Abstract
Non-technical summary
What is known about gastric electrophysiology and used in motility clinics throughout the world is mostly deduced from animal studies and extracellular recordings from human patients. Extracellular recording from gastrointestinal muscles, however, is prone to extensive motion artifact, and it is not clear that animal models can be translated directly to human physiology. Therefore, we have performed a detailed analysis of electrical activity from carefully mapped specimens of gastric muscle removed from humans during surgery for gastric cancers. Our data show several important differences in electrical activity recorded with intracellular microelectrodes and accepted gastric electrophysiological dogma. We observed ongoing electrical slow wave activity in the gastric fundus; we also found no evidence for a slow wave frequency gradient. Muscles from all regions through the thickness of the muscularis demonstrated intrinsic pacemaker activity, and this corresponded with the widespread distribution of pacemaker cells.
Abstract
Extracellular electrical recording and studies using animal models have helped establish important concepts of human gastric physiology. Accepted standards include electrical quiescence in the fundus, 3 cycles per minute (cpm) pacemaker activity in corpus and antrum, and a proximal-to-distal slow wave frequency gradient. We investigated slow wave pacemaker activity, contractions and distribution of interstitial cells of Cajal (ICC) in human gastric muscles. Muscles were obtained from patients undergoing gastric resection for cancer, and the anatomical locations of each specimen were mapped by the operating surgeon to 16 standardized regions of the stomach. Electrical slow waves were recorded with intracellular microelectrodes and contractions were recorded by isometric force techniques. Slow waves were routinely recorded from gastric fundus muscles. These events had similar waveforms as slow waves in more distal regions and were coupled to phasic contractions. Gastric slow wave frequency was significantly greater than 3 cpm in all regions of the stomach. Antral slow wave frequency often exceeded the highest frequency of pacemaker activity in the corpus. Chronotropic mechanisms such as muscarinic and prostaglandin receptor binding, stretch, extracelluar Ca2+ and temperature were unable to explain the observed slow wave frequency that exceeded accepted normal levels. Muscles from all regions through the thickness of the muscularis demonstrated intrinsic pacemaker activity, and this corresponded with the widespread distribution in ICC we mapped throughout the tunica muscularis. Our findings suggest that extracellular electrical recording has underestimated human slow wave frequency and mechanisms of human gastric function may differ from standard laboratory animal models.
Introduction
Many of the principles of human gastric physiology and pathophysiology come from animal studies, most commonly using dog, guinea-pig and mouse (Weber & Koatsu, 1970; Hinder & Kelly, 1977; el-Sharkawy et al. 1978; Morgan et al. 1981; Ozaki et al. 1991; Burns et al. 1996; Dickens et al. 1999; Ordög et al. 1999; 2000; Kito & Suzuki, 2003; Lammers et al. 2009). There have been relatively few studies of human gastric muscles (el-Sharkawy et al. 1978; Hara & Ito, 1979; Sanger, 1985; Min et al. 2010) so it is uncertain whether the mechanisms and patterns of gastric activation, receptive relaxation and peristalsis in laboratory animals translate to human gastric physiology. Standard accepted concepts in human gastric electrophysiology include: (i) electrical quiescence in the fundus, (ii) a dominant slow wave frequency of 3 cycles per minute (cpm), and (iii) an intrinsic slow wave frequency gradient where faster proximal pacemakers dominate over slower distal pacemakers. A previous study using intracellular recording, reported slow wave frequencies above what is considered normal for human gastric muscles (i.e. 6 cpm in corpus and 4 cpm in antrum), but there were too few corpus muscles in this study to contest results obtained by extracellular recordings (el-Sarkawy et al. 1978). Phasic contractions of human fundus, corpus and antrum muscles were recently shown to exceed 5 cpm (Min et al. 2010). Phasic contractions are timed by slow waves, so these studies raised several questions: (i) are slow waves present in human fundus? (ii) has slow wave frequency been underestimated by extracellular recording? and (iii) does a proximal-to-distal slow wave frequency gradient exist in human stomach? These are fundamental questions in understanding human gastric physiology and establishing parameters of ‘abnormal activity’. For example, some clinicians, utilizing electrogastrography (EGG) to evaluate symptoms of dyspepsia, gastroparesis or other motility disorders, attempt to determine whether slow wave frequency conforms to accepted ‘normal’ limits of 2–4 cpm (Koch, 2001; Lin & Chen, 2001; Leung et al. 2006; Sha et al. 2009). However, the correlation between symptoms and slow wave frequency, as assessed by EGG, is not strong (Jebbink et al. 1994; Smout et al. 1994; Chen et al. 1996; Parkman et al. 1997), and reasons for this need to be clarified.
We have characterized basal electrical rhythm of slow waves in human gastric muscles, using intracellular recording techniques. Our data suggest that the 3 cpm ‘standard’ for slow wave frequency needs reconsideration. We have found that gastric fundus muscles generate electrical slow waves that underlie phasic contractions. We also found no evidence for a proximal-to-distal frequency gradient. These findings challenge many basic concepts of human gastric electrophysiology and suggest that methods and standards for clinical evaluation of gastric electromechanical function need reconsideration.
Methods
Tissues
Tissues were obtained as surgical waste from 57 patients (40 males, 17 females with an average age of 62.1 ± 1.6 years; 32–83 years) undergoing gastric cancer surgery in Samsung Medical Center, Seoul, South Korea. The anatomical locations from which the surgical samples were removed were noted on gastric maps by the performing surgeon (Fig. 1A and B). Non-cancerous margins were placed in Krebs–Ringer buffer (KRB) and transported to the laboratory within 15 min for electrophysiological and contractile studies and processed immediately for morphological studies. The Samsung Medical Center Human Subjects Research Committee approved protocols. Written, informed consent was obtained from patients and all experiments complied with the Declaration of Helsinki. Muscles were retrieved from the fundus (12; regions 1–4), corpus (22; regions 5–12) and antrum (26; 13–16; see Fig. 1A and B).
Figure 1. Map of stomach and techniques to record electrical and contractile activity in human stomach.
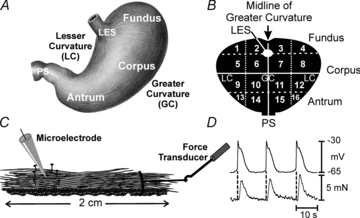
A, sketch of stomach and major regions referred to in text. B, schematic map of stomach used by surgeons to demarcate region from which surgical samples were obtained. Regions 1–4 correspond to the fundus, regions 5–12 correspond to the corpus, and regions 13–16 correspond to the antrum. PS, pyloric sphincter; LES, lower esophageal sphincter. GC and LC represent greater and lesser curvatures, respectively. C, sketch of cross-sectional muscle strip with a microelectrode to record intracellular electrical activity and attachment of a force transducer at one end to record isometric contractions or stretch. D, simultaneous recording from antrum (region 14) showing one-to-one relationship between electrical slow waves and phasic contractions.
Electrophysiology and isometric force measurements
Gastric tissues were pinned in a dissecting dish, and mucosa and submucosa were removed. Cross-sectional strips of muscle (1 × 20 mm), through the tunica muscularis, were cut transverse or parallel to the circular muscle (CM) fibres (Figs 1C and 2). The muscles were transferred to an electrophysiological chamber and pinned out in cross section with little stretch beyond resting length. Some muscles were attached to a force transducer (Gould UC3; Gould Instruments, OH, USA) for simultaneous recordings of electrical and mechanical activity (Fig. 1D). In other experiments the CM and longitudinal muscle (LM) were dissected into four layers (Fig. 2). Additional dissections included isolation of muscle bundles (≤1 mm). Intracellular recording (at 37 ± 0.5°C) was begun after 1 h equilibration as previously described (Hwang et al. 2009). Briefly, cells were impaled with microelectrodes (70–100 MΩ), and transmembrane potentials were measured with a high input impedance amplifier (Axon Instruments/Molecular Devices Corp., Sunnyvale, CA, USA). Electrical and mechanical signals were digitized (Digidata 1300 series; Axon Instruments) and stored using Axoscope 10.0 software.
