Abstract
Non-technical summary
The cardiovascular response to exercise is exaggerated in hypertension. This heightened circulatory responsiveness increases the risk of occurrence of an adverse cardiovascular event during and immediately following a bout of exercise. Accumulating evidence suggests the muscle metaboreflex, a chemically sensitive peripheral reflex originating in skeletal muscle, contributes significantly to this abnormal cardiovascular response to exercise. However, its role remains controversial. In addition, the receptor mechanisms underlying metaboreflex dysfunction in hypertension remain undetermined. To this end, the current investigation demonstrates that the metaboreflex is overactive in hypertensive rats eliciting exaggerated increases in sympathetic nerve activity and blood pressure. Importantly, the study shows, for the first time, that the metaboreflex dysfunction manifest in hypertension is mediated, in part, by activation of the skeletal muscle TRPv1 receptor. As such, the investigation identifies the muscle metaboreflex, specifically the TRPv1 receptor, as a potential target for the treatment of cardiovascular hyperexcitability during exercise in hypertension.
Abstract
The circulatory response to exercise is exaggerated in hypertension potentially increasing the risk for adverse cardiovascular events. Evidence suggests the skeletal muscle metaboreflex contributes to this abnormal circulatory response. However, as the sensitivity of this reflex has been reported to be both reduced and potentiated in hypertension, its role remains controversial. In addition, the receptor mechanisms underlying muscle metaboreflex dysfunction in this disease remain undetermined. To address these issues, metaboreflex activity was assessed during ‘supra-stimulation’ of the reflex via ischaemic hindlimb muscle contraction. This manoeuvre evoked significantly larger increases in mean arterial pressure (MAP) and renal sympathetic nerve activity (RSNA) in spontaneously hypertensive rats (SHR) compared to normotensive Wistar–Kyoto (WKY) rats. The skeletal muscle TRPv1 receptor was evaluated as a potential mediator of this metaboreflex response as it has been shown to contribute significantly to muscle reflex activation in healthy animals. Stimulation of the TRPv1 receptor by injection of capsaicin into the arterial supply of the hindlimb evoked significantly larger elevations in MAP and RSNA in SHR compared to WKY. The pressor and sympathetic responses to ischaemic muscle contraction in WKY and SHR were attenuated by the administration of the TRPv1 receptor antagonist capsazepine with the magnitude of the capsazepine-induced reductions being greater in SHR than WKY. TRPv1 protein expression in dorsal root ganglia, but not skeletal muscle, was significantly greater in SHR than WKY. The results suggest the muscle metaboreflex is overactive in hypertension. Further, this reflex overactivity can be partially normalized by antagonizing TRPv1 receptors in skeletal muscle.
Introduction
Afferent signals from working skeletal muscle are an important source of neural input to the brainstem during exercise and contribute significantly to the regulation of the cardiovascular system during physical activity (McCloskey & Mitchell, 1972). These contraction-induced signals are generated by stimulation of group III and group IV skeletal muscle afferent fibres (Kaufman et al. 1984). Group III afferents are predominantly mechanically sensitive A-δ fibres and are associated with the muscle mechanoreflex. Group IV afferents are primarily chemically sensitive C fibres and are associated with the muscle metaboreflex. Combined activation of the mechanoreflex and metaboreflex during muscle contraction, collectively termed the exercise pressor reflex (EPR), reflexively elevates arterial blood pressure and heart rate (HR) primarily via increases in efferent sympathetic nerve activity (SNA) (Mitchell et al. 1983).
In hypertension, the cardiovascular response to exercise is abnormally exaggerated (Aoki et al. 1983; Pickering, 1987; Seguro et al. 1991; Vongpatanasin et al. 2011). Since such responses have been shown to be associated with elevated risks for myocardial ischaemia, myocardial infarction, cardiac arrest and/or stroke during and after physical activity, elucidating the cause of this cardiovascular hyper-excitability is clinically important (Hoberg et al. 1990; Mittleman et al. 1993). To this end, our laboratory recently demonstrated that selective activation of the EPR elicits markedly greater increases in SNA and mean arterial pressure (MAP) in hypertensive compared to normotensive rats (Smith et al. 2006; Mizuno et al. 2011). These findings provide evidence that the exaggerated cardiovascular response to exercise in hypertension is mediated, in part, by a dysfunctional EPR.
