Abstract
Cbl family ubiquitin ligases act as key negative regulators of TCR signaling. Knockout mice lacking Cbl-b and c-Cbl show augmented T cell activation and CD28-independent IL-2 production. In order to study Cbl function directly in post-thymic T cells, a DN Cbl adenovirus was generated for transduction of T cells from Coxsackie/adenovirus receptor (CAR) transgenic (Tg) mice. We show that dominant negative (DN) Cbl-transduced CD4+ T cells exhibited enhanced IL-2 production upon TCR/CD28 engagement compared with empty adenoviral vector-transduced cells. This augmentation was reflected at both IL-2 mRNA and protein level, and correlated with increased protein phosphorylation of Vav, Akt, ERK, and p38MAPK. Our results indicate that introduction of dominant negative Cbl can potentiate activation of post-thymic CD4+ T cells, which argues for development of strategies to interfere with Cbl function as a method of immunopotentiation.
Keywords: CD4+ T cells, Cbl, CD28 costimulation, T cell activation
Introduction
Complete T cell activation requires TCR/CD3 mediated signals and additional interactions through costimulatory receptors such as CD28 (1). TCR ligation without costimulation can induce T cell anergy (2) or T cell apoptosis (3). In several in vivo pathologic settings, such as chronic infections or with a progressively growing tumor, the development of T cell dysfunction has been observed (4). Several lines of investigation have suggested that this dysfunction is mediated through immune-intrinsic negative regulatory pathways (5). For example, the receptors PD-1 and CTLA-4 expressed on activated T cells are inhibitory for T cell effector function when engaged by cognate ligands (6-11). In addition, several intracellular signaling proteins that have been shown to have negative regulatory effects are suspected to be involved in restricting continued activation of T cells following TCR ligation. These include the tyrosine phosphatases SHP1 and SHP2 (12, 13), the lipid kinases PTEN (14) and diacylglycerol kinase (15, 16), and the E3 ubiquitin ligases Cbl (17-20) and GRAIL (21). It is therefore of interest to identify candidate negative regulators that might be amenable to pharmacologic manipulation, toward the development of immunoptentiating agents for in vivo application. This requires validation that selected negative regulatory proteins are functionally inhibitory in normal, post-thymic T cells.
T cells express two highly conserved forms of Cbl protein, c-Cbl and Cbl-b (17). Both Cbl proteins have a tyrosine kinase-binding (TKB) domain in the N-terminus, a linker and RING finger domains, and a proline-rich region in the C-terminus. Cbl proteins have been shown to negatively regulate tyrosine kinase-mediated growth factor receptor signaling in multiple cell types (22, 23), in a large part through ubiquitination and degradation of numerous signaling proteins (24, 25). It appears that c-Cbl plays a critical role during T cell development (26), while Cbl-b is more important in negatively regulating peripheral T cell activation. T cells from T cell lineage specific knockout mice lacking c-Cbl and Cbl-b show the ability to produce meaningful levels of IL-2 with TCR ligation alone (17, 24), and have been suggested to be resistant to induction of anergy (27). However, as Cbl proteins are absent throughout development in these mice, it is not clear that acute interference with Cbl function in normal mature T cells would render T cells more sensitive to TCR-dependent activation.
In order to study the role of Cbl in post-thymic T cells, a strategy for interfering with Cbl function in resting peripheral T cells was needed. To this end, we generated a DN Cbl adenoviral construct, encoding the 355aa N-terminal residues of c-Cbl. This region mimics the natural v-Cbl oncogene, and because it is almost identical in both c-Cbl and Cbl-b is expected to prevent functional recruitment of both family members. This vector was utilized to transduce T cells from CAR Tg mice, which are rendered amenable to adenovirus-mediated gene expression without the need for T cell proliferation (28). We found that introduction of DN Cbl indeed potentiated cytokine production and phosphorylation of several important signaling proteins in response to CD3 ligation, indicating that inhibition of Cbl function lowers the threshold for T cell activation directly in the peripheral T cell compartment. These data support the development of strategies to interfere with Cbl function for immunopotentiation.
Materials and Methods
Mice and cells
CAR Tg mice expressing the extracellular domain of CAR under control of the Lck promoter/CD2 enhancer were generated as described (28). CAR Tg mice were interbred with IL-2 promoter-Luciferase Tg mice (29) to allow IL-2 promoter activity to be read out in transduced T cells. Mice were maintained under specific pathogen-free conditions in a barrier facility at the University of Chicago according to approved protocols and NIH guidelines. CAR Tg Th1 clones were generated from CAR Tg and CAR Tg/IL-2-Luc Tg mice with ovalbumin (OVA) immunization, and were maintained by weekly passage as previously reported (30).