Figure 2. Dissections of gastric muscles to obtain subsections for intracellular electrical recordings.
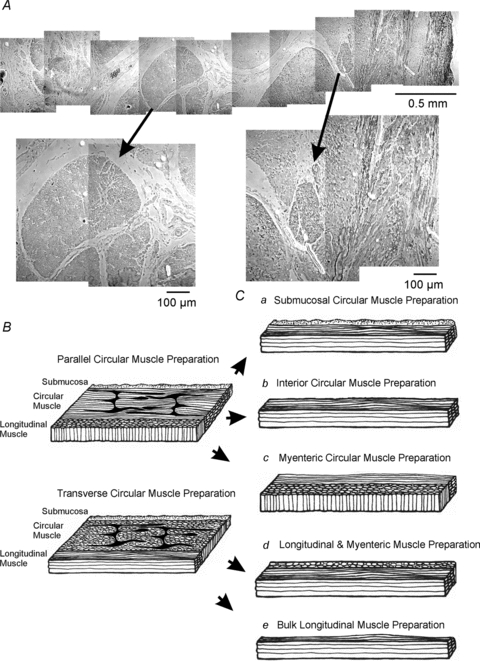
A, a phase-contrast image of a montage through the full-thickness of the human gastric antrum muscularis. Note the marked division of the circular muscle layer into bundles separated by broad septae (arrows). Higher magnifications are denoted by arrows. B, full thickness strips cut parallel to either the circular or the longitudinal muscle layers. Strips parallel to the circular muscle were used to cut subregional muscle bundles (C) from the submucosal circular, which consisted of: circular muscle adjacent to the submucosa (a), interior/bulk circular muscle bundles consisting of circular muscle fibres in the central 1/3 of the circular layer (b), or myenteric regions of the circular muscle layer (c). Longitudinal strips were used to cut subregions of longitudinal muscles which were dissected into an inner longitudinal layer with myenteric plexus attached (d) and an outer bulk longitudinal layer adjacent to the serosal surface (e).
Immunohistochemical studies
The distribution and cellular localization of Kit and Ano-1/TMEM16A were examined by immunohistochemistry on cryostat sections, as previously described (Hwang et al. 2009). Sections were examined with a Zeiss LSM 510 Meta confocal microscope (Carl Zeiss, Thornwood, NY, USA) with an excitation wavelength (488 and 594 nm). Confocal micrographs are digital composites of Z-series scans of 10–100 optical sections through a depth of 4–100 μm.
Solutions and drugs
The muscles were perfused with oxygenated Krebs–Ringer buffer (KRB) of the following composition (mm): NaCl 118.5; KCl 4.5; MgCl2 1.2; NaHCO3 23.8; KH2PO4 1.2; dextrose 11.0; CaCl2 2.5. The pH of the KRB was 7.3–7.4 when bubbled with 97% O2–3% CO2 at 37 ± 0.5°C. Atropine, tetrodotoxin and nickel (Sigma-Aldrich Corp., St Louis, MO, USA) were dissolved as stock solutions in dH2O; nifedipine and indomethacin (Sigma-Aldrich) were dissolved in ethanol before being added to the perfusion solution at the concentrations stated.
Data analysis
Data are expressed as means ± standard errors of the mean. Student's t test was used where appropriate to evaluate differences in the data. P values ≤0.05 were taken as a statistically significant difference. The n values reported in the text refer to the number of muscles used for each experimental protocol. The parameters of slow waves were tabulated as described previously (Horiguchi et al. 2001).
Results
Basal electrical rhythm in human stomach
Spontaneous phasic contractions of human fundus muscles (regions 1–4; Fig. 1) were reported previously (Min et al. 2010), but others have claimed this region is electrically quiescent based on extracellular recording (O'Grady et al. 2010). We found that fundus muscles had resting membrane potentials (RMPs; most negative potentials between slow waves) of –57 ± 3.3 mV (n = 12 muscles from 6 males and 5 females, mean age 67.1 ± 1.7 years). Electrical slow waves were recorded from 11 of 12 muscles (Table 1). The waveforms of fundus slow waves were similar to slow waves in more distal muscles: upstroke depolarization (maximal upstroke velocity (rate-of-rise or dV/dt) was 57 ± 34.5 mV s−1), partial repolarization to a ‘plateau phase’, and repolarization to RMP. The inter-slow wave interval, averaging 3.9 ± 0.9 s (P < 0.05; Fig. 3A), was shorter than slow wave duration. Recordings of electrical and mechanical activity revealed 1:1 coupling between slow waves and contractions (Fig. 3A).
Table 1.
Electrical parameters from various regions of the human stomach
| Electrical parameter | Fundus | Corpus | Antrum |
|---|---|---|---|
| RMP (mV) | −57 ± 3.3 | −70 ± 1.5 | −75 ± 1.3 |
| SW upstroke amplitude (mV) | 15 ± 3.5 | 26 ± 2.7 | 36 ± 2.0 |
| SW plateau amplitude (mV) | 10 ± 1.5 | 21 ± 2.0 | 29 ± 2.0 |
| SW frequency (cpm) | 5.1 ± 0.3 | 5.7 ± 0.5 | 7.4 ± 0.5 |
| SW duration (s) | 10.4 ± 1.7 | 9.2 ± 1.0 | 4.4 ± 0.4 |
| ½ Max amp. duration (s) | 5.4 ± 1.4 | 3.5 ± 0.6 | 1.6 ± 0.1 |
| Upstroke dV/dt (mV s−1) | 57 ± 35 | 158 ± 29 | 389 ± 46 |
RMP, resting membrane potential; SW, slow wave; cpm, cycles min−1.
Figure 3. Electrical and mechanical activities recorded from various regions of the human stomach.
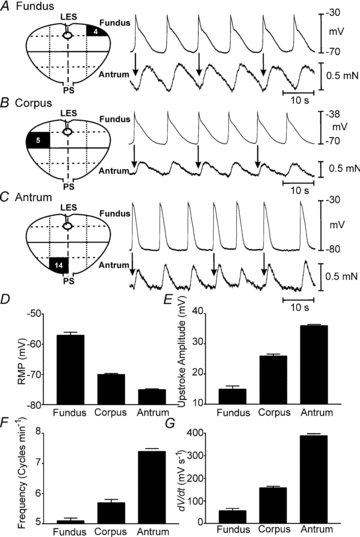
Regions from which specimens were obtained are denoted in surgical maps at left of each panel. A, recording from fundus where slow waves were routinely recorded. Each slow wave was associated with a phasic contraction (arrows) which summed to create tone. B, slow waves and corresponding phasic contractions in corpus muscle. C, slow waves and corresponding phasic contractions from an antral muscle. D–G, summary of electrical parameters (RMP, upstroke amplitude, frequency and rate of rise of the slow wave upstroke) from fundus, corpus and antrum.
Corpus cells (regions 5–12) had RMP averaging –70 ± 1.5 mV (P < 0.001 as compared to fundus; n = 22 muscles from 14 males and 3 females; mean age 64.1 ± 3.1 years). Slow wave parameters are in Table 1. The dV/dt of the upstroke depolarization averaged 158 ± 29 mV s−1, which was faster than fundus slow waves (P < 0.001). Similar to fundus, the inter-slow wave interval was shorter than the slow wave duration, averaging 3.4 ± 0.3 s (P < 0.05). Simultaneous recordings from six muscles showed 1:1 coupling between slow waves and phasic contractions (Fig. 3B).
