Abstract
In 2010, 1770 lung transplant procedures were performed in the USA, yet 2469 new candidates were added to the waiting list the same year. The shortage of suitable donor lungs requires that transplant professionals select patients for lung transplantation only if they are likely to sustain a survival benefit from the procedure. However, 20% of lung transplant recipients die within the first year of transplantation, suggesting that we are failing to identify those at high risk for severe early complications. In this perspective, we review the current guidelines for the selection of lung transplant candidates, which are based largely on expert opinion and small case series. We also propose the study of new extrapulmonary factors, such as frailty and sarcopenia, that might help improve the prediction of complications and early death after lung transplantation, leading to an improved candidate selection process.
Keywords: chronic obstructive pulmonary disease, frailty, interstitial lung disease, lung transplantation, obesity, pulmonary arterial hypertension, sarcopenia
Following the first successful lung transplantation in 1983 [1], the 1-year survival rate after lung transplantation was approximately 45% [2]. Over the past 30 years, advances in donor and recipient selection, surgical technique and the medical management of recipients have contributed to improved outcomes. 1-year survival rates now exceed 80% worldwide [3]. Despite these strides forward, the small number of available donor lungs and suboptimal long-term outcomes limit lung transplantation to a highly select group of candidates who have far-advanced disease, yet still have an acceptable long-term survival rate with transplantation. The science of identifying candidates likely to sustain a survival benefit from lung transplantation is only in its infancy. This perspective will discuss the current state of lung transplant recipient selection and provide an innovative approach that may, ultimately, improve our ability to select appropriate candidates for lung transplantation.
General indications for lung transplantation
In 2006, the International Society for Heart and Lung Transplantation (ISHLT) updated its guidelines for the selection of candidates for lung transplantation [4]. In general, lung transplantation candidates should have a chronic, progressive lung disease, such as chronic obstructive pulmonary disease (COPD), interstitial lung disease (ILD) or cystic fibrosis (CF). Table 1 shows the frequency of the diagnoses of all recipients of lung transplantation in 2010 in the USA [3]. Disease-specific guidelines are reviewed later in this article.
Table 1.
Recipients of lung transplantation in the USA in 2010.
| Diagnosis | Number (%) |
|---|---|
| Interstitial lung disease | 683 (39) |
| COPD/emphysema | 413 (23) |
| Cystic fibrosis | 218 (12) |
| Other | 152 (9) |
| Lung retransplantation | 80 (5) |
| Sarcoidosis | 54 (3) |
| α-1-antitrypsin deficiency | 43 (2) |
| Primary pulmonary hypertension | 35 (2) |
| Bronchiectasis | 30 (2) |
| Scleroderma with pulmonary hypertension | 20 (1) |
| Hypersensitivity pneumonitis | 19 (1) |
| Secondary pulmonary hypertension | 12 (1) |
| Lymphangioleiomyomatosis | 11 (1) |
| Total | 1770 |
COPD: Chronic obstructive pulmonary disease.
Data taken from [101].
Box 1 lists the absolute and relative contraindications for lung transplantation. There is widespread agreement that those with recent malignancy, life-threatening extrapulmonary disease and social or psychiatric factors that could impact on the outcome should not undergo lung transplantation. On the other hand, there is considerable variation in center-specific practices with regard to the relative contraindications listed in Box 1. In our experience, transplant recipients increasingly meet one or more relative contraindications at the time of transplantation.
Box 1. Absolute and relative contraindications for lung transplantation.
Absolute contraindications:
Malignancy in the last 2 years, with the exception of cutaneous squamous and basal cell tumors
Untreatable advanced dysfunction of another major organ system
Noncurable chronic extrapulmonary infection, including chronic active HBV, HCV and HIV
Significant chest wall or spinal deformity
Documented nonadherence or inability to follow through with medical therapy or office follow-up, or both
Untreatable psychiatric or psychologic condition associated with the inability to cooperate or comply with medical therapy
Absence of a consistent or reliable social support system
Substance addiction that is either active or within the last 6 months
Relative contraindications:
Age older than 65 years
Critical or unstable clinical condition (e.g., shock, mechanical ventilation or ECMO)
Severely limited functional status with poor rehabilitation potential
Colonization with highly resistant or highly virulent bacteria, fungi or mycobacteria
Severe obesity defined as a BMI exceeding 30 kg/m2
Chronic mechanical ventilation
Unstable extrapulmonary medical conditions that have not resulted in end-stage organ damage
ECMO: Extracorporeal membrane oxygenation; HBV: Hepatitis B virus; HCV: Hepatitis C virus.
Adapted with permission from [4].
Current system of organ allocation in the USA
The current priority-based organ allocation system in the USA allocates deceased donor lungs to candidates on the waiting list based on both the predicted risk of dying whilst on the waiting list and on the predicted survival benefit of lung transplantation. Priority is given to waiting list candidates who have a higher predicted waiting list mortality rate and a greater predicted survival benefit from transplantation. These predicted individual risks are estimated from prediction models for 1-year survival, with and without transplantation. The predictors in these models are listed in Box 2. Output from the prediction models are combined and normalized to yield a single value – the Lung Allocation Score (LAS) – which ranges from 0 to 100, with higher values connoting higher priority for transplantation. The details of the LAS system have been previously described [5].
Box 2. Variables included in the lung allocation score calculation.
Waiting list mortality predictors:
Age
BMI
Diabetes
Functional status
Forced vital capacity
Systolic pulmonary artery pressure
Supplemental oxygen requirement
6-min walk distance
Arterial partial pressure of carbon dioxide
Diagnosis
Post-transplant mortality predictors:
Age
Creatinine
Functional status
Forced vital capacity
Pulmonary capillary wedge pressure
Mechanical ventilation
Diagnosis
Data taken from the Organ Procurement and Transplantation Network.
Waiting time and waiting list mortality rates have dramatically decreased since implementation of the LAS in 2005 [6], suggesting that the LAS system has achieved its goal of reducing the number of deaths among lung transplant candidates [5]. However, the LAS system has not addressed the practice of allocating deceased donor lungs locally before offering the organs regionally and nationally, probably creating geographic disparities in access to lung transplantation. There appears to be little justification for these geographic restrictions other than concern about prolonged ischemic times. Future studies should focus on defining these disparities and evaluating the effect of a national waiting list. Furthermore, an unintended consequence of the LAS system is the prioritization of lung transplant candidates who have higher risks of death after lung transplantation [7–10]. This observation highlights the need for new predictors of poor early post-transplant outcomes that can help to identify candidates whose risk of death after lung transplantation is unacceptably high. Discriminating risk prediction models and high-quality decision-analysis tools could improve overall outcomes and help to avoid futile transplantation. Two characteristics, age and body composition, stand out as potentially important determinants of outcome that are of great interest to patients, community physicians and transplant professionals, and are discussed in greater depth below.
