Abstract
Non-technical summary
In older people, the function of the kidney deteriorates, and one possible way to slow this process down is through exercise. Exercise increases the abundance of important enzymes that are needed for optimal vessel health and kidney function, for instance the nitric oxide synthase and superoxide dismutase (SOD) enzymes. It also reduces oxidative stress, a type of cellular injury caused by highly reactive molecules. When we compared young and old sedentary rats to young and old exercise-trained (12 weeks treadmill) rats, we found that exercise was not effective in reversing the age-related kidney changes. In old rats, renal function declined as did the abundance of the protective SOD enzymes, and oxidative stress increased; interestingly, exercise did not influence these changes. Our results suggest that the cardiovascular benefits of exercise do not necessarily extend to the kidney.
Abstract
The ageing kidney exhibits slowly developing chronic kidney disease (CKD) and is associated with nitric oxide (NO) deficiency and increased oxidative stress. The impact of exercise on the ageing kidney is not well understood. Here, we determined whether 12 weeks of treadmill exercise can influence age-dependent CKD in old (22–24 months) Fisher 344 (F344) male rats by comparing sedentary (SED) and exercise (EX) trained rats; young (3 months) rats were also studied. In addition to renal structure and function, we assessed protein levels of various isoforms of the NO synthases (NOS) and superoxide dismutase (SOD) enzymes as well as markers of oxidative stress, in kidney cortex and medulla. Renal function as determined by plasma creatinine, proteinuria, and glomerular structural injury worsened with age and was unaffected by exercise. Ageing also increased the protein abundance of neuronal NOSβ and p22phox while decreasing extracellular (EC) and copper/zinc (CuZn) SOD, in kidney cortex and medulla. H2O2 content and nitrotyrosine abundance also increased in the kidney with age. None of these age-related changes were altered with exercise. However, exercise did increase renal cortical endothelial (e)NOS and EC SOD in young rats. Data indicate that exercise-induced increases in eNOS and EC SOD seen in young rats are lost with age. We conclude that chronic exercise is ineffective in reversing age-dependent CKD in the male F344 rat.
Introduction
Exercise reduces morbidity and mortality from various cardiovascular diseases in the elderly population (Larson & Bruce, 1987). It has beneficial metabolic actions including reduction in plasma triglycerides, increases in the high-density lipoprotein to low-density lipoprotein ratio and insulin sensitivity, and improves cardiac function (Heath et al. 1983; Sun et al. 2008). Physical activity may also reduce depression and mental stress thus indirectly improving blood pressure (Paluska & Schewnk, 2000).
Another beneficial response to exercise is the stimulation of endothelial nitric oxide synthase (eNOS), the main enzyme responsible for vascular NO production which is essential for optimal vascular health. An increase in endothelial shear stress as a result of increased blood flow results in (1) prolonged eNOS mRNA stability, (2) increased eNOS protein translation, and (3) increased NOS enzyme activity (Harrison et al. 2006). In addition, increased shear stress increases the antioxidant extracellular superoxide dismutase (EC SOD) enzyme by an NO-dependent mechanism (Fukai et al. 2000). The shear stress-induced up-regulation of eNOS and EC SOD enhances endothelium-dependent vasodilatation in parts of the circulation where blood flow increases during exercise, such as skeletal muscle, pulmonary and coronary circulations (Muller et al. 1994; DeSouza et al. 2000; Johnson et al. 2000; Spier et al. 2004). As a result of all these effects exercise has powerful cardiovascular protective actions.
In the kidney, blood flow decreases during exercise in an intensity-dependent manner to shunt adequate perfusion to working muscles. Renal blood flow significantly reduces with high intensity exercise (Castenfors et al. 1967). Under some circumstances a fall in blood flow leads to a reduction in shear stress, which could lead to reductions in endothelial NOS and EC SOD. Indeed, in the male Wistar rat, acute treadmill exercise in pre-trained rats reduced renal eNOS mRNA, protein, and enzyme activity, whereas increases occurred in the lungs where blood flow increases with exercise (Miyauchi et al. 2003). Blood flow also falls with exercise in the splanchic circulation and mesenteric arteries isolated from trained rats do not show enhanced flow-mediated dilatation in these vascular beds, whereas in skeletal muscle arteries exercise does improve flow-mediated dilatation (Sun et al. 1998). Thus, one direct endothelial benefit of exercise is shear stress dependent, and in organs such as the kidney where blood flow falls, depending on how intrarenal shear stress is altered, endothelial function may not be improved, or may even be impaired (Miyauchi et al. 2003).
This is of particular concern in the ageing kidney where falls in eNOS abundance and NOS activity (Erdely et al. 2003) and increased oxidative stress occur (Gomes et al. 2009) in conjunction with slowly developing glomerular and tubulointerstitial injury (Baylis & Corman, 1998). Superimposing additional eNOS and EC SOD deficits could exacerbate age-dependent kidney damage. Indeed, 6 weeks of exercise in old C57BL/6J mice magnified the age-associated renal structural injury (Lichtig et al. 1987).
The main purpose of the present study was to test the hypothesis that chronic treadmill exercise exacerbates the progression of age-dependent renal injury in the male Fisher 344 (F344) rat. We also investigated the effect of exercise on the protein levels of various isoforms of the NOS and SOD enzymes, in kidney cortex and medulla as well as aorta, in young and old rats. Furthermore, since reactive oxygen species contributes to the development of age-dependent renal changes, we assessed several markers of oxidative stress (Asghar et al. 2007).
