratify these findings in human HIV infection are currently underway and if validated will broaden our understanding of the mechanisms of HIV-induced skeletal damage.
Figure 1.
Model of HIV-induced disruption of the immune-skeletal interface: B-cell production of OPG, regulated in part through CD40 to CD40 ligand co-stimulation by T cells, counteracts the key osteoclastogenic cytokine RANKL, moderating osteoclast formation and activity. HIV infection leads to a disruption of the immuno-skeletal interface disrupting T-cell to B-cell communication and leading to elevated RANKL and diminished OPG production by B cells. The elevated RANKL/OPG ratio is biased in favor of increased osteoclast formation. (Adapted with permission from Ofotokun et al. [18].)
Evidence of ART Disruption of the Immuno-Skeletal Interface
It is also now clear that ART affects bone turnover independently of the bone loss associated with HIV infection itself [72]. Like HIV infection, studies to isolate and define the underlying mechanisms of ART-induced bone loss have been confounded by independent osteoporosis risk factors associated with traditional osteoporosis risk factors, body mass, and lifestyle factors [22, 23, 73]. Further hindrance by the wide range of classes and individual drugs combined into the ART formulations employed in modern clinical practice has added to the confusion as to the direct effects of ART on bone turnover. While answers from clinical studies have been contradictory and slow in coming, an inability to effectively replicate effects of ART in vitro or in animal models in vivo in the absence of viral infection has further confounded our understanding of this issue. In one study the protease inhibitor (PI) ritonavir, but not the related PI indinavir, was actually found to be bone sparing in mice in vivo and was found to inhibit osteoclast differentiation and abrogated bone resorption by disrupting the osteoclast cytoskeleton in vitro [74]. In another study nucleoside reverse transcriptase inhibitors (NRTIs) were found to have no effect on osteoclastogenesis but suppressed the activity of osteoblasts [75]. While there is presently no agreement on the direct effects of ART on bone cells in vivo, recently a consensus has begun to emerge that all classes of ART may be detrimental to the skeleton [21•, 76]. The evidence also suggests that the preponderance of BMD decline occurs relatively early in the course of ART initiation (typically within the first 48 weeks) [19, 20, 26] and at a time of heightened immune restoration [77], lending support to the notion that bone loss might be driven by a mechanism aligned with HIV-disease reversal, in particular immune-regeneration. Furthermore, viral suppression by ART leads to a partial recovery of depleted T cells through poorly understood mechanisms involving both peripheral expansion of existing T-cell pools, as well as IL-7–mediated thymic reactivation pathways [77, 78]. In fact, IL-7–driven thymic-dependent differentiation of bone marrow–derived progenitors and thymic-independent, peripheral expansion of mature T cells has previously been demonstrated to play a critical role in the etiology of ovariectomy-induced bone loss, a model of postmenopausal osteoporosis [79].
Based on these principles we speculate that regeneration of the immune system following ART initiation may once again promote renewed disruption of the delicate immuno-skeletal interface initiating a new wave of bone resorption and loss of BMD. A unifying mechanism for ART-induced bone loss centered on excessive osteoclastogenic cytokine production following T-cell restoration will support the current epidemiologic observation that bone loss occurs early during ART and is a common feature of almost all ART types regardless of the component drugs in the regimen. Studies to address such a mechanism are currently underway.
Conclusions
While ART has radically improved patient quality of life and longevity, some of these gains are beginning to be offset by troublesome metabolic complications including osteoporosis and elevated fracture prevalence. While skeletal deterioration has been associated with HIV and ART for more than a decade, the consequences are only now becoming apparent as the HIV population begins to shift to an older demographic, and is likely to become more acute as persistent bone loss associated with natural aging in both men and women compounds the bone loss caused by HIV infection and ART. An increase in bone fractures at an uncharacteristically young age, especially for men, is already becoming evident and is likely to increase exponentially in the future as these patients continue to age. While the mechanisms responsible for the skeletal aberrations in HIV patients appear to be extremely complex and multifactorial, recent studies have opened a window into how persistent inflammatory responses associated with HIV infection and ART may underlie disruptions to the immuno-skeletal interface that may explain in large measure the bone loss associated with both HIV infection and the exacerbation of this bone loss by ART. A better understanding of the underlying pathology and the molecular mechanisms responsible for skeletal deterioration will be essential to devise effective therapeutic interventions to safeguard the significant gains made in the long-term health of AIDS patients over the last decade.