Given that both the muscle mechanoreflex and the muscle metaboreflex contribute significantly to the EPR, it is logical to suggest that each component of the reflex mediates the EPR dysfunction that manifests in hypertension. With regard to the muscle mechanoreflex, recent reports provide evidence that activation of mechanically sensitive afferent fibres in skeletal muscle abnormally potentiates SNA and MAP (Leal et al. 2008; Mizuno et al. 2011) Findings from this work further suggest that these altered responses are mediated by skeletal muscle mechanoreceptors (Mizuno et al. 2011). However, reports in both humans and animals assessing metaboreflex function in hypertension are more controversial (Rondon et al. 2006; Leal et al. 2008; Sausen et al. 2009; Delaney et al. 2010). For example, Sausen et al. (2009) and Leal et al. (2008) have reported that the blood pressure response to preferential activation of the metaboreflex is enhanced in hypertensive patients and animals, respectively. Further, Delaney et al. (2010) recently demonstrated that the muscle SNA response to activation of the metaboreflex is augmented in older hypertensive patients as compared to normotensive individuals. In contrast, other independent studies have demonstrated the SNA response to activation of the metaboreflex is blunted while the MAP response is either unchanged or reduced in middle-aged hypertensive patients (Rondon et al. 2006). Additionally, to date, no studies have been designed to examine the receptor mechanisms underlying alterations in muscle metaboreflex function in hypertension.
Therefore, this study was designed to address the existing controversy with regard to metaboreflex regulation of SNA and MAP in hypertension as well as to determine the receptor mechanism(s) underlying abnormal metaboreflex function in this disease. With regard to the latter, we targeted the transient receptor potential vanilloid 1 (TRPv1) receptor as it has been recently demonstrated to contribute significantly to EPR activation in normotensive rats (Smith et al. 2010). In skeletal muscle, the TRPv1 receptor has been shown to be primarily localized to group IV afferent fibres and can be activated by several ligands including, but not limited to, protons and the exogenous substance capsaicin (Hoheisel et al. 2004). Additionally, in chronic heart failure (CHF), a disease state that often develops from prolonged hypertension, downregulation of mRNA (Smith et al. 2005) and protein (Wang et al. 2010) expression for the TRPv1 receptor in dorsal root ganglia (DRG) have been shown to be associated with a blunting of the muscle metaboreflex in CHF rats as compared to normal animals. This suggests that TRPv1 receptors might play an important role in the pathogenesis of abnormal muscle metaboreflex function in cardiovascular disease. Taken together, we hypothesized that activation of TRPv1 receptors mediate, in part, the alterations in muscle metaboreflex activity that manifest in hypertension. To test this hypothesis, we examined the following in decerebrate normotensive Wistar–Kyoto (WKY) and spontaneously hypertensive (SHR) rats: (i) the renal SNA (RSNA) and MAP responses to ‘supra-stimulation’ of the muscle metaboreflex via ischaemic hindlimb muscle contraction; (ii) the RSNA and MAP responses to stimulation of hindlimb TRPv1 receptors with capsaicin before and after antagonizing these receptors with capsazepine; (iii) the RSNA and MAP responses to ischaemic hindlimb muscle contraction before and after antagonizing TRPv1 receptors with capsazepine, and (iv) protein expression of TRPv1 receptors in the soleus and gastrocnemius muscles of the hindlimb as well as the DRG subserving skeletal muscle Group IV afferent fibres.
Methods
Ethical approval
Experiments were performed in age-matched (14–20 weeks) male normotensive Wistar–Kyoto (WKY; n = 46) and spontaneously hypertensive (SHR; n = 50) rats. The procedures outlined were approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center at Dallas. All studies were conducted in accordance with the US Department of Health and Human Services NIH Guide for the Care and Use of Laboratory Animals and are in accordance with the policies of the Journal of Physiology.
General surgical procedures
The surgical procedures used have been described previously (Smith et al. 2001). Briefly, rats were anaesthetized with isoflurane gas and intubated for mechanical ventilation. A pressure transducer connected to the left carotid arterial catheter was used to measure blood pressure continuously. Needle electrodes were placed on the back of the animal to obtain electrocardiograph (ECG) recordings. HR was calculated from the time between successive R waves in the ECG recording. The renal nerve was exposed and attached to a pair of stainless steel wire electrodes. The nerve and electrodes were covered with silicone glue for insulation and fixation. To quantify renal sympathetic nerve activity (RSNA), the pre-amplified nerve signal was band-pass filtered at 150–1000 Hz then full-wave rectified and low-pass filtered with a cut-off frequency of 30 Hz. Animals were held in a stereotaxic head unit, and a pre-collicular decerebration was performed rendering the animals insentient. Immediately following the decerebrate procedure, gas anaesthesia was discontinued.
Procedures for muscle contraction
A laminectomy exposing the lower lumber portions of the spinal cord (L2–L6) was performed as described previously (Smith et al. 2001). The L4 and L5 ventral roots were carefully isolated and sectioned. The cut peripheral ends of roots were placed on bipolar electrodes. The gastrocnemius and soleus muscle of the right hindlimb were isolated. The calcaneal bone of the right hindlimb was cut and the Achilles’ tendon connected to a force transducer for the measurement of muscle tension.
