Abstract
Non-technical summary
Dendritic spines of central neurons contain calcium stores, but their involvement in synaptic plasticity is not entirely clear. Synaptopodin, an actin-binding molecule, has been localized in the neck of some dendritic spines, and assumed to play a role in regulation of calcium stores in the spines. In the present study we used flash photolysis of calcium inside dendritic spines of cultured hippocampal neurons loaded with the calcium sensor Fluo-4. An ultrafast UV flash generated a rapid rise in [Ca2+]i followed by a monoexponential decay back to control level. In spines where synaptopodin was found in post hoc immunocytochemical analysis, the decay of elevated calcium was much prolonged compared to synaptopodin-negative spines. This prolongation was eliminated by blockade of release of calcium from stores. Our results provide the first direct demonstration of calcium release from stores in dendritic spines of central neurons.
Abstract
The presence of calcium stores and their function in dendritic spines is still unsettled. We have now studied the kinetics of calcium released inside dendritic spines of cultured rat hippocampal neurons by flash photolysis of caged calcium. Photolysis of calcium produced a fast rise in [Ca2+]i, followed by a variable decay. We were able to correlate the decay of elevated [Ca2+]i with the presence of synaptopodin (SP), an actin-binding protein, in the spines; spines containing SP generated the same initial [Ca2+]i transient, but their decay time was significantly slower and more complex than that of SP-negative ones. The altered decay kinetics of the flash-elevated [Ca2+]i transient was blocked by thapsigargin or cyclopiazonic acid (CPA), indicating that this kinetic change is due to compartmentalized release of calcium from intracellular stores. Thus, SP plays a pivotal role in the calcium store-associated ability of spines to locally tune calcium kinetics.
The possible release of calcium from stores within dendritic spines has been debated for some time (Emptage et al. 1999; Svoboda & Mainen, 1999; Kovalchuk et al. 2000). The physical substrate for the calcium stores, namely the endoplasmic reticulum, has been documented in dendritic spines. It forms a unique structure that extends from the dendritic smooth endoplasmic reticulum into the spine neck and is called the spine apparatus (Spacek & Harris, 1997). Synaptopodin (SP), an actin-binding protein found in renal podocytes and in dendritic spines of telencephalic neurons (Mundel et al. 1997), is associated with the spine apparatus (Deller et al. 2000). SP is linked to regulation of synaptic plasticity in that SP-deficient mice lack spine apparatus and demonstrate an impaired ability to express LTP (Deller et al. 2003).
The suggestion that SP constitutes an actin/actinin-binding/regulatory protein (Mundel et al. 1997; Kremerskothen et al. 2005; Asanuma et al. 2006) indicates that it may serve a function in shaping spines and/or linking the actin cytoskeleton with synaptic membrane proteins. Still, a mechanistic understanding of SP role in synaptic plasticity has not yet been established.
Interestingly, only a subset of spines contains SP and a spine apparatus (Spacek & Harris, 1997; Bass Orth et al. 2005) and it is not clear to which extent these spines differ from neighbouring ones which lack SP. We have recently used cultured rat hippocampal neurons transfected with a GFP-tagged SP to compare properties of SP+ and SP− spines, focusing on morphological and functional attributes of long-term synaptic plasticity at the single spine level. Our data indicate that the delivery of the glutamate receptor GluR1 into dendritic spines depends on the functioning of an internal calcium store that associates with SP (Vlachos et al. 2009). Furthermore, ryanodine receptors are associated with SP (Segal et al. 2010), and caffeine causes a rise of [Ca2+]i in association with SP (Vlachos et al. 2009). We have now examined the kinetics of free [Ca2+]i released transiently inside spines by highly localized flash photolysis of caged calcium. We wished to determine if there would be a difference between SP+ and SP− spines, and if so, it would indicate that calcium stores in dendritic spines are viable, and that SP regulates the fate of [Ca2+]i in the intracellular space, irrespective of the source of its rise. Our experiments clearly demonstrate that while the presence of SP does not affect the slope of the fast rise of flash photolysed calcium, it does retard its decay kinetics in a ryanodine receptor-dependent manner.