Adenoviral vectors
An adenoviral vector containing the gene expression unit with a dominant negative c-Cbl coding cDNA (Ad DN-Cbl) and an adenoviral vectors without a coding cDNA (EV) were generated as described (31). Briefly, DN c-Cbl mutant was PCR-amplified from a construct containing a full-length c-Cbl cDNA with forward primer, 5’ -GGGGTACCatggagcagaaactcatctctgaagaggatctggccggcaacgtgaagaag a-3’; and reverse primer, 5’ -ATAGTTTAGCGGCCGCtcaatcttgaggagttggttcacataa-3’ (capital letters represent restriction enzyme sequence; underlined letters represent myc-tag sequence). The recombinant adenoviral vectors containing a human UbC promoter were generated according to a two-cosmid system protocol as described (32).
Adenoviral transduction of CAR Tg T cells
Briefly, CAR Tg T cells were harvested and purified by centrifugation over Ficoll-Hypaque, washed twice, and resuspended in DMEM supplemented with 2% FCS at 2×107/ml. They were then mixed with 5×108 Ad particles and incubated at 37°C for 1 hour. The cell/virus mixture was then transferred to a 10-cm tissue cell culture dish, incubated overnight at 37°C in an 8% CO2 atmosphere then washed and used for experiments.
T cell stimulation
The indicated antibodies (1 μg/ml) were immobilized onto sheep anti-mouse IgG pre-coated beads (Dynal, Oslo, Norway) by overnight incubation at 4°C in bead binding buffer (0.5% bovine serum albumin (BSA, Sigma, Inc., St. Louis, MO) in Ca2+, Mg2+-free Dulbecco’s phosphate-buffered saline (DPBS, Gibco-BRL, Gaithersburg, MD). For stimulation, T cells were incubated with the antibody-coated beads for the indicated time periods at 37°C at a 5:1 bead to T cell ratio.
Flow cytometric analysis
Flow cytometry was performed on a FACScan I instrument with Cellquest software. Briefly, for the intracellular myc-tag staining, cells were fixed in PBS with 4% parafomaldehyde for 10 min at 4°C, washed with FACS buffer (PBS with 2%FCS and 0.1% NaN3), and then permeabilized with FACS buffer containing 0.15% saponin for 30 min at 4°C. Cells were then stained with FITC conjugated anti-Myc-tag or isotype control antibody for 30 min at 4°C in permeabilization buffer. Cells were then washed and resuspended in PBS for FACS analysis. For viability staining, cells were washed twice with cold PBS, then resuspended in binding buffer (10mM HEPES, pH7.4; 140mM NaCl; 2.5mM CaCl2), then exposed to 5 μl Annexin V and 2 μl Propidium Iodide (PI). Cells were gently mixed, incubated for 15 min at room temperature (RT) in the dark, and resuspended in 500 μl binging buffer for FACS analysis.
Real-time RT-PCR assays
RNA was extracted using Trizol (Invitrogen Life Technologies, Carlsbad, CA) and cDNA was synthesized using SuperScript Reverse Transcriptase (Invitrogen). Real-time PT-PCR was performed on ABI PRISM 7700 machine with Sequence Detector software. The IL-2 and the GAPDH primer/probe sets and the TaqMan Universal PCR Master Mix were purchased from Applied Biosystems (Branchburg, NJ).
Cytokine ELISAs
Cytokine production was analyzed by ELISA using NUNC Immunosorp 96-well plates and antibody pairs from Pharmingen, as described previously (33). Plates were read with a Packard (Perkin Elmer Life Sciences) plate reader using Softmax Pro software.
Western blot analysis
Briefly, cells were lysed for 30 min on ice, and then the lysates were centrifuged for 10 min at 12,000 rpm as described (34). The supernatants were transferred to a new tube, and then 5x reducing sample buffer was added to each sample. Samples were heated for 5 min at 95°C and separated on 10% SDS-PAGE gels (Bio-Rad Lab, Hercules, CA). After transferring onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA), Western blotting was performed as described (35). Anti-Myc-tag (9B11), anti-phospho-p38 MAPK, anti-phospho-Akt, anti-total-p38 MAPK, and anti-total-Akt Abs were purchased from Cell Signaling Technology (Beverly, MA). Anti-phospho-Vav and anti-total-Vav Abs were from Abcam (Cambridge, MA). Anti-β-actin was from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-phospho-extracellular signal-related kinase (ERK) was from Promega (Madison, WI). Anti-total-ERK mAb was from Zymed (South San Francisco, CA).
Luciferase assay
CAR Tg/IL-2Luc Tg Th1 clones were collected and resuspended in serum-free DMEM in luminometer cuvettes (BD Biosciences, San Diego, CA). An equal volume of Bright-Glo luciferase assay reagent (Promega, Madison, WI) was added to each sample and mixed sample thoroughly. After 2 minutes, samples were analyzed using a monolight 2010 Luminometer (BD Biosciences, San Diego, CA) in 30 minutes.