Procedures for intra-arterial injections within the hindlimb
To allow the injection of chemicals into the arterial supply of the right leg, the circulation of the hindlimb was surgically isolated as described previously (Smith et al. 2005). Briefly, a catheter was placed in the left common iliac artery with the catheter tip advanced to the bifurcation of the abdominal aorta. This allowed injection of chemicals directly into the right common iliac artery without occluding the circulation of the right hindlimb. In addition, a reversible ligature was placed around the common iliac vein emptying the right hindlimb. Use of these procedures limited the delivery of chemicals to the right hindlimb preventing their entrance into the general circulation.
Procedures for muscle ischaemia
A reversible vascular occluder was placed around right iliac artery and vein just below the aortic bifurcation.
Experimental protocols
Protocol 1: supra-stimulation of the metaboreflex during EPR activation
The EPR was stimulated in WKY (n = 13) and SHR (n = 16) by contracting the gastrocnemius and soleus muscles of the right hindlimb under freely perfused conditions for 30 s via electrical stimulation of isolated L4 and L5 ventral roots. This procedure is known to activate both the mechanically and chemically sensitive components of the EPR concomitantly (Mitchell et al. 1983). Constant current stimulation was used at a 3 times motor threshold (i.e. the minimum current required to produce a muscle twitch) with a pulse duration of 0.1 ms at 40 Hz. To assess metaboreflex function during activation of the EPR, the muscle was made ischaemic by inflating the right iliac artery vascular occluder immediately prior to muscle contraction. By reducing muscle blood flow during ischaemia, this manoeuvre impedes the removal of the metabolic by-products of muscular work and therefore serves to ‘supra-stimulate’ the metaboreflex in a physiological manner (McCloskey & Mitchell, 1972). Muscle contractions under freely perfused or ischaemic conditions were randomly assigned.
Protocol 2: pharmacological stimulation of chemically sensitive skeletal muscle afferents
Selective activation of chemically sensitive afferent fibres innervating skeletal muscle was achieved in WKY (n = 8) and SHR (n = 7) by administering graded concentrations of capsaicin into the arterial supply of the hindlimb (0.01, 0.03, 0.10, 0.30 and 1.00 μg/100 μl). Capsaicin has been shown to preferentially stimulate TRPv1 receptors which, in skeletal muscle, are predominantly expressed on Group IV afferent neurons (Jansco et al. 1977). As a result, stimulation of the TRPv1 receptor activates the afferent fibre population known to mediate metaboreflex function and is commonly used for this purpose (Kaufman et al. 1982; Li et al. 2004; Smith et al. 2005). Capsaicin was injected into the right common iliac artery while the reversible ligature placed around the right common iliac vein was occluded for 2 min. This limited drug delivery to the right hindlimb. As a control, saline was also injected into the circulation of the hindlimb. To confirm that the pressor and sympathetic responses to administration of capsaicin were mediated by TRPv1 receptor activation, WKY (n = 5) and SHR (n = 5) animals received an injection of capsaicin (1.00 μg/100 μl) in combination with capsazepine (100 μg/100 μl), a TRPv1 receptor antagonist.
Protocol 3: pharmacological blockade of the muscle metaboreflex during contraction
The TRPv1 receptor antagonist capsazepine (100 μg/100 μl) was likewise used to antagonize chemically sensitive receptors in hindlimb skeletal muscle during ischaemic muscle contraction in both WKY (n = 12 out of 13 in protocol 1) and SHR (n = 12 out of 16 in protocol 1). Hindlimb muscles were contracted during circulatory occlusion using the aforementioned protocol and stimulus parameters. As a control, the vehicle for capsazepine (isotonic saline) was also administered into the muscle circulation during separate ischaemic contraction trials. Capsazepine or saline was administered by injection into the arterial supply of the right hindlimb and trapped there for 2 min prior to ischaemic contraction. As an adjunct to this protocol, capsazepine was also administered during activation of the EPR via static muscle contraction under freely perfused conditions.
Protocol 4: quantification of TRPv1 protein expression
As a parallel line of evidence, Western blot analysis was used as a semi-quantitative approach to evaluate protein expression for the TRPv1 receptor in hindlimb skeletal muscle and the DRG of afferent neurons innervating hindlimb skeletal muscle. For Western blot analysis, 15 WKY and 17 SHR rats were anaesthetized with pentobarbital sodium (75 mg kg−1, i.p.). The soleus (containing mainly slow oxidative fibres), white gastrocnemius (containing highly glycolytic fibres) and DRGs (L4, L5 and L6) were rapidly removed. The tissue was lysed with celLytic MT mammalian tissue lysis/extraction reagent and a mixture of protease inhibitor cocktail (Sigma). The lysates were centrifuged at 10,000 g for 10 min at 4°C. Protein concentration was measured via the BCA protein assay method using bovine serum albumin as standard. Protein was denatured by heating at 100°C for 5 min and loaded onto a 10% SDS-PAGE gel along with protein standards (Bio-Rad Laboratories, Hercules, CA, USA) in a separate lane for electrophoresis and then transferred to a pure nitrocellulose membrane (0.2 μm). The membrane was blocked in 5% non-fat milk in 0.1% Tween–Tris-buffered saline (TBS) buffer for 1 h, and was probed overnight with a rabbit polyclonal antibody against TRPv1 receptors (1:100 dilutions, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Subsequently, the membrane was incubated with a secondary antibody of goat anti-rabbit IgG-HRP (1:10000 dilutions, Santa Cruz Biotechnology). Protein signals were detected by an enhanced chemiluminescent reagent (Thermo Scientific) and analysed using ImageJ software (NIH). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:48000 for skeletal muscles, 1:3000 for DRGs) was used to verify equal protein loading in each lane. Values for densities of TRPv1/GAPDH were determined from the same lane and normalized to a WKY sample.
