Abstract
Non-technical summary
The ‘arterial baroreflex’ plays an important role in the moment-to-moment regulation of blood pressure. It does this partly by eliciting changes in heart rate, but its ability to do this (i.e. sensitivity) during exercise is reduced from rest. During exercise, chemicals accumulate in the muscles (i.e. metabolites) that stimulate sensory nerves within the muscle (i.e. muscle metaboreflex). We show for the first time in humans that the stimulation of metabolically sensitive nerves within the muscles during leg cycling exercise decreases arterial baroreflex sensitivity. This new knowledge increases our understanding of the control of the human heart during exercise.
Abstract
We sought to determine whether the activation of metabolically sensitive skeletal muscle afferents (muscle metaboreflex) is a potential mechanism for the decrease in spontaneous cardiac baroreflex sensitivity (cBRS) during exercise in humans. In protocol 1, 15 male subjects (22 ± 1 years) performed steady-state leg cycling at low (26 ± 4 W) and moderate workloads (105 ± 7 W), under free-flow conditions and with partial flow restriction (bilateral thigh cuff inflation at 100 mmHg) to evoke muscle metaboreflex activation during exercise. In protocol 2, rhythmic handgrip exercise at 35% maximum voluntary contraction was performed with progressive upper arm cuff inflation (0, 80, 100 and 120 mmHg) to elicit graded metaboreflex activation. Both protocols were followed by post-exercise ischaemia (PEI) to isolate the muscle metaboreflex. Leg cycling-induced increases in HR and mean BP were augmented by partial flow restriction (P < 0.05 vs. free flow), while HR and mean BP both remained elevated during PEI (P < 0.05 vs. rest). Leg cycling evoked an intensity-dependent decrease in cBRS (16 ± 2, 7 ± 1 and 2 ± 0.2 ms mmHg−1 at rest, low and moderate workloads, respectively; P < 0.05), which was further reduced with partial flow restriction (by –2.6 ± 0.8 and –0.4 ± 0.1 ms mmHg−1 at low and moderate workloads). cBRS remained suppressed during PEI following leg cycling with partial flow restriction (4 ± 1 ms mmHg−1; P < 0.05 vs. rest). cBRS was unchanged during handgrip under free-flow conditions, handgrip with partial flow restriction and PEI following handgrip (P > 0.05 vs. rest). These data indicate that the activation of metabolically sensitive skeletal muscle afferents (muscle metaboreflex) decreases cardiac baroreflex responsiveness during leg cycling exercise in humans.
Introduction
The arterial baroreflex plays a key role in maintaining short-term arterial blood pressure (BP) homeostasis by adjusting efferent autonomic outflow to the heart and the peripheral vasculature. During dynamic exercise the arterial baroreflex is reset and remains operative around the prevailing BP and heart rate (HR) (Bevegard & Shepherd, 1966; Coote & Dodds, 1976; Potts et al. 1993; Papelier et al. 1994). This resetting is attributable to the actions and interactions of neural signals arising from higher brain centres (central command) (Iellamo et al. 1997; Gallagher et al. 2001b; McIlveen et al. 2001; Ogoh et al. 2002) and feedback from group III and IV sensory afferents in response to metabolic and mechanical stimuli within exercising skeletal muscles (muscle metaboreflex and mechanoreflex) (Iellamo et al. 1997; Gallagher et al. 2001c; McIlveen et al. 2001; Smith et al. 2003).
Concomitant with the resetting of the arterial baroreflex during dynamic exercise, cardiac baroreflex sensitivity (cBRS) appears to be reduced when estimated from the relationship between spontaneous fluctuations in BP and HR (Iellamo et al. 1999b; Ogoh et al. 2005; Sala-Mercado et al. 2007, 2010). Such measures of cBRS are associated with the operating point (i.e. point at which HR is regulated) of the full baroreflex stimulus–response curve, and during dynamic exercise the gain or sensitivity at the operating point is reduced (Ogoh et al. 2005; Fisher et al. 2007, 2009). This is because during dynamic exercise the operating point is relocated away from the point of maximal baroreflex sensitivity at the centre of the baroreflex function curve (i.e. centring point) and towards the reflex threshold to a locus of lesser gain (Ogoh et al. 2005). This phenomenon has been attributed to an exercise-induced reduction in cardiac parasympathetic tone (Ogoh et al. 2005); however, the precise mechanism(s) underlying the reduction in spontaneous cBRS reported during dynamic exercise is unclear. Notably, the effect of the muscle metaboreflex on spontaneous cBRS remains particularly controversial and incompletely understood.
Humans studies have reported that cBRS is unchanged during isolated activation of the muscle metaboreflex during a period of post-exercise ischaemia (PEI) following static handgrip (Spaak et al. 1998; Iellamo et al. 1999b; Cui et al. 2001; Ichinose et al. 2002; Fisher et al. 2008, 2010), calf plantar flexion (Drew et al. 2008) or single leg extensor exercise (Iellamo et al. 1999a). This technique involves the inflation of a suprasystolic pressure cuff proximal to the exercising muscles to arrest the circulation just prior to exercise cessation, thus trapping exercise metabolites within the muscle and sustaining the activation of metabolically sensitive muscle afferents (Alam & Smirk, 1937). In canines, muscle metaboreflex activation has been evoked by hypoperfusion of the exercising skeletal muscle, via inflation of a pneumatic occluder placed around the terminal aorta (Sala-Mercado et al. 2007, 2010). In contrast to studies in humans, activation of the muscle metaboreflex by hypoperfusion of the active skeletal muscles of dogs during treadmill running evokes a reduction in spontaneous cBRS (Sala-Mercado et al. 2007). Although species-related differences in cardiac autonomic control may contribute to such discrepant findings, an alternative explanation for these contrasting observations relates to methodological differences employed to activate the muscle metaboreflex and/or the exercise modality utilised.
Cardiac autonomic activity may be profoundly different when the muscle metaboreflex is isolated during PEI compared to when it is activated by flow restriction during exercise. Augmented muscle metaboreflex activation during dynamic exercise, at a time when central command and muscle mechanoreflex are also active, causes an elevation in HR due to an increase in cardiac sympathetic activity and/or reduction in cardiac parasympathetic activity (Bonde-Petersen et al. 1978; Wyss et al. 1983; Sundberg & Kaijser, 1992; O'Leary, 1993; Sun et al. 1993; Sala-Mercado et al. 2007, 2010). In contrast, HR has been shown to remain at baseline levels during isolated muscle metaboreflex activation with PEI following handgrip, single calf plantar flexion or single leg extensor exercise (no central command or muscle mechanoreflex) (Spaak et al. 1998; Iellamo et al. 1999a,b; Cui et al. 2001; Ichinose et al. 2002; Fisher et al. 2008, 2010; Drew et al. 2008). A potential explanation for this is that the robust reactivation of cardiac parasympathetic tone during PEI masks the potential tachycardic effect of an elevation in cardiac sympathetic activity (O'Leary, 1993; Fisher et al. 2010). Such differences in cardiac autonomic activity may mean that the mode of muscle metaboreflex activation (i.e. post vs. during exercise) differentially affects spontaneous cBRS, and the elevated cardiac parasympathetic tone during PEI may obscure the inhibitory actions of muscle metaboreflex activation on cBRS. Furthermore, the seminal work of Alam & Smirk (1938) identified that when PEI was used following dynamic calf plantar flexion of both legs, HR remained elevated, unlike following handgrip exercise where no change in HR from baseline was noted (Alam & Smirk, 1938). Such apparent specificity in the metaboreflex control of HR, indicates that the question of whether muscle metaboreflex activation reduces cBRS during dynamic exercise of a large muscle mass (e.g. leg cycling) cannot be accurately addressed by studies using small muscle mass exercise (e.g. handgrip).
To the authors’ knowledge it is presently unknown whether activation of the muscle metaboreflex by restricting skeletal muscle perfusion during exercise evokes a decrease in spontaneous cBRS in humans, as previously reported in canines. To address this, we used the sequence technique to calculate spontaneous cBRS during low and moderate leg cycling under conditions of both free flow and partial flow restriction, and during PEI. cBRS was also examined during rhythmic handgrip with free flow and partial flow restriction, and followed by PEI. We hypothesised that spontaneous cBRS would be decreased by metaboreflex activation during leg cycling with partially restricted flow and during PEI, but such muscle metaboreflex-mediated changes in cBRS would not be evident during handgrip.
Methods
Subjects
Fifteen male subjects volunteered to participate in the present study. Their mean age, weight and height (mean ± SD) was 22 ± 4 years, 78 ± 9 kg and 181 ± 5 cm, respectively. Smokers and subjects with a history or symptoms of cardiovascular, respiratory, metabolic or neurological disease were excluded from participation. None of the participants took any prescribed or over-the-counter medications and all were recreationally active, typically engaging in low- to moderate-intensity aerobic exercise activities 1–2 times per week. All experimental procedures were performed in accordance with the Declaration of Helsinki and received approval from the College of Life & Environmental Sciences ethical review committee at the University of Birmingham. All participants provided written informed consent for participation after receiving a detailed verbal and written explanation of the experimental procedures and measurements. Subjects refrained from caffeinated beverages, alcohol and strenuous physical activity for at least 12 h before each experimental session. Experiments were conducted in a laboratory with an ambient temperature of 22–24°C and with external stimuli minimized.