Acknowledgments
IO and MNW research is supported by NIAMS grant AR059364 and NIA grant AG040013. MNW is also supported by the Biomedical Laboratory Research and Development Service of the VA Office of Research and Development (5I01BX000105) and by NIAMS grants AR056090 and AR053607. IO is also supported in part by K23 A1073119 from NIAID. Authors’ translational research activities are also supported in part by the Emory Center for AIDS Research CFAR, NIH Grant P30 AI050409, and the Atlanta Clinical and Translational Science Institute (ACTSI), NIH Grant MO1RR00039.
Footnotes
Disclosure
No potential conflicts of interest relevant to this article were reported.
References
- 1.Ofotokun I, Weitzmann MN. HIV and bone metabolism. Discov Med. 2011 May;11(60):385–393. [PMC free article] [PubMed] [Google Scholar]
- 2.Kong YY, Yoshida H, Sarosi I, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999 Jan 28;397(6717):315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 3.Han X, Kawai T, Eastcott JW, Taubman MA. Bacterial-responsive B lymphocytes induce periodontal bone resorption. J Immunol. 2006 Jan 1;176(1):625–631. doi: 10.4049/jimmunol.176.1.625. [DOI] [PubMed] [Google Scholar]
- 4.Abitbol V, Roux C, Chaussade S, et al. Metabolic bone assessment in patients with inflammatory bowel disease. Gastroenterology. 1995 Feb;108(2):417–422. doi: 10.1016/0016-5085(95)90068-3. [DOI] [PubMed] [Google Scholar]
- 5.Schett G, David JP. The multiple faces of autoimmune-mediated bone loss. Nat Rev Endocrinol. 2010 Dec;6(12):698–706. doi: 10.1038/nrendo.2010.190. [DOI] [PubMed] [Google Scholar]
- 6.Merlotti D, Gennari L, Dotta F, Lauro D, Nuti R. Mechanisms of impaired bone strength in type 1 and 2 diabetes. Nutr Metab Cardiovasc Dis. 2010 Nov;20(9):683–690. doi: 10.1016/j.numecd.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi T, Sugimoto T. Bone metabolism and fracture risk in type 2 diabetes mellitus [Review] Endocr J. 2011 Jul 20; doi: 10.1507/endocrj.ej11-0063. [DOI] [PubMed] [Google Scholar]
- 8.Almeida A, Roberts I. Bone involvement in sickle cell disease. British journal of haematology. 2005 May;129(4):482–490. doi: 10.1111/j.1365-2141.2005.05476.x. [DOI] [PubMed] [Google Scholar]
- 9.Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest. 2006 May;116(5):1186–1194. doi: 10.1172/JCI28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Amelio P, Grimaldi A, Di Bella S, et al. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: A key mechanism in osteoporosis. Bone. 2008 Jul;43(1):92–100. doi: 10.1016/j.bone.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 11. Vikulina T, Fan X, Yamaguchi M, et al. Alterations in the immuno-skeletal interface drive bone destruction in HIV-1 transgenic rats. Proc Natl Acad Sci U S A. 2010 Aug 3;107(31):13848–13853. doi: 10.1073/pnas.1003020107. This study provided some of the first evidence of the disruptive impact of HIV on the immune-skeletal interface and linked immune disruption directly to skeletal perturbations.