Results and Discussion
DN Cbl protein is efficiently expressed by CAR CD4+ T cells after adenoviral transduction
We have shown previously that CAR Tg T cells are readily transducible using adenoviral-mediated gene transfer (28, 31). For the present study, an adenoviral vector encoding a myc-tagged DN Cbl protein that mimics the v-Cbl oncogene (36) was generated. We first confirmed that adenoviral transduction efficiently transferred expression of this molecule in CAR Tg CD4+ Th1 clones. As shown in Figure 1A upper panel, a dose-dependent increase in DN Cbl expression was detected by Western blotting with an anti-myc Ab. To determine relative expression of the transduced DN Cbl compared to endogenous Cbl, Western blotting using an Ab against the N-terminus of Cbl was performed. As shown in Figure 1A lower panel, the introduced DN Cbl was expressed at levels that were comparable to, or modestly higher than, the level of expression of endogenous Cbl with MOIs equal to or higher than 50. This was important, as massive over-expression of the truncated Cbl could theoretically have exerted non-specific effects on T cell function. Finally, to estimate the proportion of CAR Tg T cells successfully transduced, intracellular flow cytometry was performed on permeablized cells using the anti-myc Ab. Although this staining was of low intensity overall, the transduced population uniformly shifted to the right by FACS analysis at MOIs for 50 or greater (Figure 1B), suggesting efficient transduction as we have observed previously using GFP as an indicator gene product (28). Based on these expression data, an MOI of 50 was used for biochemical and functional experiments.
Figure 1. DN Cbl protein is expressed in CD4+ T cells after Ad transduction.
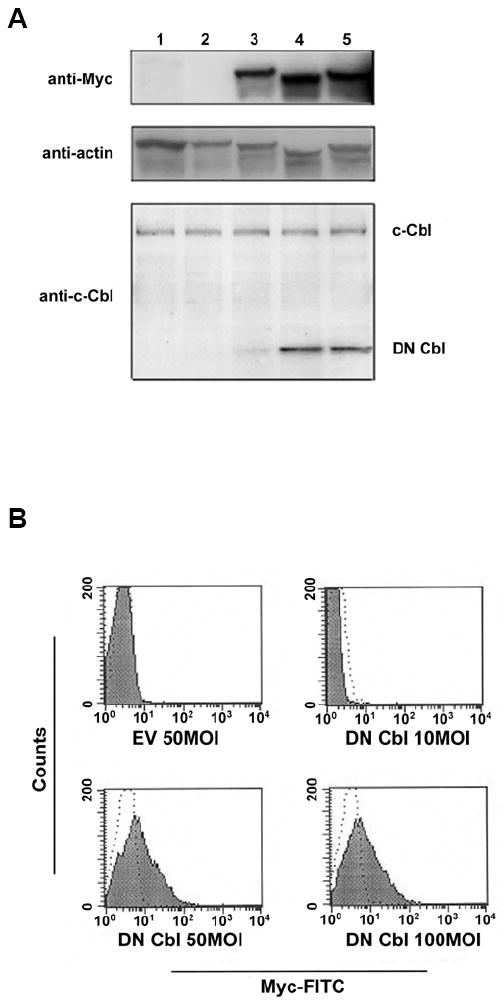
CAR Tg Th1 T cells were transduced with EV or Ad-DN Cbl and cultured overnight. On the next day, cells were analyzed for DN Cbl expression. (A) Representative image of a Western blot assay. Lane 1: Non-transduced cells. Lane 2: EV-transduced CAR Tg Th1 T cells. Lanes 3-5: Ad-DN Cbl-transduced CAR Tg Th1 T cells at an MOI of 10, 50, or 100. The upper panel was blotted with anti-myc tag antibody, the medium panel with anti-β-actin antibody as a loading control, and the lower panel with anti-c-Cbl N-terminus antibody. (B) Intracellular staining assay. Transduced CAR Tg Th1 T cells were fixed, permeabilized, and stained with a FITC-conjugated anti-myc tag antibody as shown in the solid area or isotype control antibody as shown in dotted line.
DN Cbl potentiates signaling events downstream from TCR ligation
Cbl proteins are thought to mediate an inhibitory effect on T cell activation through the ubiquitination of proximal signal transduction components, including CD3-ζ and tyrosine kinases (37). Interruption of Cbl function would thus be predicted to have consequences on a diverse set of downstream signaling events, a notion supported by the biochemical phenotype of T cells from Cbl-deficient mice (25, 38). We therefore examined phosphorylation of a panel of signaling intermediates known to be induced following TCR/CD28 ligation. As shown in Figure 2, transduction with DN Cbl increased anti-CD3/CD28-induced phosphorylation of extracellular signal-regulated kinase (ERK), p38 MAPK, Akt, and Vav1. Interestingly, phosphorylated Vav1 was detected in DN Cbl-transduced T cells even without stimulation, although CD3/CD28 ligation augmented this further. This observation is consistent with results found in Jurkat cells in which Cbl was found to negatively regulate Vav-dependent signaling (39).
Figure 2. DN Cbl increases phosphorylation of signaling intermediates in CD4 T cells upon TCR/CD28 engagement.
