Abstract
OBJECTIVES
To examine the association between life space, a measure of functional status that describes the range of movement through the environment covered during daily functioning, and the risk of mortality in older community-based persons.
DESIGN
Two ongoing, prospective observational cohort studies of aging.
SETTING
Greater metropolitan Chicago area.
PARTICIPANTS
One thousand four hundred forty-five community-based older persons without dementia.
MEASUREMENTS
Life space was measured at baseline using a series of questions designed to measure the extent of participants’ movement throughout their environment, ranging from the bedroom to out of town. The association between life space and mortality was examined using proportional hazards models adjusted for age, sex, race, and education.
RESULTS
Over up to 8 years of follow-up (mean 4.1 years), 329 of 1,445 (22.8%) participants died. In a proportional hazards model adjusted for age, sex, race, and education, a more-constricted life space was associated with a greater risk of death (hazard ratio = 1.18, 95% confidence interval = 1.09–1.27, P < .001), such that people with life spaces constricted to their immediate home environment (score = 3) were approximately 1.6 times as likely to die as those whose life spaces included trips out of town (score = 0). This association persisted after the addition of terms for several potential confounders, including physical activity, performance-based physical function, disability, depressive symptoms, social networks, body mass index, and number of chronic medical conditions.
CONCLUSION
Constricted life space is associated with greater risk of death in older community-based persons.
Keywords: life space, functional status, death, daily living, disability
Declining functional status is a frequent consequence of aging and is associated with important health outcomes, including dementia and mortality.1–8 Although functional status is most commonly measured using tests that focus on specific abilities thought to be important for daily living (e.g., bathing, dialing a telephone) or physical performance (e.g., walking), these approaches do not fully capture the complex repertoire of behavior required to maintain independence and well-being in the dynamic modern environment in which we live. Thus, some investigators have proposed that measurement of life space (the range of movement through the environment covered in daily life) may offer a complementary approach to studying functional status.7–10 Life space is a multidimensional construct that integrates physical performance with motivational, psychological, and social factors that influence how one navigates and interacts with the real world. In cross-sectional analyses, a constricted life space has been related to negative health outcomes such as depression, disability, and cognition.8,9,11,12 Data from prospective studies are limited, although one study reported an association with mortality in disabled women.13 If life space indeed provides a useful indicator of real-world functioning, it may have important prognostic implications for adverse health outcomes, including mortality, even in more-diverse and non-disabled populations.
Data were used from 1,445 older participants without dementia in the Rush Memory and Aging Project (MAP)14 and the Minority Aging Research Study (MARS)15 to test the hypothesis that a constricted life space is related to greater risk of all-cause mortality in persons free of dementia at baseline. Participants completed baseline assessments of life space and underwent detailed annual clinical evaluations for up to 8 years. The association between life space and mortality was examined using a proportional hazards model adjusted for age, sex, race, and education. Next the potential influence of several potential confounders, including physical activity, performance-based physical function, disability, depressive symptoms, social networks, body mass index, and number of chronic medical conditions, was examined. Finally, sensitivity analyses were conducted to verify that the results were not due to the influence of persons nearing death at the study baseline.
METHODS
Participants
Participants were from two ongoing studies of aging (see below) that the institutional review board of Rush University Medical Center had approved. These studies employ nearly identical data collection and operational methods, and data were combined for analytical purposes.
MAP,14 which began in 1997, is a longitudinal clinical-pathological study of common chronic conditions of aging. Participants are recruited from approximately 40 retirement communities and subsidized housing facilities around the Chicago metropolitan area. Participation requires detailed annual clinical evaluations and organ (brain) donation. Between 1997 and 2009, more than 1,300 older persons enrolled. The life space measure was added to the interview in 2001.9
MARS15 began in 2004 and is a study of risk factors for cognitive decline in older African Americans. Participants are recruited from community-based organizations and subsidized housing facilities. Participation requires annual clinical evaluations and cognitive testing. Between 2004 and 2009, more than 350 older persons enrolled. The life space measure was made at baseline.