Antral muscle cells (regions 13–16) had RMPs averaging –75 ± 1.3 mV (n = 29 muscles from 20 males and 9 females; mean age 58.6 ± 2.5 years. Slow waves parameters are shown in Table 1. The period between antral slow waves averaged 3.6 ± 0.4 s. The rate-of-rise of the upstroke component of antral slow waves averaged 389 ± 46 mV s−1, faster than slow waves in corpus (P < 0.001). Simultaneous electrical and mechanical recording (n = 11) revealed a 1:1 ratio between slow waves and phasic contractions (Fig. 3C). A summary of the electrical properties of slow waves in different regions of the stomach is shown in Fig. 3D–F.
Site of pacemaker activity within the gastric wall
Pacemaker activity in rodent and canine stomachs is generated by ICC at the level of the myenteric plexus (ICC-MY) (Dickens et al. 1999; Ordög et al. 1999; Horiguchi et al. 2001). Slow waves conduct electrotonically from ICC-MY to adjacent CM and LM (Cousins et al. 2003). In larger animals, such as dogs, ICC-MY are dominant pacemakers (Horiguchi et al. 2001), but propagation into thicker CM occurs regeneratively via ICC surrounding muscle bundles (ICC-SEP) (Lee et al. 2007). We recorded slow waves at various sites through CM and LM and in CM and LM bundles free of myenteric plexus and ICC-MY (see Fig. 2).
CM of the corpus near the myenteric border had RMPs averaging –67 ± 4.6 mV, and 24 ± 5.7 mV slow waves occurred at 5.0 ± 0.2 cpm with durations of 9.5 ± 0.6 s (n = 4). Half way through CM, RMP averaged –69 ± 1.3 mV with 29 ± 4.6 mV slow waves occurring at 5.1 ± 0.1 cpm (n = 4). Cells near the submucosal surface of CM had RMPs of –63 ± 2.1 mV (n = 3), and small slow waves (23 mV in amplitude and 11.2 s in duration, and 4.5 cpm frequency; Fig. 4A) were recorded in 2 of 3 muscles.
Figure 4. Electrical slow waves in subregions of gastric muscle denoted on surgical maps in each panel.
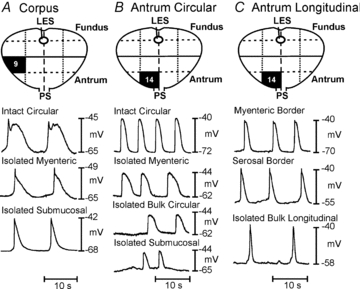
A–C, recordings from intact circular (A and B) or longtitudinal (C) muscle strips from near the myenteric border of the circular muscle layer (isolated myenteric), near the submucosal surface of the circular layer (isolated submucosal), the interior region of circular or longitudinal muscles (bulk circular), or longitudinal muscles from near the myenteric border (myenteric longitudinal) or the serosal half of the longitudinal muscle (bulk longitudinal). Slow waves were recorded from all subregions of muscles.
In antrum CM, RMP near the myenteric border averaged –68 ± 2.6 mV, and slow waves 34 ± 5.1 mV in amplitude occurred at 8.1 ± 0.8 cpm (4.5 ± 0.7 s duration; n = 7). Half way through the CM, RMP averaged –65 ± 2.5 mV, and slow waves 26 ± 3.2 mV in amplitude occurred at 7.7 ± 0.7 cpm (4.3 s ± 0.6 s durations; n = 10). Cells near the CM submucosal surface had RMPs of –64 ± 5.0 mV, and slow waves 20 ± 7.8 mV in amplitude occurred at 9.4 ± 1.7 cpm (4.1 ± 1.1 s durations; n = 6; Fig. 4B). RMP of LM cells was –66 ± 3.3 mV, and slow waves 27 ± 1.1 mV in amplitude occurred at 5.8 ± 0.7 cpm (6.5 ± 2.1 s durations; n = 5; Fig. 4C). Therefore, CM and LM both displayed intrinsic pacemaker activity.
Intrinsic pacemaker activity was further studied using muscle bundles dissected from antral CM and LM. CM bundles near the myenteric border had RMPs averaging –72 ± 2 mV, and 41 ± 4.0 mV slow waves occurred at a frequency of 8.4 ± 0.7 cpm (3.2 ± 0.9 s durations; n = 5; Fig. 4B). CM bundles, approximately 50% through the CM, had RMPs of –68 ± 6.5 mV, and 21.7 ± 6 mV slow waves occurred at a frequency of 5.7 ± 0.7 cpm (5.1 ± 0.7 s durations; n = 3). A LM bundle from the region near the myenteric plexus displayed 18 mV slow waves, 13.5 s in duration, at 5 cpm (Fig. 4C). Similar spontaneous activity was recorded from two muscles isolated from subregions of the corpus (Fig. 4A). These data suggest that active pacemaker activity occurs through the thickness of human gastric muscles.
Factors affecting slow wave frequency
Our results show that slow waves occur at higher frequencies in gastric muscles than found in studies of intact stomachs using extracellular recording (Hinder & Kelly, 1977; O'Grady et al. 2010). We investigated chronotropic mechanisms known to affect slow wave frequency to determine whether frequency was enhanced artificially in vitro. Cholinergic neurons exert chronotropic effects on gastric muscles (el-Sharkawy et al. 1978; Forrest et al. 2009), so we exposed human muscles to tetrodotoxin (TTX) and atropine to determine whether dissection increased excitatory neurotransmission. TTX (300 nm) (Supplemental Fig. 1A) and atropine had no effect on slow waves. Slow waves before atropine averaged 8.3 ± 0.6 cpm and were unchanged by atropine (1 μm; 8.5 ± 0.6 cpm; n = 6; P = 0.82; Supplemental Fig. 1B). Dissection might increase production of prostaglandins, and PGE2 has been shown to exert positive chronotropic effects on antral muscles (Sanders, 1984; Forrest et al. 2009). Therefore, muscles were treated with indomethacin (1 and 10 μm for >30 min; n = 5 each concentration; Supplemental Fig. 1C). Both concentrations caused a small decrease in frequency (i.e. 10 μm for 30–70 min reduced slow wave frequency from from 9 ± 1.0 cpm to 7.8 ± 0.9 cpm; P = 0.4, but the decrease did not bring frequency close to 3 cpm).
Stretch of antral muscles also enhances slow wave frequency (Won et al. 2005). Therefore, our recordings were performed with minimal stretch imposed on muscles (see Methods). To determine if stretch enhanced slow wave frequency in antral muscles, a force transducer was positioned on a moveable platform and repeated graded length ramps were applied (n = 5 muscles from 3 patients). In 4 of 5 muscles, stretch (60 mN) caused depolarization from –71 ± 0.8 mV to –68 ± 0.9 mV (P = 0.036) and inter-slow wave interval was decreased transiently following the onset of stretch (i.e. from 5.4 ± 0.2 s to 3.5 ± 0.4 s; P = 0.001; Supplemental Fig. 1D), and basal frequency and membrane potential were restored upon release of stretch. Many animal studies have demonstrated that slow wave frequency depends upon temperature. Our experiments were performed at 37°C, and reducing temperature caused significant reduction in slow wave frequency, dV/dt and slow wave duration (e.g. from 6.8 ± 0.9 at 36°C to 2.9 ± 0.5 at 30°C; n = 4; Q10 = 4.1; P = 0.011; Fig. 5A–F).
Figure 5. Effects of temperature on slow wave parameters.