Methods
Cultures
Animal handling was done in accordance with the guidelines published by the Institutional Animal Care and Use Committee of the Weizmann Institute and with the Israeli National guidelines on animal care. The experiments comply with the policies and regulations of The Journal of Physiology. Cultures were prepared as detailed elsewhere (Ivenshitz & Segal, 2006). Briefly, rat pups were decapitated on day of birth (P0), their brains removed, the hippocampus was dissected free and placed in a chilled (4°C), oxygenated Leibovitz L15 medium (Gibco) enriched with 0.6% glucose and gentamicin (Sigma, 20 μg ml−1). About 105 cells in 1 ml medium were plated in each well of a 24-well plate, onto a hippocampal glial feeder layer which was grown on the glass for 2 weeks prior to the plating of the neurons. Cells were left to grow in the incubator at 37°C in 5% CO2. Neurons were transfected with DsRed (a red fluorescence protein) using lipofectamine 2000 to image cell morphology, some 3–6 days before the experiment.
Imaging
Cultures were placed in the recording chamber, controlled by an automated X–Y stage (Luigs and Neumann, Ratingen, Germany). Neurons were imaged thereafter on the stage of an upright Zeiss PASCAL confocal microscope using an Olympus 63× water immersion lens (0.9 NA) and 2–4× scan zoom. Standard recording medium contained (in mm): NaCl 129, KCl 4, MgCl2 1, CaCl2 2 glucose 10, Hepes 10, TTX 0.0005 and bicuculline, 0.02, pH was adjusted to 7.4 with NaOH, and osmolarity to 310 mosmol l−1 with sucrose.
Caged calcium (o-nitrophenyl EGTA-AM) and Fluo-4 AM were purchased from Molecular Probes, Inc. (Eugene, OR, USA). Thapsigargin was from Alomone Labs (Jerusalem, Israel). All chemicals were aliquoted and frozen at –80°C. Caged EGTA-AM (6.5 μm) was incubated for 1 h at room temperature together with Fluo-4 AM (1 μm) to image calcium variations. Flash photolysis of caged molecules was described elsewhere (Korkotian & Segal, 2007). The UV spot of 1 μm2 was localized using a parallel red laser light. UV pulses at 1 Hz could be applied repeatedly without noticeable tissue damage.
Digital illustrations
Confocal image stacks were exported as 2D projections from the Zeiss LSM image browser and 3D surface-rendered images were generated with Imaris software (Bitplane, Switzerland). Figures were prepared using Photoshop CS2 graphics software (Adobe, San Jose, CA, USA).
Data quantification and analysis
Immunostained cultures were analysed on 3D image stacks of dendritic segments using the Zeiss LSM image browser to navigate through the stacks. To optimally demonstrate the subcellular localization of labelled structures, selected images were processed by 3D surface rendering. Fluorescence measurements were made by producing a 2D projection of a given segment and measuring the size of cross-sectional area as well as the length of individual dendritic spines. Fluorescence intensity was calculated using the Matlab home-made linescan acquisition program. Measurements were made in a blind procedure to assure unbiased observations. Dendritic spines that were used for flash photolysis of caged calcium were identified in the immunostained neurons, and analysed independently of the measurements of calcium transients in these same spines. Statistical comparisons including t test and other types of analysis were made using Matlab, KaleidaGraph and Origin software. Centroids were calculated using ImageJ software and all images were similarly thresholded for the determination of SP-punctum distinct border.
Results
Flash photolysis of caged calcium produced a transient rise of [Ca2+]i in a 1 μm2 sphere of the focal plane of the flash. The elevated [Ca2+]i reached a peak within 1 ms of the 4 ns flash, and decayed back to control level with a single exponential function having a time constant of 8.4 ± 0.3 ms (Fig. 1). There was no difference between 26 SP+ and 61 SP− spines in the latency to peak or magnitude of change of [Ca2+]i. However, while in SP− spines the decay kinetics was mono-exponential, that of SP+ spines was more complex, and a secondary, delayed peak of [Ca2+]i was evident (Fig. 1D). This secondary peak had different latencies following the initial peak, in different spines. Therefore, averaging of all spines in the same category turned out to ‘smear’ that secondary peak.
Figure 1. Localized responses to flash photolysis of caged calcium.