Age
Early guidelines for the selection of lung transplant candidates suggested that age limits of 65 and 60 years for candidates for single and bilateral lung transplants, respectively, be imposed based on poor post-transplant survival in older patients [11]. Nevertheless, the fraction of lung transplant procedures performed for adults 65 years and older has risen markedly in the USA from 7% of all lung transplant procedures in 2004 to 25% in 2010 (Figure 1) [4].
Figure 1. Temporal trends in recipient age at the time of lung transplantation in the USA.
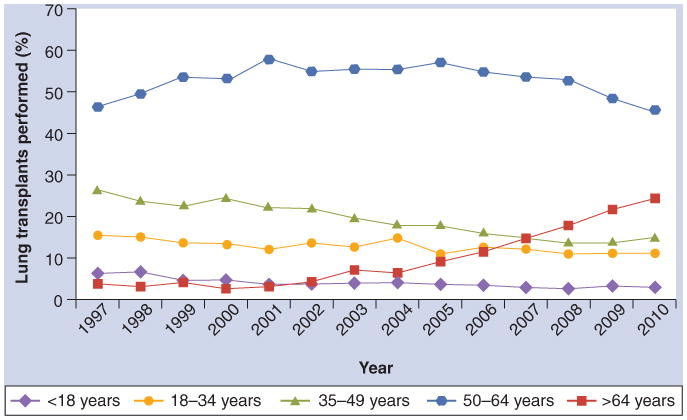
Data taken from [101].
The published experiences with older lung transplant candidates is contradictory. Some published reports suggest that older lung transplant candidates may have similar survival to younger recipients [12–14]. However, reports using ISHLT and United Network for Organ Sharing registry data demonstrate greater 1- and 5-year risks of death in recipients older than 65 years, particularly in those 70 years and older [3,15], supporting the current selection guidelines statement that:
“Although there cannot be endorsement of an upper age limit as an absolute contraindication (recognizing that advancing age alone in an otherwise acceptable candidate with few comorbidities does not necessarily compromise successful transplant outcomes), the presence of several relative contraindications can combine to increase the risks of transplantation above a safe threshold.”
– Orens et al. [4].
The basis for the observed association between older age and shorter survival time after lung transplantation has been attributed to an age-related increase in comorbidities [4]. However, the Toronto group showed that the excess mortality among lung transplant recipients over the age of 60 years exceeds that attributable to age alone [16], suggesting that the effect of age on survival time is modified – in this case magnified – by lung transplantation. Frailty, or age-related susceptibility to stressors, is a biological syndrome that has not yet been adequately investigated in the field of lung transplantation.
Frailty
Frailty is a clinical syndrome characterized by decreased reserve across multiple physiologic systems, and is associated with short- and long-term morbidity and mortality in older adults [17,18]. In 2001, Fried et al. formally defined frailty as the presence of three or more of the following: unintentional weight loss, self-reported exhaustion, weakness (low grip strength), slow walking speed and low physical activity [17]. Although frailty overlaps somewhat with disability and comorbidity, the frailty phenotype independently predicts the risk of death in community-dwelling and hospitalized elderly adults [17,19–21] and in patients with congestive heart failure [22,23] and end-stage renal disease [24].
The physiologic changes underlying frailty include loss of muscle mass (sarcopenia, see discussion below), immune senescence and a heightened inflammatory response [18]. The links between older age, frailty and poor outcomes after lung transplantation, therefore, could be mediated by ‘inflammaging’, a low-grade chronic proinflammatory state characterized by increased serum levels of proinflammatory cytokines and activation of both the innate and adaptive immune systems [25]. Although inflammaging is classically attributed to age-related increases in the burden of antigenic stimuli (e.g., presence of normal host flora and subclinical infections with common viruses) [25], the presence of chronic inflammation and recurrent infections in the setting of pulmonary disease, could accelerate inflammaging before and after lung transplantation.
The concepts of inflammaging and frailty have not yet been directly applied to patients with chronic lung diseases and lung transplantation, but existing literature suggests connections may exist. For example, frailty is characterized by increased circulating levels of IL-6 [26], which is associated with disability [27] and decreased muscle mass and strength [27,28]. IL-6 has been implicated in the major complications of lung transplantation, including primary graft dysfunction [29–34], acute rejection [32,35–37], infection [37] and bronchiolitis obliterans syndrome [38–40]. Interestingly, cytomegalovirus seropositivity in community-dwelling elderly women has also been linked to frailty [41], and higher donor age in kidney, liver and lung transplantation has been linked to higher rates of early [42,43] and late [44–46] graft dysfunction.
Frailty has been investigated as a preoperative risk stratification tool outside of the field of lung transplantation. In 594 older adults undergoing elective surgery, Makary et al. found that preoperative frailty was associated with an increased risk of postoperative complications, longer length of stay and a greater risk of discharge to a skilled nursing facility [47]. Afilalo et al. found that slow gait speed (requiring greater than or equal to 6 s to walk 5 m) independently predicted a composite end point of mortality or major morbidity in adults over the age of 70 years undergoing cardiac surgery, even after adjustment for a surgical risk score (odds ratio: 3.1; 95% CI: 1.2–7.5) [48]. Lee et al. focused on core muscle size as an objective measure of frailty [49]. In 262 patients who underwent elective open abdominal aortic aneurysm repair between 2000 and 2008, psoas muscle area measured at the level of the L4 vertebra was associated with 2-year postoperative mortality and performed at least as well as established mortality predictors, including the American Society of Anesthesiologists score.
Frailty and its related construct of sarcopenia may underlie the increased risk of death among older adults after lung transplantation. However, in the absence of data supporting this link, it is still too soon to deny lung transplantation based on the presence of frailty alone. Ongoing studies are examining the prevalence of frailty in lung transplant candidates and its impact on clinically relevant outcomes after transplantation.
Body composition
Similar to age, body composition is an evolving area of controversy in lung transplantation. The 1998 ISHLT guidelines recommended that lung transplant candidates should weigh between 70 and 130% of ideal body weight [11]. The 2006 guidelines, however, only recommend using a BMI greater than 30 as a relative contraindication to lung transplantation, but underweight recipients are not mentioned [4]. Annual ISHLT registry reports have occasionally described associations between obesity and greater multivariable-adjusted risks of death after lung transplantation [50,51]. In a study of 5978 lung transplant recipients with CF, COPD and ILD followed for a median of 4.2 years, being underweight, overweight and obese were each associated with an increased mortality rate after lung transplantation (Figure 2) [52]. A more recent study suggests that the link between obesity and poor outcomes after lung transplantation may be due to a higher risk of primary graft dysfunction (acute lung injury occurring within 72 h of lung transplantation) (Figure 3) [53]. This study also suggested that higher levels of leptin, a proinflammatory cytokine produced by adipose tissue and associated with acute lung injury and the acute respiratory distress syndrome in mouse models [54,55], might play a role in the development of primary graft dysfunction. Future studies should focus on the mechanisms underlying this association.