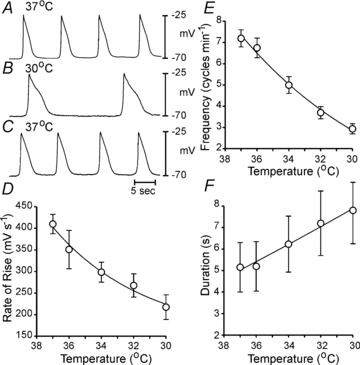
A–C, slow waves recorded during the same impalement as temperature was decreased from 37°C to 30°C and then returned to 37°C. D–F summarize the effects of temperature on the rate-of-rise of slow waves (D), frequency (E) and duration (F) (5 antral muscles of 3 patients). Data in D–F are fit with exponential functions (D and E, R2 = 0.9990; F, R2 = 0.9994).
Ca2+ entry and slow waves
Extracellular Ca2+ ([Ca2+]o) and Ca2+ entry mechanisms have been shown to regulate slow wave frequency (Ward & Sanders, 1992). KRB contains 2.5 mmol l−1[Ca2+]o, but a portion of total Ca2+ is buffered in serum. Therefore, we tested the effects of reduced [Ca2+]o on slow waves. In an initial experiment [Ca2+]o was reduced in steps from 2.5 mm to 0.1 mm, and there was little effect on slow wave parameters over this broad range (Supplemental Fig. 2). Subsequent experiments, therefore, evaluated the effects of changing [Ca2+]o from 2.5 mm to nominally Ca2+ free conditions or very low levels by buffering [Ca2+]o with EGTA (1 mm). In nominally Ca2+ free solution (20–60 min), RMP of antral muscles depolarized from –69 ± 2.5 to –59 ± 3.5 (P = 0.04), and slow waves were abolished in 5 of 8 muscles (Fig. 6A). Slow waves recovered when muscles were reperfused with KRB (Fig. 6A). Although slow waves persisted for extended time periods, contractions were rapidly abolished in nominally Ca2+ free solution (Fig. 7). In the remaining three muscles, slow waves (14.3 ± 9.8 mV in amplitude and 2.8 ± 0.4 cpm) persisted. EGTA (1 mm), added to the nominally free [Ca2+]o solution, rapidly (<5 min) abolished slow waves in all muscles (Fig. 6B). Similar results were observed in tests of three corpus muscles; RMP depolarized from –76 ± 5 mV to –62 ± 10.8 mV (P = 0.001) and was reduced in amplitude and frequency from 32.7 ± 2.8 mV and 5.2 ± 1.4 cpm to 10 ± 5 mV (P = 0.05) and 3.7 ± 0.3 cpm (N.S.), respectively. The rate of rise of the upstroke was also significantly reduced from 339 ± 88 mV s−1 to 21 ± 16.4 mV s−1 (P = 0.02; n = 3). Addition of EGTA (1 mm) also abolished slow waves in the corpus and the effect was reversible following return to 2.5 mm[Ca2+]o.
Figure 6. Effects of reduced Ca2+ and Ni2+ on slow wave parameters.
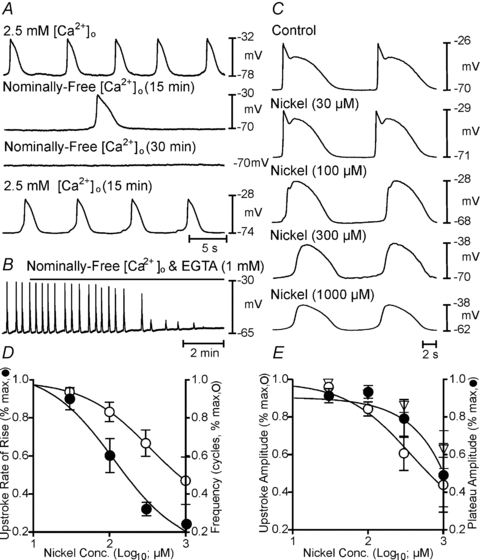
A, the time-dependent effects during a single impalement of reducing extracellular Ca2+ ([Ca2+]o) from 2.5 mm (control) to nominally free [Ca2+]o (15 and 30 min), and return to 2.5 mm[Ca2+]o. B, effects of adding EGTA (1 mm) to nominally free [Ca2+]o. Slow waves were rapidly blocked when EGTA was added. C, the effects of Ni2+ (0–1000 μm) on slow waves. D and E show summarized effects of Ni2+ on membrane potential (open diamonds in E), slow wave rate-of-rise (IC50 = 114 μm; R2 = 0.99), frequency (IC50 = 339 μm; R2 = 1), upstroke (IC50 = 372 μm; R2 = 0.99) and plateau amplitude (IC50 = 96186 μm; R2 = 0.99).
Figure 7. Effects of reduced [Ca2+]o and nifedipine on slow waves and contractions.
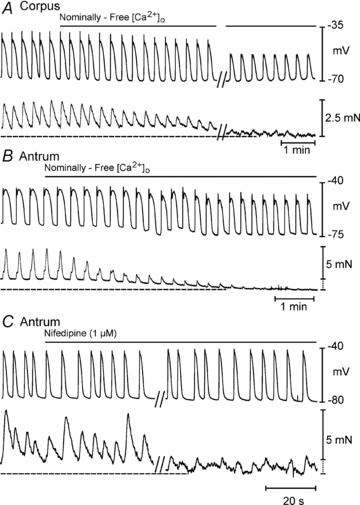
A and B, simultaneous recordings of slow waves and phasic contractions in corpus and antrum in response to reduction in [Ca2+]o from 2.5 mm to nominally free [Ca2+]o. Note nearly total inhibition of contractions and slight reduction in amplitude and durations of slow waves. Tone is also reduced in both muscles with the reduction in amplitude. Break in A represents 20 min gap in the recording during a continous impalement. C, response of an antral muscle to nifedipine (1 μm). Note reduction, but not block, of contractions with this compound and little or no effect on slow waves. Break represents 10 min gap in recording during a continuous impalement.
The L-type calcium channel antagonist nifedipine (1 μm) had little or no effect on slow wave frequency or amplitude throughout the stomach (Supplemental Fig. 3). For example RMP in corpus cells was –68.6 ± 3.4 mV, and slow wave amplitude and frequency were 29.1 ± 4 mV and 5.1 ± 1 cpm, respectively; in responses to nifedipine (1 μm), RMP was –69 ± 3.5, and slow wave amplitude and frequency were 29.4 ± 3.5 mV and 4.9 ± 0.8 cpm (P > 0.05 for all parameters, n = 8). Nifedipine also displayed little or no effect in antrum (n = 5) or fundus (n = 3).
We also tested Ni2+, a T-type Ca2+ channel antagonist. Ni2+ caused concentration-dependent reduction in slow wave upstroke velocity, amplitude and frequency, and increased slow wave duration (Fig. 6C). For example, in KRB slow waves averaged 39 ± 3.2 mV in amplitude and 7.0 ± 0.9 s in duration and occurred at a frequency of 6.3 ± 0.8 cpm. The upstroke velocity (dV/dt) averaged 445 ± 95 mV s−1 (n = 7). Ni2+ (300 μm) reduced slow waves to 28 ± 2.8 mV (P = 0.024) and increased duration to 11.8 ± 1.9 s (P = 0.05). Slow wave frequency was reduced to 3.5 ± 0.3 cpm (IC50 = 339 μm; P = 0.02), but the upstroke velocity was the most sensitive parameter and was reduced to 32 ± 6.8 mV s−1 (IC50 = 114 μm; P = 0.003; n = 6; Fig. 6D and E). Slow waves in the corpus were affected by Ni2+in a similar manner (n = 2; not shown).