Methods
Ethical approval
All animal handling was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved and monitored by the West Virginia University and University of Florida Institutional Animal Care and Use Committees.
Animal procedures
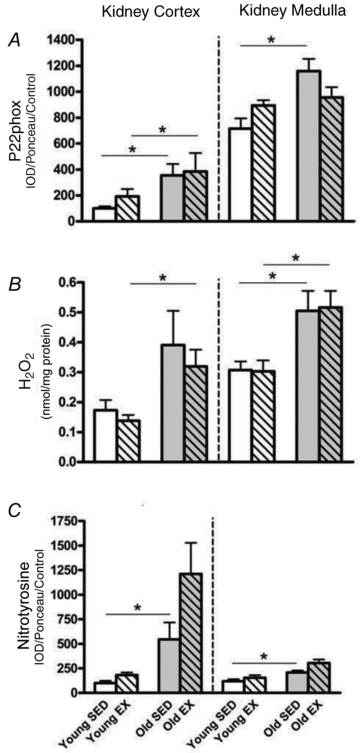
Young (3 months; n = 16) and old (22–24 months; n = 16) male Fisher 344 (F344) rats supplied from Harlan (Indianapolis, IN, USA) were purchased from the National Institute of Aging (NIA Bethesda, MD, USA). All rats were housed in a temperature/light-controlled environment and given access to standard rat chow and water ad libitum. For acclimation purposes, all rats were placed on a motor-driven treadmill for 3 day sessions of 5 min training at an intensity of 5 m min−1 and 0 deg incline. Rats were then randomly assigned to either young or old sedentary (Young SED; Old SED; n = 8 for all), or young and old exercise (Young EX; Old EX; n = 8 for all) groups. EX rats were trained for 10–12 weeks and SED were age matched. After a steady increase in treadmill training during the first 3 weeks, EX rats performed 5 days week−1 for 60 min day−1 at an intensity of 15 m min−1 and 15 deg incline for the remaining weeks. Training adherence was prompted with small bursts of compressed air and/or a low-voltage electric grid at the back of the treadmill; however, these tactics were used mainly during the first 3 weeks and minimally thereafter. This protocol is of moderate intensity, submaximal workload equivalent to an uphill walk and is likely to elicit a 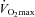 response in the range of 50–65% (Bedford et al. 1979; Wisløff et al. 2000; Musch et al. 2004), although no studies have duplicated our exercise protocol. Forty-eight hours after their last bout of training, rats were weighed, and anaesthetized with an overdose of inhaled isoflurane followed by thoracotomy (Abbott, North Chicago, IL, USA) as the method of humane killing. The kidney, aorta and soleus muscle were dissected and weighed. A transverse slice of the left kidney was saved in 10% phosphate-buffered formalin for histology (see below) and the remaining tissue separated into cortex and medulla. All tissues were flash-frozen in liquid nitrogen, then stored at –80°C until further analysis.
response in the range of 50–65% (Bedford et al. 1979; Wisløff et al. 2000; Musch et al. 2004), although no studies have duplicated our exercise protocol. Forty-eight hours after their last bout of training, rats were weighed, and anaesthetized with an overdose of inhaled isoflurane followed by thoracotomy (Abbott, North Chicago, IL, USA) as the method of humane killing. The kidney, aorta and soleus muscle were dissected and weighed. A transverse slice of the left kidney was saved in 10% phosphate-buffered formalin for histology (see below) and the remaining tissue separated into cortex and medulla. All tissues were flash-frozen in liquid nitrogen, then stored at –80°C until further analysis.
For functional measurements, a separate group of young (n = 16) and old (n = 16) F344 male rats of comparable age, supplied from Taconic Farms (Hudson, NY, USA), were purchased from the NIA. Some rats were treadmill trained (Young EX, n = 10; Old EX, n = 8) according to the same protocol described above, while others remained SED (Young SED, n = 6; Old SED, n = 8). Seven to twelve days before being humanely killed, all rats were placed on a low nitrate, complete diet for 24 h (AIN-76C, MP Biomedicals, Solon, OH, USA), then placed in metabolic cages for overnight (∼16 h) collection of urine. While in the metabolic cages, rats were fasted but were given access to distilled water. Metabolic cage collections did not interfere with the daytime training. Rats were then returned to daily training and regular diet and were killed as described above; blood was drawn from the aorta, centrifuged, and plasma collected and stored at –80°C. The left kidney was removed and weighed and a section of the kidney was fixed for histology.
Renal pathology
Fixed kidney tissue was paraffin embedded and 5 μm sections were cut and stained with periodic acid–Schiff (Sigma, St Louis, MO, USA) followed by haematoxylin as the secondary stain. All glomeruli in the section (>100) were scored, blinded, as follows: 0 = healthy glomeruli, +1 = <25% damage, +2 = 25–50% damage, +3 = 51–74% damage, +4 = >75% damage. The glomerular sclerosis index score (GSI) was calculated using the following equation: (no. of +1) + 2(no. of +2) + 3(no. of +3) + 4(no. of +4)/total glomeruli observed.
