Abstract
Non-technical summary
Like most cells, those of the kidney release protein and RNA in structures called exosomes. It is possible that the contents of exosomes released into the urine from one part of the kidney can alter the function of downstream cells. We have used a cell model to test whether exosomes act as cell-to-cell messengers within the kidney. First, cells were exposed to a hormone that regulates the body's retention of water. This increased the levels of water channels within the cells and also within their exosomes. Next, these exosomes were placed onto a separate batch of cells, which responded by increasing their transport of water. This study shows that exosomes are a new mechanism for the transfer of physiological information within the kidney.
Abstract
Exosomes are vesicles released following fusion of endosomes with the plasma membrane. Urine contains exosomes that are released from the entire length of the nephron and change in composition with kidney disease. Exosomes can shuttle information between non-renal cells via transfer of protein and RNA. In this study murine kidney collecting duct (mCCDC11) cells were used to demonstrate that exosomes can act as a signalling mechanism between cells. First, the release of exosomes by mCCDC11 cells was confirmed by multiple approaches. Following isopynic centrifugation, exosomal proteins flotillin-1 and TSG101 were identified in fractions consistent with exosomes. Electron microscopy demonstrated structures consistent in size and shape with exosomes. Exposure of mCCDC11 cells to the synthetic vasopressin analogue, desmopressin, did not affect exosomal flotillin-1 or TSG101 but increased aquaporin 2 (AQP2) in a dose- and time-dependent manner that was highly correlated with cellular AQP2 (exosomal AQP2 vs. cellular AQP2, Pearson correlation coefficient r = 0.93). To test whether the ratio of exosomal AQP2/flotillin-1 is under physiological control in vivo, rats were treated with desmopressin. The ratio of AQP2/flotillin-1 in the urinary exosome was significantly increased. Inter-cellular signalling by exosomes was demonstrated: exosomes from desmopressin-treated cells stimulated both AQP2 expression and water transport in untreated mCCDc11 cells (water flow across cells: control exosome treatment 52.8 ± 11 μl cm-2; AQP2-containing exosomes 77.4 ± 4 μl cm-2, P = 0.05, n = 4). In summary, the amount of AQP2 in exosomes released from collecting duct cells is physiologically regulated and exosomal AQP2 closely reflects cellular expression. Exosomes can transfer functional AQP2 between cells and this represents a novel physiological mechanism for cell-to-cell communication within the kidney.
Introduction
Exosomes are membrane-bound vesicles that are formed as part of the intra-cellular endosomal pathway (Thery et al. 2002). During endosomal maturation, the limiting membrane invaginates to form intra-luminal vesicles. A subset of endosomes fuses with the plasma membrane, releasing their intra-luminal vesicles into the extracellular space and are termed exosomes. Exosomes have characteristic physicochemical properties that distinguish them from other cell-derived vesicles. They are 20–100 nm in size and appear cup-shaped when visualised by transmission electron microscopy (TEM) (Pisitkun et al. 2004), have a density of 1.10 to 1.19 g ml-1 (Keller et al. 2007; Graner et al. 2009) and contain characteristic proteins that are central to their production (Thery et al. 2009). Such proteins include flotillin-1, which is associated with lipid rafts that act as the location for exosomal formation (Thery et al. 2009), and tumor susceptibility gene 101 (TSG101), a component of the endosomal sorting complex required for transport (ESCRT) protein group that mediates exosome assembly (Stoorvogel et al. 2002).
Analysis of the human urinary exosomal proteome suggests that cells of the glomerulus and each region of the renal tubule release exosomes into the urine. For example, the presence of aquaporin 2 (AQP2) in human urinary exosomes reflects release from principal cells in the collecting duct (Pisitkun et al. 2004). The urinary exosome, therefore, represents a reservoir for kidney disease biomarker discovery (Gonzales et al. 2009) and also has the potential to inform the physiological status of specific cell types within the nephron. As well as proteins, urinary exosomes also contain RNA species that may represent another potential disease biomarker reservoir (Michael et al. 2010; Miranda et al. 2010). MicroRNAs (miRNAs) are short (18–25 nucleotides) non-coding RNA molecules that function to repress a set of specific target mRNAs and thereby regulate specific cellular proteins and physiology (Bartel, 2004). The endosomal pathway is a key intra-cellular site for miRNA action (Gibbings et al. 2009; Lee et al. 2009) and a feature of exosomes is the presence of multiple miRNA species within their cargo (Camussi et al. 2010; Michael et al. 2010; Mittelbrunn et al. 2011). In non-renal cells, exosomes have been demonstrated to ‘shuttle’ protein, messenger RNA (mRNA) and miRNA between cells. This can change the proteome, and therefore function, of the ‘recipient’ cell either directly by transfer of new protein, or indirectly via translation of exosomal mRNA or miRNA interference of multiple target proteins (Valadi et al. 2007; Sheldon et al. 2010; Mittelbrunn et al. 2011). With exosomes released into the urine along the entire renal tubule, the capacity to traffic downstream protein or RNA species and thereby influence cell physiology is a novel mechanism for signalling within the kidney. This is particularly relevant to the distal renal tubule, which would be exposed to exosomes from a range of kidney cell types.