Figure 2. Continuous relationships between BMI and risk of death at 1 year and at 5 years conditional on 1-year survival after lung transplantation.
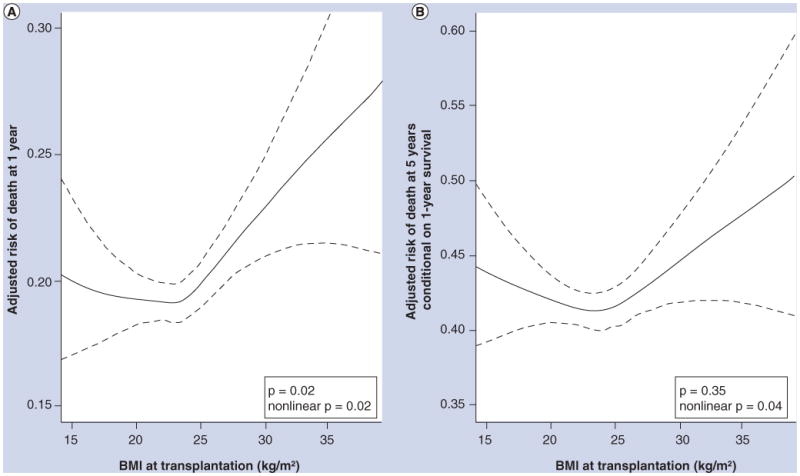
Solid lines = multivariable-adjusted smoothed regression lines. Dotted lines = 95% CI. In (A), both nonlinear (p = 0.02) and linear (p = 0.02) relationships were statistically significant. In (B), the nonlinear (p = 0.04), but not the linear (p = 0.35), relationship was statistically significant. The significant p-values for the smoothed (nonlinear) curves suggest that the relationship between BMI and risk of death after lung transplantation is nonlinear, with higher early and late mortality rates for both underweight and obese recipients. The wide confidence intervals at the extremes of BMI are due to smaller numbers of transplant recipients with these values.
Reprinted from [52] with permission of the American Thoracic Society. © American Thoracic Society.
Figure 3. Continuous association between BMI and grade 3 primary graft dysfunction adjusted for diagnosis, cardiopulmonary bypass and transplant procedure type.
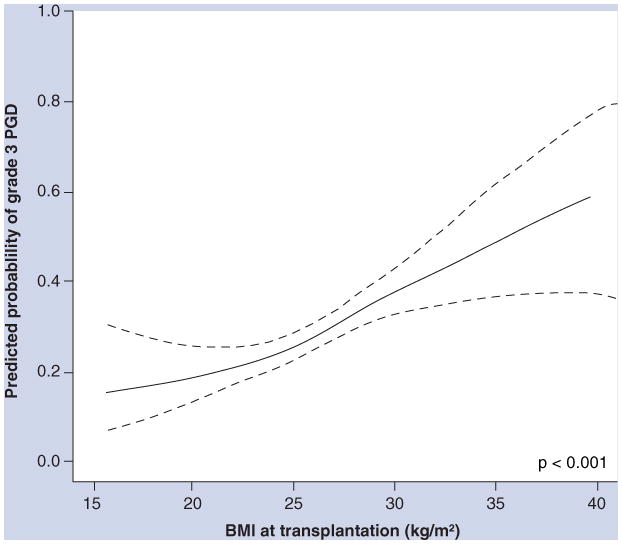
Solid line shows the effect estimate; dotted lines show the 95% CI bands. The p-value is for the association between BMI and PGD.
PGD: Primary graft dysfunction.
Reprinted from [53] with permission of the American Thoracic Society. © American Thoracic Society.
We recommend that obesity and being underweight continue to be considered relative contraindications for lung transplantation. However, the impact of these factors on survival time after lung transplantation may be relatively small compared with the risk of death without lung transplantation. Therefore, transplant clinicians should use clinical judgment to integrate body composition with other recipient, procedure and donor factors when selecting candidates for lung transplantation.
The intersection of frailty & body composition
Sarcopenia (low muscle mass) is a critical physiologic change underlying frailty [56]. While underweight adults are typically sarcopenic, sarcopenia may also be present in normal and obese adults (so-called ‘sarcopenic obesity’). The impact of sarcopenia after lung transplantation has yet to be examined, but data from liver and kidney transplant recipients suggests that sarcopenia may affect outcomes after solid organ transplantation. Englesbe et al. measured cross-sectional psoas muscle area on abdominal CT scans in 163 liver transplant recipients [57]. Psoas area correlated poorly with the Model for End-Stage Liver Disease score and serum albumin, but was strongly associated with 1- and 3-year post-transplantation survival (26% 3-year survival for the quartile with the smallest area vs 77% for those in the highest quartile). In renal transplant candidates, several studies have found that higher pretransplant muscle mass, using pretransplant creatinine as a proxy, was associated with greater post-transplant graft and patient survival [58,59].
While frailty has not specifically been studied in patients with lung disease, sarcopenia and muscle weakness measured by direct strength testing [60], mid-thigh cross-sectional area [61], mid-arm circumference [62] and bioelectrical impedance [63] have been linked to short- and long-term mortality in COPD. Indeed, anthropometric measurements, such as mid-thigh cross-sectional area and mid-arm circumference, are better predictors of mortality than BMI in COPD [61,63], probably owing to the inability of BMI to capture the loss of lean muscle mass in those who are not underweight [64].
While frailty and sarcopenia may ultimately be shown to predict outcomes after lung transplantation, it is not clear whether sarcopenia is a modifiable risk factor. The administration of growth hormone and steroids improves lean body mass in patients with COPD [65] and in patients with renal failure requiring dialysis [66,67], but the effect of these agents on mortality is unknown. Men with COPD, particularly sedentary patients with low muscle mass, frequently have low circulating levels of testosterone [68]. Testosterone supplementation increases muscle mass and strength in these patients when combined with an exercise program [69], but supplementation without exercise has not been shown to be beneficial [70], and the longer-term effects on muscle mass, mortality and safety are not known. Nutritional supplementation improves grip strength and arm circumference in at-risk liver transplant candidates, but does not alter post-transplant mortality [71]. Interestingly, there is newly emerging data regarding the potentially modifiable nature of muscle weakness at the cellular level in mouse models of aging [72,73], but this type of intervention has yet to be attempted in humans.
