SUMMARY
PIK3CA and PTEN alterations are common in human cancer, but only a fraction of such tumors are dependent upon AKT signaling. AKT-independence is associated with redundant activation of cap-dependent translation mediated by convergent regulation of the translational repressor 4E-BP1 by the AKT and ERK pathways. This provides mechanistic bases for the limited activity of AKT and MEK inhibitors in tumors with co-mutation of both pathways and the profound synergy observed with combined inhibition. Whereas such tumors are sensitive to a dominant active 4E-BP1 mutant, knockdown of 4E-BP1 expression reduces their dependence on AKT/ERK signaling for translation or survival. Thus, 4E-BP1 plays a prominent role in mediating the effects of these pathways in tumors in which they are activated by mutation.
INTRODUCTION
Mutational activation of mitogenic signaling is a frequent event in human cancer. Mutations in genes that encode components of the PI3K/AKT/mTOR and RAS/RAF/MEK/ERK pathways occur at high frequency in cancer and often coexist (McCubrey et al., 2007; Shaw and Cantley, 2006). The former pathway is activated in a majority of human cancers, due to mutations in PIK3CA, which encodes the catalytic subunit of PI3 kinase p110α, inactivation or decreased function of PTEN, or activation of receptor tyrosine kinases (Samuels et al., 2004; Vivanco and Sawyers, 2002). Activation of the PI3K pathway causes changes in metabolism, transcription, protein translation and other processes that contribute to the transformed phenotype.
The concurrent activation of the PI3K/AKT and ERK pathways by separate mutations occurs in a significant portion of human tumors (Liu et al., 2008; Simi et al., 2008; Tsao et al., 2004). The selective advantage of activating both pathways is unknown but has been thought to be due to distinct effects of each that are necessary for tumor growth. However, we and others have found that, in such tumors, inhibiting either pathway alone has negligible effects on tumor growth and survival (Hoeflich et al., 2009; She et al., 2005; Wee et al., 2009; Yu et al., 2008). One possible explanation is that these pathways activate a common set of downstream targets. If so, inhibition of neither pathway alone would be sufficient to inactivate these targets. They would thus serve to integrate the biologic effects of both pathways on transformation.
In this study, we tested this hypothesis and investigated the consequences and therapeutic implications of coexistent mutational activation of PI3K/AKT and RAS/ERK signaling in carcinomas. The 4E-BP1 protein is a target of both pathways and integrates their function at the level of regulation of translation.
RESULTS
Coexistent Mutational Activation of ERK Signaling in Tumors Is Associated with AKT Independence
We used an allosteric inhibitor of AKT (AKTi) to interrogate a panel of tumor cell lines with PIK3CA or PTEN mutation and determine their dependence on the pathway. AKTi is a non-ATP-competitive, PH-domain-dependent inhibitor of AKT1 (EC50 3.5 nM) and AKT2 (EC50 41 nM) with less potency against AKT3 (EC50 1900 nM) (Compound 17 in Bilodeau et al., 2008). It is highly selective, with no inhibition of other AGC kinases. AKTi inhibited AKT phosphorylation and downstream signaling in tissue culture and in vivo (She et al., 2008 and Figure S1A).
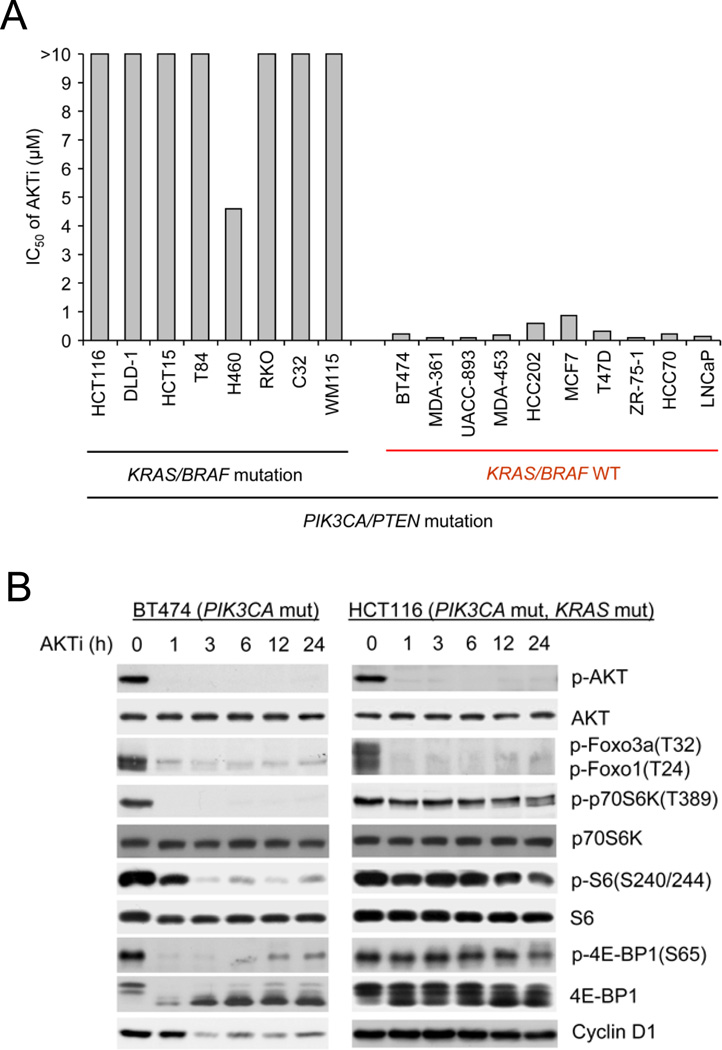
We have used this compound to show that breast cancer cells with PI3K mutation or HER2 amplification are selectively dependent on AKT signaling compared to those in which the pathway is not activated (She et al., 2008). However, not all tumor cells with PI3K or PTEN mutation are sensitive to the AKTi (Figure 1A and Figure S1B). PI3K or PTEN mutation often coexists with RAS or BRAF mutation (Simi et al., 2008; Tsao et al., 2004) or hyperactivation of EGFR (Mellinghoff et al., 2005; She et al., 2005). Analysis of this panel of cell lines showed that a significant fraction had coexistent PIK3CA and KRAS or BRAF mutations or coexistent PTEN loss and BRAF mutations (Table S1). All cells with coexistent KRAS or BRAF mutation were resistant to AKT inhibition. Ten tumor cell lines in the panel were sensitive to the drug; none of these harbored KRAS or BRAF mutation.
Figure 1. Tumor Cells with Coexistent RAS or RAF Mutations Are Resistant to AKT Inhibition.
(A) Cell growth was assessed by using the CellTiter-Glo luminescent cell viability assay after 3 days of treatment with AKTi (0–10 µM). The results are expressed as half-maximal growth inhibitory concentration (IC50) of AKTi.
(B) Cells were treated with 1 µM AKTi, and the cell lysates were immunoblotted with the indicated antibodies. See also Figure S1.
The effects of the AKTi were compared in sensitive tumor cells with PIK3CA mutation (e.g. BT474, breast cancer) and insensitive tumor cells with coexistent KRAS and PIK3CA mutations (e.g. HCT116, colorectal) (Figure 1B). Unlike ATP-competitive AKT inhibitors, the AKTi prevents the phosphorylation of AKT by preventing its association with the membrane (Cherrin et al., 2010). In all of these cell lines, 1 µM AKTi inhibited AKT phosphorylation and phosphorylation of AKT substrates Foxo1 and Foxo3a. In BT474, the phosphorylation of downstream targets of AKT signaling, p70S6K, S6 and 4E-BP1, as well as the expression of cyclin D1 were also inhibited. This is typical of tumor cell lines that are sensitive to AKT inhibition, including the three other PIK3CA mutant (T47D, MCF7, MDA-453) and two PTEN mutant (ZR-75-1, LNCaP) tumor cell lines (She et al., 2008). In contrast, in HCT116, neither p70S6K, S6, or 4E-BP1 phosphorylation nor cyclin D expression was suppressed, despite effective inhibition of AKT and Foxo phosphorylation (Figure 1B). Similar results were obtained in other tumor cells (DLD1, HCT15 and T84) with concurrent PIK3CA and KRAS mutations (Figures S3A and S5 and data not shown). The survival and proliferation of these cells were affected only marginally by AKT inhibition (Figures 1A, 2A and 2C and Figure S1B). Thus, the phosphorylation of Foxo and other proximal targets of AKT are suppressed by the AKTi in all cells tested, whether or not their growth is AKT dependent. In contrast, phosphorylation of regulators of cap-dependent translation (p70S6K, S6, 4E-BP1) and expression of cyclin D1 are suppressed by the AKTi only in tumor cells whose growth is sensitive to the drug.