In the present study we have focused on the collecting duct, which in the human releases exosomes (Pisitkun et al. 2004; Hogan et al. 2009; Rood et al. 2010). We have used a well-characterized murine cortical collecting duct (mCCDc11) cell line (Gaeggeler et al. 2005, 2011; Kortenoeven et al. 2009) to establish that exosomal protein content is specifically regulated by a physiologically relevant hormonal system, vasopressin. We have also confirmed these findings in vivo and demonstrated that exosomes can transfer physiological functions between cells.
Methods
Materials and cell culture
The mCCDC11 cell line was a kind gift from Hans-Peter Gaeggeler and Bernard Rossier (University of Lausanne, Lausanne, Switzerland; Gaeggeler et al. 2005). The cells were grown in Dulbecco's modified Eagles medium (DMEM)–F12 medium, 1:1 (Gibco, Paisley, UK), supplemented with 2% fetal calf serum (FCS; Invitrogen, Paisley, UK), 1× insulin transferrin selenium (ITS) solution (Gibco), 100 U ml-1 penicillin and 100 μg ml-1 streptomycin (Invitrogen), 50 pm dexamethasone (Sigma Aldrich, Gillingham, UK), 1 nm 3,3′,5-triiodo-l-thyronine sodium salt (Sigma Aldrich) and 10 ng ml-1 epidermal growth factor (Sigma Aldrich). Passaging was achieved by two 10 min washes with 1 mm EDTA in Dulbecco's modified phosphate-buffered saline (DPBS) followed by incubation in trypsin EDTA solution (Lonza, Basel, Switzerland).
The presence of exosomes in FCS would interfere with our study so they were depleted as follows. FCS was diluted to 20% with media and then ultracentrifuged overnight at 200,000 g. The supernatant was then centrifuged for a further hour at 200,000 g. The supernatant was again removed and filtered through a 0.22 μm cellulose acetate filter. Depletion of exosomes was confirmed by Western blotting for flotillin-1 and TSG101.
Rat urine study
All studies were performed with the appropriate Home Office (UK) license. Adult male Sprague–Dawley (n = 4) rats (∼250 g) were housed individually in metabolism cages. Urine volume was collected every 24 h and stored at –80°C. After control urine collections (days 1 and 2), desmopressin (1 μg kg-1; Sigma Aldrich) was administered sub-cutaneously at 10:00 am on both days 3 and 4.
Exosome isolation
Urine or culture media was vigorously vortexed then centrifuged at 15,000 g for 10 min to pellet any cells, large membrane fragments and other debris. The supernatant was then centrifuged at 200,000 g for 60 min to pellet the exosomal fraction. The pellet was washed with phosphate-buffered saline (PBS) and then re-centrifuged at 200,000 g for 60 min before final resuspension in PBS.
Western blot
Samples were solubilised with Laemmli sample buffer and separated on a 1-D SDS-PAGE gel (Thermo, Rockford, IL, USA) before transfer to a polyvinylidene fluoride (PVDF, Invitrogen) membrane. For studies of cell culture media, equal volumes of media were loaded in each gel lane. For studies of rat urine each lane represented 24 h of urine output. The membrane was probed with the following primary antibodies: mouse anti-flotillin-1 (BD Biosciences, Franklin Lakes, USA), mouse anti-TSG101 (Abcam, Cambridge, UK) and rabbit anti-AQP2 (Millipore, Billerica, MA, USA). After visualization of the secondary antibody's binding using enhanced chemiluminescence, the photographic films were scanned using the VersaDoc imaging system (Bio-Rad, Hercules, CA, USA) and relative band densities were measured using the Quantity One software (Bio-Rad).
Isopycnic centrifugation
Resuspended exosomes were diluted into the top fraction of a step gradient comprising layers of 2, 1.3, 1.16, 0.8, 0.5 and 0.25 m sucrose. The gradients were centrifuged for 2.5 h at 100,000 g. Six fractions were collected from the gradient and stored for density determination on an Anton Paar DMA 35N density meter and for Western blot analysis.