It appears that our best hope for improving functional status in the pretransplant population might be through pulmonary rehabilitation targeting muscle mass, strength and aerobic capacity. In heart transplant recipients, a 12-week post-transplant supervised aerobic and strength program increased lean body mass and muscle strength [74], and a promising report by Turchetta et al. suggests that a 12-week exercise training program in children with CF can improve oxygen uptake and exercise capacity [75]. Pulmonary rehabilitation may also improve functional status and reduce mortality in patients with COPD [76]. Further study is needed to determine whether modification of sarcopenia could improve outcomes after lung transplantation.
Disease-specific indications for lung transplantation
Since the timing of deceased donor lung transplantation is a random event dependent on the availability of a suitable donor, healthcare providers can only control the timing of referral for transplantation and the timing of placement on the waiting list. In general, it is recommended that referral to a lung transplant center should be considered when a patient's 2- to 3-year predicted survival is less than 50% or when they have achieved New York Heart Association class III or IV functional class [4]. Disease-specific recommendations for the timing of referral and transplantation from the 2006 ISHLT guidelines are shown in Box 3 and discussed below [4].
Box 3. Disease-specific indications for listing for lung transplantation.
Chronic obstructive pulmonary disease:
-
BODE index of 7–10 or at least one of the following:
History of hospitalization for exacerbation associated with acute hypercapnia (PaCO2 exceeding 50 mmHg)
Pulmonary hypertension, cor pulmonale or both despite oxygen therapy
FEV1 <20% and either DLCO <20% or homogeneous distribution of emphysema
Cystic fibrosis:
Oxygen-dependent respiratory failure
Hypercapnia
Pulmonary hypertension
IPF and NSIP:
Histologic or radiographic evidence of IPF and any of the following:
DLCO <39% predicted
10% or greater decrement in FVC during 6 months of follow-up
Decrease in pulse oximetry <88% during 6-min walk testing
-
Histologic evidence of NSIP and any of the following:
DLCO <35% predicted
10% or greater decrement in FVC or 15% decrease in DLCO during 6 months of follow-up
Pulmonary fibrosis associated with collagen vascular disease:
Data are insufficient to support specific guidelines
Pulmonary arterial hypertension:
Persistent NYHA class III or IV on maximal medical therapy
Low (350 m) or declining 6-min walk test
Failing therapy with intravenous epoprostenol or equivalent
Cardiac index <2 l/min/m2
Right atrial pressure >15 mm Hg
Sarcoidosis:
-
Impairment of exercise tolerance (NYHA functional class III or IV) and any of the following:
Hypoxemia at rest
Pulmonary hypertension
Elevated right atrial pressure exceeding 15 mmHg
Lymphangioleiomyomatosis:
Severe impairment in lung function and exercise capacity (e.g., VO2 max <50% predicted)
Hypoxemia at rest
Pulmonary Langerhans cell histiocytosis (eosinophilic granuloma):
Severe impairment in lung function and exercise capacity
Hypoxemia at rest
BODE: BMI, airflow obstruction, dyspnea and exercise capacity; DLCO: Diffusing capacity of carbon monoxide; FEV1: Forced expiratory volume in 1 s; FVC: Forced vital capacity; IPF: Idiopathic pulmonary fibrosis; NSIP: Nonspecific interstitial pneumonia; NYHA: New York Heart Association; PaCO2: Arterial partial pressure of carbon dioxide; VO2: Oxygen uptake.
Adapted from [4].
Chronic obstructive pulmonary disease
The 2006 guidelines for referral and transplantation of patients with COPD rely heavily on the BODE index [4]. The BODE index, a multidimensional ordinal predictor of the risk of death in COPD, is comprised of BMI (B), airflow obstruction (O) measured by forced expiratory volume in 1 s (FEV1), dyspnea (D), measured by the modified Medical Research Council Dyspnea scale, and exercise capacity (E) measured by the 6-min walk test [77]. Higher scores predict a higher rate of death in adults with COPD over a median follow-up period of 2.5 years. Based on the low risk of death over 1–2 years when the BODE index is less than five, the current selection guidelines recommend using a BODE score of at least five as the sole guideline for referral for transplant evaluation [4]. Recommended indications for the timing of listing for transplantation are: a BODE index of seven to ten (the 4-year survival of this group is only 15%), an acute exacerbation with concomitant hypercapnia, pulmonary hypertension despite appropriate supplemental oxygen use or an (FEV1) of <20%, predicted along with either a diffusing capacity of carbon monoxide of <20% predicted or homogenous emphysema on computed tomography imaging [4]. These criteria apply to patients who have deteriorated despite optimal therapy, including smoking cessation, bronchodilators, pulmonary rehabilitation, oxygen therapy and, in some cases, lung volume reduction surgery.
While these general guidelines for referral and transplantation have strong face validity for the need for transplantation, we are unaware of data showing that BODE either predicts survival time in COPD patients awaiting lung transplantation or identifies candidates who are likely to have a survival benefit from lung transplantation. Indeed, it is uncertain if lung transplantation even confers a survival benefit to COPD patients, as different observational studies have led to different conclusions [78,79]. One innovative study used simulation methods to identify predictors of the survival benefit of transplantation in adults with COPD listed for lung transplantation [80]. The authors found a great deal of variation in the survival benefit of lung transplantation in COPD. For example, 50% of single lung transplant recipients and 36% of double lung transplant recipients with COPD did not sustain a predicted survival benefit from lung transplantation [80]. The authors identified a number of predictors of the survival benefit of lung transplantation. Most notably, a survival benefit from lung transplantation was unlikely among adults older than 60 years of age, those with a predicted greater than 20% and those with a FEV1 BMI greater than 27 kg/m2 (Figure 4). While these findings have not been validated (and therefore should be cautiously integrated with clinical judgment when evaluating candidates), they highlight the limited nature of the current selection guidelines and the need for additional investigations to help refine ‘candidacy’ for lung transplantation.
Figure 4. Survival effect of double lung transplantation in patients with chronic obstructive pulmonary disease according to values of prognostic variables.
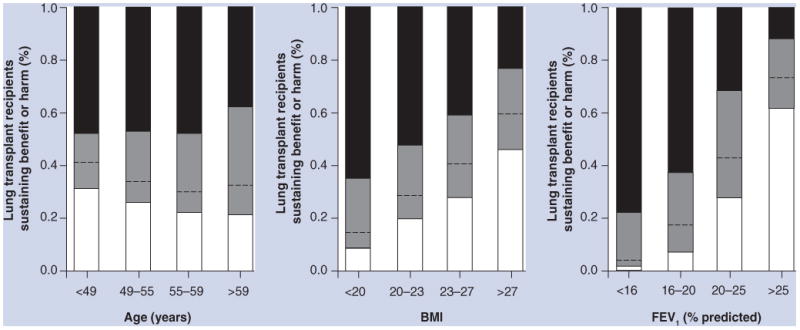
The survival effect of lung transplantation is defined as the difference between the expected median survival with lung transplantation and that without lung transplantation. The survival benefit is divided into gain of 1 year or more (black), loss of 1 year or more (white) and gain or loss of less than 1 year (gray). The dashed line separates between gain and loss of survival.