Western blot
The proteins measured by Western blot, with their specific primary antibody and concentration given in parentheses, were: (1) endothelial nitric oxide synthase (eNOS; BD Transduction; 1:250), (2) neuronal (n)NOSα (Santa Cruz; 1:50), (3) nNOSβ (Thermo Scientific, formerly ABR; 1:500), (4) extracellular superoxide dismutase (EC SOD; Abcam; 1:250), (5) cytosolic copper/zinc-containing SOD (CuZn SOD; 1:2000), (6) mitochondrial manganese-containing SOD (Mn SOD; 1:2000), (7) p22phox (Santa Cruz; 1:50), and (8) nitrotyrosine (Millipore; 1:500). Homogenized samples of kidney cortex, kidney medulla and aorta, standardized by protein concentrations (50–200 μg), were separated by electrophoresis (7.5% or 12% acrylamide gel, 200 V, 65 min) and transferred onto nitrocellulose membranes as previously described (Sasser et al. 2009). Membranes were stained with Ponceau Red (Sigma) to check for transfer efficiency/uniformity and equal loading, blocked and washed then incubated overnight while on a rocker with primary antibody at 4°C. Membranes were then incubated with the appropriate secondary antibody for one hour at room temperature, and developed with enhanced chemiluminescent reagents (Thermo Scientific). Bands were quantified by densitometry using the VersaDoc Imaging System and One Analysis Software (BioRad). Protein abundance was calculated as integrated optical density (IOD) of the protein of interest (after subtraction of background), factored for Ponceau Red stain (a marker of total protein loading) and an internal positive control value (to allow for quantitative comparisons between different membranes). To compare values between kidney cortex and kidney medulla, the protein abundance is represented as IOD/Ponceau/Control relative to the appropriate control group of the kidney cortex or medulla.
Biochemical assays
As previously described, plasma and urine creatinine concentrations were measured by HPLC, and urine protein levels were detected using the Bradford method (Sasser et al. 2009). Hydrogen peroxide (H2O2) levels in homogenates of kidney cortex, kidney medulla, and urine were measured using the Amplex Red Kit (Molecular Probes, Eugene, OR, USA) according to the manufacturer's instructions and signal specificity was confirmed by incubation with 2000 units of catalase (Sigma). Tissue H2O2 concentrations (μmol l−1) were normalized to total protein concentration (mg ml−1) and expressed as nmol (mg protein)−1. Citrate synthase activities in soleus tissue in units of μm min−1 (g wet tissue weight)−1 were based on methods adapted by Srere (1969).
Statistical analyses
Data are presented as means ± SEM and analysed using SigmaPlot software (San Jose, CA, USA). The effects of age and exercise training on functional, biochemical and protein abundance data were analysed by two-way ANOVA, and if found significant, followed by Newman–Keuls post hoc analyses. Renal pathology was analysed by the non-parametric Kruskal–Wallis test. Significance was defined as P < 0.05.
Results
The first series of studies on young and old, sedentary and exercise-trained rats were conducted on NIA colony F344 rats obtained from Harlan. Body weight was higher in old when compared to young rats and exercise reduced body weight in both age groups (Table 1). Kidney weight (factored to body weight) revealed hypertrophy with age in the EX group only. As reported previously (Sindler et al. 2009), training efficacy was confirmed with increased activities of citrate synthase in soleus skeletal muscle in both young and old (8.24 ± 0.12 vs. 10.02 ± 0.15 μm min−1 (g wet wt)−1 for Young SED and Young EX, respectively, P < 0.05; 7.08 ± 0.20 vs. 8.69 ± 0.18 μm min−1 (g wet wt)−1 for Old SED and Old EX, respectively, P < 0.05). Young rats exhibited minimal glomerular injury and this was not altered by exercise (Fig. 1). With age, the GSI (Fig. 1A) and the percentage of total damaged glomeruli (Fig. 1B) increased substantially and exercise had no effect on renal pathology.
Table 1.
Characteristics of male F344 rats obtained from Harlan, NIA
| Young SED | Young EX | Old SED | Old EX | |
|---|---|---|---|---|
| BW (g) | 367 ± 8 | 345 ± 5* | 409 ± 9† | 377 ± 9*† |
| Kidney wt (g (100 g BW)−1) | 0.32 ± 0.01 | 0.30 ± 0.00 | 0.35 ± 0.02 | 0.36 ± 0.01† |
SED, sedentary; EX, exercise; BW, body weight.
P < 0.05 vs. respective SED.
P < 0.05 vs. respective Young.
Note: kidney wt (g (100 g BW)−1) SEM values for Young EX were calculated as 0.0041.
Figure 1. Glomerular structural changes in young and old, sedentary (SED) and exercise (EX) trained rats.

Whole kidney sections were stained with periodic acid–Schiff, followed by a haematoxylin counterstain prior to blind histological grading. A, the GSI significantly increased with age and was unaffected by 12 weeks of treadmill exercise (5 days week−1, 60 min day−1 at 15 m min−1, 15 deg incline). B, the total percentage of damaged glomeruli also rose with age and exercise was without effect. *Denotes a statistical significance of P < 0.05 between the two groups.
In the kidney cortex, exercise significantly increased eNOS protein abundance in the young rat but had no effect in old (Fig. 2A). There were no differences in kidney cortex nNOSα abundance due to either age or exercise (Fig. 2B) and nNOSβ was low and unaffected by EX in young rats while there was a profound increase in nNOSβ abundance in both SED and EX old rats (Fig. 2C). In the kidney medulla, eNOS protein abundance was higher than in cortex and was similar in old and young rats and not altered by exercise (Fig. 2A). Significant increases in kidney medulla nNOSα were seen with exercise in the old only (Fig. 2B). Although age-related increases in nNOSβ protein abundance in kidney medulla were unaffected by exercise, in cortex exercise reduced the increase in nNOSβ seen with ageing (Fig. 2C). The abundance of the nNOSβ in medulla was higher than in cortex in both young groups and was further elevated in the old (Fig. 2C).