Electron microscopy
Resuspended exosomes were mixed 1:1 with 4% paraformaldehyde. A drop of this solution was placed on a Petri dish and then a Formvar-coated 200-mesh gold grid (Taab, Aldermaston, UK) floated on top for 20 min. All subsequent steps are performed in the same way. The grid was moved to PBS for two 5 min washes. Then the exosomes were re-fixed on the grid using a 1% glutaraldehyde solution for 5 min and again washed twice in PBS. Finally, the grid was transferred to a drop of 0.5% uranyl acetate–2% 25-centipoise methyl cellulose (Sigma Aldrich). Following staining for 5 min, excess fluid was removed, the grid allowed to air dry and was then examined on a Phillips CM120 BioTwin TEM.
mCCDC11 cell stimulation
Flasks of confluent cells were washed in FCS-free medium and then exosome-depleted medium added. Desmopressin (Sigma Aldrich) was diluted in serum-free medium and then added to the flasks at 316 pg ml-1, 1 ng ml-1 or 3.16 ng ml-1. The medium was collected at 48 h and then replaced. Both the cells and the medium were collected at 96 h. Cells were collected by scraping them into ice-cold RIPA solution (50 mm Tris, 150 mm NaCl, 2 mm EDTA, 1% Triton X-100, 0.1% SDS; pH 7.4) after first washing the cells in PBS. The cell suspension was then centrifuged for 5 min at 15,000 g and the supernatant stored at –80°C prior to analysis. Annexin-V-FLUOS staining kit (Roche, Burgess Hill, UK) was used for as per manufacturer's instructions for quantification of cellular apoptosis and necrosis on a FACS Calibur (BD Biosciences, Oxford, UK).
mCCDC11 cell uptake of exosomes
Cells were stimulated with 3.16 ng ml-1 desmopressin or left unstimulated with the medium changed at 48 h and then collected at 96 h. The exosomes were isolated and the protein concentration measured using the BCA assay (Roche). A different batch of cells was passaged and then grown to confluency in a 12-well plate. The purified exosomes were added to a well at a concentration of 50 μg protein ml-1. Stimulation with 3.16 ng ml-1 desmopressin was used as a control. After incubation for 48 h, the cells were washed with PBS and then scraped into ice-cold RIPA solution. The presence of AQP2 protein was determined by Western blot.
Water flow studies
Water flow was determined by gravimetry (Gaeggeler et al. 2011). Collagen-coated transwells (PTFE membranes with a 0.4 μm pore size on a 6-well insert; Corning, New York, USA) were soaked overnight in complete medium and then seeded with mCCDC11 cells. Inserts were maintained in complete medium for 5 days during which a confluent monolayer formed. The medium was then replaced with filter cup medium (DMEM:F12, 1:1, supplemented with 100 U ml-1 penicillin, 100 μg ml-1 streptomycin and 3 nm dexamethasone) and maintained for a further 5 days. During this 10 day period the medium was refreshed every second day. Steroid-free medium (DMEM:F12, 1:1, supplemented with 100 U ml-1 penicillin and 100 μg ml-1 streptomycin) was then added to the wells with exosomes at a concentration of 50 μg protein ml-1. There was an osmotic gradient across the monolayer of cells; the apical compartment's medium was diluted 1:1 with dH2O. The basolateral compartment's medium was undiluted. Following a 48 h incubation, the apical and basolateral medium was collected into pre-weighed tubes before being weighed to calculate the water flow across the membrane.
Statistical analysis
Results are presented as mean ± SEM. Groups are compared by Student's t test. A p < 0.05 was considered significant. For correlation, Pearson correlation coefficient was calculated.
Results
mCCDc11 cells release exosomes
Ultracentrifugation of medium from the mCCDc11 cells formed a pellet consistent with cellular release of low-density membrane vesicles. To establish the presence of exosomes within the pellet, the presence of two markers was investigated. Flotillin-1 and TSG101 were both present in the ultracentrifuge pellet but not the supernatant (Fig. 1A). The density of the particles containing flotillin-1 and TSG101 was determined using isopycnic centrifugation on a sucrose step gradient. The exosomal markers flotillin-1 and TSG101, together with AQP2, were localised to a density of 1.10–1.15 g ml-1 (Fig. 1B), consistent with localization to the exosomes. This distribution was consistently observed over five replicates for TSG101 and flotillin-1 and three replicates for AQP2 (data not shown). The ultracentrifugation pellet was directly visualised using TEM, and structures of the characteristic size and shape for exosomes were identified (Fig. 1C).
Figure 1. mCCDC11 cells release exosomes.