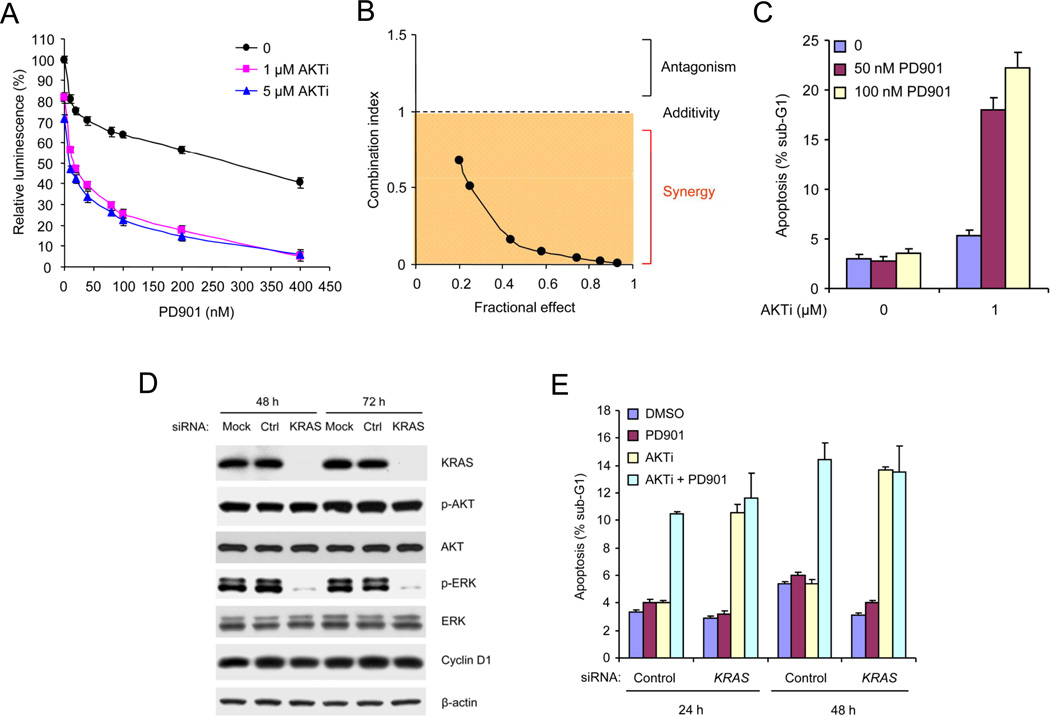
Figure 2. Tumor Cells with Coexistent PIK3CA and KRAS Mutations Are Sensitive to Combined Inhibition of AKT and KRAS/MEK/ERK Signaling.
(A) The growth of HCT116 cells was assessed after 3 days of treatment with PD0325901 (PD901) alone or in combination with AKTi. The results are expressed as the cell numbers relative to those with the DMSO-treated controls. Values represent means ± SEM (n=3).
(B) The growth of HCT116 cells were analyzed after 3 days of treatment with the drug combinations. Combination index (CI) values were determined by using the method of (Chou and Talalay, 1984) (CompuSyn software) for drug combinations with a fractional effect between 0.2 and 0.9 (20–90% of cell growth inhibition relative to control). CI values < 1 indicate drug synergy.
(C) HCT116 cells were treated with AKTi or DMSO with or without PD0325901 for 72 h. Apoptosis was assessed by sub-G1 fraction of the cells. Values represent means ± SEM (n=3).
(D) siRNAs against KRAS, control non-targeting siRNAs or transfection reagents (Mock) were transfected into HCT116 cells and incubated for 48 h and 72 h. Cell lysates were immunoblotted with the indicated antibodies.
(E) siRNAs against KRAS or control siRNAs were transfected into HCT116 cells and incubated for 48 h. The cells were then treated with 50 nM PD0325901 and 1 µM AKTi, alone or in combination or DMSO for the indicated additional times. Apoptosis was assessed by sub-G1 fraction of the cells. Values represent means ± SEM (n=3). See also Figure S2.
Combined Inhibition of AKT and ERK Signaling Causes Growth Arrest and Apoptosis in Tumors with Coexistent Pathway Activation
The results suggest that coexistent KRAS mutation may cause tumor cells to become AKT-independent. The MEK/ERK kinases are key downstream effectors of RAS signaling (McCubrey et al., 2007). A selective inhibitor of MEK (PD0325901) had only a marginal effect on growth in tumor cells with coexistent KRAS and PIK3CA mutations (HCT116, DLD-1, HCT15, T84, H460) (Figure 2A and Figure S2A). However, combined inhibition of MEK and AKT caused synergistic inhibition of proliferation and induction of apoptosis (Figures 2A–C and Figure S2B). Moreover, inhibition of KRAS expression with small interfering RNA in tumor cells with coexistent KRAS and PIK3CA mutations had no effect on the survival of HCT116, but combined inhibition of KRAS expression and AKT activity induced apoptosis synergistically (Figures 2D and 2E). MEK inhibition did not increase the apoptosis induced by KRAS knockdown in combination with AKT inhibition (Figure 2E).
Thus, tumors in which KRAS/MEK/ERK and PI3K/AKT signaling are dysregulated by mutation are dependent on neither pathway alone, but are sensitive to combined inhibition of both. If each pathway activated distinct processes necessary for tumor cell proliferation, the tumor would be suppressed by inhibiting either. The requirement for combined inhibition suggests that the two pathways activate converging targets that integrate their function.
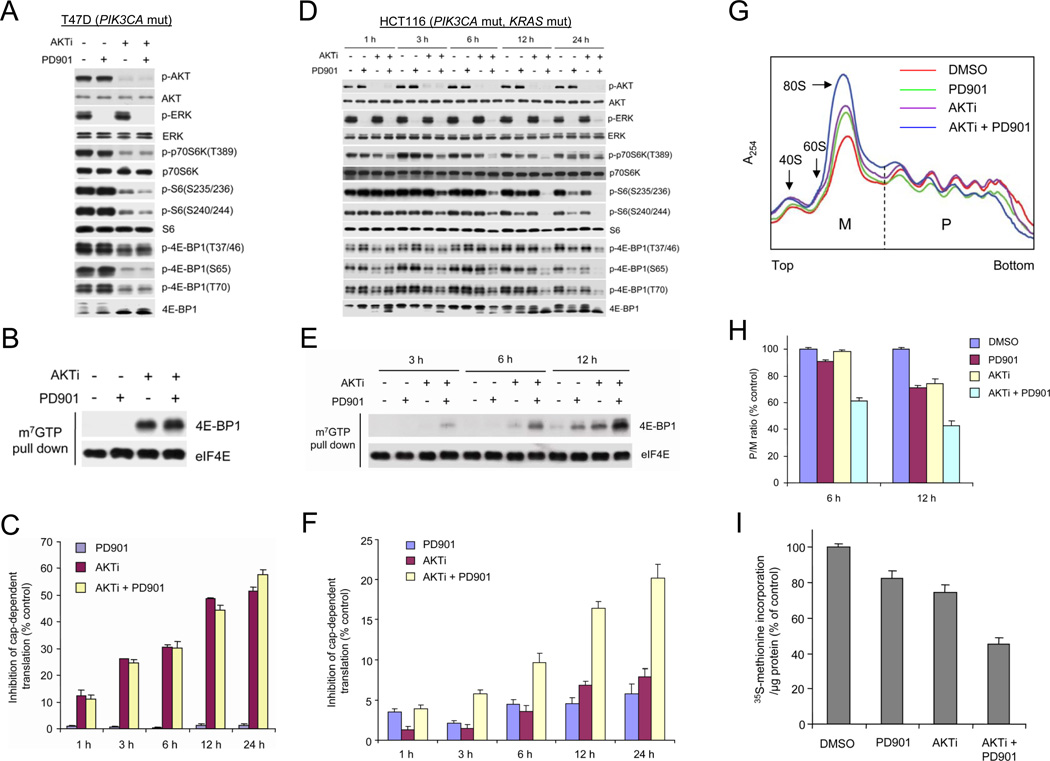
AKT or ERK Signaling Is Sufficient to Support Cap-Dependent Translation in Tumors with Coexistent Pathway Activation
In breast tumor cells with PI3K/AKT activation, phosphorylation of p70S6K, S6, and 4E-BP1 were down-regulated by the AKT inhibitor but not by MEK inhibition (Figures 1B and 3A). Dephosphorylation of 4E-BP1 allows it to bind to the eIF4E-mRNA cap complex and prevents cap-dependent translation (Richter and Sonenberg, 2005). In PIK3CA mutant, KRAS/BRAF wild-type cancer cells, inhibition of AKT, but not MEK, caused recruitment of 4E-BP1 to the mRNA cap complex and inhibited cap-dependent translation (Figures 3B and 3C). In contrast, in tumor cells with coexistent KRAS and PIK3CA mutations (HCT116), inhibition of neither AKT nor MEK was sufficient to rapidly inhibit phosphorylation of p70S6K, S6, or 4E-BP1 at any of its four phosphorylation sites, although modest inhibition was observed after exposure to the MEK inhibitor for 24 h. However, combined inhibition of MEK and AKT synergistically inhibited phosphorylation of all these sites 6 h after drug exposure and profoundly by 12 h (Figure 3D). This was associated with synergistic induction of 4E-BP1 binding to the eIF4E-mRNA cap complex within 3 h after drug addition and increasing up to 12 h after treatment (Figure 3E). Inhibition of AKT or MEK kinase alone had little effect at earlier time points, but each caused some 4E-BP1 recruitment 12 h after pathway inhibition. Similar results were observed in T84 cells with coexistent KRAS and PIK3CA mutations (Figure S3A). The degree of recruitment of 4E-BP1 to the cap-complex correlated with extent of inhibition of cap-dependent translation (Figure 3F). MEK or AKT inhibition alone had modest inhibitory effects on translation (5–7%) 12 h after drug addition, whereas combined inhibition caused 17% inhibition. The effects of combined knockdown of AKT1 and AKT2 expression on cap-dependent translation were very similar to those of the AKT inhibitor (Figures S3B and S3C).