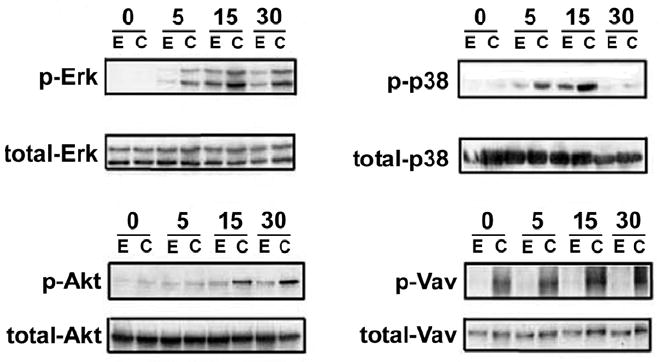
CAR Tg Th1 T cells were transduced with EV or Ad-DN Cbl. On the next day, transduced cells were stimulated with anti-CD3/CD28 antibody-coated beads for 0, 5, 15, and 30 min. Cells were then lysed for Western blotting for phospho- and total-ERK, -p38MAPK, -Akt, and -Vav. Each membrane was first blotted with the indicated phospho-antibody, then stripped and re-blotted with the total antibody.
It was of interest to determine whether DN Cbl predominantly affected TCR signaling. To address this question, we stimulated either EV- or DN Cbl-transduced CAR Th1 CD4+ T cells with titrated doses of anti-CD3 mAb, with or without the presence of anti-CD28 mAb, and examined the phosphorylation of Akt and Vav1. These targets were selected because their activation can be influenced by both TCR and CD28 ligation (40-43). As shown in Figure 3, DN Cbl significantly increased both Akt and Vav1 phosphorylation upon anti-CD3 stimulation alone. Furthermore, at the very low concentration of anti-CD3 mAb (0.01 μg/ml) used, the presence of anti-CD28 mAb could still augment both Akt and Vav1 phosphorylation in DN Cbl-transduced T cells. Together, these results suggest that the predominant augmentative effect of DN Cbl was on signals downstream from the TCR/CD3 complex, and argue that the threshold for productive TCR signaling is lowered when DN Cbl is expressed.
Figure 3. DN Cbl predominately augments a TCR signal.
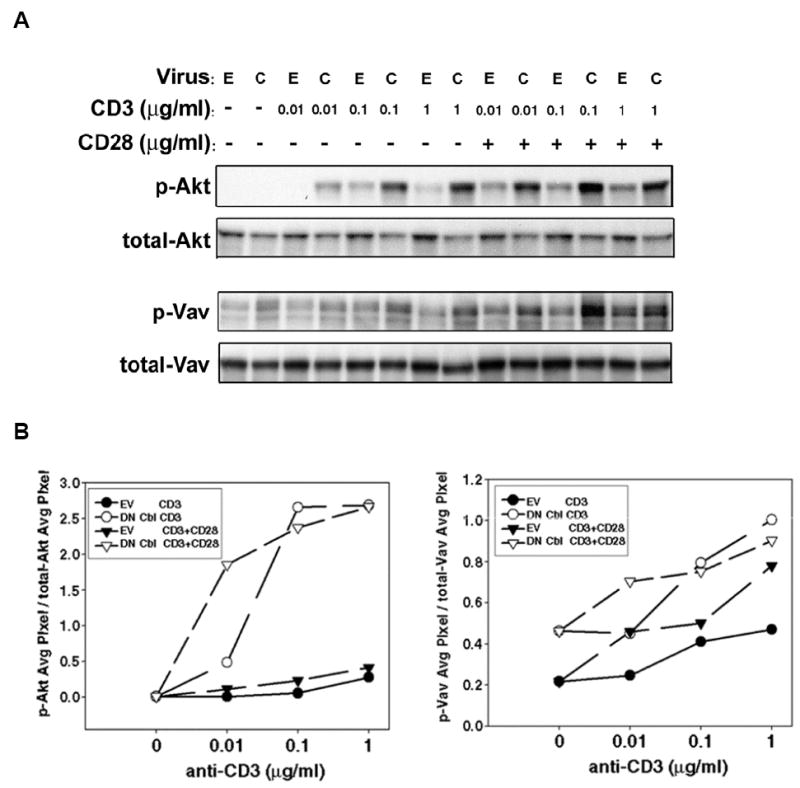
(A) CAR Tg Th1 T cells were transduced with EV or Ad-DN Cbl. On the next day, transduced cells were stimulated with serially titrated anti-CD3 antibody-coated beads with or without the presence of 1μg/ml anti-CD28 antibody on the beads, for 15 min. Cells were then lysed for Western blotting for phospho- and total-AKT (upper panels) and phosho- and total-Vav (lower panels). (B) The blots were analyzed using UN-SCAN-IT software for quantitation of the phosphorylated band intensities relative to the total amount of the protein density detected.
Blockade of endogenous Cbl augments IL-2 production in the presence of CD28 costimulation
It has been reported that T cells derived from Cbl-deficient mice showed augmented TCR-dependent IL-2 production, and even could produce some IL-2 in the absence of CD28 costimulation (44). We therefore examined whether introduction of DN Cbl to interfere with endogenous Cbl function directly in post-thymic T cells would also potentiate IL-2 production. As shown in Figure 4A, DN Cbl-transduced Th1 cells indeed produced approximately 10-fold greater IL-2 levels in response to CD3/CD28 stimulation compared with EV-transduced cells. However, unlike the Cbl-b-/- mouse system, no significant IL-2 was detected in DN Cbl-transduced cells with CD3 ligation alone. Thus, DN Cbl did not replace the need for CD28 costimulation for IL-2 production in this system, although it clearly augmented IL-2 production overall.