Protocol 5: direct electrical activation of skeletal muscle afferent fibres
In a set of corollary experiments, the sciatic nerve was electrically stimulated in WKY (n = 5) and SHR (n = 5) to directly and maximally activate afferent fibres innervating hindlimb skeletal muscle. The purpose of this manoeuvre was to assess the cardiovascular response to afferent fibre stimulation independent of their activation by nerve ending receptors (e.g. the TRPv1 receptor). The decerebrated rats were paralysed with pancuronium bromide (1 mg kg−1, i.v.). The right sciatic nerve was carefully exposed and placed on bipolar stimulating electrodes. The right sciatic nerve was electrically stimulated (20 Hz, 0.75 ms) for 30 s at current intensities which evoked a maximal pressor response (Tsuchimochi et al. 2011). Since electrical stimulation of the sciatic nerve interferes with nerve activity recordings, RSNA data were not obtained during execution of this protocol.
Data acquisition and statistical analyses
MAP, HR, RSNA and contractile force data were acquired, recorded and analysed using data acquisition software (LabChart, ADInstruments) for the Powerlab analog-to-digital convertor (Powerlab8/30, ADInstruments) at a 1 kHz sampling rate. To analyse RSNA, full-wave rectified signals of RSNA as well as background noise signals were obtained. To confirm that SNA signals were recorded from postganglionic renal sympathetic fibres, an intravenous infusion of hexamethonium bromide (60 mg kg−1) was given to abolish SNA at the conclusion of all experiments. RSNA background noise was determined over a 30 min period after the insentient decerebrated animal was humanely killed by intravenous injection of saturated potassium chloride (4 m, 2 ml kg−1). The noise signal component, which was defined as the signal recorded post mortem, was subtracted from rectified RSNA. To quantify RSNA responses to muscle contraction, basal measurements were obtained by taking the mean value of 30 s of baseline data immediately prior to the manoeuvre. This mean was considered 100% of basal RSNA. Subsequently, relative changes in RSNA (ΔRSNA,%) from this baseline were evaluated. Data sets of 1 s averages for MAP, HR, RSNA and hindlimb tension were analysed. Baseline values for all variables were determined by evaluating 30 s of recorded data before a muscle contraction. The peak response of each variable was defined as the greatest change from baseline elicited by contraction. Tension–time index (TTI, kg s) was calculated by integrating the developed tension (integrated total tension minus integrated baseline tension prior to the manoeuvre) during the contraction period.
Data were analysed using Student's unpaired t tests (WKY vs. SHR), two-way (rat group × occlusion effect, rat group × drug effect), and three-way repeated measures ANOVA (rat group × occlusion effect × time effect, rat group × drug effect × time effect). If significant interaction and main effects were observed with ANOVA, a post hoc Tukey's test was used to identify differences between specific group means. The significance level was set at P < 0.05. Results are presented as means ± SEM.
Results
Morphometric characteristics and baseline haemodynamics for WKY and SHR are presented in Table 1. Baseline MAP as well as the heart weight to body weight ratio and heart weight to tibial length ratio were significantly higher in SHR than WKY.
Table 1.
Morphometric characteristics and baseline haemodynamics
| WKY | SHR | |
|---|---|---|
| n | 31 | 33 |
| Body weight (g) | 345 ± 3 | 334 ± 3* |
| Heart weight/body weight (mg g−1) | 2.81 ± 0.06 | 3.15 ± 0.03* |
| Heart weight/tibial length (mg mm−1) | 25.3 ± 0.5 | 28.6 ± 0.4* |
| Lung weight/body weight (mg g−1) | 7.31 ± 0.27 | 6.98 ± 0.18 |
| MAP (mmHg) | 85 ± 5 | 126 ± 5* |
| HR (beats min−1) | 500 ± 7 | 486 ± 10 |
| Baseline signal to noise ratio for RSNA | 4.3 ± 0.5 | 4.0 ± 0.3 |
Values are means ± SEM.
P < 0.05 compared to WKY rats.