Figure 3. Combined Inhibition of AKT and MEK Is Required for Effective Inhibition of Cap-Dependent Translation in Tumor Cells with Coexistent PIK3CA and KRAS Mutations.
(A, B, D and E) T47D (A and B) and HCT116 (D and E) cells were treated with 50 nM PD0325901 and 1 µM AKTi, alone or in combination for 6 h (A and B) or the indicated times (D and E). Cell lysates were immunoblotted with the indicated antibodies (A and D) or precipitated with m7GTP sepharose beads followed by immunoblotting of 4E-BP1 and eIF4E (B and E).
(C and F) T47D (C) and HCT116 (F) cells were transfected with a bicistronic luciferase reporter plasmid that detects cap-dependent translation of the Renilla luciferase gene and cap-independent Polio IRES-mediated translation of the firefly luciferase gene. The transfected cells were treated with 50 nM PD0325901 and 1 µM AKTi, alone or in combination. Luciferase activities were measured by a dual-luciferase assay, and the Renilla/firefly luciferase luminescence ratio was calculated for cap-dependent translational activity. The results are expressed as the inhibition of cap-dependent translation relative to the DMSO-treated controls at each time and presented as means ± SEM (n=3).
(G and H) HCT116 cells were treated with 50 nM PD0325901 and 1 µM AKTi, alone or in combination or DMSO for 12 h (G) or the indicated times (H). The cell lysates were separated on sucrose gradients (10–50%) and fractionated to visualize the indicated ribosomal species. The absorbance of translated polysomes (P) and untranslated monosomes (M) was continuously monitored at 254 nm, and the vertical dashed line separates the polysomal and monosomal fractions. The P/M ratio, an index of translational efficiency, was calculated by comparing areas under the polysome and monosome peaks using NIH image J. The results are expressed as a percentage of the P/M ratio relative to the DMSO-treated controls at each time and presented as means ± SEM (n=2) (H).
(I) 35S-methionine incorporation into protein was determined for HCT116 cells that were treated with 50 nM PD0325901 and 1 µM AKTi, alone or in combination or DMSO for 12 h. The results are expressed as a percentage of 35S-methionine incorporation/µg protein relative to the DMSO-treated controls and presented as means ± SEM (n=2). See also Figure S3.
Actively translated mRNA is found in polysomes and changes in overall translational efficiency are reflected by changes in the polysome/monosome (P/M) ratio. The effects of MEK and AKT inhibition, alone or in combination, on P/M ratio were determined in HCT116. Figure 3G shows a representative sucrose gradient from cells treated with drug for 12 h. Inhibition of MEK or AKT kinase alone had minor effect on the P/M ratio at 6 h, although some reduction (25–29%) was observed 12 h after exposure to each drug (Figure 3H). Combined inhibition synergistically reduced the P/M ratio (39%) 6 hours after drug addition and profoundly (60%) by 12 h. Combination treatment resulted in more marked suppression of total protein synthesis (55%) than either agent alone (17–25%) (Figure 3I). Inhibition of translation was associated with loss of expression of multiple regulators of growth and survival, including D-cyclins and survivin (Figure 6A and Figure S5 and data not shown).
Figure 6. Combined Inhibition of AKT and MEK Is Required to Dephosphorylate 4E-BP1 and Suppress Tumor Growth in Vivo.
(A) Mice bearing established HCT116 xenografts were treated with PD0325901 (5 mg/kg), AKTi (100 mg/kg), combination of both drugs, or vehicle control once daily. Tumors were excised at various times after the fourth dose of drug administration. Tumor lysates were immunoblotted with the indicated antibodies.
(B–E) Mice bearing HCT116 (B), T84 (C), DLD-1 (D) and HCT15 (E) xenografts were treated with PD0325901 (5 mg/kg), AKTi (100 mg/kg), combination of both drugs, or vehicle control once daily for 5 consecutive days each week. The results are presented as the mean tumor volume ± SEM (n=5 mice/group) from two independent experiments. See also Figure S5.
Thus, tumors with PI3K mutation that are wild-type for RAS and BRAF depend upon AKT signaling for phosphorylation of various regulators of translation, including 4E-BP1, assembly of active preinitiation translation complexes, maintenance of high levels of translation, and cell growth and survival. In contrast, in tumors with coexistent RAS mutation, inhibition of AKT has only minor effects on these processes. In such tumors, either AKT or MEK/ERK signaling is sufficient to support translation, and inhibition of both pathways is necessary for its significant suppression.
To determine if KRAS mutation is responsible for loss of AKT dependence in these cells, we compared parental HCT116 and DLD-1 cells with isogenic derivatives in which the mutant KRAS allele was deleted (Shirasawa et al., 1993). The deletion of the mutant KRAS allele was sufficient to confer AKT-dependence to these PIK3CA mutant cells (Figure 4A). Unlike the parental HCT116, inhibition of AKT alone in HKh-2 and HKe-3 (wild-type RAS) cells was sufficient to inhibit phosphorylation of p70S6K, S6 and 4E-BP1, induce binding of 4E-BP1 to the eIF4E-mRNA complex and inhibit cap-dependent translation (Figures 4B and 4C and Figure S4). 4E-BP1 binding to the complex and inhibition of translation were not induced further in these cells by MEK inhibition. Conversely, deletion of the endogenous mutant PIK3CA allele in HCT116 or DLD-1 cells (Samuels et al., 2005) had the opposite effect: sensitization of these processes and cell growth and survival to MEK inhibition (Figures 4D–F).
Figure 4. Deletion of Mutant KRAS or PIK3CA Confers Dependence on the Other Pathway in Tumors in Which These Mutations Coexist.
(A) The growth of isogenic HCT116 and DLD-1 cell lines in which the mutant allele of KRAS was deleted by homologous recombination were assessed after 3 days culture in the presence of AKTi. The results are expressed as IC50 of AKTi. Values represent means ± SEM (n=3).
(B and D) Cells were treated with 50 nM PD0325901 and 1 µM AKTi, alone or in combination for 6 h. Cell lysates were immunoblotted with the indicated antibodies or precipitated with m7GTP sepharose beads followed by immunoblotting of 4E-BP1 and eIF4E.
(C) Cells were transfected with a bicistronic luciferase reporter plasmid and then treated with 50 nM PD0325901 and 1 µM AKTi, alone or in combination. The inhibition of cap-dependent translation was determined as in Figure 3C. Values represent means ± SEM (n=3).
(E) The growth of HCT116 and DLD-1 isogenic cell lines in which the mutant allele of PIK3CA was deleted by homologous recombination were assessed after 3 days culture in the presence of PD0325901. The results are expressed as IC50 of PD0325901. Values represent means ± SEM (n=3).
(F) Cells were treated with DMSO or the indicated concentration of PD0325901 for 72 h. Apoptosis was assessed by sub-G1 fraction of the cells. Values represent means ± SEM (n=2). See also Figure S4.
Thus, dysregulation of ERK by RAS mutation is responsible for the loss of AKT-dependence of translation. MAP kinase-interacting kinases (MNKs) are activated by ERK signaling and may regulate translation via phosphorylation of eIF4E (Buxade et al., 2008). Knockdown of MNK1/2 did inhibit eIF4E phosphorylation, but had no effects on phosphorylation of p70S6K, S6 and 4E-BP1, induction of 4E-BP1 binding to the eIF4E, or cap-dependent translation, nor did it enhance the effect of the AKTi on these processes (Figures S3D and S3E). In this system, therefore, the ERK effect on translation is not mediated by MNK1/2.