Figure 4. DN Cbl augments IL-2 production.
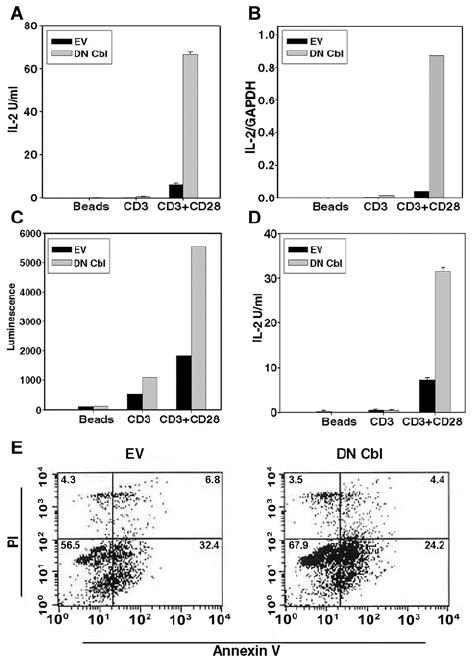
CAR Tg CD4+ Th1 T cells were transduced with EV or Ad-DN Cbl. On the next day, transduced cells were stimulated with beads alone, anti-CD3 or anti-CD3/CD28 antibody-coated beads. (A) After 20 hours, the IL-2 concentration in CAR Th1 T cells culture supernatants were analyzed by ELISA. (B) After 6 hours, the IL-2 mRNA levels in CAR Th1 T cells were analyzed by real-time RT-PCR. (C) After 20 hours, luciferase assay was performed using CAR Tg/IL-2-Luc Tg Th1 T cells. (D) A similar experiment was done with bulk CAR Tg splenic CD4+ T cells. After 20 hours of transduction, the IL-2 concentration in culture supernatents was analyzed by ELISA. (E) CAR Th1 T cells were transduced with EV or Ad-DN Cbl. On the next day, transduced cells were stimulated with anti-CD3/CD28 antibody-coated beads. After 20 hours, cells were stained with annexin V and PI, and analyzed by FACS to determine percent viability.
The mechanism of the effect of DN-Cbl on IL-2 production was examined in more detail. To determine whether increased IL-2 production in DN Cbl-transduced cells was reflected at the mRNA level, real-time RT-PCR was performed. As shown in Figure 4B, DN Cbl significantly increased IL-2 mRNA level (around 10-fold) in CAR Th1 CD4+ Th1 cells upon TCR and CD28 stimulation. To confirm that this increase could be explained by augmented transcription, DN Cbl was transduced into Th1 clones derived from CAR Tg mice interbred with mice transgenic for the IL-2 promoter driving luciferase expression. As expected, transduction with DN-Cbl in these cells resulted in increased luciferase activity in response to CD3/CD28 engagement, consistent with an effect upstream from IL-2 gene transcription. To determine whether this was a property restricted to the Th1 clones used for study, bulk splenic CAR Tg CD4+ T cells were transduced and analyzed similarly. As shown in Figure 4D, these DN Cbl-transduced cells also produced increased IL-2 in response to CD3/CD28 engagement. Finally, no significant increase in cell viability was observed in DN Cbl-transduced CAR Tg Th1 cells as assessed by annexin V plus PI staining and flow cytometric analysis (Figure 4E). Thus, these collective results indicate that introduction of DN Cbl directly into post-thymic T cells hyper-activates pathways downstream from TCR/CD28 ligation, leading to increased IL-2 gene expression.
T cells derived from Cbl-b-/- mice have been shown to be hyperactivatable through the TCR complex, and even appear to produce a meaningful level of IL-2 in response to anti-CD3 stimulation alone (17, 44). However, whether interference with Cbl function directly in peripheral T cells would augment T cell function was not clear. In our current study, we found that inhibition of Cbl function in post-thymic CD4+ T cells indeed augmented TCR-dependent signaling events, IL-2 promoter activity, IL-2 mRNA induction, and IL-2 protein secretion. These data thus support an important role for Cbl proteins in inhibiting TCR-dependent signals in post-thymic T cells, and suggest that pursuit of pharmacologic agents that disrupt Cbl functional interactions may be worthy of investigation.