Figure 2. Protein levels of the nitric oxide synthase (NOS) enzymes in the kidney cortex and kidney medulla.
Total homogenates of kidney cortex and kidney medulla from exercise (EX) trained and sedentary (SED) rats were run using Western blot and probed for endothelial nitric oxide synthase (eNOS) (A), neuronal NOS isoform α (nNOSα) (B), and nNOS isoform β (nNOSβ) (C). Relative density units were expressed as a percentage with respect to Young SED controls of the kidney cortex. *Denotes a statistical significance of P < 0.05 between the two groups.
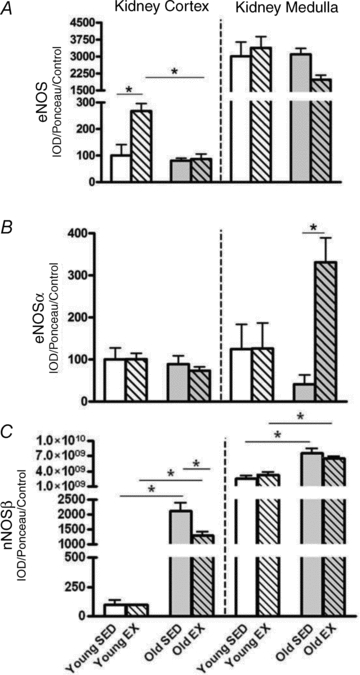
As with eNOS, exercise increased the EC SOD abundance in the kidney cortex of young but not old rats (Fig. 3A). There were no exercise effects on the other SOD isoforms in kidney cortex or medulla (Fig. 3B and C). With age there were falls in EC SOD and CuZn SOD in both kidney cortex and medulla whereas for Mn SOD, there was no impact of age or exercise in the kidney. Values of EC SOD were higher in medulla while CuZn and Mn SOD abundance values were lower in medulla vs. cortex in all groups.
Figure 3. Protein levels of the superoxide dismutase (SOD) enzymes in the kidney cortex and kidney medulla.
Total homogenates of kidney cortex and kidney medulla from exercise (EX) trained and sedentary (SED) rats were run using Western blot and probed for extracellular superoxide dismutase (EC SOD) (A), cytosolic-localized copper/zinc SOD (CuZn SOD) (B), and mitochondrial-localized manganese SOD (Mn SOD) (C). Relative density units were expressed as a percentage with respect to Young SED controls of the kidney cortex. *Denotes a statistical significance of P < 0.05 between the two groups.
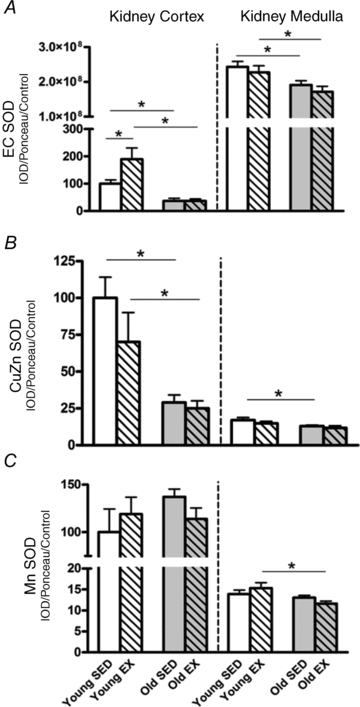
The p22phox subunit of the NADPH oxidase increased with age in both kidney cortex and medulla but exercise was without effect (Fig. 4A). Tissue H2O2 levels (Fig. 4B) and nitrotyrosine abundance (Fig. 4C) increased with age in both kidney cortex and medulla but were unaffected by exercise (Fig. 4B). In the aorta of young rats exercise training significantly increased the abundance of eNOS and age increased these levels even further (Fig. 5A); however, no differences were found between Old SED and Old EX (Fig. 5A). Aortic EC SOD abundance tended to increased with exercise in the young rats but due to high variability, this did not reach statistical significance (Fig. 5B). No further change occurred in aortic EC SOD with age or exercise (Fig. 5B). Nitrotyrosine abundance in the aorta significantly rose with age in both groups and EX was again without effect (Fig. 5C).
Figure 4. Oxidative stress markers: p22phox protein, H2O2 levels, and nitrotyrosine protein in kidney cortex and kidney medulla.
Total homogenates of kidney cortex and kidney medulla from exercise (EX) trained and sedentary (SED) rats were prepared for p22phox Western blot detection (A), H2O2 concentration levels (B), and nitrotyrosine Western blot detection (C). All values are expressed as a percentage with respect to Young SED controls of the kidney cortex. *Denotes a statistical significance of P < 0.05 between the two groups.
Figure 5. Aortic protein levels of eNOS (endothelial nitric oxide synthase), EC SOD (extracellular superoxide dismutase), and nitrotyrosine.
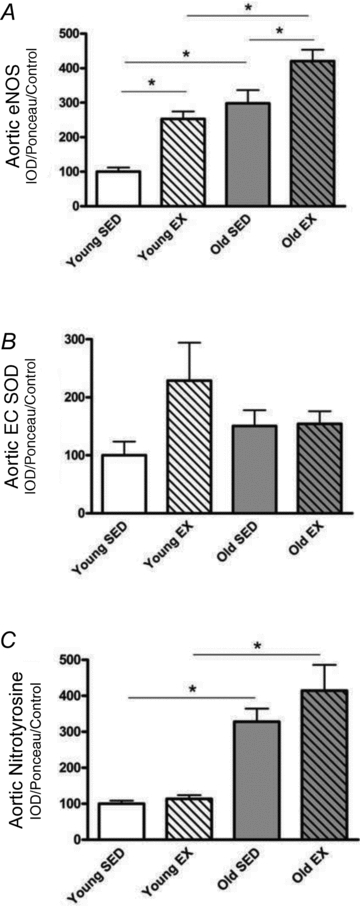
Total homogenates of aorta from exercise (EX) trained and sedentary (SED) rats were run using Western blot and probed for endothelial nitric oxide synthase (eNOS) (A), extracellular superoxide dismutase (EC SOD) (B), and nitrotyrosine (C). Relative density units were expressed as a percentage with respect to Young SED controls of the kidney cortex. *Denotes a statistical significance of P < 0.05 between the two groups.