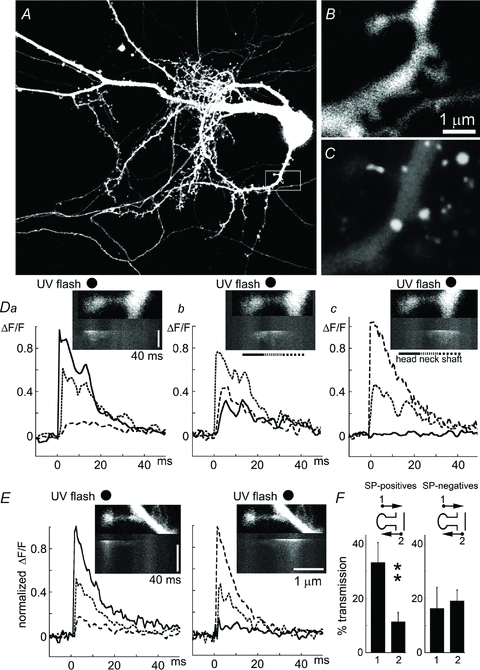
A, low-power image of a 21-day-old DsRed-transfected neuron. B, higher-magnification image of a square at the bottom right of A (white boxed area), showing a dendrite and several spines of different shapes. C, immunofluorescence of the same dendrite after its fixation, to show that the large spine contains synaptopodin (SP) primarily in its neck. D, the large spine in C was line-scanned between the spine head and the parent dendrite, and the UV flash (black dot) was aimed at either the spine head (left), neck (middle) or dendrite (right). Below each frame is a line scan, running from top to bottom, showing an increase in [Ca2+]i, primarily below the black dot of the location of the flash. Da,b,c relate to the images above, where the change in fluorescence is expressed as ΔF/F. In each of the three graphs, three lines represent regions of interest (ROI): continuous line, spine head; dotted line, neck; dashed line, parent dendrite. Note that when the spine head is activated, there is a small change in the parent dendrite, but when the dendrite is targeted (right) no change in [Ca2+]i in spine head is detected. E, a similar, but SP-negative spine, with a UV flash aimed at the spine head (left) or the parent dendrite (right). F, a summary of the relations illustrated in D: left bars for SP+ spines (n = 12); when the spine head is targeted, the parent dendrite response amounts to 32% of the response in spine head but when the parent dendrite is targeted (right column), a change of only 10% of [Ca2+]i is seen in the spine head. No similar asymmetry is seen in the SP− spines (right, n = 12).
In all cases studied, the UV flash was aimed at the spine heads. A line scanned between the spine head and the parent dendrite allowed us to detect rapid changes in [Ca2+]i in the spine head, where it was maximal, and the parent dendrite, where the response was smaller and much delayed (Fig. 1). To verify that the photolysis is rather restricted in space, we compared responses evoked when the flash was targeted to the spine head, the spine neck or the parent dendrite (Fig. 1D). Indeed, the initial responses were highly localized to the compartment to which it was aimed, and a delayed response was seen in the adjacent compartments. Interestingly, the communication between the spine head and parent dendrite was not symmetrical; for the same set of spines, photolysis of calcium in the spine head created a significantly larger response in the parent dendrite (being about 30% of the response in the spine head) than the other way around (where photolysis of calcium in the parent dendrite only created a 10% change in the spine head, Fig. 1D and E). Strikingly, in spines containing SP, the initial rise in [Ca2+]i was followed by a secondary peak (a ‘hump’) that resulted in a non-exponential decay of the elevated calcium (Fig. 1D). This ‘hump’ was not seen when the flash was aimed at the base of the same spine (Fig. 1D, right).
To examine the role of calcium stores in the secondary peak found in the heads of SP+ spines, SERCA Ca2+-ATPase was blocked with the selective antagonist thapsigargin. The same spines were examined before and after exposure to the drug. While thapsigargin did not affect the rise time or peak of calcium changes in response to the flash, the secondary peak on the falling phase of elevated [Ca2+]i was completely abolished by the drug (Fig. 2B, C and G). To verify that the calcium stores are indeed involved in the secondary peak, another drug which specifically inhibits SERCA, cyclopiazonic acid (CPA, Verkhratsky, 2005), was used (Fig. 2D). In the presence of CPA, the secondary calcium peak was also eliminated.
Figure 2. Presence of SP is correlated with an expanded increase of [Ca2+]i in response to flash photolysis of caged calcium.