- 12.Desai S, Landay A. Early immune senescence in HIV disease. Curr HIV/AIDS Rep. 2010 Feb;7(1):4–10. doi: 10.1007/s11904-009-0038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keating SM, Golub ET, Nowicki M, et al. The effect of HIV infection and HAART on inflammatory biomarkers in a population-based cohort of US women. Aids. 2011 May 12; doi: 10.1097/QAD.0b013e3283489d1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006 Nov 14;20(17):2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 15.Bonjoch A, Figueras M, Estany C, et al. High prevalence of and progression to low bone mineral density in HIV-infected patients: a longitudinal cohort study. AIDS. 2010 Nov 27;24(18):2827–2833. doi: 10.1097/QAD.0b013e328340a28d. [DOI] [PubMed] [Google Scholar]
- 16.Sharma A, Flom PL, Weedon J, Klein RS. Prospective study of bone mineral density changes in aging men with or at risk for HIV infection. AIDS. 2010 Sep 24;24(15):2337–2345. doi: 10.1097/QAD.0b013e32833d7da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone B, Dockrell D, Bowman C, McCloskey E. HIV and bone disease. Arch Biochem Biophys. 2010 Nov 1;503(1):66–77. doi: 10.1016/j.abb.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 18.Ofotokun I, Weitzmann MN. HIV-1 infection and antiretroviral therapies: risk factors for osteoporosis and bone fracture. Curr Opin Endocrinol Diabetes Obes. 2010 Dec;17(6):523–529. doi: 10.1097/MED.0b013e32833f48d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004 Jul 14;292(2):191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 20.McComsey GA, Tebas P, Shane E, et al. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clinical infectious diseases. 2010 Oct 15;51(8):937–946. doi: 10.1086/656412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr. 2009 Aug 15;51(5):554–561. doi: 10.1097/QAI.0b013e3181adce44. This study demonstrated that BMD loss following initiation of antiretroviral therapy occurred regardless of the component drugs in the regimen.
- 22.Bolland MJ, Grey A. HIV and Low Bone Density: Responsible Party, or Guilty by Association? IBMS BoneKEy. 2011 January;8(1):7–15. [Google Scholar]
- 23.Duvivier C, Kolta S, Assoumou L, et al. Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. Aids. 2009 Apr 27;27(7):817–824. doi: 10.1097/QAD.0b013e328328f789. [DOI] [PubMed] [Google Scholar]
- 24.Yin MT, Overton ET. Increasing clarity on bone loss associated with antiretroviral initiation. The Journal of infectious diseases. 2011 Jun;203(12):1705–1707. doi: 10.1093/infdis/jir184. [DOI] [PubMed] [Google Scholar]
- 25.Hansen AB, Obel N, Nielsen H, Pedersen C, Gerstoft J. Bone mineral density changes in protease inhibitor-sparing vs. nucleoside reverse transcriptase inhibitor-sparing highly active antiretroviral therapy: data from a randomized trial. HIV Med. 2011 Mar;12(3):157–165. doi: 10.1111/j.1468-1293.2010.00864.x. [DOI] [PubMed] [Google Scholar]
- 26.Stellbrink HJ, Orkin C, Arribas JR, et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis. 2010 Oct 15;51(8):963–972. doi: 10.1086/656417. [DOI] [PubMed] [Google Scholar]
- 27.Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002 Jun;23(3):279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 28. Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large US. healthcare system. J Clin Endocrinol Metab. 2008 Sep;93(9):3499–3504. doi: 10.1210/jc.2008-0828. In this large epidemiological study, the clinical significance of HIV/ART-induced bone loss was highlighted, and fracture prevalence was noted to be twofold to fourfold higher in HIV-infected subjects compared to the HIV-seronegative patients.
- 29. Young B, Dao CN, Buchacz K, Baker R, Brooks JT. Increased Rates of Bone Fracture among HIV-Infected Persons in the HIV Outpatient Study (HOPS) Compared with the US General Population, 2000–2006. Clin Infect Dis. doi: 10.1093/cid/ciq242. 2011/03/15 ed2011. This recently published large cohort study corroborated earlier reports of higher fracture prevalence rates in the setting of HIV infection.