Distribution of interstitial cells of Cajal in the human stomach
ICC are pacemakers in gastric muscles (Dickens et al. 1999; Ordög et al. 1999), so the distribution of these cells was characterized. Previous studies of ICC in human stomach have been published (Ibba et al. 2004; Yun et al. 2010), but we characterized ICC in the specific regions and same muscles used for electrophysiological recordings. Montages of cryostat sections revealed an extensive distribution of ICC throughout the gastric tunica muscularis (Fig. 8). ICC were located at the level of the myenteric plexus between the CM and LM (ICC-MY) in corpus and antrum. ICC-MY formed an anastomosing network around and between ganglia. ICC (ICC-IM) were observed in CM and LM and ran parallel to the long axis of muscle fibres (Fig. 9A and E). Unlike ICC-IM in rodents (Burns et al. 1996), human ICC-IM possessed processes extending from the cell body and contacting adjacent ICC-IM to form a loose network (Fig. 9B, C and E). ICC were also found on the outer aspects of muscle bundles, within septae that separated muscle bundles, in both CM and LM (Supplemental Figs 4–6). Processes of these ICC (ICC-SEP) extended across septae to connect adjacent muscle bundles (Supplemental Fig. 5). ICC throughout the human stomach including fundus expressed TMEM16A (Fig. 10) (Gomez-Pinilla et al. 2009; Hwang et al. 2009). Antibodies for TMEM16A protein (anoctamin 1) are more specific for ICC than Kit labelling, as mast cells were not labelled with TMEM16A antibodies (Fig. 10).
Figure 8. Montages of immunohistochemical confocal images taken through the fundus, corpus and antrum revealing Kit-immunopositive cells.
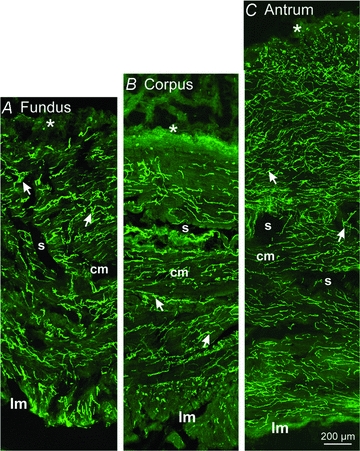
Higher resolution images (see Fig. 9 and Supplemental Figs 4–6) show that the majority of these cells are ICC, but mast cells were also identified by Kit labelling and their rounded appearance (particularly at the submucosal (s) surface of the circular muscle). A–C, a montage through the fundus region (A), the corpus region (B) and the antrum (C). Note the extensive distributions of ICC (arrows) throughout all regions of the circular (cm) and longitudial (lm) muscle bundles that were separated by septae (s). Mast cells (*) are also observed along the submucosal surface of the circular muscle layer.
Figure 9. Higher power confocal images of Kit-positive ICC in different locations through the human stomach.
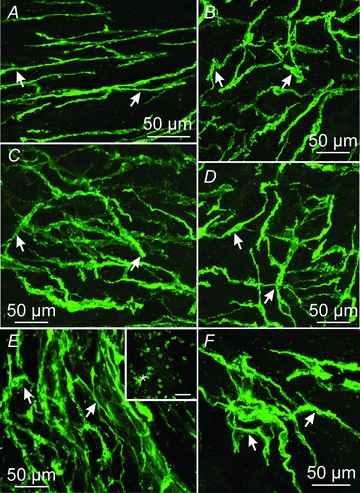
A and B, spindle shaped ICC and ICC with several projections within the gastric fundus (arrows; region 4). C and D, Kit-positive ICC within the gastric corpus (arrows; region 5). ICC formed dense networks throughout this region. E and F, Kit-positive ICC in the gastric antrum (region 13). Dense clusters of spindle shaped ICC and ICC with several projections were observed in the circular layer of this gastric region. Large numbers of small rounded Kit-positive mast cells were observed along the submucosal surface of the human stomach (*, inset in E). Scale bars = 50 μm.
Figure 10. Double labelling of Kit and Ano-1/TMEM16A in the human stomach.
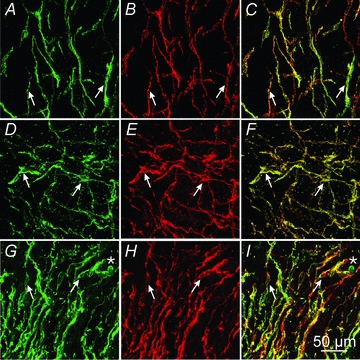
A and C, Kit (A, green), Ano-1 (B, red) and merged image (C) of ICC in the gastric fundus (arrows). D–F, Kit (D, green), Ano-1 (E, red) and merged image (F) of ICC in the gastric corpus (arrows). G–I, Kit (G, green), Ano-1 (H, red) and merged image (I) in the gastric antrum (arrows). Scale bar in I = 50 μm and represents all panels.
Discussion
Spontaneous electrical and mechanical activity throughout the human stomach was evaluated with intracellular recordings and isometric force measurements. Our data contrast with commonly held beliefs about human gastric electrophysiology and suggest that parameters considered ‘normal’ for human patients should be reconsidered. Slow wave activity was recorded in muscles from gastric fundus, an area typically considered ‘electrically quiescent’ based on animal studies (Morgan et al. 1981; Burns et al. 1996) and extracellular electrical recording from humans (O'Grady et al. 2010). Spontaneous electrical activity in human fundus was observed previously, but no characteristics of this activity were provided (Hara & Ito, 1979). The fundus does not contain an ICC-MY network, but branching septal ICC, which might provide a distributed network of pacemaker cells, were observed. Our data cannot exclude fundus smooth muscle cells as intrinsic pacemakers in human stomach, but we showed that fundus ICC express TMEM16A, which is necessary for pacemaker activity in mice (Hwang et al. 2009; Zhu et al. 2009), and this protein was not resolved in cells other than ICC. Slow wave frequencies were universally above the range considered normal in human stomach and were within the range typically considered ‘tachygastria’. Finally, we found no support for an intrinsic proximal-to-distal frequency gradient, as deduced from studies of dogs (Kelly & Code, 1969; el-Sharkawy et al. 1978). In fact, the frequency of antral slow waves often exceeded slow wave frequency in the corpus, in contrast to studies on canine muscles (el-Sharkawy et al. 1978). These results suggest significant differences in the electrophysiology of human stomachs and common laboratory animals. Our findings suggest that new concepts, tests and standards may be required for accurate clinical evaluations of gastric function.
Gastric muscles were spontaneously active in each region of the stomach and phasic contractions were associated with slow waves. Morphological studies confirmed that human gastric muscles contain several classes of interstitial cells of Cajal (ICC). ICC were present in the myenteric region (ICC-MY) between the CM and LM in corpus and antrum, within muscle bundles (ICC-IM) throughout the stomach, and in septa between muscle bundles (ICC-SEP). ICC-MY are thought to be pacemakers in the stomach because removal of these cells results in loss of slow waves (Ordög et al. 1999). However, ICC-MY are not the sole pacemakers in larger animals, as ICC-SEP can also generate and propagate electrical activity (Horiguchi et al. 2001; Lee et al. 2007). In the present study we found that muscle bundles from CM or LM, dissected to exclude ICC-MY, were spontaneously active. The specific class of ICC responsible for intrinsic pacemaker activity in the fundus will require further studies.