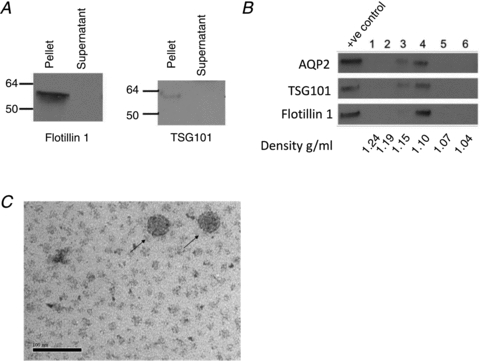
A, in the mCCDC11 cell culture medium, the exosomal marker proteins flotillin-1 and TSG101 localise to the ultracentrifugation pellet (pellet) rather than the supernatant. B, Western blot for aquaporin 2 (AQP2), flotillin-1 and TSG101 on fractions obtained following isopycnic centrifugation. The exosomal markers are present in fractions corresponding to a density of 1.10–1.15 g ml-1. The positive control was unfractioned exosomes. C, structures of exosome size and shape are visible in the cell culture medium using transmission electron microscopy. Arrows indicate 2 exosomes. Bar, 100 nm.
Exosomal AQP2 is highly correlated with cellular AQP2
Although in previous studies mCCDc11 cells had been stimulated with desmopressin at a concentration of 1 ng ml-1 without detrimental effect (Kortenoeven et al. 2009), the presence of dying cells, which release non-exosomal particles, would confound our study. We found that stimulation with 3.16 ng ml-1 desmopressin for 96 h did not cause cell necrosis as measured using flow cytometry (% necrotic cells: control 0.9 ± 0.1; desmopressin 0.7 ± 0.1, n = 3). There was an increase in cell apoptosis following exposure to desmopressin (3.16 ng ml-1 for 96 h) but apoptotic cells were still less than 1% of total (% apoptotic cells: control 0.4 ± 0.1; desmopressin 0.9 ± 0.1, n = 3, P = 0.02). In light of this small increase in apoptosis, 3.16 ng ml-1 was the highest concentration of desmopressin used in further studies.
The mCCDC11 cells were stimulated with desmopressin for 96 h and the medium collected (and replaced) at 48 h. Stimulation of the cells with desmopressin did not change expression of flotillin-1 and TSG101 (Fig. 2A). AQP2 was not detected in the exosomes released by unstimulated cells or in exosomes released by cells stimulated with desmopressin for 48 h. This is consistent with the cell biology, as we could not detect AQP2 in whole-cell extracts after stimulation for 48 h (data not shown). After 96 h of desmopressin stimulation, AQP2 was detected in both the cells and the exosomes released from those cells (Fig. 2B). The exosomal increase was dose dependent and highly correlated with the abundance of AQP2 in whole-cell extracts (Fig. 2C). To determine whether this relative change in exosomal protein composition occurs in vivo, rats were treated with desmopressin, after which AQP2 and flotillin-1 were measured in the urinary exosomes. The ratio of AQP2:flotillin-1 was increased following desmopressin treatment (Fig. 2D).
Figure 2. Stimulation increases exosomal AQP2.

A, Western blot for aquaporin 2 (AQP2), TSG101and flotillin-1 in desmopressin-stimulated cells and their exosomes. Cells were stimulated for 96 h with desmopressin (316 pg ml-1, 1 ng ml-1 or 3.16 ng ml-1). Control was no desmopressin. AQP2 in exosomes was increased by desmopressin cell stimulation for 96 h but not 48 h. Cell and exosomal TSG101 and flotillin-1 was unchanged. AQP2 was also upregulated in the cell extracts at 96 h. 1–3 are replicates. B, treatment of mCCDC11cells with desmopressin increased the cell and exosome AQP2 content. AQP2 was upregulated in the exosomes and cells after 96 h of desmopressin stimulation. *p < 0.05, **P < 0.001. n = 4. AQP2 was quantified by Western blot and band densitometry. C, the AQP2 content of exosomes was closely correlated with cellular AQP2. AQP2 was quantified by Western blot and band densitometry. Each point represents a separate experiment. D, the ratio of AQP2:flotillin-1 in rat urinary exosomes was increased by subcutaneous injection of desmopressin (1 μg kg-1) on day 3 and day 4. *P = 0.05, n = 3.
Exosomes transfer functional AQP2 between cells
Exosomes have been reported to transfer their cargo between cells in vitro (Valadi et al. 2007; Sheldon et al. 2010). To test this capability, mCCDC11 cells were co-cultured with exosomes taken from separate batches of cells that had been exposed to either desmopressin or vehicle. AQP2 was only observed in those cells (and exosomes) exposed to desmopressin (3.16 ng ml-1) for 96 h. Following 48 h of co-culture, cells exposed to exosomes derived from desmopressin-stimulated cells expressed AQP2 (Fig. 3A). Cells exposed to exosomes from unstimulated cells did not express AQP2. To determine whether induced AQP2 expression was functional, water flow across the cells was measured as previously described (Gaeggeler et al. 2011). The water flow was significantly greater following 48 h of co-culture with exosomes derived from cells that had been stimulated with desmopressin (3.16 ng ml-1) for 96 h (AQP2-expressing exosomes) compared to exosomes from cells that were exposed to desmopressin immediately prior to exosome isolation (exosomes that did not express AQP2) (Fig. 3B, Table 1). The addition of desmopressin immediately prior to exosome isolation provided a control for any desmopressin contamination of the exosomal preparation: both groups had the same desmopressin concentration in the culture medium at the time of ultracentrifugation.