- 30.Womack JA, Goulet JL, Gibert C, et al. Increased Risk of Fragility Fractures among HIV Infected Compared to Uninfected Male Veterans. PLoS One. 2011;6(2):e17217. doi: 10.1371/journal.pone.0017217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prior J, Burdge D, Maan E, et al. Fragility fractures and bone mineral density in HIV positive women: a case-control population-based study. Osteoporos Int. 2007 Oct;18(10):1345–1353. doi: 10.1007/s00198-007-0428-7. [DOI] [PubMed] [Google Scholar]
- 32.More J. Children's bone health and meeting calcium needs. J Fam Health Care. 2008;18(1):22–24. [PubMed] [Google Scholar]
- 33.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000 Apr;21(2):115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 34.Weitzmann MN, Pacifici R. T cells: unexpected players in the bone loss induced by estrogen deficiency and in basal bone homeostasis. Ann N Y Acad Sci. 2007 Nov;1116:360–375. doi: 10.1196/annals.1402.068. [DOI] [PubMed] [Google Scholar]
- 35.Clowes JA, Riggs BL, Khosla S. The role of the immune system in the pathophysiology of osteoporosis. Immunol Rev. 2005 Dec;208:207–227. doi: 10.1111/j.0105-2896.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 36.Noel D, Djouad F, Bouffi C, Mrugala D, Jorgensen C. Multipotent mesenchymal stromal cells and immune tolerance. Leukemia & lymphoma. 2007 Jul;48(7):1283–1289. doi: 10.1080/10428190701361869. [DOI] [PubMed] [Google Scholar]
- 37.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003 Oct 23;425(6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Li A, Strait K, Zhang H, Nanes MS, Weitzmann MN. Endogenous TNFalpha Lowers Maximum Peak Bone Mass and Inhibits Osteoblastic Smad Activation, through NF-kappaB. J Bone Miner Res. 2007 Jan 31;22(5):646–655. doi: 10.1359/jbmr.070121. [DOI] [PubMed] [Google Scholar]
- 39.Nanes MS. Tumor necrosis factor-alpha: molecular and cellular mechanisms in skeletal pathology. Gene. 2003 Dec 4;321:1–15. doi: 10.1016/s0378-1119(03)00841-2. [DOI] [PubMed] [Google Scholar]
- 40.Rifas L, Arackal S, Weitzmann MN. Inflammatory T cells rapidly induce differentiation of human bone marrow stromal cells into mature osteoblasts. J.Cell Biochem. 2003;88(4):650–659. doi: 10.1002/jcb.10436. [DOI] [PubMed] [Google Scholar]
- 41. Rifas L, Weitzmann MN. A novel T cell cytokine, secreted osteoclastogenic factor of activated T cells, induces osteoclast formation in a RANKL-independent manner. Arthritis Rheum. 2009 Nov;60(11):3324–3335. doi: 10.1002/art.24877. Contrary to previous opinion, this study reported activated T cells as additional sources of RANKL-independent osteoclastogenic cytokines.
- 42.Weitzmann MN, Cenci S, Rifas L, Brown C, Pacifici R. Interleukin-7 stimulates osteoclast formation by up-regulating the T- cell production of soluble osteoclastogenic cytokines. Blood. 2000;96(5):1873–1878. [PubMed] [Google Scholar]
- 43.Fry TJ, Mackall CL. Interleukin-7: master regulator of peripheral T-cell homeostasis? Trends Immunol. 2001 Oct;22(10):564–571. doi: 10.1016/s1471-4906(01)02028-2. [DOI] [PubMed] [Google Scholar]
- 44.Anderson DM, Maraskovsky E, Billingsley WL, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390(6656):175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 45.Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 46.Yasuda H, Shima N, Nakagawa N, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95(7):3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997 Apr 18;89(2):309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 48.Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Spelsberg TC, Riggs BL. Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology. 1999 Sep;140(9):4367–4370. doi: 10.1210/endo.140.9.7131. [DOI] [PubMed] [Google Scholar]
- 49.Yun TJ, Chaudhary PM, Shu GL, et al. OPG/FDCR-1, a TNF receptor family member, is expressed in lymphoid cells and is up-regulated by ligating CD40. J Immunol. 1998;161(11):6113–6121. [PubMed] [Google Scholar]