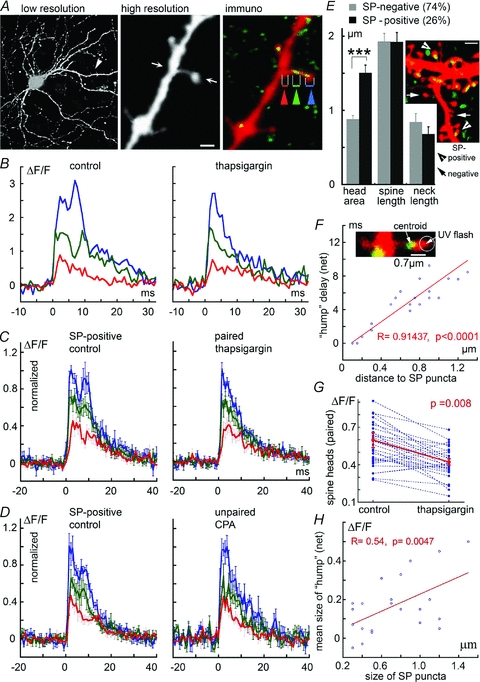
A, sample illustration of low-resolution DsRed-transfected neuron (left) and an expanded image of one of its dendrites in live imaging (middle), and following fixation and immunostaining for SP (green, right). The spine neck of this spine contains SP. Three ROIs are marked with blue (spine head), green (spine neck) and red (parent dendrite). B, changes in [Ca2+]i following flash photolysis in the spine head, of the spine illustrated in A. Left, in control conditions, and right, after incubation of the cell with thapsigargin. There is an obvious reduction in the delayed response (‘hump’) following the treatment. C, grouped data of 30 spines identified as having an SP punctum, before (left) and after (right) exposure of the same set of neurons to thapsigargin (paired, same spines before and after drug treatment). Strikingly, the ‘hump’ disappears following treatment with the drug. D, in a similar group of spines, imaged before (left) and after (right) exposure to the SERCA pump blocker CPA (n = 10 spines; unpaired, not the same group before and after the drug). E, comparison of the morphology of SP+ (n = 34) and SP− (n = 97) spines. Ordinate, μm. While the total spine and neck length are the same in both groups, SP+ spines have a significantly larger head area (measured as the largest cross-section area of the spine head, μm2) than SP− ones. Inset illustrates a dendrite with several spines endowed with a SP (arrowhead, yellow puncta) or missing one (arrow). F, the delay between the peak response to flash photolysis of caged calcium, and the peak of the secondary ‘hump’ is strongly correlated with the distance of the SP puncta from the spine head such that the closer they are, the faster the emergence of the ‘hump’ (r = 0.91, P < 0.0001, n = 20 spines). Inset, a sample illustration of the determination of the centroid point of SP, from where the distances are measured. G, reduction in [Ca2+]i following exposure to thapsigargin; same group of spines (n = 30) shown in C. The reduction in spine ‘hump’[Ca2+]i is highly significant (paired t test, P < 0.008). H, the size of the calcium ‘hump’ is positively correlated with the size of the SP puncta (n = 22 spines where the size of the SP puncta could be clearly measured (r = 0.54, P < 0.0047). Scale bars, 1 μm.
Another indication that the secondary calcium peak is related to the presence of a SP punctum in the spine head is in the highly significant correlation between the distance between the SP puncta and the spine head and the delay between the initial [Ca2+]i peak and the secondary one, such that the larger the distance, the longer the delay between the two peaks (Fig. 2F). Furthermore, there was a positive and significant correlation between the size of the SP puncta and the net size of the calcium ‘hump’ (Fig. 2H), indicating that the amount of the SP in the spine head contributes directly to the size of the calcium response. There was no significant correlation between the size of SP puncta and the size of the initial calcium transient.
To further explore the role of SP in the decay kinetics of the flash photolysed calcium transient, groups of SP+ and SP− spines, matched for size, were compared (Fig. 3). The spines that did not contain SP also did not express the ‘calcium hump’, and they were not affected by thapsigargin except for the later phase of the decay associated with calcium uptake into stores (Fig. 3Ac–f and Bc–f). There was a marked difference between spines containing SP in their heads (Fig. 3A) and those containing SP in their necks (Fig. 3B). Two groups of spines (those containing SP in their heads, group a, and those containing SP in their necks, group b) of similar length were compared (group b being shorter by 0.2 μm on average than group a). In the spines with SP in the neck, the response of the parent dendrite to the calcium surge triggered at the spine head was significantly larger than in the spines with SP in the head, or in spines not containing SP (Fig. 3Ca and b). Finally, the time constants of decay of the calcium transient was compared between SP+ and SP− spines (Fig. 3D) For groups of 72 SP− spines, the decay time in the spine heads was 9.41 ± 0.43 ms, and for their necks 10.48 ± 0.72 ms. For the SP+ spines, the comparable values were 12.44 ± 0.7 and 13.87 ± 0.87 ms. These values were affected significantly by the presence of thapsigargin (Fig. 3D).
Figure 3. Location of the SP puncta is correlated with the size of the additional calcium response (‘hump’) in association with release of calcium from stores.