FEV1: Forced expiratory volume in 1 s.
Reprinted from [80] with permission of the American Thoracic Society. © American Thoracic Society.
Interstitial lung disease
The cornerstone of the 2006 guidelines for referral and transplantation for patients with ILD is accurate diagnosis. Owing to the fact that survival without transplantation is poor among those with a fibrotic form of ILD, the current guidelines appropriately recommend referring patients with idiopathic pulmonary fibrosis or fibrotic nonspecific interstitial pneumonia for lung transplantation as soon as the diagnosis is made [4]. Guidelines for the timing of listing for transplantation include disease progression evidenced by the presence of any of a number of parameters, including diffusing capacity of carbon monoxide less than 39% predicted, oxyhemoglobin desaturation during 6-min walk test, the presence of honeycombing on chest computed tomography or a 10% reduction in forced vital capacity over 6 months [4]. We agree with these recommendations and would extend them to include ILD patients (regardless of the specific diagnosis) if they have New York Heart Association class III or IV symptoms, require supplemental oxygen or have failed to respond to a first trial of an appropriate therapy for their disease (if indeed an effective therapy exists).
Early recognition of disease is critical for transplantation and survival in patients with ILD, but frequently these patients experience long delays from the onset of symptoms to diagnosis [63]. A recent study suggested that ILD patients with delayed access to an ILD center have a shorter survival time than those who access subspecialty care sooner, even after accounting for disease severity [81]. We recommend that ILD patients should be evaluated by a multidisciplinary team with expertise in the diagnosis and management of ILD early in the course of disease. Improvement in referral practices may also improve access to lung transplantation for these patients.
Cystic fibrosis
The 2006 selection guidelines recommend that patients with CF should be referred for transplantation when one or more of the following conditions are met: FEV1 <30% predicted, rapidly declining FEV1 (particularly in young female patients), an acute exacerbation requiring intensive care, increasing frequency of exacerbations, refractory or recurrent pneumothorax, or uncontrolled recurrent hemoptysis [4]. In our experience, these guidelines are clinically useful and often also stand as indications for transplantation. Additional guidelines for transplantation include oxygen-dependent respiratory failure, hypercapnia and pulmonary hypertension [4]. Patients with recurrent pneumothoraces or hemoptysis are particularly problematic, since these factors are not accounted for in the LAS.
Pulmonary arterial hypertension
In the current era of targeted pulmonary arterial hypertension (PAH) therapy, survival rates appear to have improved and the need for lung transplantation among patients with PAH has decreased. Nevertheless, lung transplantation remains an important option for treatment-refractory PAH. Unfortunately, a recent study by Chen et al. using United Network for Organ Sharing registry data found that in contrast to other diagnostic groups, PAH patients did not experience an increase in the rate of transplantation following the introduction of the priority-based LAS system in 2005, and that waiting list mortality did not decrease for PAH patients during this same period [82]. These data suggest that the LAS may underestimate the risk of death of PAH patients awaiting lung transplantation, and that our current allocation policy needs improvement. While there are no studies specifically regarding pulmonary hypertension (PH) and body composition, available data suggest that poor functional capacity predicts mortality in PH [83] and exercise programs may improve outcomes in a nontransplant PH population [84].
Expert commentary
In the presence of an inadequate evidence base, clinical judgment and decision by consensus prevail each week at hundreds of lung transplant candidate selection meetings worldwide. The field of lung transplantation is in dire need of well-designed, prospective observational studies that carefully phenotype lung transplant candidates and recipients in order to identify predictors of (and develop clinical prediction rules for) the survival benefit of lung transplantation. We propose that frailty, sarcopenia and related constructs (e.g., disability, comorbidity and related inflammatory and immune markers) should be targets of such studies. Innovative approaches that incorporate these novel risk factors into simulated allocation strategies should be pursued. Only through collaboration between transplant centers and support by funding agencies worldwide will we be able to achieve the goal of optimizing the selection of lung transplant candidates.
Five-year view
Prospective studies are currently examining extrapulmonary factors, such as frailty and sarcopenia, that influence survival after lung transplantation. In 5 years from now, the results of these studies will start to be available, providing evidence to aid the selection of lung transplant candidates. The initial tide of data will probably suggest that frail and sarcopenic transplant candidates do not sustain a survival benefit from lung transplantation. The lung transplant community will need to exercise judicious caution in denying lung transplantation to frail or sarcopenia lung transplant candidates. Clinical trials to reduce frailty and increase muscle mass will probably be proposed in order to improve candidacy for lung transplantation. Biological mediators of the effect of frailty, such as immune senescence and IL-6, will provide innovative approaches to reduce the risk of lung transplantation.
Key issues.
Current guidelines for the selection of lung transplant candidates are based on expert opinion, registry data and single-center studies. Large-scale prospective multinational studies are needed to improve our ability to identify lung transplant candidates who are likely to sustain a survival benefit from the procedure.
Older age, obesity and being underweight are each associated with higher risks of death after lung transplantation, but the mechanisms underlying these associations (e.g., frailty and sarcopenia) require further study.
Disease-specific indications for the timing of referral and listing for lung transplantation focus on the risk of death without lung transplantation rather than the expected survival benefit of lung transplantation.
Acknowledgments
DJ Lederer is a steering committee member for the ASCEND trial of pirfenidone for idiopathic pulmonary fibrosis sponsored by Intermune, is coinvestigator in clinical trials for idiopathic pulmonary fibrosis sponsored by Gilead and Boehringer-Ingelheim and has served on advisory boards for Gilead in 2010 and 2011. DJ Lederer was supported by NIH grant numbers K23 HL086714 and R01 HL103676.