- 50.Li Y, Toraldo G, Li A, et al. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood. 2007 Jan 3;109(9):3839–3848. doi: 10.1182/blood-2006-07-037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lopez-Granados E, Temmerman ST, Wu L, et al. Osteopenia in X-linked hyper-IgM syndrome reveals a regulatory role for CD40 ligand in osteoclastogenesis. Proc Natl Acad Sci U S A. 2007 Mar 20;104(12):5056–5061. doi: 10.1073/pnas.0605715104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weitzmann MN, Cenci S, Rifas L, Haug J, Dipersio J, Pacifici R. T cell activation induces human osteoclast formation via receptor activator of nuclear factor kappaB ligand-dependent and -independent mechanisms. J Bone Miner Res. 2001;16(2):328–337. doi: 10.1359/jbmr.2001.16.2.328. [DOI] [PubMed] [Google Scholar]
- 53.Kong YY, Feige U, Sarosi I, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999 Nov 18;402(6759):304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 54.Kawai T, Matsuyama T, Hosokawa Y, et al. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol. 2006 Sep;169(3):987–998. doi: 10.2353/ajpath.2006.060180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003 Apr;111(8):1221–1230. doi: 10.1172/JCI17215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cenci S, Weitzmann MN, Roggia C, et al. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest. 2000;106(10):1229–1237. doi: 10.1172/JCI11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kotake S, Udagawa N, Takahashi N, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103(9):1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cenci S, Toraldo G, Weitzmann MN, et al. Estrogen deficiency induces bone loss by increasing T cell proliferation and lifespan through IFN-gamma-induced class II transactivator. Proc Natl Acad Sci U S A. 2003 Sep 2;100(18):10405–10410. doi: 10.1073/pnas.1533207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao Y, Grassi F, Ryan MR, et al. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J Clin Invest. 2007 Jan;117(1):122–132. doi: 10.1172/JCI30074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takayanagi H, Ogasawara K, Hida S, et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000 Nov 30;408(6812):600–605. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 61.Yadav A, Fitzgerald P, Sajadi MM, et al. Increased expression of suppressor of cytokine signaling-1 (SOCS-1): A mechanism for dysregulated T helper-1 responses in HIV-1 disease. Virology. 2009 Mar 1;385(1):126–133. doi: 10.1016/j.virol.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006 Sep 21;443(7109):350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 63.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006 Oct;12(10):1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 64.De Milito A. B lymphocyte dysfunctions in HIV infection. Curr HIV Res. 2004 Jan;2(1):11–21. doi: 10.2174/1570162043485068. [DOI] [PubMed] [Google Scholar]
- 65. Moir S, Ho J, Malaspina A, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008 Aug 4;205(8):1797–1805. doi: 10.1084/jem.20072683. This is a comprehensive review of HIV-induced changes in the B-cell subpopulation. Whereas CD4 T-cell depletion is synonymous with HIV/AIDS, the dramatic realignment in B-cell function is grossly underappreciated.
- 66.van Grevenynghe J, Halwani R, Chomont N, et al. Lymph node architecture collapse and consequent modulation of FOXO3a pathway on memory T- and B-cells during HIV infection. Semin Immunol. 2008 Jun;20(3):196–203. doi: 10.1016/j.smim.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 67.Morrow M, Valentin A, Little R, Yarchoan R, Pavlakis GN. A splenic marginal zone-like peripheral blood CD27+B220− B cell population is preferentially depleted in HIV type 1-infected individuals. AIDS Res Hum Retroviruses. 2008 Apr;24(4):621–633. doi: 10.1089/aid.2007.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Deeks SG. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med. 2009 Sep–Oct;17(4):118–123. This is an in-depth review of the metabolic complications associated with aging in HIV/AIDS that highlights the role of chronic inflammation and persistent immune activation as a driving force behind these complications.
- 69.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature medicine. 2006 Dec;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 70.Zou W, Bar-Shavit Z. Dual modulation of osteoclast differentiation by lipopolysaccharide. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2002 Jul;17(7):1211–1218. doi: 10.1359/jbmr.2002.17.7.1211. [DOI] [PubMed] [Google Scholar]