4E-BP1 Integrates the Effects of AKT and ERK Signaling on Translation and Survival
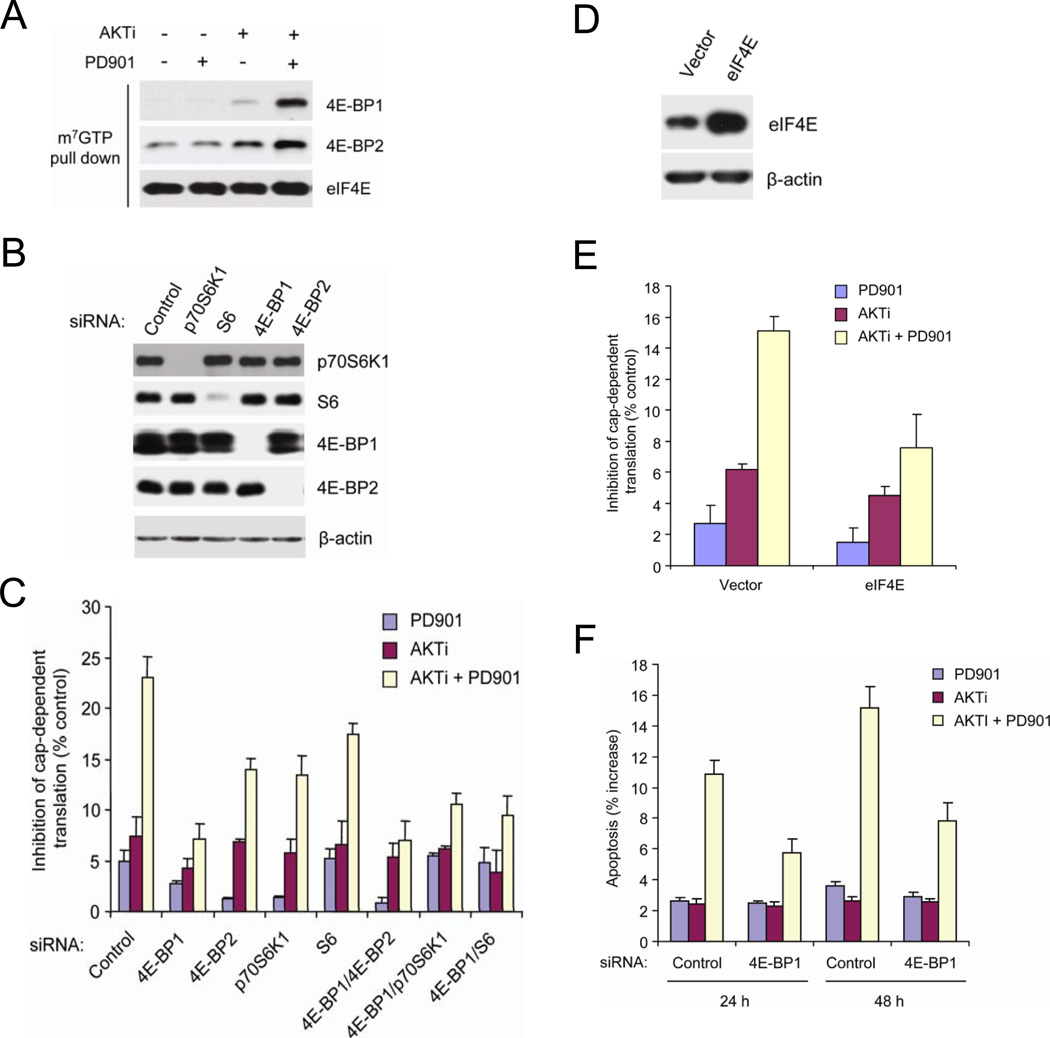
Thus, tumors with coexistent mutations depend on neither pathway alone but are sensitive to combined inhibition of both. This implies that there are downstream targets that are regulated by both activated pathways, so that inhibition of neither alone is effective. These targets may include components of the networks that regulate apoptosis such as BAD (She et al., 2005) and, as shown here, cap-dependent translation (p70S6K, S6, 4E-BP1).
Combined inhibition of AKT and MEK kinase caused the recruitment of both 4E-BP1 and 4E-BP2 to the eIF4E-mRNA cap complex (Figure 5A). Reduction of 4E-BP1 expression with siRNA knockdown markedly reduced the dependence of translation on AKT and ERK signaling (Figures 5B and 5C). Combined inhibition caused 23% inhibition of cap-dependent translation in control cells, but had only minimal effects in cells in which 4E-BP1 expression was suppressed (Figure 5C). Decreased 4E-BP2 expression had much less marked effects, and combined inhibition of 4E-BP1 and 4E-BP2 was not much more effective than 4E-BP1 loss alone (Figures 5B and 5C). The results suggest that phosphorylation of 4E-BP1 is the major effector of activation of cap-dependent translation by AKT and MEK signaling in these tumors.
Figure 5. 4E-BP1 Mediates the Effects of AKT and MEK Activation on Cap-Dependent Translation and Survival.
(A) HCT116 cells were treated with 50 nM PD0325901 and 1 µM AKTi, alone or in combination for 6 h. Cell lysates were precipitated with m7GTP sepharose beads followed by immunoblotting with the indicated antibodies.
(B) siRNAs against the indicated genes or control siRNAs were transfected into HCT116 cells and incubated for 72 h. Cell lysates were immunoblotted with the indicated antibodies.
(C) siRNAs against the indicated genes or control siRNAs were transfected into HCT116 cells and incubated for 30 h. The cells were then transfected with a bicistronic luciferase reporter plasmid for 24 h, and then treated with 50 nM PD0325901 and 1 µM AKTi, alone or in combination for an additional 12 h. The inhibition of cap-dependent translation was determined as in Figure 3C. Values represent means ± SEM (n=3).
(D) Detection of eIF4E and β-actin by immunoblot in HCT116 cells expressing the indicated transgenes.
(E) HCT116 cells expressing eIF4E or control vector were transfected with a bicistronic luciferase reporter plasmid for 24 h, and then treated with 50 nM PD0325901 and 1 µM AKTi, alone or in combination for an additional 12 h. The inhibition of cap-dependent translation was determined as in Figure 3C. Values represent means ± SEM (n=3).
(F) siRNAs against 4E-BP1 or control siRNAs were transfected into HCT116 cells and incubated for 48 h. The cells were then treated with 50 nM PD0325901 and 1 µM AKTi, alone or in combination for the indicated additional times. Apoptosis was assessed by sub-G1 fraction of the cells. The results are expressed as the increased levels of apoptosis by subtracting each of the DMSO-treated controls and presented as means ± SEM (n=3).
Phosphorylation of 70S6K and ribosomal protein S6 are also downstream of PI3K/AKT and MEK/ERK signaling and sensitive to their combined inhibition (Figures 3 and 4). Knockdown of p70S6K1 or S6 did modestly attenuate the effects of combined inhibition of AKT and ERK on translation, but to a much lesser degree than knockdown of 4E-BP1 (Figures 5B and 5C). Furthermore, knockdown of 4E-BP2, p70S6K or S6 in combination with 4E-BP1 knockdown did not further enhance the effects of the latter (Figure 5C).
The importance of 4E-BP1 dephosphorylation and binding to eIF4E in mediating the effects of combined AKT and MEK pathway inhibition was confirmed in HCT116 cells in which eIF4E protein was exogenously overexpressed (Figure 5D). In these cells, the effect of combined inhibition of MEK and AKT on cap-dependent translation was significantly reduced (Figure 5E). These data suggest that 4E-BP1 is the key integrator of the effects of ERK and PI3K/AKT activation on cap-dependent translation in tumor cells.
Disabling the inhibitory effects of 4E-BP1 by phosphorylation may exert important biologic effects in transformed cells. Downregulation of 4E-BP1 expression with siRNA significantly attenuated the apoptotic response associated with inhibition of MEK and AKT (Figure 5F). Thus, the suppression of apoptosis by mutant RAS and PI3K is mediated, in part, by phosphorylation of 4E-BP1.
Combined Inhibition of AKT and ERK Are Required to Suppress 4E-BP1 Phosphorylation and Tumor Growth in Vivo
These data suggest that inhibition of both pathways may be required to significantly affect human tumors with concurrent mutation of KRAS and PIK3CA. To explore the feasibility of this therapeutic strategy, we tested the safety and efficacy of inhibiting MEK and AKT in tumor xenografts with this genotype. As we have previously shown, AKTi 100 mg/kg and the MEK inhibitor PD0325901 5 mg/kg effectively inhibit the phosphorylation of AKT or ERK, respectively, in PIK3CA or RAS mutant xenografts (She et al., 2008; Solit et al., 2006). After four consecutive daily treatments, phosphorylation of AKT was profoundly inhibited by the AKTi and phosphorylation of ERK was inhibited by PD0325901 by 5 h after the last dose and inhibition of both pathways persisted for at least 24 h (Figure 6A). Neither drug alone caused effective inhibition of p-S6, p-4E-BP1, or D-cyclin levels nor did they induce PARP cleavage in models with concurrent mutations of both KRAS and PIK3CA. Inhibition of both pathways, however, did induce these effects synergistically. Similar results were obtained after treatment with the drugs for 4 weeks (Figure S5). These results confirm the relevance of the tissue culture data to in vivo models.