For additional functional measurements, later groups of young (n = 16) and old (n = 16) F344 rats of similar age were obtained from Taconic Farms, also from the NIA colony. Increased body weight and renal hypertrophy occurred with age (Table 2), as also seen in the Harlan NIA rats (Table 1), and the effect of exercise on body weight was similar in young and old (Table 2). Plasma creatinine (PCr) increased with age in both SED and EX groups, while creatinine clearance fell significantly with age in SED only (Table 2). Urinary protein excretion markedly increased with age, and exercise was without effect in either young or old groups (Table 2). To determine total body NO production, we measured the urinary excretion of the stable NO oxidation products, NO2 and NO3 (NOx; UNOxV). With age, UNOxV tended to fall; however, UNOxV significantly increased with exercise in both young and old rats and the magnitude of the EX-induced increase was not blunted by age (Table 2). No changes in urinary H2O2 excretion were detected among any of the groups (3.81 ± 0.56, 5.69 ± 0.99, 4.26 ± 0.80, 2.90 ± 0.75 nmol (24 h)−1 (100 g body wt)−1 for Young SED, Young EX, Old SED and Old EX, respectively). Furthermore, an age-dependent increase in glomerular injury was again observed and this was not improved by exercise (GSI scores: 0.04 ± 0.02, 0.03 ± 0.01, 0.93 ± 0.14† and 0.78 ± 0.18† for Young SED, Young EX, Old SED, and Old EX, respectively; †P < 0.05 vs. respective Young).
Table 2.
Characteristics of male F344 rats obtained from Taconic Farms, NIA
| Young SED | Young EX | Old SED | Old EX | |
|---|---|---|---|---|
| BW (g) | 403 ± 12 | 387 ± 10 | 456 ± 17† | 417 ± 23* |
| Kidney wt (g (100 g BW)−1) | 0.26 ± 0.01 | 0.28 ± 0.01 | 0.41 ± 0.04† | 0.34 ± 0.02 |
| PCr (mg dl−1) | 0.11 ± 0.02 | 0.12 ± 0.01 | 0.22 ± 0.01† | 0.20 ± 0.03† |
| CCr (ml min−1 (100 g BW)−1) | 1.98 ± 0.22 | 1.86 ± 0.12 | 0.74 ± 0.15† | 1.09 ± 0.16 |
| UpV (mg (24 h)−1 (100 g BW)−1) | 2.4 ± 0.2 | 3.8 ± 0.1 | 88.8 ± 21† | 65.1 ± 17† |
| UNOxV (μmol (24 h)−1 (100 g BW)−1) | 0.76 ± 0.01 | 1.21 ± 0.08* | 0.46 ± 0.15 | 0.88 ± 0.10*† |
SED, sedentary; EX, exercise; BW, body weight; PCr, plasma creatinine; CCr, creatinine clearance; UpV, urinary protein excretion; UNOxV, urinary nitrate/nitrite excretion
P < 0.05 vs. respective SED.
P < 0.05 vs. respective Young.
Discussion
The main finding in the present study was that 10–12 weeks of treadmill exercise increased both eNOS and EC SOD abundance in the kidney cortex of the young rat, despite the expectation of an exercise-induced fall in renal blood flow. In old rats, there was no change in renal eNOS and EC SOD with exercise. Rats developed age-dependent renal damage and exercise was not able to reverse it. In contrast to other strains there was also no loss of renal eNOS or nNOSα protein in the F344, and abundance of the nNOSβ in the kidney cortex increased >100× due to ageing, and exercise did not prevent this. The age-dependent loss of the kidney cortex antioxidants EC SOD and CuZn SOD, and age-dependent increase in p22phox were not influenced by exercise. The second series of rats indicated that with age, F344 rats develop significant proteinuria with a fall in creatinine clearance. Exercise did not worsen the age-dependent proteinuria or decline in creatinine clearance and furthermore, exercise significantly increased total NO production in both age groups. Therefore, although exercise was not efficacious in preventing age-related changes in the kidney, our data indicate that exercise did not exacerbate them.
The present studies were conducted on the National Institute of Aging's inbred F344 rat, which develops mild to moderate kidney damage with advancing age without systemic hypertension (Wei et al. 1986). There are differences in the renal response to ageing, with some strains such as the Sprague–Dawley rat being very vulnerable while others show mild to moderate injury (Munich Wistar and F344), and yet others are resistant (Wag/Rij and F344/Brown Norway cross) (Baylis & Corman, 1998; Moningka et al. 2011). There are also sex differences in the rate of loss of kidney function and development of structural damage in ageing man and rats, with the male being most vulnerable (Baylis & Corman, 1998; Baylis 2009).