Figure 3. mCCDC11 cells exposed to AQP2-containing exosomes express functional AQP2.
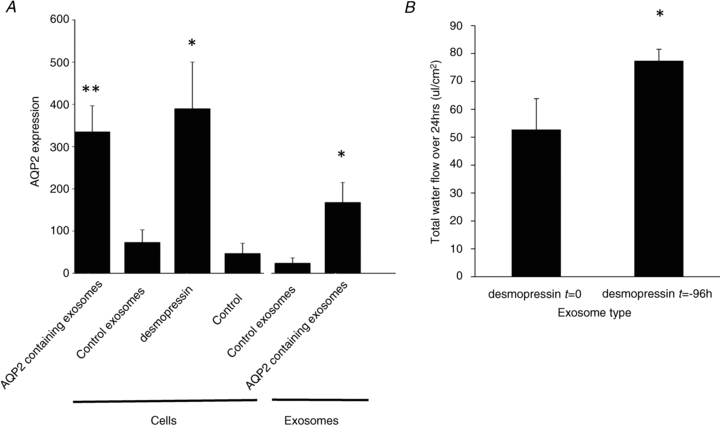
A, AQP2 protein expression was measured by Western blot and band densitometry. Cellular AQP2 expression (‘cells’) was measured after 48 h incubation with AQP2-containing exosomes (derived from mCCDC11cells treated with desmopressin (3.16 ng ml-1) for 96 h); control exosomes derived from unstimulated cells or direct treatment with desmopressin (3.16 ng ml-1). The concentration of AQP2 in the exosomes is also presented (‘exosomes’). **p < 0.02 vs. control. *p < 0.05 vs. control. n = 4. B, the water flow across mCCDC11monolayers after 48 h co-culture with exosomes from mCCDC11cells exposed to desmopressin (3.16 ng ml-1) immediately before exosome isolation (desmopressin t = 0) or 96 h before exosome isolation (desmopressin t = –96 h). Water flow was determined by weighing the medium in the apical and basolateral compartments. Water flow is expressed as μl per area of monolayer. *P = 0.05 by paired analysis. n = 4.
Table 1.
Water flow rate across mCCDC11 monolayers after co-culture with exosomes from cells exposed to desmopressin (3.16 ng ml-1) immediately before exosome isolation (t = 0) or 96 h before exosome isolation (t = −96 h)
| Experiment number | Water flow rate (μl cm−2 h−1) exosomes – desmopressin t = 0 | Water flow rate (μl cm−2 h−1) exosomes – desmopressin t = −96 h |
|---|---|---|
| 1 | 2.1 | 3.3 |
| 2 | 0.9 | 2.8 |
| 3 | 2.8 | 3.6 |
| 4 | 2.9 | 3.2 |
Water flow is expressed as microlitres per area of monolayer per hour.
Discussion
Urinary exosomes contain protein and RNA from a range of kidney cells and represent a novel reservoir for biomarker discovery (Pisitkun et al. 2004; Miranda et al. 2010). Furthermore, exosomes from non-kidney cells have been demonstrated to transfer information between cells (Valadi et al. 2007). In this study we have developed a model of exosome release from a collecting duct cell line and demonstrated that up-regulation of AQP2 in the cell is accurately reflected in the exosome. While AQP2 is up-regulated, other exosomal proteins remain constant (both in vitro and in vivo) which presents an opportunity to quantify a change in the exosome as a protein ratio. It further suggests that the content of each exosome, rather than total exosomal abundance, is physiologically regulated by vasopressin. The present study is the first to demonstrate that exosomes can transfer functional AQP2 between cells.
The mCCDC11 cell line released exosomes, as do a wide range of cell types in vitro (Thery et al. 2002) and our data are consistent with in vivo data demonstrating AQP2-containing exosomes in human urine (Pisitkun et al. 2004). The evidence for exosome release comes from a number of complementary observations: (1) proteins widely documented to be present within exosomes are enriched in the cell culture medium ultracentrifugation pellet in comparison with the supernatant; (2) following separation of the ultracentrifugation pellet based on density, the markers localise to a density range that is consistent with these proteins being contained in exosomes; (3) direct visualisation of the ultracentrifugation pellet revealed the presence of structures matching exosomes in size and shape. Together, these complementary strands of evidence confirm the release of exosomes from cultured mCCDC11 cells.