Chronic administration of both drugs together on a Monday through Friday schedule was well tolerated without weight loss in the animals (data not shown). In four tested models, the AKTi or MEK inhibitor had only marginal or modest antitumor effects (Figures 6B–E). Neither drug alone completely inhibited tumor growth. However, AKTi in combination with PD0325901 synergistically suppressed growth in all four models with tumor regression observed in HCT116 and T84 (P < 0.01 for combination of AKTi and PD0325901 vs. AKTi, PD032901 or control).
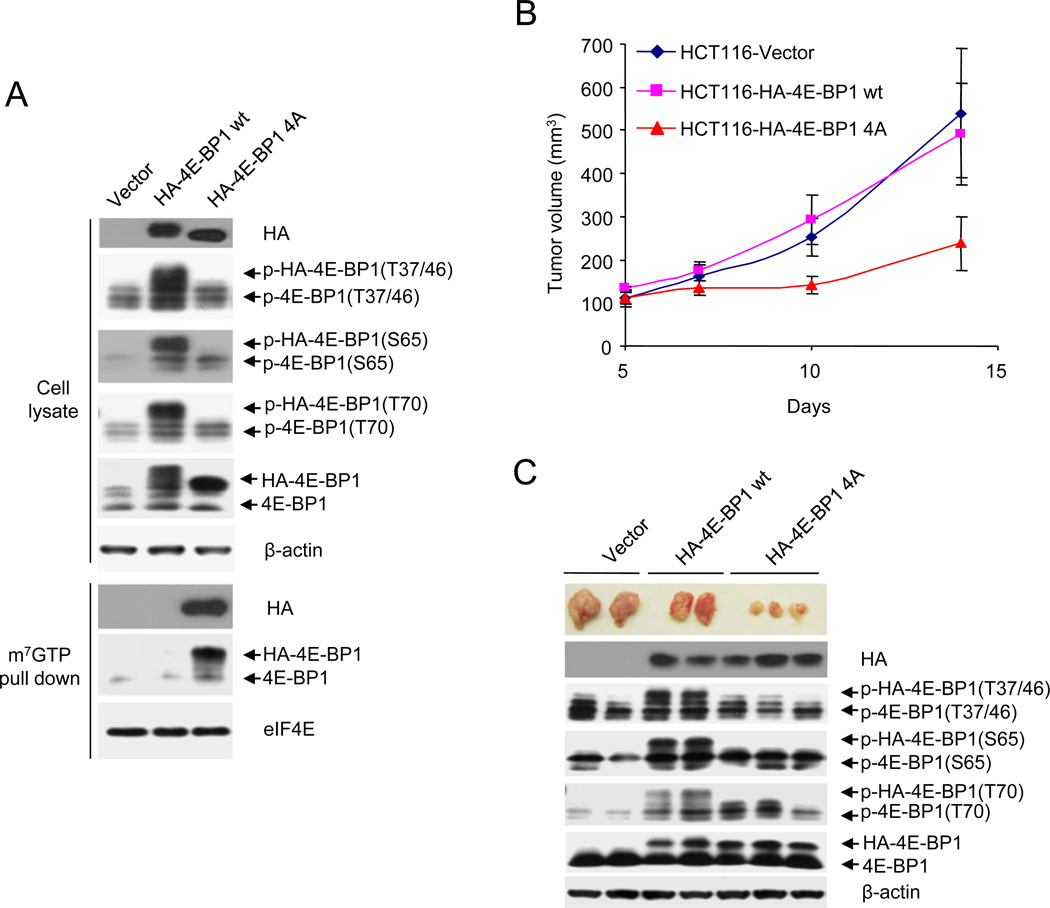
Our data imply that dephosphorylation of 4E-BP1 alone should significantly inhibit tumor growth. To test this assertion, we used a mutant 4E-BP1 (4Ala) in which its four known phosphorylation sites (Thr 37, Thr46, Ser65, Thr70) were replaced with alanine. This mutant 4E-BP1 can not be phosphorylated and binds constitutively to eIF4E (Rong et al., 2008 and Figure 7A). As compared to wild-type 4E-BP1 and vector control, expression of 4E-BP1 (4Ala) effectively suppressed tumor growth in vivo (Figures 7B and 7C; P < 0.01 for 4E-BP1 (4Ala) vs. 4E-BP1 wt or vector). These data support the hypothesis that inhibition of 4E-BP1 function by AKT and ERK signaling is required to activate translation and maintain the malignant phenotype in tumors with PI3K and RAS mutation.
Figure 7. Activated 4E-BP1 Represses PI3KCA and KRAS Mutant Tumor Growth.
(A) HCT116 cells expressing vector, HA-4E-BP1 wt or HA-4E-BP1 (4Ala) were immunoblotted with the indicated antibodies or precipitated with m7GTP sepharose beads followed by immunoblotting of HA, 4E-BP1 and eIF4E.
(B) HCT116 cells expressing vector, HA-4E-BP1 wt or HA-4E-BP1 (4Ala) were transplanted into the right flank of nude mice. Tumor volume was measured twice each week with the results presented as mean tumor volume ± SEM of 5 mice in each group.
(C) Representative tumors expressing vector, HA-4E-BP1 wt or HA-4E-BP1 (4Ala), and immunoblot analysis of the tumor lysates using indicated antibodies.
DISCUSSION
Human tumors almost invariably harbor mutations in a multitude of oncogenes and tumor suppressor genes. Mutations that result in activation of the PI3K/AKT/mTOR and RAS/RAF/MEK/ERK pathways are especially frequent. Moreover, mutations that activate these two pathways often coexist in certain tumors; thus RAS and PI3K mutation, BRAF and PI3K mutation, BRAF and PTEN mutation, and variant EGFR expression and PTEN mutation occur together in colorectal carcinoma, thyroid carcinoma, melanoma, and glioblastoma, respectively (Liu et al., 2008; Mellinghoff et al., 2005; Nosho et al., 2008; Simi et al., 2008; Tsao et al., 2004). Tumors with activation of PI3K/AKT signaling in the absence of EGFR, RAS or BRAF mutation tend to be dependent on the pathway and sensitive to selective inhibition of AKT (She et al., 2008; Yu et al., 2008). Similarly, tumors with RAS or RAF mutation tend to be sensitive to MEK inhibition if PI3K or PTEN are not also mutated (Hoeflich et al., 2009; Solit et al., 2006; Wee et al., 2009 and Figure S2A). RAS-dependent tumorigenesis in animal models has been reported to require PI3K activation by RAS, but the growth of established tumors with RAS mutation is insensitive to PI3K inhibitors (Engelman et al., 2008) and, as shown here, to AKT inhibitors.
The usual rationalization for coexistent oncogenic mutations in components of the signaling apparatus is that they mediate different aspects of the transformed phenotype that allow for their co-selection. However, in this paper and elsewhere, we and others have shown that tumors with coexistent mutation of both pathways tend to be insensitive to inhibition of either alone, but sensitive to their combined inhibition (Engelman et al., 2008; Hoeflich et al., 2009; She et al., 2005; Wee et al., 2009; Yu et al., 2008). These results suggest that neither pathway alone subserves a key function or that the original selective advantage of the first mutation has been lost. In this paper, we provide an explanation for the loss of dependence of these tumors on either pathway alone. In tumors sensitive to AKT inhibition, phosphorylation of certain downstream targets such as S6 and 4E-BP1 and cap-dependent translation are dependent on AKT signaling. In contrast, in tumors with co-activation of both AKT and ERK, inhibition of either is insufficient to adequately inhibit these processes; inhibition of both is required. Moreover, deletion of the oncogenes responsible for activation of either pathway is sufficient to confer dependence on the other. The results suggest that PI3K/AKT and MEK/ERK signaling converge on a common set of targets that integrate their function. Activation of either pathway is sufficient to affect these integrators, thus the second mutation eliminates the dependence of both the target and the tumor cell on either.