We have previously reported that development of severe chronic kidney disease (CKD) is invariably associated with loss of renal cortical nNOSα abundance, irrespective of animal model (Baylis 2009). This includes the ageing male Sprague–Dawley rat where marked glomerular injury occurs (Erdely et al. 2003). The present study demonstrates that the mild to moderate renal injury exhibited by the ageing F344 rat is not associated with a loss of eNOS or nNOSα protein. In contrast, there are large age-dependent increases in the renal cortical abundance of the nNOSβ isoform. We have also observed increased nNOSβ in experimentally induced forms of CKD, including renal mass reduction (Smith et al. 2009; Tain et al. 2011) and chronic allograft nephropathy (Tain et al 2008). We initially thought that nNOSβ activation in CKD was a secondary, compensatory response to the loss of other NOS isoforms in the damaged kidney. However, we show here that the increased nNOSβ precedes loss of the other NOS isoforms, suggesting that this may in fact be involved in causing the early, mild to moderate renal damage. There are also antioxidant and pro-oxidant changes in the ageing F344 kidney cortex which include loss of both EC SOD and CuZn SOD protein abundance, increased p22phox (NADPH oxidase subunit), increased nitrotyrosine, and increased H2O2 levels. These changes may also contribute to the early renal damage.
A main aim of this study was to determine the impact of exercise training on the kidney of the F344 rat. Here, we report that in the young adult F344, exercise increased both eNOS and EC SOD protein in the renal cortex which confirms earlier preliminary findings by us in this strain (Moningka et al. 2010). Exercise also increased aortic eNOS indicating that in the F344, these beneficial endothelial enzymes are enhanced by both increased blood flow (to aorta) and decreased blood flow (to kidney). Although not measured by us, rats of various strains including the F344 do reduce their renal blood flow with exercise (Laughlin & Armstrong, 1982; Kregel 1995; Musch et al. 2004). Possibly, in the young exercise-trained F344 increased intrarenal resistances oppose the decreased flow and lead to increased intrarenal shear stress in some locations, and hence eNOS and EC SOD activation. This is particularly interesting since we previously observed exactly opposite intrarenal effects of exercise in the young male Sprague–Dawley rat, where falls in eNOS and EC SOD were seen (Moningka et al. 2010). Marked falls in renal blood flow also occur in this strain (Laughlin & Armstrong, 1982). We speculate that in the Sprague–Dawley kidney the exercise-induced renal vasoconstriction leads to net falls in intrarenal shear stress. The directional effect of exercise on renal endothelial enzymes has profound consequences since loss of these enzymes renders the Sprague–Dawley kidney more susceptible to acute kidney injury, while the F344 strain is protected (Moningka et al. 2011). In the present study, however, since the young sedentary F344 has minimal spontaneous injury we did not see any histological effect of exercise.
Our primary goal was to establish the effect of exercise on renal endothelial enzymes as well as overall renal structure and function in the ageing F344 rat. We have already reported that similar exercise has significant general cardiovascular benefits in the ageing F344 leading to enhanced endothelial function in skeletal muscle (Spier et al. 2004; Sindler et al. 2009). Despite these cardiovascular improvements, we report here that the structural and functional kidney damage in the ageing F344 is not reversed by exercise, suggesting that the beneficial effects of the same type/duration of exercise are not inevitably transmitted to the kidney. In fact, there is considerable variability in the reported response of the ageing kidney to exercise. In the old (23 months) male Sprague–Dawley rat, life-long voluntary wheel running reduced kidney structural damage and was as effective as lifelong caloric restriction (Loupal et al. 2005). Of note, in the same study, treadmill running over the same period had no beneficial effects in the Sprague–Dawley rat (Loupal et al. 2005), similar to our present findings in the old F344 subjected to 10–12 weeks of treadmill running. In contrast, in the old C57BL/6J mouse which develops significant kidney damage, only 6 weeks of forced wheel running considerably worsened the age-associated renal structural injury (Lichtig et al. 1987). The aged F344 rat shows an exaggerated fall in renal blood flow during exercise which we speculate causes unchanged intrarenal shear stress, accounting for the lack of exercise-induced renal eNOS and EC SOD. This lack of activation of these endothelial enzymes, together with loss of CuZn SOD in the ageing kidney, which is not restored by exercise, probably contributes to the lack of exercise-induced protection against kidney damage and dysfunction.
Oxidative stress, defined as the imbalance between oxidants and antioxidants, is reported to increase with age, and can be combated with exercise. Moderate exercise reduced age-associated increases in mitochondrial oxidative stress in some organs (including kidney) of old male mice, although beneficial effects declined in senescent animals (Navarro et al. 2004). The same study found that exercise did not prevent the age-dependent decline in kidney CuZn SOD and catalase activity levels (Navarro et al. 2004). Here, we report no net effect of exercise on age-related increases in oxidative stress. The protein abundance of p22phox, a subunit of the superoxide-generating NADPH oxidase enzyme was up-regulated in both the kidney cortex and kidney medulla with age, and was unchanged with exercise. H2O2 levels, an additional marker of oxidative stress, also increased with age in the kidney medulla only, but again, was not affected by exercise. Furthermore, nitrotyrosine, also increased with age in renal cortex, medulla and aorta and was not affected by exercise.
Limitations of this study include the possible confounding impact of stress with the use of forced treadmill exercise. Further, rats were not run on a reverse light:dark cycle, thus we interrupted their normal circadian rhythm. Finally, we only initiated exercise training at 22–24 months of age and if instituted earlier in old rats, exercise may have had some benefit.