- 71.Reid W, Sadowska M, Denaro F, et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A. 2001 Jul 31;98(16):9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tebas P, Powderly WG, Claxton S, et al. Accelerated bone mineral loss in HIV-infected patients receiving potent antiretroviral therapy. Aids. 2000 Mar 10;14(4):F63–F67. doi: 10.1097/00002030-200003100-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mondy K, Yarasheski K, Powderly WG, et al. Longitudinal evolution of bone mineral density and bone markers in human immunodeficiency virus-infected individuals. Clin Infect Dis. 2003 Feb 15;36(4):482–490. doi: 10.1086/367569. [DOI] [PubMed] [Google Scholar]
- 74.Wang MW, Wei S, Faccio R, et al. The HIV protease inhibitor ritonavir blocks osteoclastogenesis and function by impairing RANKL-induced signaling. J Clin Invest. 2004 Jul;114(2):206–213. doi: 10.1172/JCI15797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taylor A, Rogers M. HIV treatments and the skeleton: Do NRTIs directly effect bone cells? [Abstract] Bone. 2010 March;46 Supplement 1:S56. [Google Scholar]
- 76.Bruera D, Luna N, David DO, Bergoglio LM, Zamudio J. Decreased bone mineral density in HIV-infected patients is independent of antiretroviral therapy. AIDS. 2003 Sep 5;17(13):1917–1923. doi: 10.1097/00002030-200309050-00010. [DOI] [PubMed] [Google Scholar]
- 77.Franco JM, Rubio A, Martinez-Moya M, et al. T-cell repopulation and thymic volume in HIV-1-infected adult patients after highly active antiretroviral therapy. Blood. 2002 May 15;99(10):3702–3706. doi: 10.1182/blood.v99.10.3702. [DOI] [PubMed] [Google Scholar]
- 78.Kalayjian RC, Spritzler J, Pu M, et al. Distinct mechanisms of T cell reconstitution can be identified by estimating thymic volume in adult HIV-1 disease. J Infect Dis. 2005 Nov 1;192(9):1577–1587. doi: 10.1086/466527. [DOI] [PubMed] [Google Scholar]
- 79.Ryan MR, Shepherd R, Leavey JK, et al. An IL-7-dependent rebound in thymic T cell output contributes to the bone loss induced by estrogen deficiency. Proc Natl Acad Sci U S A. 2005 Nov 15;102(46):16735–16740. doi: 10.1073/pnas.0505168102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pacifici R, Brown C, Puscheck E, et al. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci U S A. 1991;88(12):5134–5138. doi: 10.1073/pnas.88.12.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kimble RB, Vannice JL, Bloedow DC, et al. Interleukin-1 receptor antagonist decreases bone loss and bone resorption in ovariectomized rats. J Clin Invest. 1994;93(5):1959–1967. doi: 10.1172/JCI117187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hofbauer LC, Lacey DL, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S. Interleukin-1beta and tumor necrosis factor-alpha, but not interleukin-6, stimulate osteoprotegerin ligand gene expression in human osteoblastic cells. Bone. 1999 Sep;25(3):255–259. doi: 10.1016/s8756-3282(99)00162-3. [DOI] [PubMed] [Google Scholar]
- 83.Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. 2005 Feb;115(2):282–290. doi: 10.1172/JCI23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Passeri G, Girasole G, Jilka RL, Manolagas SC. Increased interleukin-6 production by murine bone marrow and bone cells after estrogen withdrawal. Endocrinology. 1993;133(2):822–828. doi: 10.1210/endo.133.2.8393776. [DOI] [PubMed] [Google Scholar]
- 85.Manolagas SC, Bellido T, Jilka RL. New insights into the cellular, biochemical, and molecular basis of postmenopausal and senile osteoporosis: roles of IL-6 and gp130. Int J Immunopharmacol. 1995 Feb;17(2):109–116. doi: 10.1016/0192-0561(94)00089-7. [DOI] [PubMed] [Google Scholar]
- 86.Hofbauer LC, Heufelder AE. Intercellular chatter: osteoblasts, osteoclasts and interleukin 6. Eur.J.Endocrinol. 1996;134(4):425–426. doi: 10.1530/eje.0.1340425. [DOI] [PubMed] [Google Scholar]
- 87.Hashizume M, Hayakawa N, Mihara M. IL-6 trans-signalling directly induces RANKL on fibroblast-like synovial cells and is involved in RANKL induction by TNF-alpha and IL-17. Rheumatology (Oxford) 2008 Nov;47(11):1635–1640. doi: 10.1093/rheumatology/ken363. [DOI] [PubMed] [Google Scholar]
- 88.Wong PK, Quinn JM, Sims NA, van NA, Campbell IK, Wicks IP. Interleukin-6 modulates production of T lymphocyte-derived cytokines in antigen-induced arthritis and drives inflammation-induced osteoclastogenesis. Arthritis Rheum. 2006;54(1):158–168. doi: 10.1002/art.21537. [DOI] [PubMed] [Google Scholar]