Experimental procedures
Protocol 1: Leg cycling exercise
Subjects performed leg cycling exercise in a semi-recumbent position at a constant rate of 60 revolutions per minute using a customised electrically braked cycle ergometer (Angio, Lode, Groeningen, the Netherlands). After a 3 min resting baseline period, subjects performed 10.5 min of leg cycling at a low-intensity workload (Ex90; target HR of 90 beats min−1; 26 ± 4 W), followed by 10.5 min of leg cycling at a moderate-intensity workload (Ex120; target HR of 120 beats min−1; 105 ± 7 W) (Fig. 1). The first 3.5 min of each workload were used to adjust the resistance in order to reach the target HR, following which the workload was kept constant for 7 min. Two trials were conducted in a counterbalanced order and separated by at least 30 min to ensure the baseline physiological status was re-established. In one trial, bilateral thigh cuffs were inflated to 100 mmHg (E20, Hokanson, Bellevue, WA, USA) during the last 3.5 min of each exercise workload (at low and moderate intensity) in order to partially restrict the blood flow to the exercising muscles and engage the muscle metaboreflex. The other trial served as a time control, as the thigh cuffs were not inflated and exercise was performed under free-flow conditions. Ratings of perceived exertion (RPE) were obtained during leg cycling using the standard 6–20 Borg scale (Borg, 1998). Ten seconds before the end of exercise in both trials, thigh cuff pressure was increased to 230 mmHg and a 3.5 min period of PEI was undertaken to isolate muscle metaboreflex activation. In a subset of eight subjects, two identical trials of moderate-intensity leg cycling exercise were performed in a counterbalanced order (3.5 min warm-up, followed by 3.5 min at 139 ± 14 W). In one trial a period of PEI followed exercise, while in the other trial recovery was conducted under free-flow conditions.
Figure 1. Schematic representation of experimental protocol 1, consisting of leg cycling exercise under free-flow conditions (Trial A) and with partial flow restriction (Trial B).
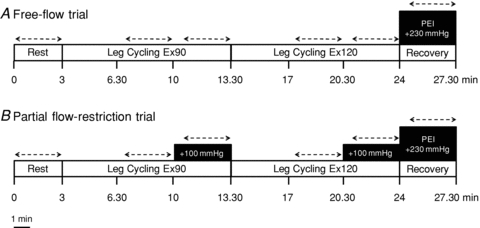
Dashed lines indicate time of data acquisition.
Protocol 2: Rhythmic handgrip exercise
Subjects performed rhythmic handgrip exercise in a semi-recumbent position using a custom-built handgrip dynamometer held in the right hand, while the arm was supported on an adjustable bedside table. Maximum voluntary contraction (MVC) was determined as the highest force produced during three to five efforts, each separated by 1 min. The force exerted by the subject during the experimental protocol, expressed as a percentage of maximum, was continuously recorded and displayed on a computer screen positioned in front of the subject at eye level. Following instrumentation and assessment of MVC, subjects rested for 15 min. Rhythmic handgrip exercise was performed at a duty cycle of 1 s contraction–2 s relaxation (20 contractions per minute) to allow blood velocity and diameter measurements during exercise (Dinenno & Joyner, 2003; Hartwich et al. 2010). After a 3 min resting baseline period, subjects performed 17.5 min of rhythmic handgrip exercise at 35% of MVC (Fig. 2). The first 3.5 min were used to attain steady-state exercise conditions. Two trials were conducted in a counterbalanced order and separated by at least 20 min, to ensure the baseline physiological status was re-established. In one trial, after 7 min of handgrip exercise a pressure-cuff around the exercising upper arm was rapidly inflated to 80 mmHg (E20, Hokanson). After 3.5 min arm cuff pressure was increased to 100 mmHg, and after a further 3.5 min arm cuff pressure was increased to 120 mmHg. This manoeuvre was performed in order to restrict the blood flow to the exercising muscles and engage the muscle metaboreflex. The other trial served as a time control, as the pressure cuff was not inflated during handgrip, and exercise was performed under free-flow conditions. Ten seconds before the end of exercise, the arm cuff pressure was increased to 230 mmHg and a 3.5 min period of PEI undertaken. An RPE was obtained during handgrip exercise using the standard 6–20 Borg scale (Borg, 1998).
Figure 2. Schematic representation of experimental protocol 2, consisting of rhythmic handgrip exercise under free-flow (Trial A) and partial flow restriction (Trial B).
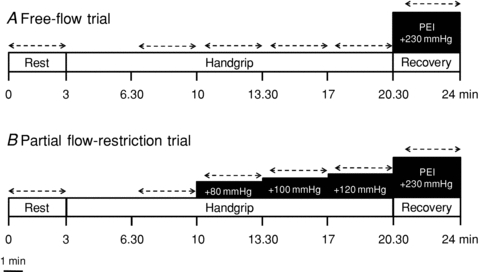
Dashed lines indicate time of data acquisition.
Experimental measurements
HR was continuously monitored using a lead II electrocardiogram (Diascope DS 512, S&W Medioteknik AS, Albertslund, Denmark). Beat-to-beat arterial BP was obtained from the finger using photoplethysmography (PortaPres Model-2, TNO Biomedical Instrumentation, Amsterdam, the Netherlands) (Imholz, 1996). In addition, brachial artery BP was measured using an automated sphygmomanometer (SunTech Tango+, SunTech Medical, Morrisville, USA) (Cameron et al. 2004). Mean BP was calculated as: mean BP = diastolic BP + 1/3(systolic BP – diastolic BP). During protocol 2 (rhythmic handgrip exercise), forearm blood flow velocity (FBV) from the brachial artery of the right arm was obtained by Doppler ultrasound (Philips Envisor, Andover, MA, USA). With an insonation angle of 60 deg maintained relative to the skin, a linear array Doppler ultrasound probe was placed on the medial aspect of the upper arm approximately 5–8 cm proximal to the antecubital fossa over the brachial artery (Dinenno & Joyner, 2003). FBV was measured in Duplex mode from the velocity waveform. Online tracing of the waveform allowed for beat-to-beat recordings of the time-averaged mean velocity, which were stored on a videotape for offline analysis. Velocity measurements were taken as an average over 10 cardiac cycles, and three measures made at each experimental phase. The diameter of the brachial artery was determined in 2-dimensional B-Mode at the end of each experimental phase and was recorded in loops over three cardiac cycles and stored on the ultrasound device for offline analysis. The average of three measurements of arterial diameter, made at diastole, was then taken as the diameter for that experimental phase (Schrage et al. 2005; Hartwich et al. 2010). During exercise, diastolic cross-sectional measurements were obtained between contractions. Forearm blood flow (FBF; ml min−1) was calculated using the formula: FBF = FBV ×π× (diameter/2)2× 60. FBF was normalized to the lean forearm mass, assessed by measurements of forearm length and circumferences corrected for skinfold thickness using established formulae (Jones & Pearson, 1969; Donato et al. 2006).
Data analysis
Physiological data were digitised at 1 kHz (1401plus, Cambridge Electronic Design, Cambridge, UK) and stored on a personal computer. Customized Spike 2 script files were used offline to determine beat-to-beat values for systolic BP, diastolic BP, mean BP, HR and R-R interval (RRI). Spontaneous cBRS was calculated using the sequence technique (Iellamo et al. 1997; Fisher et al. 2009). In brief, a customized Spike 2 script file was used to detect sequences of three or more consecutive beats, where systolic BP and RRI changed in the same direction, or systolic BP and HR changed in an opposite direction (i.e. arterial baroreflex sequences). A linear regression was applied to each individual sequence and only those sequences in which r2 was >0.85 were accepted. To estimate cardiac parasympathetic nerve activity, time domain HR variability was performed using the square root of the mean of successive differences in RRI (RMSSD) (Task Force, 1996; Fisher et al. 2010). For each trial, cBRS and cardiovascular data were averaged over a 3 min period for each of the experimental phases (indicated with dashed lines in Figs 1 and 2). Physiological data were statistically analysed using two-way repeated measures analysis of variance in which the main factors were trial (free flow or partial flow restriction) and phase (protocol 1: Rest, Ex90, Ex90 free flow or partially restricted flow, Ex120 and Ex120 free flow or partially restricted flow, PEI; protocol 2: Rest, Ex, Ex free flow or partially restricted flow +80, Ex free flow or partially restricted flow +100, and Ex free flow or partially restricted flow +120). Post hoc analysis was employed using Student's t tests with Bonferoni correction to investigate significant main effects and interactions. The level of statistical significance was set at P < 0.05. SPSS for Windows (IBM Corporation, Somers, NY, USA) was used for all statistical analyses. Data are presented as means ± SEM.
Results
Protocol 1: Leg cycling exercise
Resting HR, RRI, BP, RMSSD and cBRS were similar in both the free-flow and restricted-flow trials (Figs 3 and 4, Table 1). Dynamic leg cycling at a low-intensity workload elicited a significant increase in HR (+22 ± 2 beats min−1) and systolic BP, while diastolic BP and mean BP remained unchanged from rest (Fig. 3, Table 1). During moderate-intensity leg cycling, HR (+55 ± 3 beats min−1), systolic BP and mean BP (+12 ± 2 mmHg) were all significantly elevated above resting levels, while diastolic BP tended to fall. RRI was significantly decreased during exercise in an intensity-dependent manner (Table 1). cBRS was decreased from rest during low-intensity exercise, and further decreased from rest during moderate-intensity exercise (RRI-cBRS, 16 ± 2, 7 ± 1 and 2 ± 0.2 ms mmHg−1 at rest, low and moderate leg cycling, respectively; P < 0.05; Fig. 4). RMSSD was similarly reduced in an exercise intensity-dependent manner (Table 1). RPE was significantly increased from low- (8 ± 0.4 au) to moderate- (13 ± 0.5 au) intensity leg cycling.
Figure 3. Mean arterial blood pressure (Mean BP, A) and heart rate (HR, B) at rest and during dynamic leg cycling under free-flow conditions (open bars) and with partial flow restriction (filled bars).