Electrical slow waves induce phasic contractions in gastric muscles in animal models (Morgan & Szurszewski 1980). A well-developed theory has been proposed explaining how membrane potentials during electrical slow waves couple to contractile events (Szurszewski, 1987), and in the present study we confirmed coupling between slow waves and contractions. Action potentials are not a typical feature of gastric electrical activity, except in the most distal reaches of the terminal antrum and pylorus (el-Sharkawy et al. 1978; Sanders & Vogalis 1989). Excitatory agonists (ACh, gastrin, etc.) increased the amplitude and durations of slow waves, but failed to elicit action potentials (Morgan & Szurszewski 1980; Ozaki et al. 1991). In fact, gastric slow waves exceed the threshold for activation of voltage-dependent Ca2+ channels, and Ca2+ entry persists during the plateau phase and the build-up of cytoplasmic Ca2+ ([Ca2+]i) correlates with contractions (Ozaki et al. 1991). Our results support these concepts; human gastric slow waves couple to contractions in the absence of action potentials and contractions were sensitive to blocking Ca2+ entry.
Our results show that authentic slow waves occur at a higher frequency than commonly acknowledged. Most gastroenterologists would currently agree that the normal dominant gastric pacemaker frequency is about 3 cpm in human stomach (Hamilton et al. 1986; Lin et al. 2000; Hocke et al. 2009; Sha et al. 2009; O'Grady et al. 2010). Conditions above and below this frequency are referred to as tachygastria and bradygastria, respectively. Such gastric dysrhythmias are thought to be counterproductive to gastric emptying and may contribute to symptoms of gastroparesis and/or dyspepsia (Leahy et al. 1999). Slow wave frequency in humans is typically measured by a variety of extracellular recording techniques – fixed metal electrodes, surface array electrodes and EGG – but signals from these techniques are highly correlated with gastric movement (Hocke et al. 2009; Bayguinov et al. 2011), and it is difficult to disassociate movement from electrical activity in living animals or patients. Thus, it is uncertain whether electrical signals detected by extracellular recording techniques are due to slow waves or to movement. Gastric peristaltic frequency was first established at about 3 cpm in human patients by manometry techniques (Hightower et al. 1949), but recent studies suggest that it is the contractions at 3 cpm that cause biopotentials rather than the 3 cpm biopotentials causing contractions (Bayguinov et al. 2011). Our data suggest that biopotentials recorded by extracellular techniques occur at a frequency much below that of authentic slow waves.
A previous study using sucrose gap and intracellular recording measured slow waves at 2.88 cpm in human corpus muscles (Hara & Ito, 1979). However, as we demonstrated, slow wave frequency is quite temperature sensitive (Q10 = 4.1 in present study). Slow waves were measured at 33°C in the previous study (Hara & Ito, 1979), so it is likely that frequency would have been enhanced significantly at physiological temperature. Another study, conducted at 37°C, reported considerably higher gastric slow wave frequency in corpus muscles (el-Sharkawy et al. 1978). However, recordings were made from only three corpus muscles in that study, and the authors did not raise questions about the frequency differences in extracellular and intracellular recordings. From a more extensive study of human gastric muscles, we showed that pacemaker frequency is consistently much higher than the 3 cpm slow wave rhythm considered normal in human stomach.
We could not reduce slow wave frequency to 3 cpm by blocking neural, paracrine and mechanical mechanisms known to have chronotropic effects in the stomach. It might be suggested that slow waves may have been modified by paraneoplastic effects, such as pacemaker remodelling, but our results are consistent with slow wave frequencies in patients undergoing surgery for reasons unrelated to gastric carcinomas (el-Sharkawy et al. 1978). Stretch can also affect gastric slow wave frequency (Won et al. 2005); however, muscles were stretched minimally beyond the resting length in this study. Stretch beyond the resting level had positive chronotropic effects. Dissection of muscles might activate or inhibit neural regulation or induce production of autocoids. For example, prostaglandins and cholinergic nerve stimulation have positive chronotropic effects in gastric muscles (el-Sharkawy et al. 1978; Sanders, 1984; Forrest et al. 2006, 2009). Treating muscles with TTX, atropine or indomethacin, however, did not reduce slow wave frequency close to 3 cpm. Finally, tonically active regulatory neural or hormonal inputs might be lost when in vitro muscle preparations are utilized. It is possible, for example, that tonic vagal activation of enteric inhibitory neurons might suppress spontaneous slow wave frequency in vivo, but previous studies on human patients showed no effect of vagotomy on slow wave frequency (Hinder & Kelly, 1977). There are additional factors, such as circulating hormones, that might influence gastric slow wave frequency, and it is admittedly difficult to simulate conditions in vivo. Common postprandial hormones, however, have either positive chronotropic effects (e.g. gastrin or cholecystokinin; Ohkawa & Watanabe, 1977; Morgan et al., 1978; el-Sharkawy et al., 1978) or no effect on slow wave frequency (e.g. secretin; Ohkawa & Watanabe, 1977). Thus, the slow wave frequencies observed in this study were unlikely to be abnormally high due to depletion of one of these hormones.
Fundus muscles of laboratory animals do not display slow waves (Morgan et al. 1981; Burns et al. 1996), and extracellular recording would appear to corroborate this conclusion (Kelly et al. 1969; Kelly & Code, 1971; Hinder & Kelly, 1977). Recent studies using extracellular recording techniques measured propagating electrical activity in the corpus and antrum of human patients, but failed to measure slow waves in the fundus (O'Grady et al. 2010). Our data show that spontaneous slow waves are a prominent feature of human gastric fundus muscles and phasic contractions were coupled to slow waves. Summation of phasic contractions driven by slow waves (e.g. like a partial tetanus) appears to be a source of mechanical tone in human stomach (Min et al. 2010). It is important to question why slow waves can be recorded by extracellular techniques from antrum and corpus but not from fundus (O'Grady et al., 2010). It is possible that fundus slow waves are more difficult to resolve because of their slower upstroke velocities compared to corpus or antrum (Table 1). However, we have shown that biopotentials, recorded from GI muscles with extracellular electrodes, are largely movement artifacts (Bayguinov et al. 2011). Thus, it is also possible that biopotentials cannot be resolved in fundus muscles because in this region phasic contractions summate to tonic contraction, but biopotentials are resolved in antrum and corpus where large amplitude phasic contractions cause rhythmic distortions of the tunica muscularis. The presence of slow waves in fundus suggests that novel mechanisms may be responsible for receptive relaxation and storage of food (e.g. suppression of electrical rhythmicity) and emptying the proximal stomach (e.g. enhancement in phasic contractions and/or propagation of contractions) will need additional investigation.
A major characteristic of the stomach deduced from animal studies is a proximal-to-distal slow wave frequency gradient in which faster proximal pacemakers entrain distal pacemakers. This is thought to be the basis for the directionality of slow wave propagation and spread of gastric peristaltic contractions driving the antral pump (Carlson et al. 1966; Kelly et al. 1969; Hinder & Kelly, 1977; Szurszewski, 1987). We could not identify an intrinsic slow wave frequency gradient in muscles isolated from human stomach. In fact frequently antral muscles displayed higher intrinsic frequencies (range 2–15 cpm) than corpus muscles (range 4–11 cpm). Many of the antral muscles might have been classified as having ‘tachygastria’ (Telander et al. 1978; Sha et al. 2009), but blinded patient records revealed no abnormal symptoms of gastric emptying disorders or gastroparesis. It appears that intrinsic antral pacemakers function at higher intrinsic frequencies than currently considered acceptable, and this observation raises questions about the clinical definitions of gastric dysrhythmias. Our data do not provide an obvious explanation for proximal-to-distal spread of peristaltic contractions in the human stomach. This may result from juxtaposition of additional regulatory mechanisms upon the intrinsic pacemaker frequency in the distal stomach.
In summary this study reveals several features of human gastric electrophysiology that contrast with the ‘standard model’ used by most investigators and clinicians. Intrinsic gastric pacemakers occur at nearly double the frequency considered normal for human gastric slow waves. Fundus muscles generate slow waves, and are not electrically quiescent. Finally, we found no evidence for a proximal-to-distal frequency gradient, which is considered fundamental to the directionality of peristaltic contractions in the stomach (el-Sharkawy et al. 1978; Forrest et al. 2009). Our data suggest that many current concepts of gastric electrophysiology and motility, deduced from experiments on laboratory animals, need reconsideration.