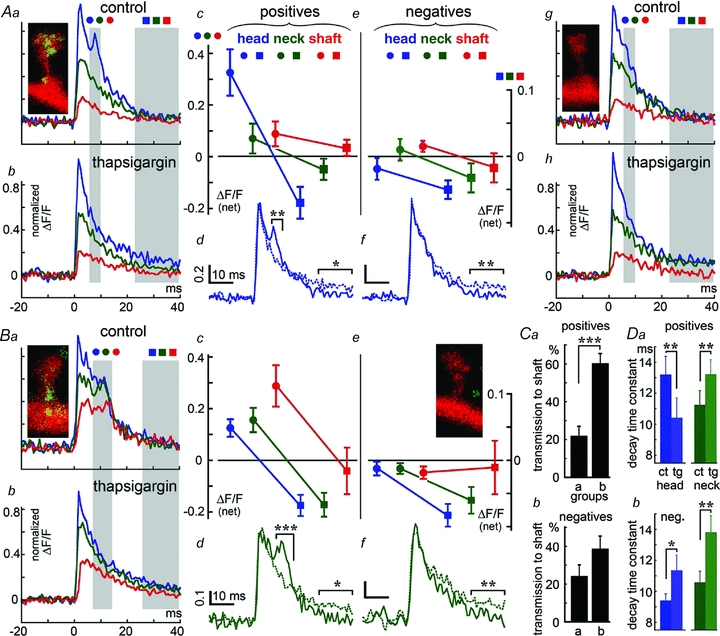
A, comparison between SP+ (Aa–d) (n = 9) and SP− (Ae–h) (n = 9) spines. Spines are of the same size in both groups, except that the first one contains SP puncta in or near their heads. Aa is an average of the calcium transient seen after flash photolysis of caged calcium in the spine heads of the group. The three traces represent the three compartments: spine head (blue), neck (green) and shaft (red). The shaded area is the ROI where analysis was conducted. Ab, same spines as in Aa, after exposure to thapsigargin, which smoothes the decay of the calcium transient. Ac, analysis of the net size of the calcium transient before and after thapsigargin at early stage of response (first ROI, circles) and the later stage (second ROI, squares), showing that only the spine head calcium compartment is drastically reduced by the drug (scale ΔF/F (net) in all). Ad, spine head calcium shown alone, before and after exposure to thapsigargin; Ae–h, same as Aa–d, except in SP− spines (n = 9). No ‘hump’ is found, and no effect of thapsigargin is seen during the initial stage (first ROI, circles) but becoming evident at the second stage (second ROI, squares). B, same as in A; analysis of 17 slightly shorter spines (by 0.2 μm on average), with SP puncta localized in the spine neck (Ba–d) or spines of similar dimensions, lacking SP (Be–h, n = 17). There are two clear distinctions between the spines in A and B: first, the response in the parent dendrites is far larger in B than in A, and second, the secondary rise in [Ca2+]i (the ‘hump’) is slower in B than in A, while it is seen in all 3 compartments, the head, the neck and the dendrite (Bc, circles, and Bd, neck). The late effect of thapsigargin is seen both in the heads and the necks in SP+ as well as SP− spines (Bc and Be, squares; and Bd and Bf, dots versus continuous lines). In either case, thapsigargin reduced the secondary [Ca2+]i surge in both the spine head and the parent dendrite. No such change was seen in any of the 17 control spines lacking SP (Be–h). C, the ratio between [Ca2+]i changes in the parent dendrite and the spine head was calculated for groups of nine spines matched for their length. Comparisons were made between spines containing SP in their heads (group a) and those containing SP in their necks (group b). There was a significantly larger response in the parent dendrites of the latter group compared to the former group, and compared to spines at same length and neck parameters as in groups a and b, not containing SP (Cb). Ordinate, transmission ration to the parent dendrite, as in Fig. 1F. D, analysis of decay time constants for the SP+ (Da) and SP− spines (Db), in control, left (ct, darker bars) and after exposure to thapsigargin, right (tg, lighter bars), in the heads and necks of 34 SP+ and 72 SP− longer (not stubby) spines. The decay time of only the SP+ spine heads is significantly reduced by the drug, whereas in the SP− as well as the spine necks of the SP+ spines, the drug slows down the decay time constant significantly. Ad, Af, Bd and Bf express control conditions versus thapsigargin treatment as continuous versus dashed lines.
In a subgroup of eight short spines, where SP was found in all compartments (Fig. 4), there was a large secondary [Ca2+]i peak as well as a large surge in the parent dendrite, as expected from the short distance between the two compartments. This secondary peak was also eliminated in the presence of thapsigargin.
Figure 4. A subgroup of short spines where SP puncta are found in both spine head, neck and parent dendrite compartment (n = 8 spines) compared to a group of 8 similar spines, where no SP was found.