At the time of these analyses, 1,530 persons (1,188 MAP, 342 MARS) had undergone their baseline clinical evaluation, including assessment of life space since 2001. Of those, 85 with dementia were excluded (75 MAP, 10 MARS), leaving 1,445 eligible persons (1,113 MAP, 332 MARS); analyses are based on this group. They were followed for up to 8 years (MAP mean 4.3 ± 2.0, range 0–8; MARS mean 3.6, range 0–5), with a mean of 4.1 ± 2.0 years of follow-up; these data were frozen for analyses on December 14, 2009. At baseline, participants had a mean age of 78.5 ± 7.9, a mean of 14.5 ± 3.3 years of education, and a mean score of 27.9 ± 2.2 on the Mini-Mental State Examination16; 73.7% (1,065) were women, and 71.1% (1,028) were white and non-Hispanic.
Clinical Evaluation
Participants from both studies underwent detailed annual clinical evaluations that included medical history, neurological examinations, and cognitive function testing, as previously described.14,17 A physician classified persons with respect to dementia using the criteria of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association.18 Cognitive impairment was determined in the same way for both studies.16,17,19
Assessment of Life Space
Life space was assessed using a modified version of the Life Space Questionnaire, a self-report measure of the range of movement through specified zones of the environment.9 Each zone represents a concentric enlargement of life space ranging from the bedroom to the porch or patio, parking lot or yard, neighborhood, outside of the neighborhood, outside of town. Participants were asked whether they had been in each of the six specific zones within their environment in the past week. The life space score is the sum of yes (scored as a 1, vs no = 0) responses, and the score was reverse-coded so that the reference group (score = 0) included individuals with the least-restricted life space and the largest number of persons (as opposed to the smallest) to obtain a more-stable estimate of the association between life space and mortality; thus, higher scores indicate a smaller life space. The two most-restricted life space categories were combined because of small cell sizes, resulting in a life space measure with scores ranging from 0 (travel outside of town) to 5 (homebound). The mean score was 0.64 ± 1.16, and the distribution of scores was as follows: 0, n = 955 (66%), 1, n = 281 (19%), 2, n = 83 (6%), 3, n = 57 (4%), 4, n = 36 (3%), 5, n = 33 (2%).
Other Covariates
Disability was assessed according to the Katz activity of daily living (ADL) and Lawton–Brody instrumental activity of daily living (IADL) scales.20,21 The Katz scale includes six basic ADLs: walking across a small room, bathing, dressing, eating, transferring from a bed to a chair, and toileting.20 A composite measure was created by summing the items on which participants reported the need for assistance; higher scores indicate greater disability (mean 0.18 ± 0.65).
IADLs were assessed using items adapted from the Duke Older Americans Resources and Services project.21 Items assessed include eight activities: telephone use, meal preparation, money management, medication management, light and heavy housekeeping, shopping, and local travel. A composite measure was created by summing the number of items on which participants reported the need for assistance, with higher scores indicating greater disability (mean 0.91 ± 1.39).
Gait was assessed using a performance-based test adapted from the procedures of the Established Populations for Epidemiologic Studies of the Elderly.22 Participants were asked to walk 8 feet and turn 360°, and the time and number of steps taken to complete each task were measured. A composite measure was computed by converting scores on the component measures to z-scores using the baseline mean and standard deviation for the entire cohort and averaging the z-scores (mean 0.03 ± 0.82).23
Physical activity was assessed using three questions adapted from the 1985 National Health Interview Survey.24 Participants were asked whether they had walked for exercise, done gardening or yard work, or done calisthenics or general exercise within the past 2 weeks. The number of activities reported was summed (mean = 1.20 ± 0.87).
Depressive symptoms were assessed using a 10-item version of the Center for Epidemiologic Studies Depression (CES-D) scale.25,26 Persons were asked whether they had experienced each of 10 symptoms in the past week; the score was the number of symptoms reported (mean = 1.35 ± 1.79).
Social network size was quantified using standard questions regarding the number of children, family, and friends participants had and how often they interacted with them.27 Social network size was the number of these individuals seen at least once a month (mean 6.66 ± 6.20).
Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared.
History of seven medical conditions was self-reported (diabetes mellitus, heart disease, hypertension, thyroid disease, cancer, head injury, and stroke). The total number of conditions present was used as an index of chronic illness (mean = 1.40 ± 1.05).14
Other variables were age, sex, education (years of schooling completed), and race.
Determination of Vital Status
The autopsy rate for MAP exceeds 80%.14 Thus, for most participants, the exact date of death was the day an autopsy was performed. Participants from both cohorts are also contacted quarterly to determine vital status and changes in health, and death is occasionally learned of then and confirmed by documentation from family or other contacts. Finally, research assistants regularly search the Social Security Death Index for the small number of persons it was not possible to contact. At the time of these analyses, vital status was known within the past 4 months for more than 95% of participants.
Data Analysis
The crude associations between life space and age, sex, education and race were first examined. Next, the relationship between life space and mortality was examined using a proportional hazards model adjusted for age, sex, education, and race.28 In subsequent models, several potential confounders of the association between life space and mortality weer examined and terms added for the interactions between age, sex, education, and race and life space. Finally, sensitivity analyses excluding persons who died before the first or second year of follow-up were conducted. Model validation was performed graphically and analytically, and there was no evidence of nonlinearity or non-proportionality. Programming was done in SAS (SAS Institute, Inc., Cary, NC).
RESULTS
Psychometric Properties of Life Space
Scores on the life space measure ranged from 0 to 5 (median 0, mean 0.64 ± 1.16), with higher scores indicating greater constriction of life space. At baseline, life space was modestly associated with age (correlation coefficient (r) = 0.18) education (r = −0.16), depressive symptoms (r = 0.17), disability (ADLs, r = 0.27; IADLs, r = 0.34), gait (r = −0.29), social networks (r = −0.16), and physical activity (r = −0.13); all P < .001); life space was not related to BMI or number of chronic medical conditions. Women showed a trend toward reporting a more-constricted life space than men (P = .06), and white participants reported more-constricted life space than African Americans (P = .001).
Life Space and Mortality
Over up to 8 years of follow-up (mean 4.1), 329 of 1,445 (22.8%) persons died. Table 1 provides crude baseline data on those who died and survived. Those who died were older and had more-constricted life spaces, lower MMSE scores, more depressive symptoms, more disability, and lower BMI; were less physically active; had poorer gait and smaller social networks; and were more likely to be female and white.
Table 1.
Participant Baseline Characteristics
| Characteristic | Survived (n = 1,116) | Died (n = 329) | P-Value |
|---|---|---|---|
| Age | 76.9 | 83.9 | <.001 |
| Female, n (%) | 858 (76) | 198 (64) | <.001* |
| White, n (%) | 738 (65) | 282 (97) | <.001* |
| Education, years, mean | 14.5 | 14.3 | .19 |
| Mini-Mental State Examination score, mean (range 0–30) | 28.1 | 27.4 | <.001 |
| Life space score, mean | 0.52 | 1.04 | <.001 |
| Number of depressive symptoms, mean | 1.26 | 1.64 | <.001 |
| Disability* | |||
| Activity of daily living score, mean (range 0–6) | 0.13 | 0.36 | <.001 |
| Instrumental activity of daily living score, mean ± standard deviation (range 0–8) | 0.68 ± 1.12 | 1.67 ± 1.83 | .006 |
| Gait score, mean | 0.13 | −0.29 | <.001 |
| Physical activity score, mean | 1.24 | 1.09 | .005 |
| Number of social networks, mean | 6.87 | 5.95 | .006 |
| Body mass index, kg/m2, mean | 28.1 | 27.1 | .006 |
| Number of medical conditions, mean | 1.36 | 1.39 | .70 |
Statistical significance is based on t-tests or
chi-square tests.