Acknowledgments
This project was supported by Samsung Medical Center, NIH DK57236 to S.M.W. and DK40569 to K.M.S. Morphological studies were performed in a Core laboratory supported by NIH P01 DK41315. The authors are extremely grateful to the excellent technical support provided by Yulia Bayguinov and Catrina J. Mullan.
Glossary
Abbreviations
- EGG
electrogastrography
- ICC
interstitial cells of Cajal
- RMP
resting membrane potential
Author contributions
P.-L.R., J.Y.L., H.J.S., J.J.K., J.C.R., S.D.K., S.J.H., K.M.S. and S.M.W. designed and performed experiments. S.K. performed surgery and provided tissues. P.-L.R., K.M.S. and S.M.W. drafted and critically revised the manuscript. The work was carried out at Samsung Biomedical Research Institute and the Department of Physiology and Cell Biology, University of Nevada School of Medicine, Reno, NV. The authors have no conflicts of interest for this study.
Supplementary material
Supplementary Fig. 1
Supplementary Fig. 2
Supplementary Fig. 3
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Bayguinov O, Hennig GW, Sanders KM. Movement based artifacts in extracellular electrical recording from GI muscles. Neurogastroenterol Motil. 2011 doi: 10.1111/j.1365-2982.2011.01784.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci U S A. 1996;93:12008–12013. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson HC, Code CF, Nelson RA. Motor action of the canine gastroduodenal function: a cineradiographic, pressure and electric study. Am J Dig Dis. 1966;11:155–172. doi: 10.1007/BF02239239. [DOI] [PubMed] [Google Scholar]
- Chen JDZ, Lin Z, Pan J, McCallum RW. Abnormal gastric myoelectrical activity and delayed gastric emptying in patients with symptoms suggestive of gastroparesis. Dig Dis Sci. 1996;41:1538–1545. doi: 10.1007/BF02087897. [DOI] [PubMed] [Google Scholar]
- Cousins HM, Edwards FR, Hickey H, Hill CE, Hirst GD. Electrical coupling between the myenteric interstitial cells of Cajal and adjacent muscle layers in the guinea-pig gastric antrum. J Physiol. 2003;550:829–844. doi: 10.1113/jphysiol.2003.042176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens EJ, Hirst GD, Tomita T. Identification of rhythmically active cells in guinea-pig stomach. J Physiol. 1999;514:515–531. doi: 10.1111/j.1469-7793.1999.515ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Sharkawy TY, Morgan KG, Szurszewski JH. Intracellular electrical activity of canine and human gastric smooth muscle. J Physiol. 1978;279:291–307. doi: 10.1113/jphysiol.1978.sp012345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Sharkawy TY, Szurszewski JH. Modulation of canine antral circular smooth muscle by acetylcholine, noradrenaline and pentagastrin. J Physiol. 1978;279:309–320. doi: 10.1113/jphysiol.1978.sp012346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest AS, Ordög T, Sanders KM. Neural regulation of slow-wave frequency in the murine gastric antrum. Am J Physiol Gastrointest Liver Physiol. 2006;290:G486–G495. doi: 10.1152/ajpgi.00349.2005. [DOI] [PubMed] [Google Scholar]
- Forrest AS, Hennig GW, Jokela-Willis S, Park CD, Sanders KM. Prostaglandin regulation of gastric slow waves and peristalsis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1180–G1190. doi: 10.1152/ajpgi.90724.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, Lorincz A, Pozo MJ, Pasricha PJ, Van de Rijn M, West RB, Sarr MG, Kendrick ML, Cima RR, Dozois EJ, Larson DW, Ordog T, Farrugia G. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1370–G1381. doi: 10.1152/ajpgi.00074.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JW, Bellahsene BE, Reichelderfer M, Webster JG, Bass P. Human electrogastrograms. Comparison of surface and mucosal recordings. Dig Dis Sci. 1986;31:33–39. doi: 10.1007/BF01347907. [DOI] [PubMed] [Google Scholar]
- Hara Y, Ito Y. The electrical activity recorded from smooth muscle of the circular layer of the human stomach. Pflugers Arch. 1979;382:145–153. doi: 10.1007/BF00584216. [DOI] [PubMed] [Google Scholar]
- Hightower J, Code CF, Maher TM. A method for the study of gastro-intestinal motor activity in human beings. Mayo Clin Proc. 1949;24:453–462. [PubMed] [Google Scholar]
- Hinder RA, Kelly KA. Human gastric pacesetter potential. Site of origin, spread, and response to gastric transection and proximal gastric vagotomy. Am J Surgery. 1977;133:29–33. doi: 10.1016/0002-9610(77)90187-8. [DOI] [PubMed] [Google Scholar]
- Hocke M, Schöne U, Richert H, Hocke M, Gömert P, Keller J, Layer P, Stallmach A. Every slow-wave impulse is associated with motor activity of the human stomach. Am J Physiol Gastrointest Liver Physiol. 2009;296:G709–G716. doi: 10.1152/ajpgi.90318.2008. [DOI] [PubMed] [Google Scholar]
- Horiguchi K, Semple GS, Sanders KM, Ward SM. Distribution of pacemaker function through the tunica muscularis of the canine gastric antrum. J Physiol. 2001;537:237–250. doi: 10.1111/j.1469-7793.2001.0237k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SJ, Blair PJ, Britton FC, O'Driscoll KE, Hennig G, Bayguinov YR, Rock JR, Harfe BD, Sanders KM, Ward SM. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol. 2009;587:4887–4904. doi: 10.1113/jphysiol.2009.176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibba Manneschi L, Pacini S, Corsani L, Bechi P, Faussone-Pellegrini MS. Interstitial cells of Cajal in the human stomach: distribution and relationship with enteric innervation. Histol Histopathol. 2004;19:1153–1164. doi: 10.14670/HH-19.1153. [DOI] [PubMed] [Google Scholar]
- Jebbink HJ, Bruijs PP, Bravenboer B, Akkermans LM, vanBerge-Henegouwen GP, Smout AJ. Gastric myoelectrical activity in patients with type I diabetes mellitus and autonomic neuropathy. Dig Dis Sci. 1994;39:2376–2383. doi: 10.1007/BF02087654. [DOI] [PubMed] [Google Scholar]
- Kelly KA, Code CF. Effect of transthoracic vagotomy on canine gastric electrical activity. Gastroenterology. 1969;57:51–58. [PubMed] [Google Scholar]
- Kelly KA, Code CF. Canine gastric pacemaker. Am J Physiol. 1971;220:112–118. doi: 10.1152/ajplegacy.1971.220.1.112. [DOI] [PubMed] [Google Scholar]
- Kelly KA, Code CF, Elveback LR. Patterns of canine gastric electrical activity. Am J Physiol. 1969;217:461–470. doi: 10.1152/ajplegacy.1969.217.2.461. [DOI] [PubMed] [Google Scholar]
- Kelly KA, Woodward ER, Code CF. Effect of secretin and cholecystokinin on canine gastric electrical activity. Proc Soc Exp Biol Med. 1969;130:1060–1063. doi: 10.3181/00379727-130-33720. [DOI] [PubMed] [Google Scholar]
- Kito Y, Suzuki H. Electrophysiological properties of gastric pacemaker potentials. J Smooth Muscle Res. 2003;39:163–173. doi: 10.1540/jsmr.39.163. [DOI] [PubMed] [Google Scholar]
- Koch KL. Electrogastrography: physiological basis and clinical application in diabetic gastropathy. Diabetes Technol Ther. 2001;3:51–62. doi: 10.1089/152091501750220019. [DOI] [PubMed] [Google Scholar]