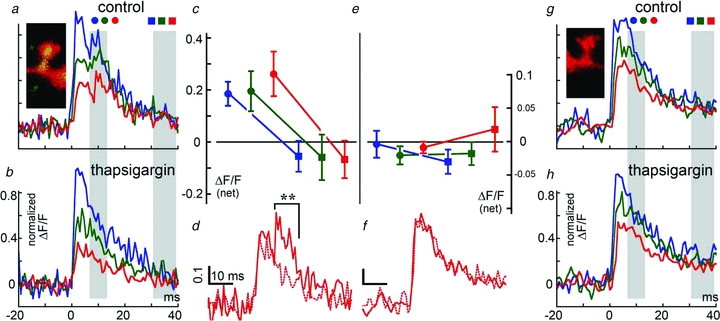
As in previous figures, a–d comprises SP+ spines, and e–h are SP− spines. In these spines, the response of the parent dendrite to flash photolysis of caged calcium released in the spine head was much larger compared to the other groups shown in Fig. 3. As in the other groups, thapsigargin removed the secondary rise in [Ca2+]i and smoothened its decay. The largest effect was seen in the shaft compartment, where the secondary ‘hump’ was even larger than the initial response. Thapsigargin did not change the large [Ca2+]i transient in the parent dendrite in either case, but completely eliminated the ‘hump’ in all 3 compartments (c versus e, circles, and d versus f for dendrites). The late decay of calcium response was also not affected by thapsigargin, unlike the data shown in Fig. 3A and B, probably due to easier calcium leak from the short spine to the shaft.
Taken together, these data suggest that SP is associated with flash photolysed calcium-induced calcium release, in that it boosts the communication between spine heads and parent dendrite, shown before to be instructive in delivery of glutamate receptors into the spine heads (Korkotian & Segal, 2007).
Discussion
The present experiments demonstrate a clear correlation between the presence of SP and calcium-induced release of calcium from stores in dendritic spines. We also extend our earlier observations that demonstrated that SP accumulates in strategic locations at the base of the head of some dendritic spines in cultured hippocampal neurons. The presence of SP in spines correlates with the presence of functional GluRs of the AMPA type, and its presence is upregulated by a transient activation of the NMDA receptor that causes a long-lasting potentiation of synaptic connectivity (Ivenshitz & Segal, 2006).
Synaptopodin has been associated with the spine apparatus, a membranous extension of the dendritic smooth endoplasmic reticulum. This neuronal organelle is found in a subpopulation of spines of telencephalic neurons (Spacek & Harris, 1997) and, interestingly, its regional distribution pattern mirrors the expression pattern of SP (Mundel et al. 1997; Deller et al. 2003). Using immunogold electron microscopy analysis, antibodies against SP were found in close association with the spine apparatus (Deller et al. 2003). The striking evidence for the critical role of SP in the formation of spine apparatus came from studies using a synaptopodin-deficient mouse, where the spine apparatus was absent (Deller et al. 2003). The involvement of the spine apparatus in the functional properties of the spine is still enigmatic, and a role in local protein synthesis, as well as in regulation of intracellular calcium concentrations, have been suggested (Burgoyne et al. 1983; Fifkova et al. 1983; Pierce et al. 2000), consistent with the association of endoplasmic reticulum with calcium stores (Verkhratsky 2005).
Since spine motility via Rho signalling is likely to be associated with spine head plasticity (Fischer et al. 2000; Pilpel & Segal, 2004; Korkotian & Segal, 2007), SP could provide a link between the actin cytoskeleton, growth of spine head volume and synaptic GluRs. However, the strategic and restricted location of SP, at the base of the spine head, indicates that it does not serve merely as a binding protein for the ubiquitous actin cytoskeleton but that it links actin to calcium stores.
Our results link SP with RyR/SERCA calcium stores: the ryanodine receptors are co-localized with SP (Segal et al. 2010), and depleting internal calcium stores with CPA suppresses the association of SP-containing puncta with the response to glutamate (Vlachos et al. 2009). In addition, CPA blocks plasticity of spine shape and GluR1 evoked by conditioning, as seen in SP-shRNA (small hairpin ribonucleic acid or short hairpin ribonucleic acid) treated cells. Blockade of the calcium pump with thapsigargin or CPA eliminated the secondary peak in the evoked calcium rise following its release from cage, and reduced the dendritic response to the calcium released in the spine head. These observations indicate that flash-photolysed calcium causes an evoked release of calcium from stores, which boosts the dendritic responses to an evoked calcium transient produced in the spine head. The increase in dendritic calcium transient is likely to be instrumental in the delivery of glutamate receptors into the spine head, as described before (Korkotian & Segal, 2007).