- 89.Weitzmann MN, Cenci S, Roggia C, Toraldo G, Weitzmann L, Pacifici R. Increased production of IL-7 uncouples bone formation from bone resorption during estrogen deficiency. J Clin Invest. 2002 Dec;110(11):1643–1650. doi: 10.1172/JCI15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Toraldo G, Roggia C, Qian WP, Pacifici R, Weitzmann MN. IL-7 induces bone loss in vivo by induction of receptor activator of nuclear factor kappa B ligand and tumor necrosis factor alpha from T cells. Proc Natl Acad Sci U S A. 2003 Jan 7;100(1):125–130. doi: 10.1073/pnas.0136772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000 Sep 1;289(5484):1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 92.Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001 Dec;142(12):5050–5055. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- 93.Arai F, Miyamoto T, Ohneda O, et al. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. The Journal of experimental medicine. 1999 Dec 20;190(12):1741–1754. doi: 10.1084/jem.190.12.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matsuzaki K, Udagawa N, Takahashi N, et al. Osteoclast differentiation factor (ODF) induces osteoclast-like cell formation in human peripheral blood mononuclear cell cultures. Biochem Biophys Res Commun. 1998;246(1):199–204. doi: 10.1006/bbrc.1998.8586. [DOI] [PubMed] [Google Scholar]
- 95.Gao Y, Qian WP, Dark K, et al. Estrogen prevents bone loss through transforming growth factor {beta} signaling in T cells. Proc Natl Acad Sci U S A. 2004 Nov 23;101(47):16618–16623. doi: 10.1073/pnas.0404888101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weitzmann MN, Cenci S, Haug J, Brown C, DiPersio J, Pacifici R. B lymphocytes inhibit human osteoclastogenesis by secretion of TGFbeta. J Cell Biochem. 2000;78(2):318–324. doi: 10.1002/(sici)1097-4644(20000801)78:2<318::aid-jcb13>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 97.Hughes DE, Dai A, Tiffee JC, Li HH, Mundy GR, Boyce BF. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-beta. Nat Med. 1996;2(10):1132–1136. doi: 10.1038/nm1096-1132. [DOI] [PubMed] [Google Scholar]
- 98.Takai H, Kanematsu M, Yano K, et al. Transforming growth factor-beta stimulates the production of osteoprotegerin/osteoclastogenesis inhibitory factor by bone marrow stromal cells. J Biol Chem. 1998;273(42):27091–27096. doi: 10.1074/jbc.273.42.27091. [DOI] [PubMed] [Google Scholar]
- 99.Thirunavukkarasu K, Miles RR, Halladay DL, et al. Stimulation of osteoprotegerin (OPG) gene expression by transforming growth factor-beta (TGF-beta). Mapping of the OPG promoter region that mediates TGF-beta effects. J Biol Chem. 2001 Sep 28;276(39):36241–36250. doi: 10.1074/jbc.M104319200. [DOI] [PubMed] [Google Scholar]
- 100.Horwood NJ, Kartsogiannis V, Quinn JM, Romas E, Martin TJ, Gillespie MT. Activated T Lymphocytes Support Osteoclast Formation in Vitro. Biochem Biophys Res Commun. 1999;265(1):144–150. doi: 10.1006/bbrc.1999.1623. [DOI] [PubMed] [Google Scholar]
- 101.Janssens K, Ten Dijke P, Janssens S, Van Hul W. Transforming Growth Factor-{beta}1 to the Bone. Endocr Rev. 2005 Oct;26(6):743–774. doi: 10.1210/er.2004-0001. [DOI] [PubMed] [Google Scholar]
- 102.Tang Y, Wu X, Lei W, et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009 Jul;15(7):757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roggia C, Gao Y, Cenci S, et al. Up-regulation of TNF-producing T cells in the bone marrow: A key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci U S A. 2001 Nov 20;98(24):13960–13965. doi: 10.1073/pnas.251534698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang YH, Heulsmann A, Tondravi MM, Mukherjee A, Abu-Amer Y. Tumor Necrosis Factor-alpha (TNF) Stimulates RANKL-induced Osteoclastogenesis via Coupling of TNF Type 1 Receptor and RANK Signaling Pathways. J Biol Chem. 2001;276(1):563–568. doi: 10.1074/jbc.M008198200. [DOI] [PubMed] [Google Scholar]
- 105.Fuller K, Murphy C, Kirstein B, Fox SW, Chambers TJ. TNFalpha potently activates osteoclasts, through a direct action independent of and strongly synergistic with RANKL. Endocrinology. 2002 Mar;143(3):1108–1118. doi: 10.1210/endo.143.3.8701. [DOI] [PubMed] [Google Scholar]
- 106.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000 Dec;106(12):1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]