Representative tracings from SHR of arterial blood pressure and RSNA responses to static muscle contraction under freely perfused (free) and ischaemic conditions both without (occlusion) and with the intra-arterial administration of capsazepine (occlusion+capz) are presented in Fig. 1. Under freely perfused conditions, the pressor, tachycardic and sympathetic responses (Fig. 2A–I) to stimulation of the EPR during static muscle contraction were exaggerated in hypertensive compared to normotensive rats (rats group × time effect, MAP: P < 0.05; HR: P < 0.05, RSNA: P < 0.05). Importantly, the magnitude of the increases in MAP and RSNA during circulatory occlusion compared to freely perfused conditions was markedly greater in SHR compared to WKY (Fig. 2A and G; rat group × occlusion effect; MAP: P < 0.05, RSNA: P < 0.05). In hypertensive rats, the peak and integrated MAP responses to contraction, but not HR and RSNA, were significantly augmented by circulatory occlusion (Fig. 2B–C, E–F and H–I). Table 2 summarizes peak tension and TTI developed during muscle contraction under freely perfused or ischaemic conditions in WKY and SHR. These variables were not significantly different between groups or trials.
Figure 1.
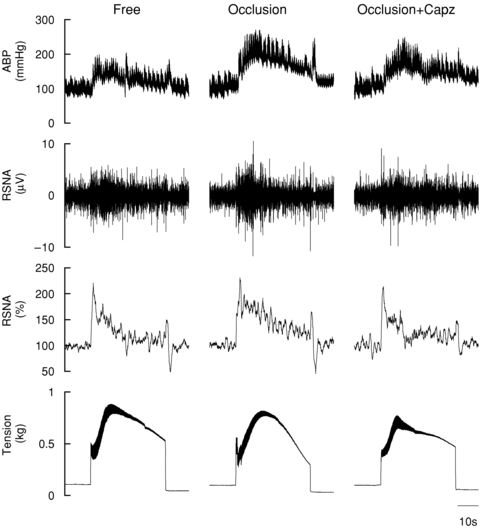
Original tracing demonstrating the arterial blood pressure (ABP) and RSNA responses to static muscle contraction during freely perfused (free) and ischaemic conditions both without (occlusion) and with the intra-arterial administration of capsazepine (occlusion+capz) in SHR
Figure 2. MAP, HR and RSNA responses to static muscle contraction during freely perfused (free) and ischaemic (occlusion) conditions in WKY and SHR.
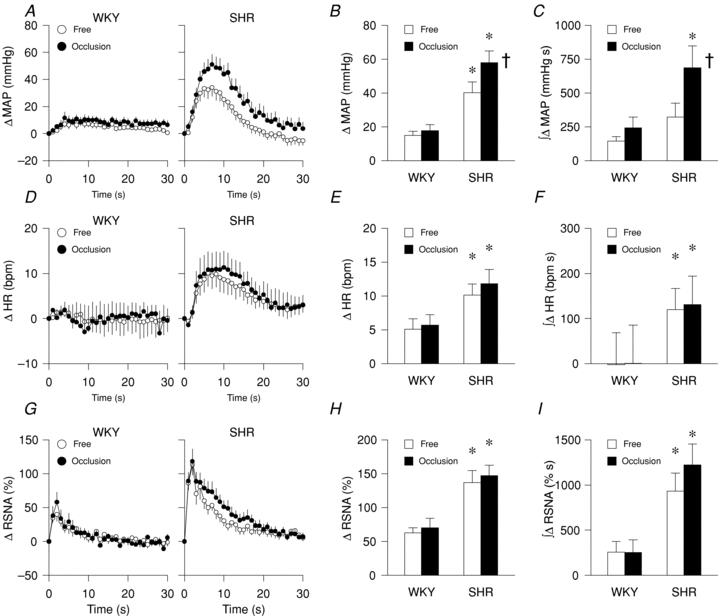
A–C, MAP: time course (A), peak (B) and integrated (C). D–F, HR: time course (D), peak (E) and integrated (F). G–I, RSNA: time course (G), peak (H) and integrated (I). WKY: n = 13, SHR: n = 16; *P < 0.05 compared to WKY; †P < 0.05 compared to freely perfused condition.
Table 2.
The peak tension and TTI during muscle contraction under freely perfused or ischaemic conditions (protocol 1)
| WKY | SHR | |
|---|---|---|
| Peak tension (g) | ||
| Freely perfused | 725 ± 46 | 615 ± 40 |
| Occluded | 716 ± 48 | 664 ± 47 |
| TTI (kg s) | ||
| Freely perfused | 15.2 ± 1.1 | 14.1 ± 0.9 |
| Occluded | 14.5 ± 1.2 | 15.0 ± 1.1 |
Values are means ± SEM. There were no significant differences among groups and trials.