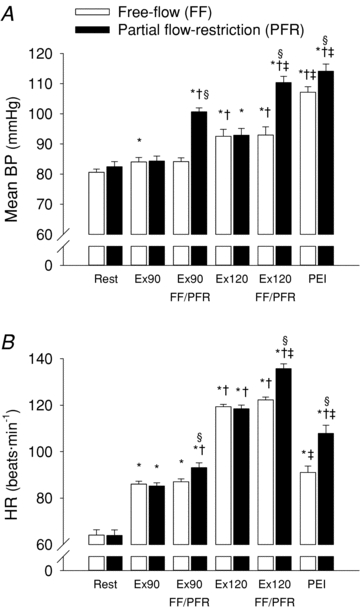
FF, free flow; PFR, partial flow restriction; BP, blood pressure; RRI, R-R interval; Ex90, leg cycling at target HR of 90 beats min−1; Ex120, leg cycling at target HR of 120 beats min−1; PEI, post-exercise ischaemia. *P < 0.05 vs. rest of corresponding trial; †P < 0.05 vs. Ex90 of corresponding trial; ‡P < 0.05 vs. Ex120 of corresponding trial; §P < 0.05 vs. FF trial at corresponding time point.
Figure 4. Spontaneous cardiac baroreflex sensitivity (cBRS) at rest and during dynamic leg cycling under free-flow conditions (open bars) and with partial flow restriction (filled bars).
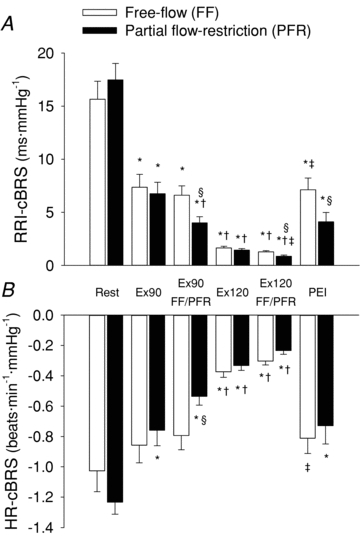
Spontaneous cBRS calculated using either R-R interval (RRI-cBRS, A) or heart rate (HR-cBRS, B). FF, free flow; PFR, partial flow restriction; BP, blood pressure; RRI, R-R interval; Ex90, leg cycling at target HR of 90 beats min−1; Ex120, leg cycling at target HR of 120 beats min−1; PEI, post-exercise ischaemia. *P < 0.05 vs. rest of corresponding trial; †P < 0.05 vs. Ex90 of corresponding trial; ‡P < 0.05 vs. Ex120 of corresponding trial; §P < 0.05 vs. FF trial at corresponding time point.
Table 1.
Selected physiological variables at rest and low and moderate leg cycling during the free-flow and partial flow restriction experimental trials
| Rest | Ex90 | Ex90 FF/PFR | Ex120 | Ex120 FF/PFR | PEI | |
|---|---|---|---|---|---|---|
| Systolic BP (mmHg) | ||||||
| FF | 121 ± 3 | 132 ± 3* | 134 ± 3* | 163 ± 5*† | 164 ± 5*† | 161 ± 5*† |
| PFR | 119 ± 2 | 132 ± 3* | 148 ± 3*†# | 163 ± 5*† | 185 ± 4*†‡# | 170 ± 4*†# |
| Diastolic BP (mmHg) | ||||||
| FF | 60 ± 1 | 60 ± 2 | 59 ± 1 | 57 ± 2 | 57 ± 2 | 82 ± 2*†‡ |
| PFR | 64 ± 2# | 62 ± 3 | 77 ± 2*†# | 58 ± 2 | 74 ± 2‡# | 86 ± 3*†‡ |
| RRI (ms) | ||||||
| FF | 961 ± 37 | 701 ± 10* | 693 ± 10* | 504 ± 5*† | 492 ± 5*†‡ | 673 ± 20*‡ |
| PFR | 965 ± 39 | 710 ± 12* | 651 ± 15*†# | 508 ± 7*† | 444 ± 7*†‡# | 569 ± 20*†‡# |
| RMSSD (ms) | ||||||
| FF | 54 ± 5 | 25 ± 4* | 24 ± 3* | 6 ± 1*† | 5 ± 1*† | 26 ± 4*‡ |
| PFR | 59 ± 6 | 26 ± 3* | 17 ± 3*†# | 6 ± 1*† | 5 ± 2*† | 16 ± 3*‡# |
Values are mean ± SEM. FF, free flow; PFR, partial flow restriction; BP, blood pressure; RRI, R-R interval; RMSSD, square root of the mean of the sum of successive differences in R-R interval; Ex90, leg cycling at target HR of 90 beats min−1; Ex120, leg cycling at target HR of 120 beats min−1; PEI, post-exercise ischaemia.
P < 0.05 vs. rest of corresponding trial
P < 0.05 vs. Ex90 of corresponding trial
P < 0.05 vs. Ex120 of corresponding trial
P < 0.05 vs. FF trial at corresponding time point.
Muscle metaboreflex activation, with partial flow restriction to the exercising skeletal muscles, evoked a significant decrease in RRI and increase in HR during both low- and moderate-intensity exercise (+6 ± 2 beats min−1 and +13 ± 2 beats min−1, P < 0.05 vs. free-flow trial; Fig. 3). Muscle metaboreflex activation also increased mean BP by +17 ± 1 mmHg and +17 ± 2 mmHg at low and moderate exercise intensities (P < 0.05 vs. free-flow trial; Fig. 4). cBRS was significantly attenuated by muscle metaboreflex activation during low- and moderate-intensity leg cycling (RRI-cBRS, –2.6 ± 0.8 and –0.4 ± 0.1 ms mmHg−1; P < 0.05 vs. free-flow trial; Figs 4 and 5). Notably, the muscle metaboreflex-mediated reduction in cBRS was more pronounced during low-intensity leg cycling than during moderate-intensity leg cycling (Fig. 5). Muscle metaboreflex activation also reduced RMSSD during low- but not moderate-intensity leg cycling (Table 1). The relationship between RRI and RRI-cBRS, and HR and HR-cBRS were non-linear (Fig. 6). The effect of dynamic exercise with or without partial flow restriction on cBRS were similar irrespective of whether cBRS was analysed using either HR or RRI. Partial flow restriction during exercise evoked a marked increase in RPE at both low (11 ± 0.5 au) and moderate (16 ± 0.4 au) exercise intensities (P < 0.05 vs. free-flow trial).
Figure 5. Change in spontaneous cardiac baroreflex sensitivity (cBRS) induced by partial flow restriction vs. free-flow trial at low (grey bars) and moderate (grey hatched bars) exercise intensities.
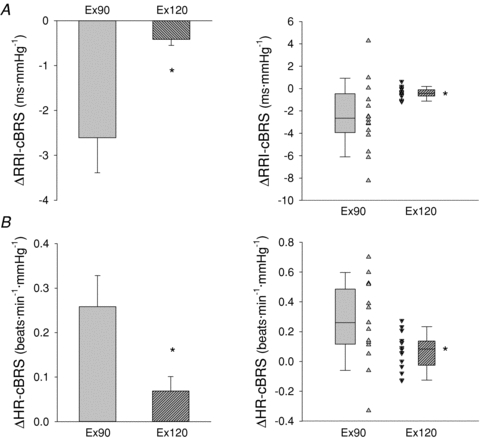
Boxes on right indicate the average reduction in cBRS at low (grey box) and moderate (grey hatched box) exercise intensities. Whiskers indicate the 5th and 95th percentile. Individual data are presented in triangles during low- (upward triangles) and moderate- (downward triangles) intensity leg cycling. Spontaneous cBRS calculated using either R-R interval (RRI-cBRS, A) or heart rate (HR-cBRS, B). *P < 0.05, significantly different from Ex90.
Figure 6. Relationship between heart rate (HR) or R-R interval (RRI) and spontaneous cardiac baroreflex sensitivity (HR-cBRS, A; RRI-cBRS, B).
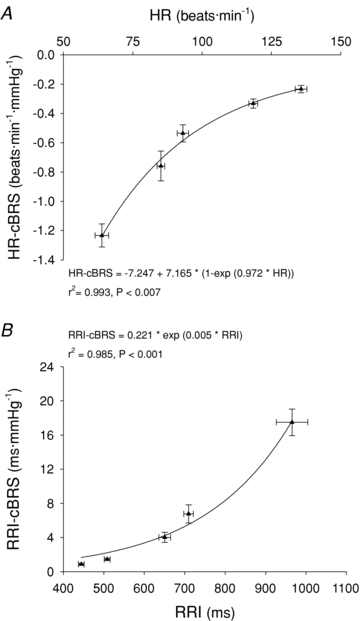
Triangles represent group average data during the partial flow restriction trial.
Isolated muscle metaboreflex activation during PEI following leg cycling with partial flow restriction maintained a pronounced elevation in mean BP (+32 ± 2 mmHg) and HR (+44 ± 3 beats min−1) from rest (P < 0.05), while cBRS remained suppressed (P < 0.05 vs. rest) irrespective of whether cBRS was analysed using HR or RRI. RMSSD also remained suppressed during PEI following leg cycling with partial flow restriction (Table 1). In the subset of subjects who performed separate trials of leg cycling under free-flow conditions followed either PEI or recovery under free-flow conditions, cBRS and RMSSD were significantly reduced during PEI (RRI-cBRS, 6 ± 2 and 15 ± 4 ms mmHg−1; RMSSD, 26 ± 12 vs. 54 ± 15 ms; P < 0.05 PEI vs. free-flow recovery). In contrast, the increase from rest in mean BP and HR was significantly greater during PEI compared to recovery under free-flow conditions (+32 ± 2 vs.+3 ± 2 mmHg, +29 ± 6 vs.+15 ± 3 beats min−1; P < 0.05 PEI vs. free-flow recovery).