- Lammers WJ, Ver Donck L, Stephen B, Smets D, Schuurkes JA. Origin and propagation of the slow wave in the canine stomach: the outlines of a gastric conduction system. Am J Physiol Gastrointest Liver Physiol. 2009;296:1200–1210. doi: 10.1152/ajpgi.90581.2008. [DOI] [PubMed] [Google Scholar]
- Leahy A, Beshredas K, Clayman C, Mason I, Epstein O. Abnormalities of the electrogastrogram in functional gastrointestinal disorders. Am J Gastroenterol. 1999;94:1023–1028. doi: 10.1111/j.1572-0241.1999.01007.x. [DOI] [PubMed] [Google Scholar]
- Lee HT, Hennig GW, Fleming NW, Keef KD, Spencer NJ, Ward SM, Sanders KM, Smith TK. Septal interstitial cells of Cajal conduct pacemaker activity to excite muscle bundles in human jejunum. Gastroenterology. 2007;133:907–917. doi: 10.1053/j.gastro.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung MW, Wong BP, Chao NS, Chung KW, Kwok WK, Liu KK. Electrogastrography in the management of pediatric functional dyspepsia and motility disorder. J Pediatr Surg. 2006;41:2069–2072. doi: 10.1016/j.jpedsurg.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Lin Z, Chen JD, Schirmer BD, McCallum RW. Postprandial response of gastric slow waves: correlation of serosal recordings with the electrogastrogram. Dig Dis Sci. 2000;45:645–651. doi: 10.1023/a:1005434020310. [DOI] [PubMed] [Google Scholar]
- Lin X, Chen JZ. Abnormal gastric slow waves in patients with functional dyspepsia assessed by multichannel electrogastrography. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1370–G1375. doi: 10.1152/ajpgi.2001.280.6.G1370. [DOI] [PubMed] [Google Scholar]
- Min B-H, Sinn DH, Ko E-J, Lee JY, Kim JJ, Rhee JC, Kim S, Ward SM, Rhee PL. Regional differences of the effects of acetylcholine in the human gastric circular muscle. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1198–G1203. doi: 10.1152/ajpgi.00523.2009. [DOI] [PubMed] [Google Scholar]
- Morgan KG, Schmalz PF, Go VL, Szurszewski JH. Electrical and mechanical effects of molecular variants of CCK on antral smooth muscle. Am J Physiol Endocrinol Metab. 1978;235:E324–E329. doi: 10.1152/ajpendo.1978.235.3.E324. [DOI] [PubMed] [Google Scholar]
- Morgan KG, Muir TC, Szurszewski JH. The electrical basis for contraction and relaxation in canine fundal smooth muscle. J Physiol. 1981;311:475–88. doi: 10.1113/jphysiol.1981.sp013599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan KG, Szurszewski JH. Mechanisms of phasic and tonic actions of pentagastrin on canine gastric smooth muscle. J Physiol. 1980;301:229–242. doi: 10.1113/jphysiol.1980.sp013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Grady G, Du P, Cheng LK, Lammers WJ, Windsor JA, Pullan AJ. Origin and propagation of human gastric slow-wave activity defined by high-resolution mapping. Am J Physiol Gastrointest Liver Physiol. 2010;299:G585–G592. doi: 10.1152/ajpgi.00125.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H, Watanabe M. Effects of gastrointestinal hormones on the electrical and mechanical activity of the cat stomach. Tohoku J Exp Med. 1977;122:287–298. doi: 10.1620/tjem.122.287. [DOI] [PubMed] [Google Scholar]
- Ordög T, Ward SM, Sanders KM. Interstitial cells of Cajal generate electrical slow waves in the murine stomach. J Physiol. 1999;518:257–269. doi: 10.1111/j.1469-7793.1999.0257r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordög T, Takayama I, Cheung WK, Ward SM, Sanders KM. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49:1731–1739. doi: 10.2337/diabetes.49.10.1731. [DOI] [PubMed] [Google Scholar]
- Ozaki H, Stevens RJ, Blondfield DP, Publicover N, Sanders KM. Simultaneous measurement of membrane potential, cytosolic Ca2+, and tension in intact smooth muscles. Am J Physiol Cell Physiol. 1991;260:C917–C925. doi: 10.1152/ajpcell.1991.260.5.C917. [DOI] [PubMed] [Google Scholar]
- Parkman HP, Miller MA, Trate D, Knight LC, Urbain JL, Maurer AH, Fisher RS. Electrogastrography and gastric emptying scintigraphy are complementary for assessment of dyspepsia. J Clin Gastroenterol. 1997;24:214–219. doi: 10.1097/00004836-199706000-00006. [DOI] [PubMed] [Google Scholar]
- Sanders KM. Role of prostaglandins in regulating gastric motility. Am J Physiol Gastrointest Liver Physiol. 1984;247:G117–G126. doi: 10.1152/ajpgi.1984.247.2.G117. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Vogalis F. Organization of electrical activity in the canine pyloric canal. J Physiol. 1989;416:49–66. doi: 10.1113/jphysiol.1989.sp017748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger GJ. Effects of metoclopramide and domperidone on cholinergically mediated contractions of human isolated stomach muscle. J Pharm Pharmacol. 1985;37:661–664. doi: 10.1111/j.2042-7158.1985.tb05108.x. [DOI] [PubMed] [Google Scholar]
- Sha W, Pasricha PJ, Chen JD. Rhythmic and spatial abnormalities of gastric slow waves in patients with functional dyspepsia. J Clin Gastroenterol. 2009;43:123–129. doi: 10.1097/MCG.0b013e318157187a. [DOI] [PubMed] [Google Scholar]
- Smout AJPM, Jebbink HJ, Akkermans LMA, Bruijs PPM. Role of electrogastrography and gastric impedance measurements in evaluating gastric emptying and motility. Dig Dis Sci. 1994;39:110S–113S. doi: 10.1007/BF02300387. [DOI] [PubMed] [Google Scholar]
- Szurszewski JH. Electrical basis for gastrointestinal motility. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1987. pp. 393–422. [Google Scholar]
- Telander RL, Morgan KG, Kreulen DL, Schmalz PF, Kelly KA, Szurszewski JH. Human gastric atony with tachygastria and gastric retention. Gastroenterology. 1978;75:497–501. [PubMed] [Google Scholar]
- Weber J, Koatsu S. Pacemaker localization and electrical conduction patterns in the canine stomach. Gastroenterology. 1970;59:717–726. [PubMed] [Google Scholar]
- Ward SM, Sanders KM. Dependence of electrical slow waves of canine colonic smooth muscle on calcium gradient. J Physiol. 1992;455:307–319. doi: 10.1113/jphysiol.1992.sp019303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won KJ, Sanders KM, Ward SM. Interstitial cells of Cajal mediate mechanosensitive responses in the stomach. Proc Natl Acad Sci U S A. 2005;102:14913–14918. doi: 10.1073/pnas.0503628102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun HY, Sung R, Kim YC, Choi W, Kim HS, Kim H, Lee GJ, You RY, Park SM, Yun SJ, Kim MJ, Kim WS, Song YJ, Xu WX, Lee SJ. Regional distribution of interstitial cells of Cajal (ICC) in human stomach. Korean J Physiol Pharmacol. 2010;14:317–324. doi: 10.4196/kjpp.2010.14.5.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MH, Kim TW, Ro S, Yan W, Ward SM, Koh SD, Sanders KM. A Ca2+-activated Cl− conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol. 2009;587:4905–4918. doi: 10.1113/jphysiol.2009.176206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