AKT and ERK signaling affect many common downstream targets and processes, including regulators of cell cycle progression, apoptosis, transcription and translation (Manning and Cantley, 2007; McCubrey et al., 2007). In normal cells, these functions are regulated by a complex signaling network, but, in tumor cells, ‘oncogene addiction’ implies that they have become dependent on a single, dominant, oncoprotein activated pathway. Mutational activation of the second pathway would then serve to reduce dependency on either. The downstream convergence of PI3K/AKT and ERK signaling may account for the significant frequency of coexistent mutations in these pathways. The selective advantage for the second mutation is not certain; it may lie in divergent effects of the second pathway but it is also possible that the dependence of key processes such as translation on a single oncogene-activated pathway may result in decreased fitness of the cell in certain environments. In support of this possibility, the growth of tumor xenografts with mutant RAS is slowed in calorie restricted mice and this effect is rescued by coexistent PIK3CA mutation (Kalaany and Sabatini, 2009). This interpretation is consistent with that of Ericson et al. who report that in tumors with coexistent RAS and PI3K mutations, AKT was required for growth only in challenging microenvironments, such as growth factor depletion and during the metastatic process (Ericson et al., 2010). Whatever the mechanism of selection, it is clear that the second mutation reduces or eliminates the dependency or ‘addiction’ of the tumor to the first mutation. Whether this loss of dependency is responsible for the selection or is a neutral byproduct of the second hit, it has important clinical and biologic implications.
The data reported here support recent studies that show that activation of cap-dependent translation plays an important role in induction and maintenance of the transformed phenotype (Mamane et al., 2004; Polunovsky and Bitterman, 2006). The phosphorylation of two components of the translation machinery, S6 and 4E-BP1 was shown to be dependent on AKT signaling in tumors in which the PI3K/AKT pathway is dysregulated, but not in those in which there is coexistent mutational activation of ERK signaling. In such tumors, combined inhibition of both pathways is required to affect their phosphorylation and to significantly inhibit cap-dependent translation. Thus, these two proteins are candidate integrators of AKT and ERK signaling that may play a role in mediating transformation and oncoprotein dependency.
In particular, 4E-BP1 is identified as a key downstream target of both mutant PI3K and RAS-activated signaling in human cancer cells. Knockdown of this inhibitor of translation in tumor cells markedly reduces their dependence on activated signaling for translation and survival. This is somewhat surprising, given that these pathways also activate the phosphorylation of the S6K, S6 ribosomal protein and other regulators of translation, including other members of the 4E-BP family (Pause et al., 1994). However, in the experiments reported here, knockdown of either S6K, S6 or 4E-BP2, alone or in combination with 4E-BP1 has more than a marginal effect. This suggests that 4E-BP1 inhibition is responsible for much of the activation of translation by RAS and PI3K/AKT in these cells and this in turn plays an important part in mediating the effects of these pathways in the tumor. It is consistent with recent clinical findings that expression of high levels of phosphorylated 4E-BP1 are associated with poor prognosis in several tumor types, independent of specific upstream oncogenic alterations (Armengol et al., 2007). The AKT dependence of phosphorylation of 4E-BP1 and of tumor growth is closely correlated. These data suggest that this relationship is causal. This is supported by our finding that a dominant negative 4E-BP1 incapable of being phosphorylated in response to upstream pathways is sufficient to suppress the growth of HCT116 tumors in vivo. Others have found that the non-phosphorylated (activated) 4E-BP1 is capable of suppressing tumorigenesis in PTEN-mutant breast cancer (Avdulov et al., 2004) and KRAS mutant non-small cell lung cancer (Jacobson et al., 2006). We thus show that tumor cells in which both pathways are activated are insensitive to inhibition of either, but sensitive to their combined inhibition or to dominant activated 4E-BP1. Furthermore, tumors in which eIF4E is overexpressed or 4E-BP1 expression is knocked down lose dependence on AKT and ERK signaling. Taken together, these data support the conclusion that inhibition of 4E-BP1 function by activation of AKT and ERK signaling plays a crucial role in maintaining the transformed phenotype and add support to the idea that the eIF4E complex represents a valid and intriguing target for drug development (Graff et al., 2008).
The mTOR kinase is another downstream target of both AKT and ERK signaling that integrates their function. This may occur via phosphorylation of TSC2 and perhaps other proteins by both pathways (Ma et al., 2005; Manning et al., 2002). The mTOR-containing TORC1 complex is responsible for phosphorylation of S6K and 4E-BP1 by the enzyme. Rapamycin is a selective inhibitor of the TORC1 complex, but is much less effective than combined inhibition of AKT and MEK in downregulating 4E-BP1 phosphorylation and its binding to eIF4E, or inducing apoptosis in tumor cells with coexistent RAS and PI3K mutations (data not shown). This suggests that the effects of AKT and MEK inhibition are mediated by other targets in addition to mTOR. However, this result is complicated by the recent report that rapamycin is only a modest inhibitor of TORC1 activity and that mTOR kinase inhibitors are much more efficient downregulators of 4E-BP1 phophorylation (Feldman et al., 2009; Thoreen et al., 2009). However, the TORC2 complex is also an upstream activator of AKT (Sarbassov et al., 2005) and T70 phosphorylation of 4E-BP1 is sensitive to AKT/MEK inhibition and reported to be insensitive to mTOR kinase inhibition (Thoreen et al., 2009). Furthermore, phosphorylation of 4E-BP1 and its activity have also been shown to be regulated by the PP2A phosphatase and other kinases independent of mTOR (Herbert et al., 2002; Imai et al., 2008; Michlewski et al., 2008). Thus, it is still unclear whether all of the effects of AKT and ERK signaling on 4E-BP1 are integrated by mTOR.
It is likely that some of the effects of combined inhibition of AKT and ERK are mediated by other targets, including components of the apoptotic machinery (She et al., 2005). We have previously shown that BAD is a downstream target that can integrate EGFR/ERK and PI3K signaling in PTEN-negative/EGFR amplified tumors and that knocking down BAD significantly (~50%) attenuates the effects of combined pathway inhibition in MDA-468 breast cancer cells (She et al., 2005). In HCT116 cells, knockdown of BAD expression reduces induction of apoptosis in response to combined pathway inhibition by approximately 25% (data not shown). How activation of cap-dependent translation interacts with regulation of apoptotic regulators to mediate oncogenic survival signaling is likely to be complex and a matter for further investigation.
These are important questions because relatively selective inhibitors of RAF, MEK, PI3K, AKT and mTOR kinases are now available and many are in early clinical testing. This work suggests that the tumors from patients in these trials should be evaluated for mutations in components of both pathways and tumors with coexistent mutations in both pathways will not respond to inhibition of one alone. This hypothesis should now be tested in these clinical trials. Furthermore, dephosphorylation of 4E-BP1 in response to drug should be an important biomarker for predicting response to therapy.
The tolerability of the combined inhibition of AKT and ERK and its synergistic effects on cap-dependent translation and on tumor growth suggest that this strategy might be useful in the variety of metastatic tumors in which these pathways are co-activated. There is currently no therapeutic agent that directly and effectively inhibits RAS function. Since RAF and PI3K are two of the key effectors of the transforming activity of mutant RAS, the combined inhibition of MEK and AKT may constitute an anti-RAS therapeutic strategy as well, of potential utility in diseases (pancreatic, colon, lung carcinoma) with mutated RAS for which there are few and only marginally effective therapies. Given the importance of 4E-BP1 in integrating the effects of AKT and ERK on protein translation and apoptosis, mTOR kinase inhibitors currently in development may also be useful for treating these tumors. However, these inhibitors release the feedback inhibition of receptor tyrosine kinases and activate both ERK and PI3K/AKT in tumors (N Rosen, unpublished data and Carracedo et al., 2008). Combined inhibition of ERK and AKT both effectively inhibits 4E-BP1 phosphorylation and prevents reactivation of ERK and AKT and thus may have a therapeutic advantage.
EXPERIMENTAL PROCEDURES
Cell Culture and Inhibitors
Human tumor cell lines were obtained from the American Type Culture Collection (ATCC) and maintained in the appropriate medium supplemented with 2 mM glutamine, 50 units/ml each of penicillin and streptomycin, and 10% FBS as suggested by ATCC. The isogenic cell lines with deletion of mutant alleles of KRAS (Shirasawa et al., 1993) or PIK3CA (Samuels et al., 2005) from HCT116 or DLD-1 cells were grown similarly in McCoy’s 5A medium. The AKTi was obtained from Merck (Bilodeau et al., 2008). The MEK inhibitor PD0325901 was synthesized as described (Barrett et al., 2008). Both inhibitors were dissolved in dimethyl sulfoxide.
Cell Viability/Proliferation and Apoptosis Assays
Cells were seeded in 96-well plates at a density of 2,000–5,000 cells in triplicates. After 24 h, cells were treated with different concentrations of the indicated kinase inhibitors and incubated at 37°C. The cells were cultured for 3 days and then the number of viable cells was measured by CellTiter-Glo luminescent cell viability assay (Promega) (She et al., 2008). Cell proliferation was detected by a chemiluminescent immunoassay based on the measurement of bromodeoxyuridine incorporation during DNA synthesis according to the manufacturer’s standard protocol (Roche Applied Science). For in vitro combination studies, the synergy was assessed using the combination index (CI) of Chou and Talalay method using CompuSyn software (Chou and Talalay, 1984). Generally, CI values of <1 are taken to indicate synergistic interaction between drugs, and CI values of >1 indicate no interaction (drug antagonism). To measure apoptosis, both adherent and floating cells were harvested after drug treatment, and the cell nuclei were stained with ethidium bromide (She et al., 2008). Detection and quantitation of apoptotic cells (sub-G1 fraction) were performed by flow cytometric analysis.