Capsaicin-induced increases in MAP but not HR (Fig. 3A and B) were significantly greater in SHR as compared to WKY. In the present study, a similar response pattern as that demonstrated for MAP was observed for RSNA (Fig. 3C). Capsazepine attenuated the MAP (Fig. 3D), HR (Fig. 3E) and RSNA (Fig. 3F) responses to capsaicin administration. These reductions were statistically significant in SHR except with regard to HR. The magnitude of reduction in MAP and RSNA was significantly greater in SHR compared to WKY (rat group × drug effect: P < 0.05).
Figure 3. MAP (A and D), HR (B and E) and RSNA (C and F) responses to the intra-arterial administration of capsaicin (A–C) and co-administration of capsaicin with capsazepine (D–F) in the hindlimb of WKY and SHR.
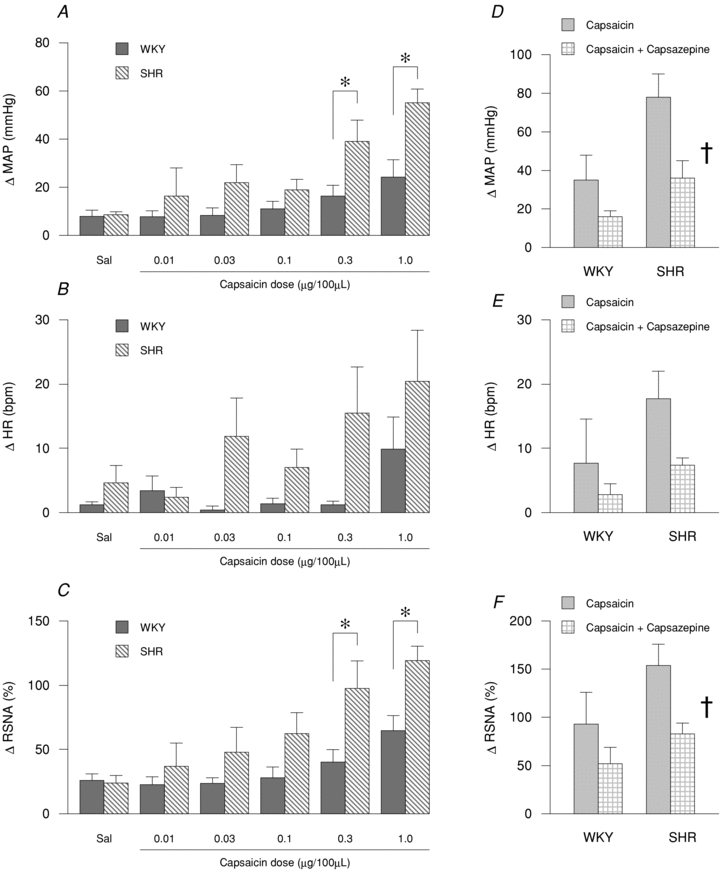
A–C, WKY: n = 8, SHR: n = 7; *P < 0.05 compared to WKY. D–F, WKY: n = 5, SHR: n = 5; †P < 0.05 compared to capsaicin trial.
The administration of capsazepine into the arterial supply of the hindlimb during ischaemic muscle contraction significantly attenuated the increases in MAP and RSNA, but not HR, as compared to saline control trials performed under ischaemic conditions (Fig. 4A–I; drug effect, MAP: P < 0.05; HR: P = 0.29; RSNA: P < 0.05). Notably, the magnitude of the capsazepine-induced reduction in MAP, HR and RSNA was significantly greater in SHR compared to WKY (Fig. 4A, D and G; rat group × drug effect, MAP: P < 0.05; HR: P < 0.05; RSNA: P < 0.05). In hypertensive rats, the peak MAP response to ischaemic contraction, but not the HR and RSNA response, was significantly reduced by pharmacologically antagonizing the TRPv1 receptor with capsazepine (Fig. 4B, E and H). The integrated RSNA response to ischaemic contraction, but not the integrated MAP (P = 0.08) and HR responses, was significantly attenuated by capsazepine (Fig. 4C, F and I). Table 3 summarizes peak tension and TTI developed during muscle contraction under saline or capsazepine conditions in WKY and SHR. These variables were not significantly different between groups or trials. Compared to saline administration, capsazepine injection likewise attenuated the MAP response to contraction under freely perfused conditions (SHR: 40 ± 6 vs. 35 ± 6 mmHg, respectively; WKY: 15 ± 2 vs. 9 ± 2 mmHg, respectively; data not shown in figures). Similarly, RSNA responses to contraction under freely perfused conditions during saline injection were reduced by TRPv1 blockade during capsazepine administration (SHR: 137 ± 18 vs. 117 ± 13%, respectively; WKY: 63 ± 7 vs. 37 ± 6%, respectively; data not shown in figures).
Figure 4. MAP, HR and RSNA responses to ischaemic static muscle contraction in WKY and SHR after the intra-arterial administration of saline or capsazepine in the hindlimb.