Protocol 2: Rhythmic handgrip exercise
Resting BP, HR, FBF, RRI, RMSSD and cBRS were similar in both the free-flow and restricted-flow trials (Figs 7 and 8, Table 2). Rhythmic handgrip exercise elicited a significant increase in BP, HR and FBF from rest, under free-flow conditions, while RRI was reduced and cBRS remained unchanged (Figs 7 and 8, Table 2). Arm cuff inflation during rhythmic handgrip exercise evoked a graded reduction in FBF compared to the free-flow trial, such that FBF during rhythmic handgrip was reduced by –126 ± 24, –187 ± 21 and –211 ± 19 ml min−1 kg−1 during the Ex+80, Ex+100 and Ex+120 conditions (P < 0.05; equivalent to a reduction of –24 ± 4, –35 ± 2 and –38 ± 2%, from the respective free-flow value, during the Ex+80, Ex+100 and Ex+120 conditions; Fig. 7). Muscle metaboreflex activation, during incremental partial flow restriction to the exercising skeletal muscles, evoked an increase in mean BP and HR (P < 0.05 vs. free-flow trial; Fig. 7), whereas cBRS was unchanged (P > 0.05 vs. free-flow trial; Fig. 8). RPE was 10 ± 1 au during the early phase of rhythmic handgrip under free-flow conditions and increased progressively to 12 ± 1, 13 ± 1 and 15 ± 0.5 au, during the Ex+80, Ex+100 and Ex+120 conditions, respectively. Isolated muscle metaboreflex activation, during PEI following rhythmic handgrip exercise, partially maintained the exercise-induced increase in mean BP, while HR returned to baseline (Fig. 7). cBRS and RMSSD were unchanged from rest during PEI following rhythmic handgrip exercise (Table 2, Fig. 8).
Figure 7. Mean arterial blood pressure (Mean BP, A), heart rate (HR, B) and forearm blood flow (FBF, C) at rest, during rhythmic handgrip exercise under free-flow conditions (open bars) and with partial flow restriction (filled bars).
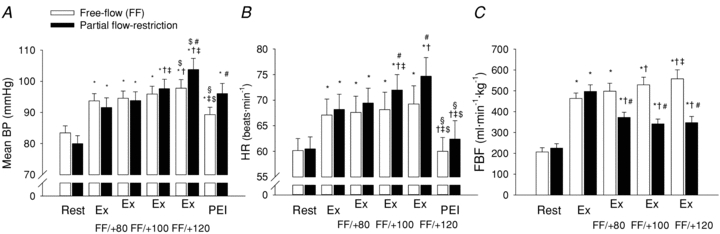
FF, free flow; PFR, partial flow restriction; BP, blood pressure; RRI, R-R interval; Ex, rhythmic handgrip exercise at 35% of maximum voluntary contraction; Ex FF/+80, Ex FF/+100 and Ex FF/+120, handgrip exercise without or with upper arm cuff inflation to 80, 100 and 120 mmHg, respectively; PEI, post-exercise ischaemia. *P < 0.05 vs. rest of corresponding trial; †P < 0.05 vs. Ex of corresponding trial; ‡P < 0.05 vs. Ex FF/+80 of corresponding trial; $P < 0.05 vs. Ex FF/+100 of corresponding trial; §P < 0.05 vs. Ex FF/+120 of corresponding trial; #P < 0.05 vs. FF trial at corresponding time point.
Figure 8. Spontaneous cardiac baroreflex sensitivity (cBRS) at rest, during handgrip exercise under free-flow conditions (open bars) and with graded partial flow restriction (filled bars).
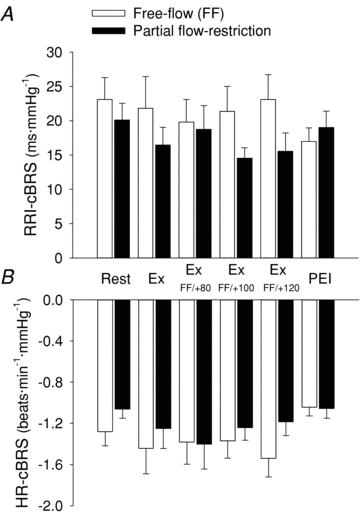
Spontaneous cBRS calculated using either R-R interval (RRI-cBRS, A) or heart rate (HR-cBRS, B). FF, free flow; PFR, partial flow restriction; RRI, R-R interval; Ex, rhythmic handgrip exercise at 35% of maximum voluntary contraction; Ex FF/+80, Ex FF/+100 and Ex FF/+120, handgrip exercise without or with upper arm cuff inflation to 80, 100 and 120 mmHg, respectively; PEI, post-exercise ischaemia. Statistical analysis using 2 × 6 repeated measures ANOVA indicated no significant main effects of phase (P = 0.486 and P = 0.246 for RRI-cBRS and HR-cBRS, respectively) and trial (P = 0.113 and P = 0.159 for RRI-cBRS and HR-cBRS, respectively) and no significant interactions (P = 0.183 and P = 0.664 for RRI-cBRS and HR-cBRS, respectively).
Table 2.
Selected physiological variables at rest, handgrip exercise and post-exercise ischaemia during the free-flow and graded partial flow restriction experimental trials
| Rest | Ex | Ex FF/+80 | Ex FF/+100 | Ex FF/+120 | PEI | |
|---|---|---|---|---|---|---|
| Systolic BP (mmHg) | ||||||
| FF | 127 ± 4 | 145 ± 4* | 146 ± 4* | 148 ± 5* | 152 ± 5*$ | 140 ± 4*§ |
| PFR | 126 ± 4 | 145 ± 5* | 149 ± 5* | 153 ± 5*†‡ | 162 ± 6*†‡$# | 152 ± 5*# |
| Diastolic BP (mmHg) | ||||||
| FF | 63 ± 2 | 72 ± 2* | 72 ± 2* | 73 ± 2* | 75 ± 2* | 68 ± 2*†‡$§ |
| PFR | 60 ± 2# | 69 ± 2*# | 71 ± 2*† | 74 ± 2*†‡ | 79 ± 3*†‡$# | 72 ± 3*# |
| RRI (ms) | ||||||
| FF | 1024 ± 44 | 927 ± 48* | 920 ± 47* | 916 ± 48* | 903 ± 48* | 1034 ± 49 †‡$§ |
| PFR | 1016 ± 40 | 906 ± 39# | 888 ± 36*† | 858 ± 36*† | 831 ± 37*†‡$ | 1009 ± 54*†‡$# |
| RMSSD (ms) | ||||||
| FF | 59 ± 6 | 60 ± 8 | 62 ± 9 | 67 ± 9 | 67 ± 9 | 61 ± 7 |
| PFR | 56 ± 6 | 58 ± 8 | 58 ± 9 | 56 ± 8 | 57 ± 9 | 63 ± 8 |
Values are mean ± SEM. FF, free flow; PFR, partial flow restriction; BP, blood pressure; RRI, R-R interval; RMSSD, square root of the mean of the sum of successive differences in R-R interval; Ex, rhythmic handgrip exercise at 35% of maximum voluntary contraction; Ex FF/+80, Ex FF/+100 and Ex FF/+120, handgrip exercise with or without upper arm cuff inflation to 80, 100 and 120 mmHg, respectively; PEI, post-exercise ischaemia.
P < 0.05 vs. rest of corresponding trial
P < 0.05 vs. Ex of corresponding trial
P < 0.05 vs. Ex FF/+80 of corresponding trial
P < 0.05 vs. Ex FF/+100 of corresponding trial
P < 0.05 vs. Ex FF/+120 of corresponding trial
P < 0.05 vs. FF trial at corresponding time point.
Discussion
The major novel findings of the present investigation are twofold. First, activation of the muscle metaboreflex during leg cycling exercise by partial restriction of blood flow to the active skeletal muscles evoked a decrease in cardiac baroreflex responsiveness (cBRS). Second, while pronounced reductions in cBRS were observed during isolated activation of the muscle metaboreflex with PEI following leg cycling exercise, cBRS was unchanged during PEI following rhythmic handgrip exercise. Collectively these data indicate that activation of metabolically sensitive muscle afferents contributes to the exercise-induced decrease in cBRS during leg cycling exercise in humans, and reveal that the effect of muscle metaboreflex activation on cBRS is specific to the exercise modality studied.
An exercise intensity-dependent decrease in cBRS was observed during dynamic leg cycling in support of previous work in dogs (Sala-Mercado et al. 2007, 2010) and humans (Ogoh et al. 2005; Iellamo et al. 1998). Such a reduction in cBRS is potentially attributable to the activation of central command, the muscle mechanoreflex and the muscle metaboreflex, which are powerful modulators of cardiac autonomic activity and baroreflex function, both independently and interactively. The aim of the present study was to investigate whether the activation of the muscle metaboreflex is a potential mechanism for the exercise-mediated reduction in spontaneous cBRS during dynamic exercise of a large muscle mass (i.e. leg cycling). Previous work in humans from our group (Fisher et al. 2008, 2010) and others (Spaak et al. 1998; Iellamo et al. 1999b; Cui et al. 2001; Ichinose et al. 2002) have demonstrated that cBRS is unchanged from baseline during isolated muscle metaboreflex during a period of PEI following handgrip exercise. This is seemingly in contrast to the findings of the present study and those undertaken in exercising canines (Sala-Mercado et al. 2007, 2010), where augmented activation of the muscle metaboreflex elicited by partial restriction of blood flow to the active skeletal muscles has been shown to reduce cBRS. Thus, it appears that methodological differences in the mode of muscle metaboreflex activation (post-exercise vs. during exercise) or indeed exercise modality can differentially affect spontaneous cBRS.