Immunoblot Analysis
Protein extracts were prepared by cell lysis in buffer containing protease and phosphatase inhibitors, subjected to SDS-PAGE and analyzed by immunoblot using primary antibodies as indicated throughout. Methodological details are provided in Supplemental Experimental Procedures.
Cap-Binding Assay
Cell lysates as prepared above were incubated with m7GTP sepharose beads (GE Healthcare) to capture eIF4E and its binding partners. Precipitates were washed three times with lysis buffer, resuspended in 2× Laemmli sample buffer, and resolved by SDS-PAGE followed by immunoblot with the indicated antibodies.
Quantification of Cap-Dependent Translation
Cells (250,000) were transfected with a bicistronic luciferase reporter plasmid (2 µg), pcDNA3-rLuc-PolioIRES-fLuc, which directs cap-dependent translation of the Renilla luciferase gene and cap-independent Polio IRES-mediated translation of the firefly luciferase gene (Pause et al., 1994), in 6-well plates using Lipofectamine 2000 (Invitrogen). After 24 h transfection, cells were treated with kinase inhibitors for the indicated times. Cell were rinsed with PBS and incubated with the passive lysis buffer (Promega) for 15 min. Cell debris was pelleted by centrifugation, and triplicate supernatant samples were assayed for Renilla luciferase and firefly luciferase activities in an Analyst AD (Molecular Devices) using a dual-luciferase reporter assay system (Promega). Cap-dependent Renilla activity was normalized against cap-independent firefly activity as the internal control. The Renilla/firefly luciferase luminescence ratio was calculated for cap-dependent translational activity (Roux et al., 2007).
Polysome Analysis
Sucrose density gradient centrifugation was employed to separate the ribosome fractions following treatment of cells with drugs. Fifteen minutes before collection, cycloheximide (100 µg/ml) was added to the culture medium. Cells were washed in ice-cold PBS containing 100 µg/ml cycloheximide, and harvested in polysome lysis buffer (5 mM Tris-HCl, pH7.5, 2.5 mM MgCl2, 1.5 mM KCl, 2 mM DTT, 0.5% Triton X-100, 0.5% sodium deoxycholate, 100 µg/ml cycloheximide, RNAsin inhibitor, protease and phosphatase inhibitors). Cells were incubated on ice for 15 min and then centrifuged at 10,000 × g for 10 min at 4 °C. The supernatant (4 mg of protein) was layered on a pre-chilled 10–50% linear sucrose gradient preparing in 5 mM Tris-HCl, pH7.5, 2.5 mM MgCl2 and 1.5 mM KCl, and then centrifuged in a Beckman SW40Ti rotor at 35,000 rpm for 2.5 h at 4 °C. Gradients were fractionated while monitoring absorbance at A254 with a Density Gradient Fractionation System (Teledyne ISCO).
35S-Methionine Incorporation Assay
Cells were labeled with 100 µCi of 35S-methionine per ml (Perkin Elmer) in methionine-free medium (Invitrogen) for 1 h, washed twice with PBS, and lysed in the NP-40 lysis buffer as above. Lysates were clarified by centrifugation for 10 min at 10,000 × g. Labeled proteins were precipitated with trichloroacetic acid and resuspended in 0.5 N NaOH. The proteins were transferred to glass microfiber filters (Whatman) and counted in a scintillation counter. 35S-methionine incorporation was normalized to protein amount.
Gene Silencing by siRNA
siRNAs were purchased from Dharmacon. Cells were seeded in 6-well plates at a density of 150,000 cells/well. In the next day, cells were transfected with 20 nM siRNA pool against human KRAS (L-005069), AKT1 (L-003000), AKT2 (L-003001), MNK1 (L-004879), MNK2 (L-004908), 4E-BP1 (L-003005), 4E-BP2 (L-018671), p70S6K1 (L-003616), S6 (L-003024), BAD (L-003870) or non-targeting control siRNA pool (D-001810-10) using Lipofectamine RNAiMAX reagent according to the manufacturer’s instructions (Invitrogen). After 48 h transfection, cell were treated with kinase inhibitors for the indicated times and subjected to immunoblot analysis and assays for cap-dependent translation and apoptosis.
DNA Constructs, Virus Production and Infection
Retroviral constructs including MSCV-eIF4E and empty vector MSCV-GFP (Wendel et al., 2004), pBABE-HA-4E-BP1, pBABE-HA-4E-BP1 (4Ala) and pBABE empty vector (Rong et al., 2008) were transfected into amphotropic phoenix 293T packaging cells. After 48 h, virus-containing medium was filtered, collected and used to infect HCT116 cells in the presence of 8 µg/ml of polybrene (Millipore) for 3 times at 4–5 h intervals. Cell population expressing eIF4E were obtained by sorting infected cells according to GFP intensity at 488 nm laser emission using a Beccton Dickinson FACS AriaII (BD Biosciences) with a 530/30 optical filter, followed by assessment by immunoblot. The stable transfectants with expression of HA-4E-BP1 and its mutant (4Ala) were obtained by selection with puromycin (2 µg/ml, Sigma) for 1 week and further analyzed by immunoblot.
Animal Studies
Six-week-old nu/nu athymic female mice (NCI-Frederick Cancer Center) were maintained in pressurized ventilated cages. Experiments were carried out under an IACUC approved protocol and institutional guidelines for the proper and humane use of animals in research were followed. Tumors were generated by transplanting 1.5–3 × 106 tumor cells in a 1:1 mixture of media and Matrigel (BD Biosciences) into the right flank (200 µl/mouse). Prior to initiation of treatment, mice were randomized among control and treated groups (n=5 per group). AKTi was formulated in 25% hydroxypropyl β-cyclodextrin (pH 4–5), and administered subcutaneously at a dose of 100 mg/kg per day for 5 consecutive days each week. PD0325901 was formulated in 0.5% hydroxypropyl methyl-cellulose plus 0.2% Tween 80, and administered orally at a dose of 5 mg/kg per day for 5 consecutive days each week. For combination treatment, both drugs were given concurrently. Control mice received vehicle alone for both drugs. The average tumor diameter (two perpendicular axes of the tumor were measured) was measured in control and treated groups using a caliper. The data are expressed as the increase or decrease in tumor volume in mm3 (mm3 = π/6 × (larger diameter) × (smaller diameter)2). Unpaired, two-tailed Student’s t-test was used to assess statistical significance. To prepare lysates, tumor tissue was homogenized in 2% SDS lysis buffer and then processed for immunoblot.
SIGNIFICANCE.
In tumors with coexistent mutational activation of PI3K/AKT and ERK pathways, inhibition of both is required to inhibit transformation, whereas deletion of either causes the tumor to become dependent on the other. The data suggests that the second mutation abrogates addiction of the tumor to the first by causing redundant activation of targets that integrate the effects of both pathways. 4E-BP1 is a key integrator of these pathways that mediates their effects on transformation and is thus a potential target for therapy. We find that combined inhibition of AKT and MEK is feasible in vivo and effectively inhibits 4E-BP1 phosphorylation and tumor growth. This is, therefore, a therapeutic strategy for the many malignancies in which both pathways are activated.