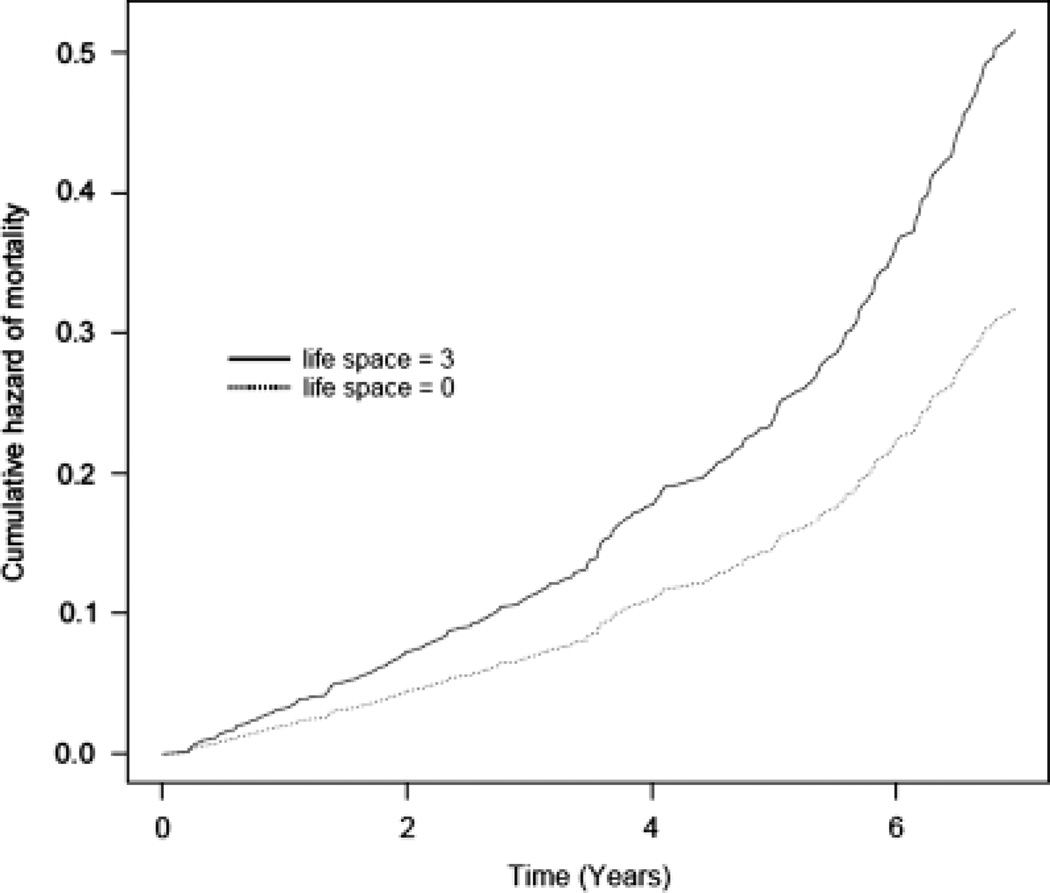
The association between life space and risk of mortality was examined in a core proportional hazards model adjusted for age, sex, race, and education. In this analysis, more-constricted life space was associated with greater risk of mortality (hazard ratio (HR) = 1.18, 95% confidence interval (CI) = 1.09–1.27). Thus, as illustrated in Figure 1, people with life spaces constricted to their immediate home environments (life spaces that involved travel no further than their driveway or yard; score = 3) were approximately 1.6 times as likely to die than those whose life spaces included travel beyond their home town (score = 0). Furthermore, because MARS consists only of African Americans, and there are differences in recruitment strategies for African Americans and white non-Hispanics (the majority of MAP participants), analyses of the association between life space and mortality were conducted separately according to cohort. Results of these analyses demonstrated that the associations were similar (MAP HR = 1.17, 95% CI = 1.08–1.27; MARS HR = 1.24, 95% CI = 0.86–1.81), although the finding in MARS did not reach significance, probably because of limited power.
Figure 1.
Cumulative hazard of mortality for participants with less- (dotted line) and more- (solid line) constricted life space.
Next, because there are several potential confounders of the association between life space and mortality, the analysis described above was repeated with additional terms for gait, disability, depressive symptoms, social networks, BMI, and number of chronic medical conditions (Table 2). Physical activity, which could be a confounder or mediator of the association between life space and mortality, was also examined, and the estimate was not attenuated (Table 2).