Footnotes
Financial & competing interests disclosure: The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Unilateral lung transplantation for pulmonary fibrosis. Toronto Lung Transplant Group. N Engl J Med. 1986;314(18):1140–1145. doi: 10.1056/NEJM198605013141802. [DOI] [PubMed] [Google Scholar]
- 2.Grossman RF, Frost A, Zamel N, et al. Results of single-lung transplantation for bilateral pulmonary fibrosis The Toronto Lung Transplant Group. N Engl J Med. 1990;322(11):727–733. doi: 10.1056/NEJM199003153221104. [DOI] [PubMed] [Google Scholar]
- 3.Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult lung and heart-lung transplant report – 2010. J Heart Lung Transplant. 2010;29(10):1104–1118. doi: 10.1016/j.healun.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 4•.Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update – a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25(7):745–755. doi: 10.1016/j.healun.2006.03.011. These are the most recent international guidelines for the selection of lung transplant candidates. A balanced discussion of risk factors for mortality without transplantation in each disease state is given. [DOI] [PubMed] [Google Scholar]
- 5.Egan TM, Murray S, Bustami RT, et al. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6(5 Pt 2):1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 6.Yusen RD, Shearon TH, Qian Y, et al. Lung transplantation in the United States, 1999–2008. Am J Transplant. 2010;10(4 Pt 2):1047–1068. doi: 10.1111/j.1600-6143.2010.03055.x. [DOI] [PubMed] [Google Scholar]
- 7.Liu V, Zamora MR, Dhillon GS, Weill D. Increasing lung allocation scores predict worsened survival among lung transplant recipients. Am J Transplant. 2010;10(4):915–920. doi: 10.1111/j.1600-6143.2009.03003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merlo CA, Weiss ES, Orens JB, et al. Impact of U.S. Lung Allocation Score on survival after lung transplantation. J Heart Lung Transplant. 2009;28(8):769–775. doi: 10.1016/j.healun.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 9.Weiss ES, Allen JG, Merlo CA, Conte JV, Shah AS. Lung allocation score predicts survival in lung transplantation patients with pulmonary fibrosis. Ann Thorac Surg. 2009;88(6):1757–1764. doi: 10.1016/j.athoracsur.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Russo MJ, Iribarne A, Hong KN, et al. High lung allocation score is associated with increased morbidity and mortality following transplantation. Chest. 2010;137(3):651–657. doi: 10.1378/chest.09-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maurer JR, Frost AE, Estenne M, Higenbottam T, Glanville AR. International guidelines for the selection of lung transplant candidates The International Society for Heart and Lung Transplantation the American Thoracic Society, the American Society of Transplant Physicians, the European Respiratory Society. J Heart Lung Transplant. 1998;17(7):703–709. [PubMed] [Google Scholar]
- 12.Mahidhara R, Bastani S, Ross DJ, et al. Lung transplantation in older patients? J Thorac Cardiovasc Surg. 2008;135(2):412–420. doi: 10.1016/j.jtcvs.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 13.Vadnerkar A, Toyoda Y, Crespo M, et al. Age-specific complications among lung transplant recipients 60 years and older. J Heart Lung Transplant. 2011;30(3):273–281. doi: 10.1016/j.healun.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 14.Smith PW, Wang H, Parini V, et al. Lung transplantation in patients 60 years and older: results, complications, and outcomes. Ann Thorac Surg. 2006;82(5):1835–1841. doi: 10.1016/j.athoracsur.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 15.Weiss ES, Merlo CA, Shah AS. Impact of advanced age in lung transplantation: an analysis of United Network for Organ Sharing data. J Am Coll Surg. 2009;208(3):400–409. doi: 10.1016/j.jamcollsurg.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Gutierrez C, Al-Faifi S, Chaparro C, et al. The effect of recipient's age on lung transplant outcome. Am J Transplant. 2007;7(5):1271–1277. doi: 10.1111/j.1600-6143.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- 17••.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. Landmark paper describing the frailty phenotype as a five-component construct including its predictive validity for mortality in community-dwelling older adults. [DOI] [PubMed] [Google Scholar]
- 18.Fried LP, Xue QL, Cappola AR, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64(10):1049–1057. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10):881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 20.Graham JE, Snih SA, Berges IM, Ray LA, Markides KS, Ottenbacher KJ. Frailty and 10-year mortality in community-living Mexican American older adults. Gerontology. 2009;55(6):644–651. doi: 10.1159/000235653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purser JL, Kuchibhatla MN, Fillenbaum GG, Harding T, Peterson ED, Alexander KP. Identifying frailty in hospitalized older adults with significant coronary artery disease. J Am Geriatr Soc. 2006;54(11):1674–1681. doi: 10.1111/j.1532-5415.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- 22.Boxer R, Kleppinger A, Ahmad A, Annis K, Hager D, Kenny A. The 6-minute walk is associated with frailty and predicts mortality in older adults with heart failure. Congest Heart Fail. 2010;16(5):208–213. doi: 10.1111/j.1751-7133.2010.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cacciatore F, Abete P, Mazzella F, et al. Frailty predicts long-term mortality in elderly subjects with chronic heart failure. Eur J Clin Invest. 2005;35(12):723–730. doi: 10.1111/j.1365-2362.2005.01572.x. [DOI] [PubMed] [Google Scholar]
- 24.Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18(11):2960–2967. doi: 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]
- 25.Franceschi C, Capri M, Monti D, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128(1):92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Leng S, Chaves P, Koenig K, Walston J. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: a pilot study. J Am Geriatr Soc. 2002;50(7):1268–1271. doi: 10.1046/j.1532-5415.2002.50315.x. [DOI] [PubMed] [Google Scholar]
- 27.Ferrucci L, Penninx BW, Volpato S, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50(12):1947–1954. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 28.Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57(5):M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman SA, Wang L, Shah CV, et al. Plasma cytokines and chemokines in primary graft dysfunction post-lung transplantation. sAm J Transplant. 2009;9(2):389–396. doi: 10.1111/j.1600-6143.2008.02497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreno I, Vicente R, Ramos F, Vicente JL, Barbera M. Determination of interleukin-6 in lung transplantation: association with primary graft dysfunction. Transplant Proc. 2007;39(7):2425–2426. doi: 10.1016/j.transproceed.2007.07.056. [DOI] [PubMed] [Google Scholar]