Previous studies support the role of calcium stores in excitatory synaptic responses, as well as in boosting back-propagating action potential-induced calcium transients (Markram et al. 1995; Emptage et al. 1999; Sandler & Barbara, 1999; Kovalchuk et al. 2000). These studies did not detect a similar secondary rise in [Ca2+]i following the initial rise, and they report much slower kinetics of the calcium decay. The difference between our results and these early ones are mainly methodological, e.g. we are using low concentration of the calcium sensor, much higher time resolution (1 ms) in an easily accessible, low-noise monolayer of neurons. In addition, the fast flash that we used (about 1 ns flash) allows a fast and momentary rise (less than 1 ms) of the calcium surge, followed by its rapid dissipation. In addition, we are separating between SP+ and SP− spines, so as to detect the additional calcium response in only the former group. The response to a transient flash photolysis of caged calcium is a unique case where calcium is not released initially from stores or influx through the membrane, which are slow and heterogeneous processes, but is released in an artificial manner from the cage, yet it can trigger the further release of calcium from stores. The advantage of such a test system is in its independence of mechanisms that are responsible for the initial influx of calcium, and allows detection of calcium-induced calcium release, as well as detection of mechanisms for handling excess calcium. In that context, the secondary peak in response to flash photolysis clearly indicates the presence of a Ca2+-induced Ca2+ release (CICR) mechanism, which is affected by SP.
The use of a fast-flash photolysis of caged [Ca2+]i allows detection of a difference in direction of communication between spine head and the parent dendrite (e.g. Fig. 1F), which is asymmetrical in favour of spine head to dendrite in the SP+ spines, unlike the case for SP− spines, where the communication is symmetrical. Thus, such an observation contributes to the understanding of the role of SP in spine–dendrite communication.
Finally, the involvement of ryanodine receptor-associated calcium stores in synaptic plasticity has been alluded to in the past decade, and some molecular mechanisms associated with the store-related plasticity have been suggested (Harvey & Collingridge, 1992; Emptage et al. 1999; Korkotian & Segal, 1999). Our current studies provide a further link between synaptopodin, calcium stores and synaptic plasticity, at the single synapse level.
Acknowledgments
We thank Ms E. Biton for production and maintenance of the cultures. This work was supported by a grant from the Israel Science Foundation.
Glossary
Abbreviations
- CPA
cyclopiazonic acid
- RyR
ryanodine receptor
- SP
synaptopodin
Author contributions
E.K. and M.S. designed the experiments, E.K. conducted the experiments, E.K. and M.S. wrote the manuscript, and both authors approved the final version.
References
- Asanuma K, Yanagida-Asanuma E, Faul C, Tomino Y, Kim T, Mundel P. Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat Cell Biol. 2006;8:485–491. doi: 10.1038/ncb1400. [DOI] [PubMed] [Google Scholar]
- Bas Orth C, Vlachos A, Del Turco D, Burbach GJ, Haas CA, Mundel P, Feng G, Frotscher M, Deller T. Lamina-specific distribution of synaptopodin, an actin-associated molecule essential for the spine apparatus, in identified principal cell dendrites of the mouse hippocampus. J Comp Neurol. 2005;487:227–239. doi: 10.1002/cne.20539. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Barron J, Geisow MJ. Cytochemical localisation of calcium binding sites in adrenal chromaffin cells and their relation to secretion. Cell Tissue Res. 1983;229:207–217. doi: 10.1007/BF00217893. [DOI] [PubMed] [Google Scholar]
- Deller T, Korte M, Chabanis S, Drakew A, Schwegler H, Stefani GG, Zuniga A, Schwarz K, Bonhoeffer T, Zeller R, Frotscher M, Mundel P. Synaptopodin-deficient mice lack a spine apparatus and show deficits in synaptic plasticity. Proc Natl Acad Sci U S A. 2003;100:10494–10499. doi: 10.1073/pnas.1832384100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deller T, Merten T, Roth SU, Mundel P, Frotscher M. Actin-associated protein synaptopodin in the rat hippocampal formation: localization in the spine neck and close association with the spine apparatus of principal neurons. J Comp Neurol. 2000;418:164–181. doi: 10.1002/(sici)1096-9861(20000306)418:2<164::aid-cne4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Emptage N, Bliss TV, Fine A. Single synaptic events evoke NMDA receptor-mediated release of calcium from internal stores in hippocampal dendritic spines. Neuron. 1999;22:115–124. doi: 10.1016/s0896-6273(00)80683-2. [DOI] [PubMed] [Google Scholar]