Table 2.
Association Between Life Space and Mortality After Adjustment for Individual Confounders
| Covariate | Hazard Ratio (95% Confidence Interval) |
P-Value |
|---|---|---|
| Depressive symptoms (n = 1,443) | 1.16 (1.07–1.25) | <.001 |
| Activity of daily living disability (n = 1,443) | 1.15 (1.06–1.25) | .001 |
| Instrumental activity of daily living disability (n = 1,443) | 1.10 (1.01–1.19) | .03 |
| Gait (n = 1,444) | 1.09 (1.00–1.18) | .04 |
| Physical activity (n = 1,444) | 1.16 (1.08–1.26) | <.001 |
| Social networks (n = 1,440) | 1.17 (1.08–1.26) | <.001 |
| Body mass index (n = 1,412) | 1.16 (1.07–1.26) | <.001 |
| Number of medical conditions (n = 1,444) | 1.18 (1.09–1.27) | <.001 |
All models controlled for age, sex, education, and race.
Finally, the core analysis above was repeated with terms for the interactions between age, sex, education, and race and life space in separate models. The association between life space and mortality was found to vary with age and sex, such that it decreased slightly as age increased (estimate for the age × life space interaction = 0.99, 95% CI = 0.98–0.99) and was somewhat stronger in men (estimate for the sex × life space interaction = 1.20, 95% CI = 1.03–1.40). No interactions were found with race or education.
Sensitivity Analyses
In sensitivity analyses to examine the robustness of the association between life space and mortality, the core model was repeated after excluding persons who died before the first follow-up (Model 1) and then after excluding persons who died before the first or second follow-up (Model 2). The HRs were not substantially different (Model 1 HR = 1.17, 95% CI = 1.08–1.27; Model 2 HR = 1.15, 95% CI = 1.05–1.25), although power was reduced because of the smaller number of deaths.
DISCUSSION
In 1,445 community-based older persons free of dementia, it was found that constricted life space was associated with greater risk of mortality. That is, a person who had not been to an area beyond their yard or driveway in the previous week was approximately 1.6 times as likely to die as a person with a life space involving travel outside town. The association between life space and mortality was robust in that it persisted even after the inclusion of a wide variety of potential confounding variables, including traditional measures of physical performance and disability, and in sensitivity analyses examining the potential influence of persons nearing death at study baseline. These findings suggest that life space captures aspects of functional status beyond those assessed using traditional measures and may have important prognostic implications for health outcomes in advanced age.
Recently, investigators have posited that a focus on more-comprehensive measures of functional status than those commonly used may provide new information about health and longevity,8–10 but the literature on life space is limited, consisting mostly of cross-sectional studies with outcomes such as depressive symptoms and disability.8,9,11 The authors are aware of only one study that prospectively examined the relationship between life space and mortality. 13 That study involved a cohort of disabled women and focused on incident frailty (frailty-free mortality was considered a competing risk), but a constricted life space was associated with a three times greater risk of frailty-free mortality over 3 years. The present study included men and women and persons without disability and was restricted to persons without dementia, which increases the validity of the use of this self-report measure. Furthermore, although the prior study considered self-reported mobility, the current study included performance-based measures of physical function. That the association between life space and mortality persisted even after controlling for these covariates suggests that life space may reflect the complex array of physical and psychosocial factors necessary for independent functioning in the real world and, consequently, for optimal health outcomes. Thus, the current study extends prior studies in several important respects and shows an association between life space and risk of death in a large and diverse cohort of community-based men and women who underwent detailed clinical assessments and were free of dementia.
Perhaps surprisingly, the relationship between life space and mortality varied slightly according to age and sex, such that it was weaker at older ages and stronger in men than women. It is possible that the effect is attenuated at older ages because the influence of other health variables becomes more pronounced as age increases (i.e., competing risk). The finding that the association was somewhat greater in men than women was unexpected. Men may maintain a larger life space longer than women in old age because of practical considerations (e.g., men tend to work longer; many older women do not drive), and such factors may influence the relationship between space and death, but this is speculative, but overall, the volunteer nature of the cohort limits the inferences one can draw from differential findings according to demographic variables, and it will be important for future studies to examine such issues.