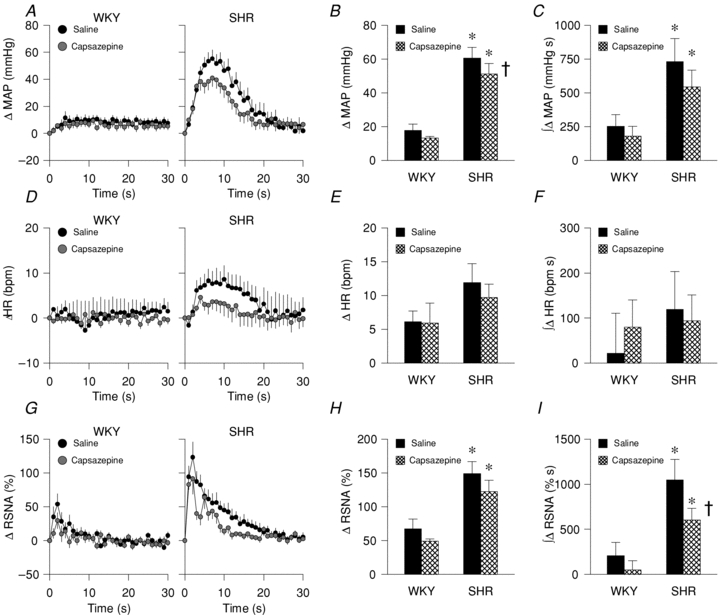
A–C, MAP: time course (A), peak (B), and integrated (C). D–F, HR: time course (D), peak (E), and integrated (F). G–I, RSNA: time course (G), peak (H) and integrated (I). WKY: n = 12, SHR: n = 12; *P < 0.05 compared to WKY; †P < 0.05 compared to saline trial.
Table 3.
The peak tension and TTI during ischaemic muscle contraction under saline or capsazepine conditions (protocol 3)
| WKY | SHR | |
|---|---|---|
| Peak tension (g) | ||
| Saline | 709 ± 52 | 664 ± 59 |
| Capsazepine | 645 ± 49 | 601 ± 57 |
| TTI (kg s) | ||
| Saline | 14.6 ± 1.3 | 14.8 ± 1.4 |
| Capsazepine | 12.8 ± 1.1 | 13.8 ± 1.5 |
Values are means ± SEM. There were no significant differences among groups and trials.
Western blot analysis demonstrated that the density of TRPv1 expression was significantly increased by 90 ± 27% in the DRG of SHR compared to WKY (Fig. 5A). However, no significant differences in TRPv1 protein expression were detected in the soleus (Fig. 5B) and the gastrocnemius muscles of the two animal groups (Fig. 5C).
Figure 5. Protein expression of the TRPv1 receptor in the DRG (A), soleus (B) and gastrocnemius (C).
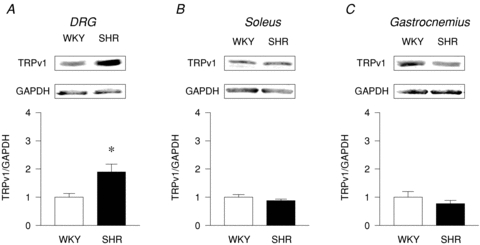
A, DRG: WKY: n = 7, SHR: n = 9; B, soleus: WKY: n = 8, SHR: n = 8; C, gastrocnemius: WKY: n = 8, SHR: n = 8. *P < 0.05 compared to WKY.
The peak pressor response evoked by direct electrical stimulation of the sciatic nerve was significantly greater in SHR compared to WKY (Fig. 6A). The peak tachycardic response between the two groups was not significantly different in response to this perturbation (Fig. 6B).
Figure 6. MAP (A) and HR (B) responses to sciatic nerve stimulation in WKY and SHR.
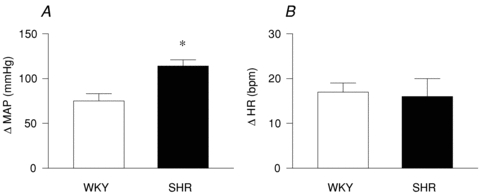
WKY: n = 5, SHR: n = 5. *P < 0.05 compared to WKY.