One of the exciting possibilities for exosome research is that their cargo might change to reflect alterations in cell physiology and the onset of disease. However, at the RNA level, exosomal mRNA is different from cellular mRNA with regard to the relative abundance of different species and, therefore, changes in exosomal mRNA may not reflect cellular changes (Valadi et al. 2007). Moreover, the inclusion of proteins into intra-cellular endosomes and ultimately exosomes, is a complex process and may prevent changes in the cellular proteome being faithfully reflected in the exosome (Hanson et al. 2009). To the best of our knowledge there are no published studies that explore the relationship between a protein change in a kidney cell and the exosome derived from that cell population but there are studies that demonstrate differences in urinary exosome proteins with kidney disease. Fetuin-A is up-regulated in cisplatin-induced acute kidney injury (AKI) (Zhou et al. 2006a), transcription factors increase in abundance following AKI and podocyte injury (Zhou et al. 2008), whereas aquaporin 1 is decreased following ischaemia–reperfusion kidney injury (Sonoda et al. 2009) and AQP2 is decreased in patients with American cutaneous leishmaniasis (Oliveira et al. 2011). As the mCCDC11 cell line up-regulates AQP2 in response to stimulation and AQP2 is an archetypal exosomal protein (Pisitkun et al. 2004; Gaeggeler et al. 2011), we measured cellular and exosomal AQP2 before and after stimulation with the synthetic vasopressin analogue desmopressin. Our data demonstrate that cellular and exosomal AQP2 were highly correlated, with the exosomal AQP2 being a faithful reflection of cellular concentration. This validates the use of the urinary exosome as a reservoir for protein biomarker discovery, but given the complex process of protein recruitment into the exosome, our data should be extrapolated with caution. Studies are needed that compare multiple exosomal and cellular proteins to clearly define how the exosomal proteome relates to changes in the kidney proteome.
While exosomal AQP2 concentration was increased by cellular stimulation with desmopressin, flotillin-1 and TSG101 did not change. This is consistent with the number of exosomes released remaining constant before and after stimulation (but with an increase in their AQP2 ‘cargo’). An alternative explanation is that desmopressin stimulation releases AQP2-containing exosomes that do not express flotillin-1 or TSG101 but have the same density on isopycnic centrifugation. This second explanation is unlikely as flotillin-1 and TSG101 are central to exosome assembly. In rats, treatment with desmopressin increased the ratio of AQP2:flotillin-1 in urinary exosomes, which is consistent with our in vitro data. When quantifying an exosomal protein, there are limitations in the current approaches used to adjust for the wide variability in urine concentration. The mass of urinary protein excreted over time requires a timed urine collection, which is often not available in the clinical setting, and does not allow for technical variability in exosome recovery from different samples. The use of urinary creatinine overcomes the need for a timed urine collection but is often not valid in AKI (when creatinine excretion is not at steady state) and still does not adjust for variability in exosome isolation. As flotillin-1 and TSG101 remain constant, investigators could quantify a change in protein abundance with regard to these exosomal proteins. Such an approach adjusts for variability in sample preparation, for example, differences in the percentage of exosomes isolated from a given urine sample. This is an important issue as differences between urine samples, such as the amount of Tamm–Horsfall protein (THP), can significantly affect exosome recovery (Fernandez-Llama et al. 2010). THP itself has been suggested as a protein that can normalise changes in the exosome because the amount of THP in the ultra-centrifugation pellet is highly correlated with exosomal marker proteins such as TSG101 (Fernandez-Llama et al. 2010). THP is easily measured; however, inter-individual variation in urinary THP concentration (Lau et al. 2008) may complicate comparisons across people and direct measurement of exosomal proteins such as TSG101 may still be necessary. For the successful development of biomarkers, these critical issues need to be resolved by large studies in human samples.
Exosomes can transfer information between cells in the form of protein, mRNA and miRNA (Valadi et al. 2007; Sheldon et al. 2010; Mittelbrunn et al. 2011). In our study we demonstrated that mCCDC11 cell-derived exosomes can transfer functional AQP2 to cells that did not previously express the protein. There was a significant increase in AQP2 protein in cells exposed to AQP2-containing exosomes, which resulted in a significant increase in water flow. To the best of our knowledge exosomal transfer of information has not been demonstrated in kidney cells and there are two possible explanations. First, the exosomes contained AQP2, which could have been transferred to the cells as a functioning membrane water channel. Second, the exosomes could have transferred mRNA or miRNA that increased the expression of endogenous AQP2. On the basis of our experiments, we cannot distinguish between these possibilities. Further studies are needed to establish the physiological relevance of our proof-of-concept studies. For example, we exposed the cell line to exosomes at 50 μg protein ml-1, which has previously been shown to transfer cellular information in vitro (Sheldon et al. 2010). Whether this represents a physiological concentration that collecting cells are exposed to in vivo is unknown but, in human urine, the exosomal protein concentration is around 2 μg ml-1 (Zhou et al. 2006b).