It is well established that activation of metabolically sensitive afferents elicits an increase in sympathetic nerve activity to the heart and peripheral vasculature (Mark et al. 1985; O'Leary, 1993; Fisher et al. 2010). These autonomic adjustments cause pronounced increases in HR and BP when the muscle metaboreflex is engaged by hypoperfusion of the dynamically exercising muscles using partial terminal aortic occlusion in dogs (Wyss et al. 1983; O'Leary, 1993; Sala-Mercado et al. 2007, 2010), or using lower body positive pressure in cycling humans (Bonde-Petersen et al. 1978; Sundberg & Kaijser, 1992; Sun et al. 1993). However, during isolated muscle metaboreflex activation with PEI following handgrip exercise, BP remains elevated while HR returns to resting levels (Mark et al. 1985; Fisher et al. 2008, 2010). Our group (Fisher et al. 2010) and another (O'Leary, 1993) have provided evidence to suggest that the restoration of HR from end-exercise levels under such circumstances is related to the loss of the inhibitory actions of central command and/or the muscle mechanoreflex on cardiac parasympathetic activity, at the end of exercise. In addition, the elevation of BP during PEI may facilitate the robust reactivation of cardiac parasympathetic tone via the arterial baroreflex, thus masking the potential tachycardic effects of an elevation in cardiac sympathetic activity. Thus, it is possible that during PEI the elevated cardiac parasympathetic tone obscures the inhibitory actions of muscle metaboreflex activation on cBRS. In the present study we replicated previous observations that cBRS was unchanged during PEI following handgrip exercise (Spaak et al. 1998; Iellamo et al. 1999b; Cui et al. 2001; Ichinose et al. 2002; Fisher et al. 2008, 2010), and further document that cBRS is not altered by augmented muscle metaboreflex activation during handgrip exercise (partial flow restriction). However, in contrast, we found that cBRS was attenuated by both muscle metaboreflex activation during leg cycling (partial flow restriction) and PEI following leg cycling. Our observations are congruent with the ground-breaking work of Alam & Smirk (1938), who demonstrated that HR remained elevated during PEI following dynamic calf plantar flexion of both legs, but not during PEI following handgrip (Alam & Smirk, 1938), and Blonde-Petersen et al. (1978) who demonstrated that HR also remained elevated during PEI following leg cycling exercise (Bonde-Petersen et al. 1978). Taken together, these findings indicate that when examining the interaction between the muscle metaboreflex and cBRS, the exercise modality employed may be more important than the means by which muscle metaboreflex activation is achieved. As such, one cannot accurately elucidate the effect of muscle metaboreflex activation on cBRS during dynamic exercise of a large muscle mass (e.g. leg cycling) using studies using small muscle mass exercise (e.g. handgrip). Of note, Iellamo et al. (2006) reported a maintained cBRS during PEI after leg cycling (Iellamo et al. 2006). However, these observations should be interpreted with care since the study sample comprised a unique cohort of four astronauts. As the authors acknowledge, possibly due to the timing of the experiments (e.g. close to launch) and the age of the participants (45 years compared with 22 years in the present study), resting cBRS was remarkably low (∼4 ms mmHg−1 compared with ∼16 ms mmHg−1 in the present study). This may have reduced the chances of detecting a reduction in cBRS with metaboreflex activation, and means that direct comparisons with the present study are difficult.
We used the sequence technique to provide an estimate of the integrated cBRS (i.e. carotid and aortic baroreceptors). This method uses the natural and spontaneous beat-to-beat fluctuations in BP, and the corresponding short-term, baroreflex-mediated change in HR (identified according to established criteria). There is strong evidence to suggest that spontaneous measures of cBRS predominantly represent alterations in cardiac parasympathetic efferent activity (Parlow et al. 1995; Ogoh et al. 2005; Fisher et al. 2010). Indeed, cBRS estimated using the sequence technique is virtually abolished by administration of an anti-cholinergic drug (e.g. atropine or glycopyrrolate) (Parlow et al. 1995; Fisher et al. 2010). Importantly, reductions in cBRS during dynamic exercise are also linked to cardiac parasympathetic withdrawal (Ogoh et al. 2005). Thus, it is tempting to speculate that activation of the muscle metaboreflex during leg cycling and PEI following leg cycling attenuates cBRS via a direct reduction in cardiac parasympathetic tone. O'Leary (1993) reported that hypoperfusion of the hind limbs of dogs running on a treadmill increased HR despite pharmacological blockade of β-adrenoreceptors, supporting the notion that muscle metaboreflex activation during leg cycling reduces cardiac parasympathetic activity, thus increasing HR and potentially decreasing cBRS (O'Leary, 1993; Sun et al. 1993). In the present study we observed that decreases in spontaneous cBRS and RMSSD with muscle metaboreflex activation were more marked at low-intensity exercise (HR ∼90 beats min−1) than during moderate-intensity exercise (HR ∼120 beats min−1). This possibly reflects the greater cardiac parasympathetic activity available to be inhibited at lower exercise intensities by the muscle metaboreflex (Robinson et al. 1966). Furthermore, both cBRS and RMSSD were also reduced during isolated activation of the muscle metaboreflex during PEI. Skeletal muscle afferents have been reported to converge on barosensitive cells within the nucleus tractus solitarii and cardiac vagal motoneurons within the brainstem, and can act to decrease cardiac baroreflex responsiveness (Iwamoto & Kaufman, 1987; McWilliam & Yang, 1991; Potts & Mitchell, 1998; Potts, 2006). However, the available evidence in humans indicates that those skeletal muscle afferents evoking alterations in cardiac parasympathetic activity and cBRS are mechanosensitive rather than metabolically sensitive (Gladwell & Coote, 2002; Gladwell et al. 2005). Thus, whether the muscle metaboreflex alters cBRS during leg cycling via a direct effect remains incompletely understood.
An alternative mechanism by which the bilateral thigh cuff inflation manoeuvre, used to evoke a hypoperfusion of the exercising muscle during leg cycling, may indirectly modulate cBRS is via an increase in intramuscular pressure and consequent stimulation of mechanically sensitive muscle afferents (Kaufman & Rybicki, 1987). Isolated activation of the muscle mechanoreflex by passive stretch of the calf muscles has been reported to cause a reduction in spontaneous cBRS (Drew et al. 2008) and an increase in HR (+5 ± 3 beats min−1) due to a reduction in cardiac parasympathetic nerve activity (Gladwell & Coote, 2002; Gladwell et al. 2005). However, an increase in HR with passive stretch was not evident when cardiac parasympathetic tone was reduced with either glycopyrrolate administration, mild rhythmic handgrip exercise or carotid baroreceptor unloading (Gladwell et al. 2005). Therefore, it is unlikely that mechanoreflex activation could wholly account for the reduction in cBRS observed in the present study when blood flow was obstructed to the exercising muscles during low and moderate leg cycling (target HR of 90 and 120 beats min−1, respectively), as cardiac parasympathetic tone would be expected to be significantly withdrawn at these workloads (Robinson et al. 1966). Furthermore, it has been suggested that stretch-sensitive group III and IV muscle afferents are a distinct population from those activated by muscular contraction (Hayes et al. 2005). Muscle mechanoreflex activation has also been experimentally elicited by external calf muscle compression (Bell & White, 2005): however, the cuff inflation pressure previously used was much greater than in the present study (300 vs. 100 mmHg) and did not cause any HR increase. Muscle afferent responses to mechanical stimuli are also suggested to be augmented by concomitant accumulation of metabolites in the interstitium (Kaufman & Rybicki, 1987). Thus, augmenting metabolite accumulation within the exercising muscle may increase the firing of mechanosensitive muscle afferents. In the present study, we observed HR and BP increases with bilateral thigh cuff inflation during leg cycling, in excess of the modest cardiovascular responses previously observed in response to experimental sensitization of muscle mechanoreflex (Middlekauff & Chiu, 2004; Bell & White, 2005; Fisher et al. 2005; Cui et al. 2008; Drew et al. 2008). Furthermore, we observed that cBRS was attenuated during PEI following leg cycling, when the exercise-induced activation of mechanically sensitive muscle afferents was presumably absent. In light of these collective findings we suggest that the activation of mechanically sensitive muscle afferents is unlikely to explain the decrease in cBRS with partial restriction of blood flow to the active skeletal muscles during leg cycling observed in the present study; nevertheless, we cannot definitively rule out their possible contribution.
An alternative explanation for the reduction in cardiac baroreflex responsiveness elicited by hypoperfusion of the active skeletal muscles during leg cycling is an increase in central command. It is possible that bilateral thigh cuff inflation during leg cycling may decrease mechanical efficiency, thus altering motor unit recruitment strategies and augmenting central command. Moreover, skeletal muscle afferent feedback may exert an inhibitory influence to spinal and supraspinal areas of the central nervous system (Gandevia, 2001). Activation of metabolically sensitive muscle afferents during exercise may inhibit alpha motor neurons innervating the skeletal muscle, reducing their excitability and meaning that additional central drive is required to maintain the requisite exercise intensity (Amann et al. 2009). In support of this, we observed that RPE, a measure of the participants’ sense of effort, historically related to central command (Mitchell, 1990), was significantly increased by bilateral thigh cuff inflation during leg cycling. Central command predominantly alters HR in humans via withdrawal of parasympathetic tone (Mitchell et al. 1989), and evokes a movement of the operating point towards the threshold of the carotid baroreflex function curve (i.e. a point of reduced sensitivity) (Gallagher et al. 2001b; Ogoh et al. 2002). As such, a muscle afferent-induced increase in central command provides a plausible explanation for the further reduction in parasympathetic tone and spontaneous cBRS observed during partial flow restriction during leg cycling in the present study. However, it should be noted that in exercising dogs, muscle afferent blockade abolishes the cardiovascular response to unilateral iliac arterial occlusion during exercise (Pomeroy et al. 1986; Kozelka et al. 1987). Whether such observations can be translated to humans remains unclear. Thus, on the basis of the available evidence, it is possible that muscle metaboreflex activation may indirectly reduce spontaneous cBRS during leg cycling in humans via an increase in central neural drive.