The biological basis of the association between life space and mortality is unknown. It is possible that persons with a constricted life space are sicker and nearer to death, although the association with mortality persisted even after controlling for chronic diseases and excluding persons who died during the early follow-up years. Another possibility is that life space encompasses psychosocial factors or personality traits that are related to mortality. For example, persons with a constricted life space may be less able to adapt to the challenges of aging, such as loss of physical and cognitive function, and have difficulty navigating the world. Furthermore, having a greater life space may contribute to the effective functioning of physiological systems, as may be the case of psychosocial factors such as optimism and wellbeing, which are related to disease biomarkers such as cortisol, inflammation, and cardiovascular disease.29 Whether this is the case for life space remains uncertain. Future studies are needed to examine the biological basis of the association between life space and mortality.
This study has many strengths, including the assessment of life space in two large cohorts of racially diverse community-dwelling older adults free of dementia; the ability to examine the role of several potentially important confounders; and sensitivity analyses, ensuring that the findings were not due to the inclusion of persons very near death at baseline. Limitations were the selected nature of this cohort, which may have restricted the range of life space scores and limit the generalizability of findings, and the assessment of life space at only a single point in time. Future studies are needed to examine the association between life space and additional health outcomes and to characterize the trajectory of life space in advanced age.
ACKNOWLEDGMENTS
We are indebted to the participants and the staff of the Rush MAP, the MARS, and the Rush Alzheimer’s Disease Center for this work and to Sue Leurgans, Woojeong Bang, and Bryan James for help with statistical programming. This work was supported by the Illinois Department of Public Health (James); National Institute on Aging Grants R01AG17917 (Bennett), R01AG022018 (Barnes), R01AG24480 (Buchman, Bennett), R01AG34374 (Boyle), and R01AG33678 (Boyle); and the Robert C. Borwell Endowment Fund (Bennett).
Sponsor’s Role: The organizations funding this study had no role in the design or conduct of the study; in the collection, management, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: Patricia Boyle had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. She was involved with study concept and design, analysis and interpretation of data, and preparation of the manuscript. Drs. Buchman, Barnes, and Bennett were involved in study concept and acquisition of data, assisted with the analysis and interpretation of the data, and critically revised the manuscript for important intellectual content. Dr. James was involved in the data analysis and interpretation and critically revised the manuscript for important intellectual content. All authors have seen and approved the final version.
REFERENCES
- 1.Strawbridge WJ, Kaplan GA, Camacho T, et al. The dynamics of disability and functional change in an elderly cohort: Results from the Alameda County Study. J Am Geriatr Soc. 1992;40:799–806. doi: 10.1111/j.1532-5415.1992.tb01852.x. [DOI] [PubMed] [Google Scholar]
- 2.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 3.Landi F, Liperoti R, Russo A, et al. Disability, more than multimorbidity, was predictive of mortality among older persons aged 80 years and older. J Clin Epidemiol. 2010;63:752–759. doi: 10.1016/j.jclinepi.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Marengoni A, von Strauss E, Rizzuto D, et al. The impact of chronic multi-morbidity and disability on functional decline and survival in elderly persons. A community-based, longitudinal study. J Intern Med. 2008;265:1365–2796. doi: 10.1111/j.1365-2796.2008.02017.x. [DOI] [PubMed] [Google Scholar]