There are a number of situations in which exosomes could be an important mechanism of information transfer along the nephron. Increased cellular demand, such as an increase in the urinary sodium concentration in the proximal tubule, could cause release of exosomes that alter tubular function downstream, for example, by changing distal tubular sodium transport. If this exosomal transfer of information were at the level of functional proteins then this mechanism could result in rapid cellular changes, a potential advantage compared to other signalling systems that may take more time to produce an effect. This is speculative, although there is evidence that proximal tubular proteins can be detected in downstream cells, and transfer of these proteins by exosomes has been suggested (van Balkom et al. 2011). There is stronger evidence that exosomes can deliver therapeutic interventions: systemically administered exosomes, engineered to express a neuron-specific protein, can deliver short interfering RNA (siRNA) to the mouse brain with a high degree of tissue specificity (Alvarez-Erviti et al. 2011). This suggests exosomes may represent vehicles for delivery of therapies specifically to the kidney tubular cells. However, fundamental questions remain and need answering. Our data are from a cell line model; do exosomes transfer information between primary human kidney cells? What is the mechanism of exosome uptake by kidney tubular cells, specifically are there receptors that can be exploited to manufacture kidney specific exosomes? What is the key functional cargo of the exosome: protein, mRNA or miRNA? Finally, it is still unclear as to whether circulating exosomes are filtered by the glomerulus and actually enter the urine.
In summary, exosomes are released in vitro and changes in their proteome closely reflect changes in the cell, at least for AQP2. The concentration of flotillin-1 and TSG101 in the exosome is constant allowing relative changes in a protein of interest to be quantified. Exosomes can transfer functional AQP2 between cells and this study demonstrates that exosomes have the potential to mediate cell-to-cell communication along the nephron.
Acknowledgments
This project was funded by the British Heart Foundation (BHF). J.M.S. and R.I.M. were BHF 4-year PhD students. M.A.B. is funded by BHF and Kidney Research UK. D.J.W. is funded by the Wellcome Trust, Medical Research Council and Chief Scientist Office. J.W.D. acknowledges the contribution of the British Heart Foundation Centre of Research Excellence Award.
Glossary
Abbreviations
- AQP2
aquaporin 2
- AKI
acute kidney injury
- mCCDC11
murine cortical collecting duct cell line
- miRNA
microRNA
- TEM
transmission electron microscopy
- TSG101
tumor susceptibility gene 101
Author contributions
Experiments were performed in the laboratory of author J.W.D. J.M.S. performed the majority of the studies. Immunoblotting was performed partly by W.B. The rat studies were performed by R.I.M. The studies were designed by J.M.S., D.J.W., M.A.B. and J.W.D. Most of the data analysis was performed by J.M.S. The article was drafted by J.W.D. and revised critically by all authors. All the authors approve the final version.
References
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78:838–848. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- Fernandez-Llama P, Khositseth S, Gonzales PA, Star RA, Pisitkun T, Knepper MA. Tamm-Horsfall protein and urinary exosome isolation. Kidney Int. 2010;77:736–742. doi: 10.1038/ki.2009.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaeggeler HP, Gonzalez-Rodriguez E, Jaeger NF, Loffing-Cueni D, Norregaard R, Loffing J, et al. Mineralocorticoid versus glucocorticoid receptor occupancy mediating aldosterone-stimulated sodium transport in a novel renal cell line. J Am Soc Nephrol. 2005;16:878–891. doi: 10.1681/ASN.2004121110. [DOI] [PubMed] [Google Scholar]
- Gaeggeler HP, Guillod Y, Loffing-Cueni D, Loffing J, Rossier BC. Vasopressin-dependent coupling between sodium transport and water flow in a mouse cortical collecting duct cell line. Kidney Int. 2011;79:843–852. doi: 10.1038/ki.2010.486. [DOI] [PubMed] [Google Scholar]
- Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol. 2009;11:1143–1149. doi: 10.1038/ncb1929. [DOI] [PubMed] [Google Scholar]