Overall, this study demonstrates that in the young male F344 rat, 12 weeks of treadmill exercise increases kidney cortex eNOS and EC SOD abundance, and that with age, this response is lost. We observed several other age-related changes in the kidney including worsening of renal structural injury, increased renal oxidative stress as detected by increase protein abundance in p22phox, H2O2 content and nitrotyrosine, and decreased antioxidant defences reflected by loss of EC and CuZn SOD. Interestingly, exercise did not prevent any of these adverse changes in the kidney. Therefore, we conclude that although 10–12 weeks of chronic treadmill exercise was ineffective in reversing the age-associated declines in renal function and renal antioxidant status in the male F344 rat, it did not worsen age-related renal injury.
Acknowledgments
The authors thank Harold Snellen, Kevin Engels and Paul Harton for excellent technical assistance. This work was funded by NIH RO1 DK56843 (C.B.) and NIH RO1 HL077224 (J.M.M.-D). N.C.M. was supported by the Endocrine, Metabolic, and Prenatal Basis of Chronic Kidney Disease Training Program (NIH T32DK076541).
Glossary
Abbreviations
- CKD
chronic kidney disease
- CuZn SOD
copper/zinc superoxide dismutase
- EC SOD
extracellular superoxide dismutase
- eNOS
endothelial nitric oxide synthase
- EX
exercise
- F344
Fisher 344 rat
- GSI
glomerular sclerosis index
- Mn SOD
manganese superoxide dismutase
- NIA
National Institute of Aging
- nNOS
neuronal nitric oxide synthase
- NOS
nitric oxide synthase
- PCr
plasma creatinine
- SED
sedentary
Author contributions
This study was a collaboration between J.M.M.-D. and C.B., and all experiments were conducted in their laboratories. N.C.M. was involved in data collection, analysis, interpretation, manuscript preparation, and provided intellectual content. Co-author A.L.S. was involved in some data collection, provided intellectual contact and helped with the final approval of the manuscript. Co-author J.M.M.-D. was involved in the conception and design of the experiment, provided intellectual content, and revised the manuscript critically prior to submission. C.B. was responsible for the conception and design of the study, and provided critical assessment of all aspects of the data analyses and preparation of the manuscript. All authors approved the final version of the manuscript.
References
- Asghar M, George L, Lokhandwala MF. Exercise decreases oxidative stress and inflammation and restores renal dopamine D1 receptor function in old rats. Am J Physiol Renal Physiol. 2007;293:F914–F919. doi: 10.1152/ajprenal.00272.2007. [DOI] [PubMed] [Google Scholar]
- Baylis C. Sexual dimorphism in the aging kidney: differences in the nitric oxide system. Nat Rev Nephrol. 2009;5:384–396. doi: 10.1038/nrneph.2009.90. [DOI] [PubMed] [Google Scholar]
- Baylis C, Corman B. The aging kidney: insights from experimental studies. J Am Soc Nephrol. 1998;9:699–709. doi: 10.1681/ASN.V94699. [DOI] [PubMed] [Google Scholar]
- Bedford TG, Tipton CM, Wilson NC, Oppliger RA, Gisolfi CV. Maximum oxygen consumption of rats and its changes with various experimental procedures. J Appl Physiol. 1979;6:1278–1283. doi: 10.1152/jappl.1979.47.6.1278. [DOI] [PubMed] [Google Scholar]
- Castenfors J, Mossfeld F, Pscator M. Effect of prolonged heavy exercise on renal function and urinary protein excretion. Acta Physiol Scand. 1967;70:194–206. doi: 10.1111/j.1748-1716.1967.tb03615.x. [DOI] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Erdely A, Greenfeld Z, Wagner L, Baylis C. Sexual dimorphism in the aging kidney: effects on injury and nitric oxide system. Kidney Int. 2003;63:1021–1026. doi: 10.1046/j.1523-1755.2003.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukai T, Siegfried MR, Ushio-Fukai M, Cheng Y, Kojda G, Harrison DG. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J Clin Invest. 2000;105:1631–1639. doi: 10.1172/JCI9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes P, Simao S, Silva E, Pinto V, Amaral JS, Afonso J, Serrao MP, Pinho MJ, Soares-da-Silva P. Aging increases oxidative stress and renal expression of oxidant and antioxidant enzymes that are associated with an increased trend in systolic blood pressure. Oxid Med Cell Longev. 2009;2:138–145. doi: 10.4161/oxim.2.3.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DG, Widder J, Grumbach I, Chen W, Weber M, Searles C. Endothelial mechanotransduction, nitric oxide, and vascular inflammation. J Intern Med. 2006;259:351–363. doi: 10.1111/j.1365-2796.2006.01621.x. [DOI] [PubMed] [Google Scholar]
- Heath GW, Ehsani AA, Hagberg JM, Hinderliter JM, Goldberg AP. Exercise training improves lipoprotein lipid profiles in patients with coronary artery disease. Am Heart J. 1983;105:889–895. doi: 10.1016/0002-8703(83)90385-x. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Parker JL, Laughlin MH. Chronic exercise training improves ACh-induced vasorelaxation in pulmonary arteries of pigs. J Appl Physiol. 2000;88:443–451. doi: 10.1152/jappl.2000.88.2.443. [DOI] [PubMed] [Google Scholar]