- Fifkova E, Markham JA, Delay RJ. Calcium in the spine apparatus of dendritic spines in the dentate molecular layer. Brain Res. 1983;266:163–168. doi: 10.1016/0006-8993(83)91322-7. [DOI] [PubMed] [Google Scholar]
- Fischer M, Kaech S, Wagner U, Brinkhaus H, Matus A. Glutamate receptors regulate actin-based plasticity in dendritic spines. Nat Neurosci. 2000;3:887–894. doi: 10.1038/78791. [DOI] [PubMed] [Google Scholar]
- Harvey J, Collingridge GL. Thapsigargin blocks the induction of long-term potentiation in rat hippocampal slices. Neurosci Lett. 1992;139:197–200. doi: 10.1016/0304-3940(92)90551-h. [DOI] [PubMed] [Google Scholar]
- Ivenshitz M, Segal M. Simultaneous NMDA-dependent long-term potentiation of EPSCs and long-term depression of IPSCs in cultured rat hippocampal neurons. J Neurosci. 2006;26:1199–1210. doi: 10.1523/JNEUROSCI.2964-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkotian E, Segal M. Release of calcium from stores alters the morphology of Dendritic spines in cultured hippocampal neurons. Proc Nat Acad Sci U S A. 1999;96:12068–12072. doi: 10.1073/pnas.96.21.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkotian E, Segal M. Morphological constraints on calcium dependent glutamate receptor trafficking into individual dendritic spine. Cell Calcium. 2007;42:41–57. doi: 10.1016/j.ceca.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Kovalchuk Y, Eilers J, Lisman J, Konnerth A. NMDA receptor-mediated subthreshold Ca2+ signals in spines of hippocampal neurons. J Neurosci. 2000;20:1791–1799. doi: 10.1523/JNEUROSCI.20-05-01791.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremerskothen J, Plaas C, Kindler S, Frotscher M, Barnekow A. Synaptopodin, a molecule involved in the formation of the dendritic spine apparatus, is a dual actin/α-actinin binding protein. J Neurochem. 2005;92:597–606. doi: 10.1111/j.1471-4159.2004.02888.x. [DOI] [PubMed] [Google Scholar]
- Markram H, Helm PJ, Sakmann B. Dendritic calcium transients evoked by single back-propagating action potentials in rat neocortical pyramidal neurons. J Physiol. 1995;485:1–20. doi: 10.1113/jphysiol.1995.sp020708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundel P, Heid HW, Mundel TM, Kruger M, Reiser J, Kriz W. Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol. 1997;139:193–204. doi: 10.1083/jcb.139.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo-Suzuki R, Okada D, Sekiguchi M, Inokuchi K. Synaptopodin maintains the neural activity-dependent enlargement of dendritic spines in hippocampal neurons. Mol Cell Neurosci. 2008;38:266–276. doi: 10.1016/j.mcn.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Pierce JP, van Leyen K, McCarthy JB. Translocation machinery for synthesis of integral membrane and secretory proteins in dendritic spines. Nat Neurosci. 2000;3:311–313. doi: 10.1038/73868. [DOI] [PubMed] [Google Scholar]
- Pilpel Y, Segal M. Activation of PKC induces rapid morphological plasticity in dendrites of hippocampal neurons via Rac and Rho-dependent mechanisms. Eur J Neurosci. 2004;19:3151–3164. doi: 10.1111/j.0953-816X.2004.03380.x. [DOI] [PubMed] [Google Scholar]
- Sandler VM, Barbara JG. Calcium-induced calcium release contributes to action potential-evoked calcium transients in hippocampal CA1 pyramidal neurons. J Neurosci. 1999;19:4325–4336. doi: 10.1523/JNEUROSCI.19-11-04325.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M, Vlachos A, Korkotian E. The spine apparatus, synaptopodin, and dendritic spine plasticity. Neuroscientist. 2010;16:125–131. doi: 10.1177/1073858409355829. [DOI] [PubMed] [Google Scholar]
- Spacek J, Harris KM. Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J Neurosci. 1997;17:190–203. doi: 10.1523/JNEUROSCI.17-01-00190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda K, Mainen ZF. Synaptic [Ca2+]i: intracellular stores spill their guts. Neuron. 1999;22:427–430. doi: 10.1016/s0896-6273(00)80698-4. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev. 2005;85:201–279. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- Vlachos A, Korkotian E, Schonfeld E, Copanaki E, Deller T, Segal M. Synaptopodin regulates plasticity of dendritic spines in hippocampal neurons. J Neurosci. 2009;29:1017–1033. doi: 10.1523/JNEUROSCI.5528-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