- Gonzales PA, Pisitkun T, Hoffert JD, Tchapyjnikov D, Star RA, Kleta R, et al. Large-scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol. 2009;20:363–379. doi: 10.1681/ASN.2008040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graner MW, Alzate O, Dechkovskaia AM, Keene JD, Sampson JH, Mitchell DA, Bigner DD. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J. 2009;23:1541–1557. doi: 10.1096/fj.08-122184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson PI, Shim S, Merrill SA. Cell biology of the ESCRT machinery. Curr Opin Cell Biol. 2009;21:568–574. doi: 10.1016/j.ceb.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MC, Manganelli L, Woollard JR, Masyuk AI, Masyuk TV, Tammachote R, et al. Characterization of PKD protein-positive exosome-like vesicles. J Am Soc Nephrol. 2009;20:278–288. doi: 10.1681/ASN.2008060564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S, Rupp C, Stoeck A, Runz S, Fogel M, Lugert S, et al. CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney Int. 2007;72:1095–1102. doi: 10.1038/sj.ki.5002486. [DOI] [PubMed] [Google Scholar]
- Kortenoeven ML, Li Y, Shaw S, Gaeggeler HP, Rossier BC, Wetzels JF, Deen PM. Amiloride blocks lithium entry through the sodium channel thereby attenuating the resultant nephrogenic diabetes insipidus. Kidney Int. 2009;76:44–53. doi: 10.1038/ki.2009.91. [DOI] [PubMed] [Google Scholar]
- Lau WH, Leong WS, Ismail Z, Gam LH. Qualification and application of an ELISA for the determination of Tamm Horsfall protein (THP) in human urine and its use for screening of kidney stone disease. Int J Biol Sci. 2008;4:215–222. doi: 10.7150/ijbs.4.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Pressman S, Andress AP, Kim K, White JL, Cassidy JJ, et al. Silencing by small RNAs is linked to endosomal trafficking. Nat Cell Biol. 2009;11:1150–1156. doi: 10.1038/ncb1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael A, Bajracharya SD, Yuen PS, Zhou H, Star RA, Illei GG, Alevizos I. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010;16:34–38. doi: 10.1111/j.1601-0825.2009.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda KC, Bond DT, McKee M, Skog J, Paunescu TG, Da Silva N, et al. Nucleic acids within urinary exosomes/microvesicles are potential biomarkers for renal disease. Kidney Int. 2010;78:191–199. doi: 10.1038/ki.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira RA, Diniz LF, Teotonio LO, Lima CG, Mota RM, Martins A, Sanches TR, Seguro AC, Andrade L, Silva GB, Jr, Liborio AB, Daher EF. Renal tubular dysfunction in patients with American cutaneous leishmaniasis. Kidney Int. 2011;80:1099–1106. doi: 10.1038/ki.2011.251. [DOI] [PubMed] [Google Scholar]
- Pisitkun T, Shen R-F, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A. 2004;101:13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood IM, Deegens JK, Merchant ML, Tamboer WP, Wilkey DW, Wetzels JF, Klein JB. Comparison of three methods for isolation of urinary microvesicles to identify biomarkers of nephrotic syndrome. Kidney Int. 2010;78:810–816. doi: 10.1038/ki.2010.262. [DOI] [PubMed] [Google Scholar]
- Sheldon H, Heikamp E, Turley H, Dragovic R, Thomas P, Oon CE, et al. New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes. Blood. 2010;116:2385–2394. doi: 10.1182/blood-2009-08-239228. [DOI] [PubMed] [Google Scholar]
- Sonoda H, Yokota-Ikeda N, Oshikawa S, Kanno Y, Yoshinaga K, Uchida K, et al. Decreased abundance of urinary exosomal aquaporin-1 in renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2009;297:F1006–F1016. doi: 10.1152/ajprenal.00200.2009. [DOI] [PubMed] [Google Scholar]
- Stoorvogel W, Kleijmeer MJ, Geuze HJ, Raposo G. The biogenesis and functions of exosomes. Traffic. 2002;3:321–330. doi: 10.1034/j.1600-0854.2002.30502.x. [DOI] [PubMed] [Google Scholar]
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- van Balkom BW, Pisitkun T, Verhaar MC, Knepper MA. Exosomes and the kidney: prospects for diagnosis and therapy of renal diseases. Kidney Int. 2011 doi: 10.1038/ki.2011.292. DOI: 10.1038/ki.2011.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Cheruvanky A, Hu X, Matsumoto T, Hiramatsu N, Cho ME, et al. Urinary exosomal transcription factors, a new class of biomarkers for renal disease. Kidney Int. 2008;74:613–621. doi: 10.1038/ki.2008.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Pisitkun T, Aponte A, Yuen PS, Hoffert JD, Yasuda H, et al. Exosomal Fetuin-A identified by proteomics: a novel urinary biomarker for detecting acute kidney injury. Kidney Int. 2006a;70:1847–1857. doi: 10.1038/sj.ki.5001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Yuen PS, Pisitkun T, Gonzales PA, Yasuda H, Dear JW, et al. Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int. 2006b;69:1471–1476. doi: 10.1038/sj.ki.5000273. [DOI] [PMC free article] [PubMed] [Google Scholar]