Supplementary Material
ACKNOWLEDGMENTS
We thank N. Sonenberg for providing 4E-BP1 constructs, H. G. Wendel for the eIF4E construct, B. Vogelstein and V. E. Velculescu for providing the HCT116 and DLD-1 isogenic cell lines, and E. DeStanchina, H. Zhao and D. Domingo for technical assistance. This work was supported by NIH Grants P01-CA094060 and P01-CA129243, the Breast Cancer Research Foundation, the Taub Foundation, and the William H. and Alice Goodwin Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Armengol G, Rojo F, Castellvi J, Iglesias C, Cuatrecasas M, Pons B, Baselga J, Ramon y Cajal S. 4E-binding protein 1: a key molecular "funnel factor" in human cancer with clinical implications. Cancer Res. 2007;67:7551–7555. doi: 10.1158/0008-5472.CAN-07-0881. [DOI] [PubMed] [Google Scholar]
- Avdulov S, Li S, Michalek V, Burrichter D, Peterson M, Perlman DM, Manivel JC, Sonenberg N, Yee D, Bitterman PB, Polunovsky VA. Activation of translation complex eIF4F is essential for the genesis and maintenance of the malignant phenotype in human mammary epithelial cells. Cancer Cell. 2004;5:553–563. doi: 10.1016/j.ccr.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Barrett SD, Bridges AJ, Dudley DT, Saltiel AR, Fergus JH, Flamme CM, Delaney AM, Kaufman M, LePage S, Leopold WR, et al. The discovery of the benzhydroxamate MEK inhibitors CI-1040 and PD 0325901. Bioorg. Meed. Chem. Lett. 2008;18:6501–6504. doi: 10.1016/j.bmcl.2008.10.054. [DOI] [PubMed] [Google Scholar]
- Bilodeau MT, Balitza AE, Hoffman JM, Manley PJ, Barnett SF, Defeo-Jones D, Haskell K, Jones RE, Leander K, Robinson RG, et al. Allosteric inhibitors of Akt1 and Akt2: a naphthyridinone with efficacy in an A2780 tumor xenograft model. Bioorg. Meed. Chem. Lett. 2008;18:3178–3182. doi: 10.1016/j.bmcl.2008.04.074. [DOI] [PubMed] [Google Scholar]
- Buxade M, Parra-Palau JL, Proud CG. The Mnks: MAP kinase-interacting kinases (MAP kinase signal-integrating kinases) Front Biosci. 2008;13:5359–5373. doi: 10.2741/3086. [DOI] [PubMed] [Google Scholar]
- Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J. Clin. Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrin C, Haskell K, Howell B, Jones R, Leander K, Robinson R, Watkins A, Bilodeau M, Hoffman J, Sanderson P, et al. An allosteric Akt inhibitor effectively blocks Akt signaling and tumor growth with only transient effects on glucose and insulin levels in vivo. Cancer Biol. Ther. 2010 doi: 10.4161/cbt.9.7.11100. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, Maira M, McNamara K, Perera SA, Song Y, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nature Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson K, Gan C, Cheong I, Rago C, Samuels Y, Velculescu VE, Kinzler KW, Huso DL, Vogelstein B, Papadopoulos N. Genetic inactivation of AKT1, AKT2, and PDPK1 in human colorectal cancer cells clarifies their roles in tumor growth regulation. Proc. Natl. Acad. Sci. USA. 2010;107:2598–2603. doi: 10.1073/pnas.0914018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, Shokat KM. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff JR, Konicek BW, Carter JH, Marcusson EG. Targeting the eukaryotic translation initiation factor 4E for cancer therapy. Cancer Res. 2008;68:631–634. doi: 10.1158/0008-5472.CAN-07-5635. [DOI] [PubMed] [Google Scholar]
- Herbert TP, Tee AR, Proud CG. The extracellular signal-regulated kinase pathway regulates the phosphorylation of 4E-BP1 at multiple sites. J. Biol. Chem. 2002;277:11591–11596. doi: 10.1074/jbc.M110367200. [DOI] [PubMed] [Google Scholar]
- Hoeflich KP, O'Brien C, Boyd Z, Cavet G, Guerrero S, Jung K, Januario T, Savage H, Punnoose E, Truong T, et al. In vivo antitumor activity of MEK and phosphatidylinositol 3-kinase inhibitors in basal-like breast cancer models. Clin Cancer Res. 2009;15:4649–4664. doi: 10.1158/1078-0432.CCR-09-0317. [DOI] [PubMed] [Google Scholar]
- Imai Y, Gehrke S, Wang HQ, Takahashi R, Hasegawa K, Oota E, Lu B. Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. EMBO J. 2008;27:2432–2443. doi: 10.1038/emboj.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson BA, Alter MD, Kratzke MG, Frizelle SP, Zhang Y, Peterson MS, Avdulov S, Mohorn RP, Whitson BA, Bitterman PB, et al. Repression of cap-dependent translation attenuates the transformed phenotype in non-small cell lung cancer both in vitro and in vivo. Cancer Res. 2006;66:4256–4262. doi: 10.1158/0008-5472.CAN-05-2879. [DOI] [PubMed] [Google Scholar]
- Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458:725–731. doi: 10.1038/nature07782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Hou P, Ji M, Guan H, Studeman K, Jensen K, Vasko V, El-Naggar AK, Xing M. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J. Clin. Eendocrinol. Metab. 2008;93:3106–3116. doi: 10.1210/jc.2008-0273. [DOI] [PubMed] [Google Scholar]
- Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–193. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Mamane Y, Petroulakis E, Rong L, Yoshida K, Ler LW, Sonenberg N. eIF4E--from translation to transformation. Oncogene. 2004;23:3172–3179. doi: 10.1038/sj.onc.1207549. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol. Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellinghoff IK, Wang MY, Vivanco I, Haas-Kogan DA, Zhu S, Dia EQ, Lu KV, Yoshimoto K, Huang JH, Chute DJ, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N. Engl. J. Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- Michlewski G, Sanford JR, Caceres JF. The splicing factor SF2/ASF regulates translation initiation by enhancing phosphorylation of 4E-BP1. Mol. Cell. 2008;30:179–189. doi: 10.1016/j.molcel.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Nosho K, Kawasaki T, Ohnishi M, Suemoto Y, Kirkner GJ, Zepf D, Yan L, Longtine JA, Fuchs CS, Ogino S. PIK3CA mutation in colorectal cancer: relationship with genetic and epigenetic alterations. Neoplasia. 2008;10:534–541. doi: 10.1593/neo.08336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pause A, Belsham GJ, Gingras AC, Donze O, Lin TA, Lawrence JC, Jr, Sonenberg N. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5'-cap function. Nature. 1994;371:762–767. doi: 10.1038/371762a0. [DOI] [PubMed] [Google Scholar]
- Polunovsky VA, Bitterman PB. The cap-dependent translation apparatus integrates and amplifies cancer pathways. RNA Biol. 2006;3:10–17. doi: 10.4161/rna.3.1.2718. [DOI] [PubMed] [Google Scholar]
- Richter JD, Sonenberg N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature. 2005;433:477–480. doi: 10.1038/nature03205. [DOI] [PubMed] [Google Scholar]
- Rong L, Livingstone M, Sukarieh R, Petroulakis E, Gingras AC, Crosby K, Smith B, Polakiewicz RD, Pelletier J, Ferraiuolo MA, Sonenberg N. Control of eIF4E cellular localization by eIF4E-binding proteins, 4E-BPs. RNA. 2008;14:1318–1327. doi: 10.1261/rna.950608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux PP, Shahbazian D, Vu H, Holz MK, Cohen MS, Taunton J, Sonenberg N, Blenis J. RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J. Biol. Chem. 2007;282:14056–14064. doi: 10.1074/jbc.M700906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels Y, Diaz LA, Jr, Schmidt-Kittler O, Cummins JM, Delong L, Cheong I, Rago C, Huso DL, Lengauer C, Kinzler KW, et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005;7:561–573. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- She QB, Chandarlapaty S, Ye Q, Lobo J, Haskell KM, Leander KR, DeFeo-Jones D, Huber HE, Rosen N. Breast tumor cells with PI3K mutation or HER2 amplification are selectively addicted to Akt signaling. PLoS ONE. 2008;3:e3065. doi: 10.1371/journal.pone.0003065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She QB, Solit DB, Ye Q, O'Reilly KE, Lobo J, Rosen N. The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer Cell. 2005;8:287–297. doi: 10.1016/j.ccr.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasawa S, Furuse M, Yokoyama N, Sasazuki T. Altered growth of human colon cancer cell lines disrupted at activated Ki-ras. Science. 1993;260:85–88. doi: 10.1126/science.8465203. [DOI] [PubMed] [Google Scholar]
- Simi L, Pratesi N, Vignoli M, Sestini R, Cianchi F, Valanzano R, Nobili S, Mini E, Pazzagli M, Orlando C. High-resolution melting analysis for rapid detection of KRAS, BRAF, and PIK3CA gene mutations in colorectal cancer. Am. J. Clin. Pathol. 2008;130:247–253. doi: 10.1309/LWDY1AXHXUULNVHQ. [DOI] [PubMed] [Google Scholar]
- Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, Ye Q, Lobo JM, She Y, Osman I, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–362. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J. Biol. Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao H, Goel V, Wu H, Yang G, Haluska FG. Genetic interaction between NRAS and BRAF mutations and PTEN/MMAC1 inactivation in melanoma. J. Invest. Dermatol. 2004;122:337–341. doi: 10.1046/j.0022-202X.2004.22243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nature Rev. Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Wee S, Jagani Z, Xiang KX, Loo A, Dorsch M, Yao YM, Sellers WR, Lengauer C, Stegmeier F. PI3K pathway activation mediates resistance to MEK inhibitors in KRAS mutant cancers. Cancer Res. 2009;69:4286–4293. doi: 10.1158/0008-5472.CAN-08-4765. [DOI] [PubMed] [Google Scholar]
- Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, Cordon-Cardo C, Pelletier J, Lowe SW. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- Yu K, Toral-Barza L, Shi C, Zhang WG, Zask A. Response and determinants of cancer cell susceptibility to PI3K inhibitors: combined targeting of PI3K and Mek1 as an effective anticancer strategy. Cancer Biol. Ther. 2008;7:307–315. doi: 10.4161/cbt.7.2.5334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.