- 31.Mathur A, Baz M, Staples ED, et al. Cytokine profile after lung transplantation: correlation with allograft injury. Ann Thorac Surg. 2006;81(5):1844–1849. doi: 10.1016/j.athoracsur.2005.11.053. [DOI] [PubMed] [Google Scholar]
- 32.Rolfe MW, Kunkel SL, Demeester SR, et al. Expression of interleukin-6 in association with rat lung reimplantation and allograft rejection. Am Rev Respir Dis. 1993;147(4):1010–1016. doi: 10.1164/ajrccm/147.4.1010. [DOI] [PubMed] [Google Scholar]
- 33.Pham SM, Yoshida Y, Aeba R, et al. Interleukin-6, a marker of preservation injury in clinical lung transplantation. J Heart Lung Tranpslant. 1992;11(6):1017–1024. [PubMed] [Google Scholar]
- 34.Kaneda H, Waddell TK, de Perrot M, et al. Pre-implantation multiple cytokine mRNA expression analysis of donor lung grafts predicts survival after lung transplantation in humans. Am J Transplant. 2006;6(3):544–551. doi: 10.1111/j.1600-6143.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 35.Iacono A, Dauber J, Keenan R, et al. Interleukin 6 and interferon-gamma gene expression in lung transplant recipients with refractory acute cellular rejection: implications for monitoring and inhibition by treatment with aerosolized cyclosporine. Transplantation. 1997;64(2):263–269. doi: 10.1097/00007890-199707270-00015. [DOI] [PubMed] [Google Scholar]
- 36.Magnan A, Mege JL, Escallier JC, et al. Balance between alveolar macrophage IL-6 and TGF-beta in lung-transplant recipients Marseille and Montreal Lung Transplantation Group. Am J Respir Crit Care Med. 1996;153(4 Pt 1):1431–1436. doi: 10.1164/ajrccm.153.4.8616577. [DOI] [PubMed] [Google Scholar]
- 37.Yoshida Y, Iwaki Y, Pham S, et al. Benefits of posttransplantation monitoring of interleukin 6 in lung transplantation. Ann Thorac Surg. 1993;55(1):89–93. doi: 10.1016/0003-4975(93)90479-2. [DOI] [PubMed] [Google Scholar]
- 38.Nawrot TS, Vos R, Jacobs L, et al. The impact of traffic air pollution on bronchiolitis obliterans syndrome and mortality after lung transplantation. Thorax. 2011;66(9):748–754. doi: 10.1136/thx.2010.155192. [DOI] [PubMed] [Google Scholar]
- 39.Lu KC, Jaramillo A, Lecha RL, et al. Interleukin-6 and interferon-gamma gene polymorphisms in the development of bronchiolitis obliterans syndrome after lung transplantation. Transplantation. 2002;74(9):1297–1302. doi: 10.1097/00007890-200211150-00017. [DOI] [PubMed] [Google Scholar]
- 40.Scholma J, Slebos DJ, Boezen HM, et al. Eosinophilic granulocytes and interleukin-6 level in bronchoalveolar lavage fluid are associated with the development of obliterative bronchiolitis after lung transplantation. Am J Respir Crit Care Med. 2000;162(6):2221–2225. doi: 10.1164/ajrccm.162.6.9911104. [DOI] [PubMed] [Google Scholar]
- 41.Schmaltz HN, Fried LP, Xue QL, Walston J, Leng SX, Semba RD. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005;53(5):747–754. doi: 10.1111/j.1532-5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]
- 42.Botha P, Trivedi D, Weir CJ, et al. Extended donor criteria in lung transplantation: impact on organ allocation. J Thorac Cardiovasc Surg. 2006;131(5):1154–1160. doi: 10.1016/j.jtcvs.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 43.Pilcher DV, Snell GI, Scheinkestel CD, Bailey MJ, Williams TJ. High donor age, low donor oxygenation, and high recipient inotrope requirements predict early graft dysfunction in lung transplant recipients. J Heart Lung Transplant. 2005;24(11):1814–1820. doi: 10.1016/j.healun.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Veroux M, Grosso G, Corona D, et al. Age is an important predictor of kidney transplantation outcome. Nephrol Dial Transplant. 2011 doi: 10.1093/ndt/gfr524. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 45.Kwon OJ, Lee HG, Kwak JY. The impact of donor and recipient age on the outcome of kidney transplantation. Transplant Proc. 2004;36(7):2043–2045. doi: 10.1016/j.transproceed.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 46.Avolio AW, Cillo U, Salizzoni M, et al. Balancing donor and recipient risk factors in liver transplantation: the value of D-MELD with particular reference to HCV recipients. Am J Transplant. 2011;11(12):2724–2736. doi: 10.1111/j.1600-6143.2011.03732.x. [DOI] [PubMed] [Google Scholar]
- 47••.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210(6):901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. Single-center study demonstrating that frailty is independently associated with a higher risk of surgical complications in older adults undergoing ambulatory surgery. [DOI] [PubMed] [Google Scholar]
- 48.Afilalo J, Eisenberg MJ, Morin JF, et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56(20):1668–1676. doi: 10.1016/j.jacc.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 49.Lee JS, He K, Harbaugh CM, et al. Frailty, core muscle size, and mortality in patients undergoing open abdominal aortic aneurysm repair. J Vasc Surg. 2011;53(4):912–917. doi: 10.1016/j.jvs.2010.10.111. [DOI] [PubMed] [Google Scholar]
- 50.Christie JD, Edwards LB, Aurora P, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fifth official adult lung and heart/lung transplantation report – 2008. J Heart Lung Transplant. 2008;27(9):957–969. doi: 10.1016/j.healun.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 51.Trulock EP, Christie JD, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult lung and heart-lung transplantation report – 2007. J Heart Lung Transplant. 2007;26(8):782–795. doi: 10.1016/j.healun.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 52•.Lederer DJ, Wilt JS, D’Ovidio F, et al. Obesity and underweight are associated with an increased risk of death after lung transplantation. Am J Respir Crit Care Med. 2009;1(9):887–895. doi: 10.1164/rccm.200903-0425OC. Nationwide cohort study demonstrating that obesity and being underweight are associated with higher risks of death after lung transplantation independent of potential confounders. Obesity and being underweight accounted for up to 12% of all deaths in the first year after lung transplantation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lederer DJ, Kawut SM, Wickersham N, et al. Obesity and primary graft dysfunction after lung transplantation: the LTOG obesity study. Am J Respir Crit Care Med. 2011 doi: 10.1164/rccm.201104-0728OC. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jain M, Budinger GR, Lo A, et al. Leptin promotes fibroproliferative acute respiratory distress syndrome by inhibiting peroxisome proliferator-activated receptor-gamma. Am J Respir Crit Care Med. 2011;183(11):1490–1498. doi: 10.1164/rccm.201009-1409OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bellmeyer A, Martino JM, Chandel NS, Scott Budinger GR, Dean DA, Mutlu GM. Leptin resistance protects mice from hyperoxia-induced acute lung injury. Am J Respir Crit Care Med. 2007;175(6):587–594. doi: 10.1164/rccm.200603-312OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54(6):991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 57.Englesbe MJ, Patel SP, He K, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211(2):271–278. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Streja E, Molnar MZ, Kovesdy CP, et al. Associations of pretransplant weight and muscle mass with mortality in renal transplant recipients. Clin J Am Soc Nephrol. 2011;6(6):1463–1471. doi: 10.2215/CJN.09131010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oterdoom LH, van Ree RM, de Vries AP, et al. Urinary creatinine excretion reflecting muscle mass is a predictor of mortality and graft loss in renal transplant recipients. Transplantation. 2008;86(3):391–398. doi: 10.1097/TP.0b013e3181788aea. [DOI] [PubMed] [Google Scholar]