It was interesting to note in our study that DN Cbl was only capable of inducing IL-2 production when CD28 co-ligation was provided. This result is not exactly the same as that seen with Cbl-b-/- mice, as in the latter case meaningful IL-2 production could be seen without CD28 costimulation. There are several hypotheses to explain the different results. It is conceivable that our DN-Cbl adenovirus did not inhibit the function of Cbl proteins completely. It is also possible the functions of c-Cbl and Cbl-b are differentially inhibited. However, it is also conceivable that in the knockout mouse setting the continuous absence of Cbl protein throughout mouse development may have different consequences than interfering with Cbl function directly in post-thymic T cells after development, as suggested by previous studies with other genes (45) (46) (47) 48). T cells from Cbl-deficient mice have also been suggested to be resistant to anergy induction (27). However, we have recently reported that DN-Cbl transduction in vitro also failed to prevent or reverse T cell anergy (16). Further differentiation between the possibilities could benefit from strategies to conditionally ablate the genes for c-Cbl and/or Cbl-b directly in post-thymic T cells. Nonetheless, our data clearly support the notion that interfering with Cbl function in peripheral T cells can potentiate T cell activation, making it an attractive target for development of pharmacologic strategies for immune stimulation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 2.Fields P, Fitch FW, Gajewski TF. Control of T lymphocyte signal transduction through clonal anergy. J Mol Med. 1996;74:673–683. doi: 10.1007/s001090050071. [DOI] [PubMed] [Google Scholar]
- 3.Kishimoto H, Sprent J. Strong TCR ligation without costimulation causes rapid onset of Fas-dependent apoptosis of naive murine CD4+ T cells. J Immunol. 1999;163:1817–1826. [PubMed] [Google Scholar]
- 4.Frumento G, Piazza T, Di Carlo E, Ferrini S. Targeting tumor-related immunosuppression for cancer immunotherapy. Endocr Metab Immune Disord Drug Targets. 2006;6:233–237. doi: 10.2174/187153006778250019. [DOI] [PubMed] [Google Scholar]
- 5.Inman BA, Frigola X, Dong H, Kwon ED. Costimulation, coinhibition and cancer. Curr Cancer Drug Targets. 2007;7:15–30. doi: 10.2174/156800907780006878. [DOI] [PubMed] [Google Scholar]
- 6.Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. 2005;54:307–314. doi: 10.1007/s00262-004-0593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1757–1761. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 8.Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother. 2007;56:739–745. doi: 10.1007/s00262-006-0272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blank C, Kuball J, Voelkl S, Wiendl H, Becker B, Walter B, Majdic O, Gajewski TF, Theobald M, Andreesen R, Mackensen A. Blockade of PD-L1 (B7-H1) augments human tumor-specific T cell responses in vitro. Int J Cancer. 2006;119:317–327. doi: 10.1002/ijc.21775. [DOI] [PubMed] [Google Scholar]
- 10.Contardi E, Palmisano GL, Tazzari PL, Martelli AM, Fala F, Fabbi M, Kato T, Lucarelli E, Donati D, Polito L, Bolognesi A, Ricci F, Salvi S, Gargaglione V, Mantero S, Alberghini M, Ferrara GB, Pistillo MP. CTLA-4 is constitutively expressed on tumor cells and can trigger apoptosis upon ligand interaction. Int J Cancer. 2005;117:538–550. doi: 10.1002/ijc.21155. [DOI] [PubMed] [Google Scholar]
- 11.Sabel MS, Hess SD, Egilmez NK, Conway TF, Jr, Chen FA, Bankert RB. CTLA-4 blockade augments human T lymphocyte-mediated suppression of lung tumor xenografts in SCID mice. Cancer Immunol Immunother. 2005;54:944–952. doi: 10.1007/s00262-005-0668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen TV, Ke Y, Zhang EE, Feng GS. Conditional deletion of Shp2 tyrosine phosphatase in thymocytes suppresses both pre-TCR and TCR signals. J Immunol. 2006;177:5990–5996. doi: 10.4049/jimmunol.177.9.5990. [DOI] [PubMed] [Google Scholar]
- 13.Brockdorff J, Williams S, Couture C, Mustelin T. Dephosphorylation of ZAP-70 and inhibition of T cell activation by activated SHP1. Eur J Immunol. 1999;29:2539–2550. doi: 10.1002/(SICI)1521-4141(199908)29:08<2539::AID-IMMU2539>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 14.Buckler JL, Walsh PT, Porrett PM, Choi Y, Turka LA. Cutting edge: T cell requirement for CD28 costimulation is due to negative regulation of TCR signals by PTEN. J Immunol. 2006;177:4262–4266. doi: 10.4049/jimmunol.177.7.4262. [DOI] [PubMed] [Google Scholar]