- Kregel KC. Augmented mesenteric and renal vasoconstriction during exercise in senescent Fischer 344 rats. J Appl Physiol. 1995;79:706–712. doi: 10.1152/jappl.1995.79.3.706. [DOI] [PubMed] [Google Scholar]
- Larson EB, Bruce RA. Health benefits of exercise in an aging society. Arch Intern Med. 1987;147:353–356. [PubMed] [Google Scholar]
- Laughlin MH, Armstrong RB. Muscular blood flow distribution patterns as a function of running speed in rats. Am J Physiol Heart Circ Physiol. 1982;43:H296–H306. doi: 10.1152/ajpheart.1982.243.2.H296. [DOI] [PubMed] [Google Scholar]
- Lichtig C, Levy J, Gershon D, Reznick AZ. Effect of aging and exercise on the kidney. Anatomical and morphological studies. Gerontology. 1987;33:40–48. doi: 10.1159/000212852. [DOI] [PubMed] [Google Scholar]
- Loupal G, Url A, Skalicky M, Viidik A. Physical exercise retards the development of chronic nephropathy in the ageing rat as efficiently as food restriction does. Gerontology. 2005;51:83–93. doi: 10.1159/000082193. [DOI] [PubMed] [Google Scholar]
- Miyauchi T, Maeda S, Lemitsu M, Kobayashi T, Kumagai Y, Yamaguchi I, Matsuda M. Exercise causes a tissue-specific change of NO production in the kidney and lung. J Appl Physiol. 2003;94:60–68. doi: 10.1152/japplphysiol.00269.2002. [DOI] [PubMed] [Google Scholar]
- Moningka N, Cunningham M, Jr, Sterling M, Baylis C. Impact of 12 wks exercise on renal nitric oxide and antioxidant status: a strain difference comparison. FASEB J. 2010;24:1059.1. [Google Scholar]
- Moningka NC, Sasser JM, Croker B, Carter C, Baylis C. Protection against age-dependent renal injury in the F344xBrown Norway male rat is associated with maintained nitric oxide synthase. Mech Ageing Dev. 2011;132:1–7. doi: 10.1016/j.mad.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JM, Myers PR, Laughlin MH. Vasodilator responses of coronary resistance arteries of exercise-trained pigs. Circulation. 1994;89:2308–2314. doi: 10.1161/01.cir.89.5.2308. [DOI] [PubMed] [Google Scholar]
- Musch TI, Eklund KE, Hageman KS, Poole DC. Altered regional blood flow responses to submaximal exercise in older rats. J Appl Physiol. 2004;96:81–88. doi: 10.1152/japplphysiol.00729.2003. [DOI] [PubMed] [Google Scholar]
- Navarro A, Gomez C, Lopez-Cepero JM, Boveris A. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am J Physiol Regul Integr Comp Physiol. 2004;286:R505–R511. doi: 10.1152/ajpregu.00208.2003. [DOI] [PubMed] [Google Scholar]
- Paluska SA, Schewnk TL. Physical activity and mental health: current concepts. Sports Med. 2000;29:167–180. doi: 10.2165/00007256-200029030-00003. [DOI] [PubMed] [Google Scholar]
- Sasser JM, Moningka NC, Cunningham MW, Croker BP, Baylis C. Asymmetric dimethylarginine in angiotensin II induced hypertension. Am J Physiol Regul Integr Comp Physiol. 2009;298:R740–R746. doi: 10.1152/ajpregu.90875.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol. 2009;587:3885–3897. doi: 10.1113/jphysiol.2009.172221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Merchant M, Fekete A, Nyugen H-L, Oh P, Tain Y-L, Klein JB, Baylis C. Splice variants of neuronal nitric oxide synthase are present in the rat kidney. Nephrol Dial Transplant. 2009;24:1422–1428. doi: 10.1093/ndt/gfn676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol. 2004;556:947–958. doi: 10.1113/jphysiol.2003.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srere PA. Citrate synthase. Methods Enzymol. 1969;13:3–5. [Google Scholar]
- Sun D, Huang A, Koller A, Kaley G. Adaptation of flow-induced dilation of arterioles to daily exercise. Microvasc Res. 1998;56:54–61. doi: 10.1006/mvre.1998.2083. [DOI] [PubMed] [Google Scholar]
- Sun MW, Qian FL, Wang J, Tao T, Guo J, Wang L, Lu AY, Chen H. Low-intensity voluntary running lowers blood pressure with simultaneous improvement in endothelium-dependent vasodilatation and insulin sensitivity in aged spontaneously hypertensive rats. Hypertens Res. 2008;31:543–552. doi: 10.1291/hypres.31.543. [DOI] [PubMed] [Google Scholar]
- Tain YL, Ghosh S, Krieg RJ, Baylis C. Reciprocal changes of renal neuronal nitric oxide synthase-α and β associated with renal progression in a neonatal 5/6 nephrectomized rat model. Pediatr Neonatol. 2011;52:66–72. doi: 10.1016/j.pedneo.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tain YL, Muller V, Szabo AJ, Erdely A, Smith C, Baylis C. Sex differences in response to rapamycin in kidney transplantation: Impact on constitutive nitric oxide synthase. Nitric Oxide. 2008;18:80–86. doi: 10.1016/j.niox.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei JY, Mendelowitz D, Anastasi N, Rowe JW. Maintenance of carotid baroreflex function in advanced age in the rat. Am J Physiol Regul Integr Comp Physiol. 1986;250:R1047–R1051. doi: 10.1152/ajpregu.1986.250.6.R1047. [DOI] [PubMed] [Google Scholar]
- Wisløff U, Helgerud J, Kemi OJ, Ellingsen Ø. Intensity-controlled treadmill running in rats: VO2max and cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2000;280:H1301–H1310. doi: 10.1152/ajpheart.2001.280.3.H1301. [DOI] [PubMed] [Google Scholar]