- 5.Peres K, Helmer C, Amieva H, et al. Natural history of decline in instrumental activities of daily living performance over the 10 years preceding the clinical diagnosis of dementia: A prospective population-based study. J Am Geriatr Soc. 2008;56:37–44. doi: 10.1111/j.1532-5415.2007.01499.x. [DOI] [PubMed] [Google Scholar]
- 6.Wang L, Larson EB, Bowen JD, et al. Performance-based physical function and future dementia in older people. Arch Intern Med. 2006;166:1115–1120. doi: 10.1001/archinte.166.10.1115. [DOI] [PubMed] [Google Scholar]
- 7.Glass TA. Conjugating the “tenses” of function: Discordance among hypothetical, experimental, and enacted function in older adults. Gerontologist. 1998;38:101–112. doi: 10.1093/geront/38.1.101. [DOI] [PubMed] [Google Scholar]
- 8.Murata C, Kondo T, Tamakoshi K, et al. Factors associated with life space among community-living rural elders in Japan. Public Health Nurs. 2006;23:324–331. doi: 10.1111/j.1525-1446.2006.00568.x. [DOI] [PubMed] [Google Scholar]
- 9.Barnes LL, Wilson RS, Bienias JL, et al. Correlates of life space in a volunteer cohort of older adults. Exp Aging Res. 2007;33:77–93. doi: 10.1080/03610730601006420. [DOI] [PubMed] [Google Scholar]
- 10.Stalvey BT, Owsley C, Sloane ME, et al. The Life Space Questionnaire: A measure of the extent of mobility of older adults. J Appl Gerontol. 1999;18:460–478. [Google Scholar]
- 11.Baker PS, Bodner EV, Allman RM. Measuring life-space mobility in community-dwelling older adults. J Am Geriatr Soc. 2003;51:1610–1614. doi: 10.1046/j.1532-5415.2003.51512.x. [DOI] [PubMed] [Google Scholar]
- 12.Allman RM, Sawyer P, Roseman JM. The UAB Study of Aging: Background and insights into life-space mobility among older Americans in rural and urban settings. Aging Health. 2006;2:417–429. [Google Scholar]
- 13.Xue QL, Fried LP, Glass TA, et al. Life-space constriction, development of frailty, and the competing risk of mortality: The Women’s Health and Aging Study I. Am J Epidemiol. 2008;167:240–248. doi: 10.1093/aje/kwm270. [DOI] [PubMed] [Google Scholar]
- 14.Bennett DA, Schneider JA, Buchman AS, et al. The Rush Memory and Aging Project: Study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25:163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 15.Lewis TT, Aiello AE, Leurgans S, et al. Self-reported experiences of everyday discrimination are associated with elevated C-reactive protein levels in older African-American adults. Brain Behav Immun. 2010;24:438–443. doi: 10.1016/j.bbi.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- 18.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 19.Boyle PA, Wilson RS, Aggarwal NT, et al. Mild cognitive impairment: Risk of Alzheimer disease and rate of cognitive decline. Neurology. 2006;67:441–445. doi: 10.1212/01.wnl.0000228244.10416.20. [DOI] [PubMed] [Google Scholar]
- 20.Katz S, Akpom C. A measure of primary sociobiological functions. Int J Health Serv. 1976;6:493–508. doi: 10.2190/UURL-2RYU-WRYD-EY3K. [DOI] [PubMed] [Google Scholar]
- 21.Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 22.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyle PA, Wilson RS, Buchman AS, et al. Lower extremity motor function and disability in mild cognitive impairment. Exp Aging Res. 2007;33:355–371. doi: 10.1080/03610730701319210. [DOI] [PubMed] [Google Scholar]
- 24.US Dept. of Health and Human Services. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 1996. Physical Activity and Health: A report of the Surgeon General. [Google Scholar]
- 25.Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Measure. 1977;1:385–401. [Google Scholar]
- 26.Kohout FJ, Berkman LF, Evans DA, et al. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 27.Bennett DA, Schneider JA, Tang Y, et al. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: A longitudinal cohort study. Lancet Neurol. 2006;5:406–412. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- 28.Gail MH, Lubin JH, Rubinstein LV. Likelihood calculations for matched case-control studies and survival studies with tied death times. Biometrika. 1981;68:703–707. [Google Scholar]
- 29.Cacioppo JT. Social neuroscience: Autonomic, neuroendocrine, and immune response to stress. Psychophysiology. 1994;31:113–128. doi: 10.1111/j.1469-8986.1994.tb01032.x. [DOI] [PubMed] [Google Scholar]