It is possible that muscle metaboreflex-mediated elevations in plasma noradrenaline may attenuate parasympathetic control of HR and consequently reduce cBRS (Miyamoto et al. 2003). Miyamoto et al. (2003) demonstrated that spontaneous cBRS was attenuated by noradrenaline infusion during vagus nerve stimulation in anaesthetized rabbits. Although a directly comparable study has not been performed in humans, Taylor et al. (2001) reported that administration of the β-adrenergic receptor antagonist atenolol augmented respiratory sinus arrhythmia, indicating that cardiac sympathetic nerve activity may oppose cardiac parasympathetic nerve activity in resting humans. However, Ogoh et al. (2005) demonstrated that β-adrenergic blockade has a minimal affect on spontaneous cBRS during dynamic exercise. As such, we feel that it is unlikely that muscle metaboreflex-mediated sympathoexcitation reduced cBRS in the present study.
Since the sequence technique involves analysis of spontaneous fluctuations in BP and HR (or RRI), we have been unable to evaluate the full arterial baroreflex stimulus–response curve. For this reason, we cannot conclude whether the maximal sensitivity of the baroreflex has been manipulated by our intervention, or whether the operating point of the reflex has shifted to a non-linear region of the baroreflex function curve. It has been suggested that spontaneous measures of cBRS provide the same data as the sensitivity of the baroreflex at the operating point (Parati et al. 2000; Ogoh et al. 2005). For this reason, our findings are important as they imply that the physiologically active region of the baroreflex (i.e. the operating point) operates with a reduced sensitivity during dynamic exercise, possibly due to activation of metabolically sensitive muscle afferents.
A reduction in blood flow to the exercising skeletal muscles has been effectively shown to evoke increases in HR and BP using either graded clamping of the terminal aorta blood flow in treadmill running dogs (Sheriff et al. 1993; Sala-Mercado et al. 2007, 2010) or non-invasively using lower body positive pressure in cycling humans (Eiken et al. 1992; Sundberg & Kaijser, 1992; Sun et al. 1993; Gallagher et al. 2001a). In the present study, a comparable cardiovascular response was elicited by bilateral thigh cuff inflation to 100 mmHg during dynamic leg cycling exercise. Previous reports indicate that this manoeuvre evokes a reduction in limb blood flow, a mismatch between oxygen delivery and demand, an accumulation of workload-related muscle metabolites and the activation of metabolically sensitive skeletal muscle afferents (Eiken & Bjurstedt, 1987; Eiken et al. 1992). Our measurements of FBF during rhythmic handgrip confirmed that inflation of a cuff proximal to the exercising muscles significantly reduced their perfusion. As we did not have access to a suitable methodology (e.g. femoral venous thermodilution) we were unable to measure leg blood flow during cycling exercise, and thus a limitation of the present study is that we are not able to quantify or compare the magnitude of the flow restriction during low and moderate cycling workloads. In addition, we acknowledge that restriction of venous outflow from the exercising muscle via thigh cuff inflation to 100 mmHg is also likely to evoke venous congestion, which may stimulate mechanosensitive afferents located in the walls of the vasculature within the skeletal muscle (McClain et al. 1993; Haouzi et al. 1999; Cui et al. 2009). Although stimulation of such sensory afferents has been shown to evoke a cardiovascular response (McClain et al. 1993; Haouzi et al. 1999; Cui et al. 2009), it is presently unclear if they modulate cBRS.
The present study examines the effect of muscle metaboreflex activation on the arterial baroreflex; however, it is recognised that this is a two-way interaction. Work by Waldrop & Mitchell (1985) and Sheriff et al. (1990) indicated that the arterial baroreflex attenuates the pressor response evoked by muscle afferent activation (Waldrop & Mitchell, 1985; Sheriff et al. 1990). More recently, Kim et al. (2005) demonstrated that following barodeinervation (in dogs) the muscle metaboreflex-induced increase in cardiac output was attenuated (due to a decrease in stroke volume), and that the pressor response was greater, as compared to the baro-intact condition (Kim et al. 2005). Further studies in humans are required to examine the impact of the arterial baroreflex on the strength and mechanisms by which the muscle metaboreflex modulates BP during exercise (i.e. cardiac output vs. total peripheral resistance).
In chronic disease conditions characterised by a hypoperfusion of the active skeletal muscles (e.g. peripheral vascular disease, chronic heart failure) the muscle metaboreflex is not activated in isolation from central command and the muscle mechanoreflex. Thus, experimental reductions in muscle blood flow to augment the muscle metaboreflex during exercise may more realistically mimic such clinical conditions. As such, in light of the cardioprotective properties of parasympathetic tone (Billman, 2006), the muscle metaboreflex-mediated reduction in cBRS observed in the present study may have clinical significance for patient populations in whom increased muscle metaboreflex sensitivity (Piepoli et al. 1996; Scott et al. 2002) and decreased cBRS (Grassi et al. 1995) have been identified.
In summary, the findings of the present study indicate that the activation of the muscle metaboreflex during dynamic leg cycling exercise using partial restriction of the blood flow to the active skeletal muscles, or during PEI, elicits a decrease in cardiac baroreflex responsiveness. Overall, the present findings suggest that the activation of metabolically sensitive muscle afferents plays an important role in the decrease in cBRS during dynamic exercise involving a large muscle mass in humans.
Acknowledgments
The time and effort expended by all volunteer subjects is greatly appreciated. We thank Dr David McIntyre, for writing Spike 2 script files and Tyrone Kerr and Tom Grice for their excellent technical assistance.
Glossary
Abbreviations
- BP
blood pressure
- cBRS
cardiac baroreflex sensitivity
- ECG
electrocardiogram
- Ex90/120
leg cycling at target heart rate of 90/120 beats min−1
- Ex +80/+100/+120
rhythmic handgrip exercise with arm cuff at 80/100/120 mmHg
- FBF
forearm blood flow
- FBV
forearm blood velocity
- HR
heart rate
- HR-cBRS
spontaneous cardiac baroreflex sensitivity calculated using heart rate
- FF
free flow
- MVC
maximum voluntary contraction
- PEI
post-exercise ischaemia
- PFR
partial flow restriction
- RMSSD
square root of the mean of successive differences in R-R interval
- RPE
rating of perceived exertion
- RRI
R-R interval
- RRI-cBRS
spontaneous cardiac baroreflex sensitivity calculated using R-R interval
Author contributions
D.H. contributed to study design, data acquisition, data analysis, data interpretation, writing the first draft and critical review of the manuscript. W.E.D. contributed to study design, data acquisition, data analysis and interpretation and writing the first draft of the manuscript. J.L.W. contributed to study design, data acquisition and data analysis. J.P.F. contributed to study design, data acquisition, data interpretation, writing the first draft and critical review of the manuscript. All authors approved the final version of the manuscript.