- 15.Sanjuan MA, Pradet-Balade B, Jones DR, Martinez AC, Stone JC, Garcia-Sanz JA, Merida I. T cell activation in vivo targets diacylglycerol kinase alpha to the membrane: a novel mechanism for Ras attenuation. J Immunol. 2003;170:2877–2883. doi: 10.4049/jimmunol.170.6.2877. [DOI] [PubMed] [Google Scholar]
- 16.Zha Y, Marks R, Ho AW, Peterson AC, Janardhan S, Brown I, Praveen K, Stang S, Stone JC, Gajewski TF. T cell anergy is reversed by active Ras and is regulated by diacylglycerol kinase-alpha. Nat Immunol. 2006;7:1166–1173. doi: 10.1038/ni1394. [DOI] [PubMed] [Google Scholar]
- 17.Naramura M, Jang IK, Kole H, Huang F, Haines D, Gu H. c-Cbl and Cbl-b regulate T cell responsiveness by promoting ligand-induced TCR down-modulation. Nat Immunol. 2002;3:1192–1199. doi: 10.1038/ni855. [DOI] [PubMed] [Google Scholar]
- 18.Loeser S, Loser K, Bijker MS, Rangachari M, van der Burg SH, Wada T, Beissert S, Melief CJ, Penninger JM. Spontaneous tumor rejection by cbl-b-deficient CD8+ T cells. J Exp Med. 2007;204:879–891. doi: 10.1084/jem.20061699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loeser S, Penninger JM. Regulation of peripheral T cell tolerance by the E3 ubiquitin ligase Cbl-b. Semin Immunol. 2007;19:206–214. doi: 10.1016/j.smim.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Chiang JY, Jang IK, Hodes R, Gu H. Ablation of Cbl-b provides protection against transplanted and spontaneous tumors. J Clin Invest. 2007;117:1029–1036. doi: 10.1172/JCI29472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueller DL. E3 ubiquitin ligases as T cell anergy factors. Nat Immunol. 2004;5:883–890. doi: 10.1038/ni1106. [DOI] [PubMed] [Google Scholar]
- 22.Panchamoorthy G, Fukazawa T, Miyake S, Soltoff S, Reedquist K, Druker B, Shoelson S, Cantley L, Band H. p120cbl is a major substrate of tyrosine phosphorylation upon B cell antigen receptor stimulation and interacts in vivo with Fyn and Syk tyrosine kinases, Grb2 and Shc adaptors, and the p85 subunit of phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:3187–3194. doi: 10.1074/jbc.271.6.3187. [DOI] [PubMed] [Google Scholar]
- 23.Miyake S, Mullane-Robinson KP, Lill NL, Douillard P, Band H. Cbl-mediated negative regulation of platelet-derived growth factor receptor-dependent cell proliferation. A critical role for Cbl tyrosine kinase-binding domain. J Biol Chem. 1999;274:16619–16628. doi: 10.1074/jbc.274.23.16619. [DOI] [PubMed] [Google Scholar]
- 24.Bachmaier K, Krawczyk C, Kozieradzki I, Kong YY, Sasaki T, Oliveira-dos-Santos A, Mariathasan S, Bouchard D, Wakeham A, Itie A, Le J, Ohashi PS, Sarosi I, Nishina H, Lipkowitz S, Penninger JM. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–216. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- 25.Naramura M, Kole HK, Hu RJ, Gu H. Altered thymic positive selection and intracellular signals in Cbl-deficient mice. Proc Natl Acad Sci U S A. 1998;95:15547–15552. doi: 10.1073/pnas.95.26.15547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy MA, Schnall RG, Venter DJ, Barnett L, Bertoncello I, Thien CB, Langdon WY, Bowtell DD. Tissue hyperplasia and enhanced T-cell signalling via ZAP-70 in c-Cbl-deficient mice. Mol Cell Biol. 1998;18:4872–4882. doi: 10.1128/mcb.18.8.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeon MS, Atfield A, Venuprasad K, Krawczyk C, Sarao R, Elly C, Yang C, Arya S, Bachmaier K, Su L, Bouchard D, Jones R, Gronski M, Ohashi P, Wada T, Bloom D, Fathman CG, Liu YC, Penninger JM. Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity. 2004;21:167–177. doi: 10.1016/j.immuni.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Wan YY, Leon RP, Marks R, Cham CM, Schaack J, Gajewski TF, DeGregori J. Transgenic expression of the coxsackie/adenovirus receptor enables adenoviral-mediated gene delivery in naive T cells. Proc Natl Acad Sci U S A. 2000;97:13784–13789. doi: 10.1073/pnas.250356297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abraham C, Miller J. Molecular mechanisms of IL-2 gene regulation following costimulation through LFA-1. J Immunol. 2001;167:5193–5201. doi: 10.4049/jimmunol.167.9.5193. [DOI] [PubMed] [Google Scholar]
- 30.Gajewski TF, Joyce J, Fitch FW. Antiproliferative effect of IFN-gamma in immune regulation. III. Differential selection of TH1 and TH2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-gamma. J Immunol. 1989;143:15–22. [PubMed] [Google Scholar]