- 60.Swallow EB, Reyes D, Hopkinson NS, et al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax. 2007;62(2):115–120. doi: 10.1136/thx.2006.062026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marquis K, Debigare R, Lacasse Y, et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(6):809–813. doi: 10.1164/rccm.2107031. [DOI] [PubMed] [Google Scholar]
- 62.Soler-Cataluna JJ, Sanchez-Sanchez L, Martinez-Garcia MA, Sanchez PR, Salcedo E, Navarro M. Mid-arm muscle area is a better predictor of mortality than body mass index in COPD. Chest. 2005;128(4):2108–2115. doi: 10.1378/chest.128.4.2108. [DOI] [PubMed] [Google Scholar]
- 63.Slinde F, Gronberg A, Engstrom CP, Rossander-Hulthen L, Larsson S. Body composition by bioelectrical impedance predicts mortality in chronic obstructive pulmonary disease patients. Respir Med. 2005;99(8):1004–1009. doi: 10.1016/j.rmed.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 64.Mador MJ. Muscle mass, not body weight, predicts outcome in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(6):787–789. doi: 10.1164/rccm.2206003. [DOI] [PubMed] [Google Scholar]
- 65.Burdet L, de Muralt B, Schutz Y, Pichard C, Fitting JW. Administration of growth hormone to underweight patients with chronic obstructive pulmonary disease A prospective, randomized, controlled study. Am J Respir Crit Care Med. 1997;156(6):10–16. doi: 10.1164/ajrccm.156.6.9704142. [DOI] [PubMed] [Google Scholar]
- 66.Johannsson G, Bengtsson BA, Ahlmen J. Double-blind, placebo-controlled study of growth hormone treatment in elderly patients undergoing chronic hemodialysis: anabolic effect and functional improvement. Am J Kidney Dis. 1999;33(4):709–717. doi: 10.1016/s0272-6386(99)70223-4. [DOI] [PubMed] [Google Scholar]
- 67.Johansen KL, Painter PL, Sakkas GK, Gordon P, Doyle J, Shubert T. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: a randomized, controlled trial. J Am Soc Nephrol. 2006;17(8):2307–2314. doi: 10.1681/ASN.2006010034. [DOI] [PubMed] [Google Scholar]
- 68.Debigare R, Marquis K, Cote CH, et al. Catabolic/anabolic balance and muscle wasting in patients with COPD. Chest. 2003;124(1):83–89. doi: 10.1378/chest.124.1.83. [DOI] [PubMed] [Google Scholar]
- 69.Casaburi R, Bhasin S, Cosentino L, et al. Effects of testosterone and resistance training in men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170(8):870–878. doi: 10.1164/rccm.200305-617OC. [DOI] [PubMed] [Google Scholar]
- 70.Sharma S, Arneja A, McLean L, et al. Anabolic steroids in COPD: a review and preliminary results of a randomized trial. Chron Respir Dis. 2008;5(3):169–176. doi: 10.1177/1479972308092350. [DOI] [PubMed] [Google Scholar]
- 71.Le Cornu KA, McKiernan FJ, Kapadia SA, Neuberger JM. A prospective randomized study of preoperative nutritional supplementation in patients awaiting elective orthotopic liver transplantation. Transplantation. 2000;69(7):1364–1369. doi: 10.1097/00007890-200004150-00026. [DOI] [PubMed] [Google Scholar]
- 72.Andersson DC, Betzenhauser MJ, Reiken S, et al. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 2011;14(2):196–207. doi: 10.1016/j.cmet.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bellinger AM, Reiken S, Dura M, et al. Remodeling of ryanodine receptor complex causes ‘leaky’ channels: a molecular mechanism for decreased exercise capacity. Proc Natl Acad Sci USA. 2008;105(6):2198–2202. doi: 10.1073/pnas.0711074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haykowsky M, Taylor D, Kim D, Tymchak W. Exercise training improves aerobic capacity and skeletal muscle function in heart transplant recipients. Am J Transplant. 2009;9(4):734–739. doi: 10.1111/j.1600-6143.2008.02531.x. [DOI] [PubMed] [Google Scholar]
- 75.Turchetta A, Salerno T, Lucidi V, Libera F, Cutrera R, Bush A. Usefulness of a program of hospital-supervised physical training in patients with cystic fibrosis. Pediatr Pulmonol. 2004;38(2):115–118. doi: 10.1002/ppul.20073. [DOI] [PubMed] [Google Scholar]
- 76.Cote CG, Celli BR. Pulmonary rehabilitation and the BODE index in COPD. Eur Respir J. 2005;26(4):630–636. doi: 10.1183/09031936.05.00045505. [DOI] [PubMed] [Google Scholar]
- 77.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 78•.Titman A, Rogers CA, Bonser RS, Banner NR, Sharples LD. Disease-specific survival benefit of lung transplantation in adults: a national cohort study. Am J Transplant. 2009;9(7):1640–1649. doi: 10.1111/j.1600-6143.2009.02613.x. Evidence that lung transplantation confers a survival benefit in chronic obstructive pulmonary disease (COPD). [DOI] [PubMed] [Google Scholar]
- 79.Hosenpud J, Bennett LE, Keck BM, Edwards EB, Novick RJ. Effect of diagnosis on survival benefit of lung transplantation for end-stage lung disease. Lancet. 1998;351:24–27. doi: 10.1016/S0140-6736(97)06405-2. [DOI] [PubMed] [Google Scholar]
- 80••.Thabut G, Ravaud P, Christie JD. Determinants of the survival benefit of lung transplantation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177(10):1156–1163. doi: 10.1164/rccm.200708-1283OC. Innovative approach to identifying predictors of the survival benefit of lung transplantation in COPD. Most lung transplant recipients with COPD did not sustain a survival benefit. [DOI] [PubMed] [Google Scholar]
- 81.Lamas DJ, Kawut SM, Bagiella E, Philip N, Arcasoy SM, Lederer DJ. Delayed access and survival in idiopathic pulmonary fibrosis: a cohort study. Am J Respir Crit Care Med. 2011;184(7):842–847. doi: 10.1164/rccm.201104-0668OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen H, Shiboski SC, Golden JA, et al. Impact of the lung allocation score on lung transplantation for pulmonary arterial hypertension. Am J Respir Crit Care Med. 2009;1(5):468–474. doi: 10.1164/rccm.200810-1603OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010;122(2):164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 84.Mereles D, Ehlken N, Kreuscher S, et al. Exercise and respiratory training improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension. Circulation. 2006;114(14):1482–1489. doi: 10.1161/CIRCULATIONAHA.106.618397. [DOI] [PubMed] [Google Scholar]
Website
- 101.Organ Procurement and Transplantation Network. [Accessed 11 May 2011]; http://optn.transplant.hrsa.gov.