References
- Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol. 1937;89:372–383. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M, Smirk FH. Observations in man on a pulse-accelerating reflex from the voluntary muscles of the legs. J Physiol. 1938;92:167–177. doi: 10.1113/jphysiol.1938.sp003592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol. 2009;587:271–283. doi: 10.1113/jphysiol.2008.163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MP, White MJ. Cardiovascular responses to external compression of human calf muscle vary during graded metaboreflex stimulation. Exp Physiol. 2005;90:383–391. doi: 10.1113/expphysiol.2004.029140. [DOI] [PubMed] [Google Scholar]
- Bevegard BS, Shepherd JT. Circulatory effects of stimulating the carotid arterial stretch receptors in man at rest and during exercise. J Clin Invest. 1966;45:132–142. doi: 10.1172/JCI105317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billman GE. A comprehensive review and analysis of 25 years of data from an in vivo canine model of sudden cardiac death: Implications for future anti-arrhythmic drug development. Pharmacol Ther. 2006;111:808–835. doi: 10.1016/j.pharmthera.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Bonde-Petersen F, Rowell LB, Murray RG, Blomqvist GG, White R, Karlsson E, Campbell W, Mitchell JH. Role of cardiac output in the pressor responses to graded muscle ischemia in man. J Appl Physiol. 1978;45:574–580. doi: 10.1152/jappl.1978.45.4.574. [DOI] [PubMed] [Google Scholar]
- Borg GA. Human Kinetics. Champaign, IL: 1998. Borg's perceived exertion and pain scales. [Google Scholar]
- Cameron JD, Stevenson I, Reed E, McGrath BP, Dart AM, Kingwell BA. Accuracy of automated auscultatory blood pressure measurement during supine exercise and treadmill stress electrocardiogram-testing. Blood Press Monit. 2004;9:269–275. doi: 10.1097/00126097-200410000-00007. [DOI] [PubMed] [Google Scholar]
- Coote JH, Dodds WN. Baroreceptor reflex and cardiovascular changes associated with sustained muscular-contraction in cat. Pflugers Arch. 1976;363:167–173. doi: 10.1007/BF01062286. [DOI] [PubMed] [Google Scholar]
- Cui J, McQuillan P, Moradkhan R, Pagana C, Sinoway LI. Sympathetic responses during saline infusion into the veins of an occluded limb. J Physiol. 2009;587:3619–3628. doi: 10.1113/jphysiol.2009.173237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Mascarenhas V, Moradkhan R, Blaha C, Sinoway LI. Effects of muscle metabolites on responses of muscle sympathetic nerve activity to mechanoreceptor(s) stimulation in healthy humans. Am J Physiol Regul Integr Comp Physiol. 2008;294:R458–R466. doi: 10.1152/ajpregu.00475.2007. [DOI] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Shibasaki M, Hodges NA, Crandall CG. Baroreflex modulation of muscle sympathetic nerve activity during posthandgrip muscle ischemia in humans. J Appl Physiol. 2001;91:1679–1686. doi: 10.1152/jappl.2001.91.4.1679. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: Is nitric oxide obligatory? J Physiol. 2003;553:281–292. doi: 10.1113/jphysiol.2003.049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol. 2006;290:H272–H278. doi: 10.1152/ajpheart.00405.2005. [DOI] [PubMed] [Google Scholar]
- Drew RC, Bell MP, White MJ. Modulation of spontaneous baroreflex control of heart rate and indexes of vagal tone by passive calf muscle stretch during graded metaboreflex activation in humans. J Appl Physiol. 2008;104:716–723. doi: 10.1152/japplphysiol.00956.2007. [DOI] [PubMed] [Google Scholar]
- Eiken O, Bjurstedt H. Dynamic exercise in man as influenced by experimental restriction of blood flow in the working muscles. Acta Physiol Scand. 1987;131:339–345. doi: 10.1111/j.1748-1716.1987.tb08248.x. [DOI] [PubMed] [Google Scholar]
- Eiken O, Convertino VA, Doerr DF, Dudley GA, Morariu G, Mekjavic IB. Characteristics of the carotid baroreflex in man during normal and flow-restricted exercise. Acta Physiol Scand. 1992;144:325–331. doi: 10.1111/j.1748-1716.1992.tb09301.x. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Ogoh S, Ahmed A, Aro MR, Gute D, Fadel PJ. Influence of age on cardiac baroreflex function during dynamic exercise in humans. Am J Physiol Heart Circ Physiol. 2007;293:H777–H783. doi: 10.1152/ajpheart.00199.2007. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Ogoh S, Junor C, Khaja A, Northrup M, Fadel PJ. Spontaneous baroreflex measures are unable to detect age-related impairments in cardiac baroreflex function during dynamic exercise in humans. Exp Physiol. 2009;94:447–458. doi: 10.1113/expphysiol.2008.044867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JP, Sander M, MacDonald I, White MJ. Decreased muscle sympathetic nerve activity does not explain increased vascular conductance during contralateral isometric exercise in humans. Exp Physiol. 2005;90:377–382. doi: 10.1113/expphysiol.2004.028761. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Seifert T, Hartwich D, Young CN, Secher NH, Fadel PJ. Autonomic control of heart rate by metabolically sensitive skeletal muscle afferents in humans. J Physiol. 2010;588:1117–1127. doi: 10.1113/jphysiol.2009.185470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JP, Young CN, Fadel PJ. Effect of muscle metaboreflex activation on carotid-cardiac baroreflex function in humans. Am J Physiol Heart Circ Physiol. 2008;294:H2296–H2304. doi: 10.1152/ajpheart.91497.2007. [DOI] [PubMed] [Google Scholar]
- Gallagher KM, Fadel PJ, Smith SA, Norton KH, Querry RG, Olivencia-Yurvati A, Raven PB. Increases in intramuscular pressure raise arterial blood pressure during dynamic exercise. J Appl Physiol. 2001a;91:2351–2358. doi: 10.1152/jappl.2001.91.5.2351. [DOI] [PubMed] [Google Scholar]
- Gallagher KM, Fadel PJ, Stromstad M, Ide K, Smith SA, Querry RG, Raven PB, Secher NH. Effects of partial neuromuscular blockade on carotid baroreflex function during exercise in humans. J Physiol. 2001b;533:861–870. doi: 10.1111/j.1469-7793.2001.t01-1-00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher KM, Fadel PJ, Stromstad M, Ide K, Smith SA, Querry RG, Raven PB, Secher NH. Effects of exercise pressor reflex activation on carotid baroreflex function during exercise in humans. J Physiol. 2001c;533:871–880. doi: 10.1111/j.1469-7793.2001.t01-2-00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Gladwell VF, Coote JH. Heart rate at the onset of muscle contraction and during passive muscle stretch in humans: A role for mechanoreceptors. J Physiol. 2002;540:1095–1102. doi: 10.1113/jphysiol.2001.013486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwell VF, Fletcher J, Patel N, Elvidge LJ, Lloyd D, Chowdhary S, Coote JH. The influence of small fibre muscle mechanoreceptors on the cardiac vagus in humans. J Physiol. 2005;567:713–721. doi: 10.1113/jphysiol.2005.089243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Cattaneo BM, Lanfranchi A, Vailati S, Giannattasio C, Delbo A, Sala C, Bolla GB, Pozzi M, Mancia G. Sympathetic activation and loss of reflex sympathetic control in mild congestive-heart-failure. Circulation. 1995;92:3206–3211. doi: 10.1161/01.cir.92.11.3206. [DOI] [PubMed] [Google Scholar]
- Haouzi P, Hill JM, Lewis BK, Kaufman MP. Responses of group iii and iv muscle afferents to distension of the peripheral vascular bed. J Appl Physiol. 1999;87:545–553. doi: 10.1152/jappl.1999.87.2.545. [DOI] [PubMed] [Google Scholar]
- Hartwich D, Fowler KL, Wynn LJ, Fisher JP. Differential responses to sympathetic stimulation in the cerebral and brachial circulations during rhythmic handgrip exercise in humans. Exp Physiol. 2010;95:1089–1097. doi: 10.1113/expphysiol.2010.054387. [DOI] [PubMed] [Google Scholar]
- Hayes SG, Kindig AE, Kaufman MP. Comparison between the effect of static contraction and tendon stretch on the discharge of group iii and iv muscle afferents. J Appl Physiol. 2005;99:1891–1896. doi: 10.1152/japplphysiol.00629.2005. [DOI] [PubMed] [Google Scholar]
- Ichinose M, Saito M, Wada H, Kitano A, Kondo N, Nishiyasu T. Modulation of arterial baroreflex dynamic response during muscle metaboreflex activation in humans. J Physiol. 2002;544:939–948. doi: 10.1113/jphysiol.2002.024794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iellamo F, Di Rienzo M, Lucini D, Legramante JM, Pizzinelli P, Castiglioni P, Pigozzi F, Pagani M, Parati G. Muscle metaboreflex contribution to cardiovascular regulation during dynamic exercise in microgravity: Insights from mission STS-107 of the space shuttle Columbia. J Physiol. 2006;572:829–838. doi: 10.1113/jphysiol.2005.102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iellamo F, Legramante JM, Raimondi G, Peruzzi G. Baroreflex control of sinus node during dynamic exercise in humans: Effects of central command and muscle reflexes. Am J Physiol Heart Circ Physiol. 1997;272:H1157–H1164. doi: 10.1152/ajpheart.1997.272.3.H1157. [DOI] [PubMed] [Google Scholar]
- Iellamo F, Massaro M, Legramante JM, Raimondi G, Peruzzi G, Galante A. Spontaneous baroreflex modulation of heart rate during incremental exercise test in humans. FASEB J. 1998;12:4012. [Google Scholar]
- Iellamo F, Massaro M, Raimondi G, Peruzzi G, Legramante JM. Role of muscular factors in cardiorespiratory responses to static exercise: Contribution of reflex mechanisms. J Appl Physiol. 1999a;86:174–180. doi: 10.1152/jappl.1999.86.1.174. [DOI] [PubMed] [Google Scholar]
- Iellamo F, Pizzinelli P, Massaro M, Raimondi G, Peruzzi G, Legramante JM. Muscle metaboreflex contribution to sinus node regulation during static exercise: Insights from spectral analysis of heart rate variability. Circulation. 1999b;100:27–32. doi: 10.1161/01.cir.100.1.27. [DOI] [PubMed] [Google Scholar]