- 31.Marks RE, Ho AW, Rivas F, Marshall E, Janardhan S, Gajewski TF. Differential Ras signaling via the antigen receptor and IL-2 receptor in primary T lymphocytes. Biochem Biophys Res Commun. 2003;312:691–696. doi: 10.1016/j.bbrc.2003.10.168. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Zeng W, Murakawa M, Freeman MW, Seed B. Episomal segregation of the adenovirus enhancer sequence by conditional genome rearrangement abrogates late viral gene expression. J Virol. 2000;74:11296–11303. doi: 10.1128/jvi.74.23.11296-11303.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griffin MD, Hong DK, Holman PO, Lee KM, Whitters MJ, O’Herrin SM, Fallarino F, Collins M, Segal DM, Gajewski TF, Kranz DM, Bluestone JA. Blockade of T cell activation using a surface-linked single-chain antibody to CTLA-4 (CD152) J Immunol. 2000;164:4433–4442. doi: 10.4049/jimmunol.164.9.4433. [DOI] [PubMed] [Google Scholar]
- 34.Rivas FV, O’Herrin S, Gajewski TF. CD28 is not required for c-Jun N-terminal kinase activation in T cells. J Immunol. 2001;167:3123–3128. doi: 10.4049/jimmunol.167.6.3123. [DOI] [PubMed] [Google Scholar]
- 35.Fields PE, Gajewski TF. Biochemical analysis of activated T lymphocytes. Protein phosphorylation and Ras, ERK, and JNK activation. Methods Mol Biol. 2000;134:307–317. doi: 10.1385/1-59259-682-7:307. [DOI] [PubMed] [Google Scholar]
- 36.Langdon WY, Hartley JW, Klinken SP, Ruscetti SK, Morse HC., 3rd v-cbl, an oncogene from a dual-recombinant murine retrovirus that induces early B-lineage lymphomas. Proc Natl Acad Sci U S A. 1989;86:1168–1172. doi: 10.1073/pnas.86.4.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang HY, Altman Y, Fang D, Elly C, Dai Y, Shao Y, Liu YC. Cbl promotes ubiquitination of the T cell receptor zeta through an adaptor function of Zap-70. J Biol Chem. 2001;276:26004–26011. doi: 10.1074/jbc.M010738200. [DOI] [PubMed] [Google Scholar]
- 38.Thien CB, Bowtell DD, Langdon WY. Perturbed regulation of ZAP-70 and sustained tyrosine phosphorylation of LAT and SLP-76 in c-Cbl-deficient thymocytes. J Immunol. 1999;162:7133–7139. [PubMed] [Google Scholar]
- 39.Miura-Shimura Y, Duan L, Rao NL, Reddi AL, Shimura H, Rottapel R, Druker BJ, Tsygankov A, Band V, Band H. Cbl-mediated ubiquitinylation and negative regulation of Vav. J Biol Chem. 2003;278:38495–38504. doi: 10.1074/jbc.M305656200. [DOI] [PubMed] [Google Scholar]
- 40.Salazar-Fontana LI, Barr V, Samelson LE, Bierer BE. CD28 engagement promotes actin polymerization through the activation of the small Rho GTPase Cdc42 in human T cells. J Immunol. 2003;171:2225–2232. doi: 10.4049/jimmunol.171.5.2225. [DOI] [PubMed] [Google Scholar]
- 41.Rudd CE, Raab M. Independent CD28 signaling via VAV and SLP-76: a model for in trans costimulation. Immunol Rev. 2003;192:32–41. doi: 10.1034/j.1600-065x.2003.00005.x. [DOI] [PubMed] [Google Scholar]
- 42.Raab M, Pfister S, Rudd CE. CD28 signaling via VAV/SLP-76 adaptors: regulation of cytokine transcription independent of TCR ligation. Immunity. 2001;15:921–933. doi: 10.1016/s1074-7613(01)00248-5. [DOI] [PubMed] [Google Scholar]
- 43.Kane LP, Weiss A. The PI-3 kinase/Akt pathway and T cell activation: pleiotropic pathways downstream of PIP3. Immunol Rev. 2003;192:7–20. doi: 10.1034/j.1600-065x.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- 44.Chiang YJ, Kole HK, Brown K, Naramura M, Fukuhara S, Hu RJ, Jang IK, Gutkind JS, Shevach E, Gu H. Cbl-b regulates the CD28 dependence of T-cell activation. Nature. 2000;403:216–220. doi: 10.1038/35003235. [DOI] [PubMed] [Google Scholar]
- 45.Davies JA, Ladomery M, Hohenstein P, Michael L, Shafe A, Spraggon L, Hastie N. Development of an siRNA-based method for repressing specific genes in renal organ culture and its use to show that the Wt1 tumour suppressor is required for nephron differentiation. Hum Mol Genet. 2004;13:235–246. doi: 10.1093/hmg/ddh015. [DOI] [PubMed] [Google Scholar]
- 46.Seddon B, Legname G, Tomlinson P, Zamoyska R. Long-term survival but impaired homeostatic proliferation of Naive T cells in the absence of p56lck. Science. 2000;290:127–131. doi: 10.1126/science.290.5489.127. [DOI] [PubMed] [Google Scholar]
- 47.Swan KA, Alberola-Ila J, Gross JA, Appleby MW, Forbush KA, Thomas JF, Perlmutter RM. Involvement of p21ras distinguishes positive and negative selection in thymocytes. Embo J. 1995;14:276–285. doi: 10.1002/j.1460-2075.1995.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamashita M, Kimura M, Kubo M, Shimizu C, Tada T, Perlmutter RM, Nakayama T. T cell antigen receptor-mediated activation of the Ras/mitogen-activated protein kinase pathway controls interleukin 4 receptor function and type-2 helper T cell differentiation. Proc Natl Acad Sci U S A. 1999;96:1024–1029. doi: 10.1073/pnas.96.3.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]