- Imholz BP. Automated blood pressure measurement during ergometric stress testing: Possibilities of Finapres. Z Kardiol. 1996;85:76–80. [PubMed] [Google Scholar]
- Iwamoto GA, Kaufman MP. Caudal ventrolateral medullary cells responsive to muscular contraction. J Appl Physiol. 1987;62:149–157. doi: 10.1152/jappl.1987.62.1.149. [DOI] [PubMed] [Google Scholar]
- Jones PR, Pearson J. Anthropometric determination of leg fat and muscle plus bone volumes in young male and female adults. J Physiol. 1969;204:63P–66P. [PubMed] [Google Scholar]
- Kaufman MP, Rybicki KJ. Discharge properties of group iii and iv muscle afferents: Their responses to mechanical and metabolic stimuli. Circ Res. 1987;61:I60–I65. [PubMed] [Google Scholar]
- Kim JK, Sala-Mercado JA, Rodriguez J, Scislo TJ, O'Leary DS. Arterial baroreflex alters strength and mechanisms of muscle metaboreflex during dynamic exercise. Am J Physiol Heart Circ Physiol. 2005;288:H1374–H1380. doi: 10.1152/ajpheart.01040.2004. [DOI] [PubMed] [Google Scholar]
- Kozelka JW, Christy GW, Wurster RD. Ascending pathways mediating somatoautonomic reflexes in exercising dogs. J Appl Physiol. 1987;62:1186–1191. doi: 10.1152/jappl.1987.62.3.1186. [DOI] [PubMed] [Google Scholar]
- McClain J, Hardy C, Enders B, Smith M, Sinoway L. Limb congestion and sympathoexcitation during exercise. Implications for congestive heart failure. J Clin Invest. 1993;92:2353–2359. doi: 10.1172/JCI116840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlveen SA, Hayes SG, Kaufman MP. Both central command and exercise pressor reflex reset carotid sinus baroreflex. Am J Physiol Heart Circ Physiol. 2001;280:H1454–H1463. doi: 10.1152/ajpheart.2001.280.4.H1454. [DOI] [PubMed] [Google Scholar]
- McWilliam PN, Yang T. Inhibition of cardiac vagal component of baroreflex by group iii and iv afferents. Am J Physiol Heart Circ Physiol. 1991;260:H730–H734. doi: 10.1152/ajpheart.1991.260.3.H730. [DOI] [PubMed] [Google Scholar]
- Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res. 1985;57:461–469. doi: 10.1161/01.res.57.3.461. [DOI] [PubMed] [Google Scholar]
- Middlekauff HR, Chiu J. Cyclooxygenase products sensitize muscle mechanoreceptors in healthy humans. Am J Physiol Heart Circ Physiol. 2004;287:H1944–H1949. doi: 10.1152/ajpheart.00329.2004. [DOI] [PubMed] [Google Scholar]
- Mitchell JH. Neural control of the circulation during exercise. Med Sci Sports Exerc. 1990;22:141–154. [PubMed] [Google Scholar]
- Mitchell JH, Reeves DR, Jr, Rogers HB, Secher NH, Victor RG. Autonomic blockade and cardiovascular responses to static exercise in partially curarized man. J Physiol. 1989;413:433–445. doi: 10.1113/jphysiol.1989.sp017662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Kawada T, Takaki H, Inagaki M, Yanagiya Y, Jin Y, Sugimachi M, Sunagawa K. High plasma norepinephrine attenuates the dynamic heart rate response to vagal stimulation. Am J Physiol Heart Circ Physiol. 2003;284:H2412–H2418. doi: 10.1152/ajpheart.00660.2002. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Fisher JP, Dawson EA, White MJ, Secher NH, Raven PB. Autonomic nervous system influence on arterial baroreflex control of heart rate during exercise in humans. J Physiol. 2005;566:599–611. doi: 10.1113/jphysiol.2005.084541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogoh S, Wasmund WL, Keller DM, A O-Yurvati, Gallagher KM, Mitchell JH, Raven PB. Role of central command in carotid baroreflex resetting in humans during static exercise. J Physiol. 2002;543:349–364. doi: 10.1113/jphysiol.2002.019943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary DS. Autonomic mechanisms of muscle metaboreflex control of heart rate. J Appl Physiol. 1993;74:1748–1754. doi: 10.1152/jappl.1993.74.4.1748. [DOI] [PubMed] [Google Scholar]
- Papelier Y, Escourrou P, Gauthier JP, Rowell LB. Carotid baroreflex control of blood pressure and heart rate in men during dynamic exercise. J Appl Physiol. 1994;77:502–506. doi: 10.1152/jappl.1994.77.2.502. [DOI] [PubMed] [Google Scholar]
- Parati G, Di Rienzo M, Mancia G. How to measure baroreflex sensitivity: From the cardiovascular laboratory to daily life. J Hypertens. 2000;18:7–19. [PubMed] [Google Scholar]
- Parlow J, Viale JP, Annat G, Hughson R, Quintin L. Spontaneous cardiac baroreflex in humans – comparison with drug-induced responses. Hypertension. 1995;25:1058–1068. doi: 10.1161/01.hyp.25.5.1058. [DOI] [PubMed] [Google Scholar]
- Piepoli M, Clark AL, Volterrani M, Adamopoulos S, Sleight P, Coats AJS. Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure – effects of physical training. Circulation. 1996;93:940–952. doi: 10.1161/01.cir.93.5.940. [DOI] [PubMed] [Google Scholar]
- Pomeroy G, Ardell JL, Wurster RD. Spinal opiate modulation of cardiovascular reflexes in the exercising dog. Brain Res. 1986;381:385–389. doi: 10.1016/0006-8993(86)90095-8. [DOI] [PubMed] [Google Scholar]
- Potts JT. Inhibitory neurotransmission in the nucleus tractus solitarii: Implications for baroreflex resetting during exercise. Exp Physiol. 2006;91:59–72. doi: 10.1113/expphysiol.2005.032227. [DOI] [PubMed] [Google Scholar]
- Potts JT, Mitchell JH. Rapid resetting of carotid baroreceptor reflex by afferent input from skeletal muscle receptors. Am J Physiol Heart Circ Physiol. 1998;275:H2000–H2008. doi: 10.1152/ajpheart.1998.275.6.H2000. [DOI] [PubMed] [Google Scholar]
- Potts JT, Shi XR, Raven PB. Carotid baroreflex responsiveness during dynamic exercise in humans. Am J Physiol Heart Circ Physiol. 1993;265:H1928–H1938. doi: 10.1152/ajpheart.1993.265.6.H1928. [DOI] [PubMed] [Google Scholar]
- Robinson BF, Epstein SE, Beiser GD, Braunwal E. Control of heart rate by autonomic nervous system – studies in man on interrelation between baroreceptor mechanisms and exercise. Circ Res. 1966;19:400–411. doi: 10.1161/01.res.19.2.400. [DOI] [PubMed] [Google Scholar]
- Sala-Mercado JA, Ichinose M, Coutsos M, Li Z, Fano D, Ichinose T, Dawe EJ, O'Leary DS. Progressive muscle metaboreflex activation gradually decreases spontaneous heart rate baroreflex sensitivity during dynamic exercise. Am J Physiol Heart Circ Physiol. 2010;298:H594–H600. doi: 10.1152/ajpheart.00908.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala-Mercado JA, Ichinose M, Hammond RL, Ichinose T, Pallante M, Stephenson LW, O'Leary DS, Iellamo F. Muscle metaboreflex attenuates spontaneous heart rate baroreflex sensitivity during dynamic exercise. Am J Physiol Heart Circ Physiol. 2007;292:H2867–H2873. doi: 10.1152/ajpheart.00043.2007. [DOI] [PubMed] [Google Scholar]
- Schrage WG, Wilkins BW, Dean VL, Scott JP, Henry NK, Wylam ME, Joyner MJ. Exercise hyperemia and vasoconstrictor responses in humans with cystic fibrosis. J Appl Physiol. 2005;99:1866–1871. doi: 10.1152/japplphysiol.00616.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott AC, Davies LC, Coats AJS, Piepoli M. Relationship of skeletal muscle metaboreceptors in the upper and lower limbs with the respiratory control in patients with heart failure. Clin Sci. 2002;102:23–30. [PubMed] [Google Scholar]
- Sheriff DD, O'Leary DS, Scher AM, Rowell LB. Baroreflex attenuates pressor response to graded muscle ischemia in exercising dogs. Am J Physiol Heart Circ Physiol. 1990;258:H305–H310. doi: 10.1152/ajpheart.1990.258.2.H305. [DOI] [PubMed] [Google Scholar]
- Sheriff DD, Rowell LB, Scher AM. Is rapid rise in vascular conductance at onset of dynamic exercise due to muscle pump? Am J Physiol Heart Circ Physiol. 1993;265:H1227–H1234. doi: 10.1152/ajpheart.1993.265.4.H1227. [DOI] [PubMed] [Google Scholar]
- Smith SA, Querry RG, Fadel PJ, Gallagher KM, Stromstad M, Ide K, Raven PB, Secher NH. Partial blockade of skeletal muscle somatosensory afferents attenuates baroreflex resetting during exercise in humans. J Physiol. 2003;551:1013–1021. doi: 10.1113/jphysiol.2003.044925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaak J, Sundblad P, Linnarsson D. Human carotid baroreflex during isometric lower arm contraction and ischemia. Am J Physiol Heart Circ Physiol. 1998;275:H940–H945. doi: 10.1152/ajpheart.1998.275.3.H940. [DOI] [PubMed] [Google Scholar]
- Sun JC, Eiken O, Mekjavic IB. Autonomic nervous control of heart rate during blood-flow restricted exercise in man. Eur J Appl Physiol Occup Physiol. 1993;66:202–206. doi: 10.1007/BF00235094. [DOI] [PubMed] [Google Scholar]
- Sundberg CJ, Kaijser L. Effects of graded restriction of perfusion on circulation and metabolism in the working leg; quantification of a human ischaemia-model. Acta Physiol Scand. 1992;146:1–9. doi: 10.1111/j.1748-1716.1992.tb09386.x. [DOI] [PubMed] [Google Scholar]
- Task Force. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Taylor JA, Myers CW, Halliwill JR, Seidel H, Eckberg DL. Sympathetic restraint of respiratory sinus arrhythmia: Implications for vagal-cardiac tone assessment in humans. Am J Physiol Heart Circ Physiol. 2001;280:H2804–H2814. doi: 10.1152/ajpheart.2001.280.6.H2804. [DOI] [PubMed] [Google Scholar]
- Waldrop TG, Mitchell JH. Effects of barodenervation on cardiovascular-responses to static muscular-contraction. Am J Physiol Heart Circ Physiol. 1985;249:H710–H714. doi: 10.1152/ajpheart.1985.249.4.H710. [DOI] [PubMed] [Google Scholar]
- Wyss CR, Ardell JL, Scher AM, Rowell LB. Cardiovascular-responses to graded reductions in hindlimb perfusion in exercising dogs. Am J Physiol Heart Circ Physiol. 1983;245:H481–H486. doi: 10.1152/ajpheart.1983.245.3.H481. [DOI] [PubMed] [Google Scholar]