Discussion
The major findings from this investigation were (i) the MAP and RSNA responses to ‘supra-stimulation’ of the muscle metaboreflex during ischaemic muscle contraction are markedly augmented in hypertensive compared to normotensive animals; (ii) the pressor and sympathetic responses to selective stimulation of chemically sensitive afferent fibres in skeletal muscle via stimulation of TRPv1 receptors are enhanced in SHR compared to WKY; (iii) blockade of skeletal muscle TRPv1 receptors during ‘supra-stimulation’ of the metaboreflex (i.e. ischaemic contraction) and full activation of the EPR (i.e. freely perfused contraction) significantly reduces reflex-mediated increases in MAP and RSNA in hypertensive rats; (iv) protein expression of the TRPv1 receptor in the DRG, but not soleus and gastrocnemius muscles, is significantly greater in hypertensive compared to normotensive rats; and (v) the pressor response to direct activation of skeletal muscle afferent fibres via electrical sciatic nerve stimulation is significantly greater in SHR compared to WKY. Collectively, these findings support the contention that muscle metaboreflex function is exaggerated in hypertension. Further, this investigation provides evidence that the abnormally large increases in SNA and MAP mediated by the metaboreflex in this disease are evoked, in part, by activation of TRPv1 receptors in skeletal muscle.
As stated previously, initial investigations in humans assessing metaboreflex function in hypertension have produced conflicting results reporting both reductions and enhancements in metaboreflex sensitivity with the manifestation of disease (Rondon et al. 2006; Sausen et al. 2009; Delaney et al. 2010). The current investigation was designed to address these discrepancies with the results supporting the conclusion that the metaboreflex is exaggerated in hypertension. It must be noted, however, that other factors may have contributed to the disparate results in humans. For example, in the study in which Rondon et al. (2006) demonstrated that the SNA and MAP responses to metaboreflex activation were blunted, the patient population was middle-aged (mean age 42 years). In studies by Sausen et al. (2009) and Delaney et al. (2010) in which the metaboreflex was demonstrated to be overactive, the patient population was older (mean age ≥60 years). As hypertension is a progressively developing disease, it is quite possible that metaboreflex dysfunction presents differently at various stages of disease progression and may be the key to reconciling the apparent conflicting results. Additional factors such as the type and length of anti-hypertensive treatment and time since disease onset could also play a role. Additional studies in both animals and humans are warranted to address these important questions.
This study was also designed to identify a receptor mechanism underlying metaboreflex dysfunction in hypertension. To this end, blockade of the TRPv1 receptor partially corrected metaboreflex overactivity in SHR supporting a role for this receptor in the manifestation of abnormal reflex function. Interestingly, the role of the TRPv1 receptor in the development of metaboreflex dysfunction appears to be different in hypertension as compared to the closely related cardiovascular disease heart failure. It has been demonstrated previously that the expression of mRNA for the TRPv1 receptor is decreased in the DRG of CHF rats as compared to sham-treated animals (Smith et al. 2005). In agreement, Wang et al. (2010) recently reported that protein expression for the TRPv1 receptor was likewise decreased in the DRG of CHF rats compared to control animals. Further, compared with normal rats, both the pressor response (Smith et al. 2005) and the discharge of chemically sensitive group IV afferents (Wang et al. 2010) were reduced in response to the intra-arterial administration of capsaicin in CHF animals. In contrast, TRPv1 protein expression in the DRG was upregulated and the muscle metaboreflex augmented in hypertensive compared to normotensive animals in the current study. The reasons for these differences in metaboreflex function between hypertension and heart failure are at present not clear. Speculatively, it seems plausible to suggest that the heightened metaboreflex function that manifests, most likely progressively, in hypertension is reversed as heart failure develops from prolonged exposure to chronic high blood pressure. Decreases in cardiac output and peripheral blood flow are hallmark features of heart failure. Reductions in skeletal muscle blood flow could abrogate the ability to remove metabolites produced by muscle contraction leading to, over the long term, a down-regulation of receptors responsible for activating the metaboreflex. As such, the metaboreflex may become desensitized in heart failure. The mechanisms underlying the sequalae of events in both hypertension and heart failure remain to be thoroughly investigated. Collectively, these studies suggest that the skeletal muscle TRPv1 receptor is likely to play an important role in determining metaboreflex function in both hypertension and heart failure.
The mechanisms underlying the generation of augmented metaboreflex activity in hypertension remain unclear. It is known that a mismatch between blood/oxygen supply and demand activates the muscle metaboreflex (Mitchell et al. 1983). To this end, essential hypertension is associated with a reduction in the microvascular network of several tissues (including skeletal muscle) which could increase vascular resistance within muscle during exercise (Hansen et al. 2010). Further, functional sympatholysis, the normal blunting of sympathetic vasoconstriction in exercising muscle (Remensnyder et al. 1962), has recently been shown to be impaired in hypertensive patients (Vongpatanasin et al. 2011). These factors alone could reduce blood flow to working skeletal muscle and impede the removal of metabolites produced during physical activity. As such, the receptors responsible for activation of the metaboreflex could be ‘supra-stimulated’ leading to an augmentation in reflex function.
An increase in the density of chemically sensitive skeletal muscle receptors is another plausible explanation for the enhanced metaboreflex sensitivity demonstrated in hypertension. In support of this possibility, protein expression for the TRPv1 receptor was found to be increased in the DRG of hypertensive rats in the current study. Surprisingly, however, TRPv1 protein expression in the soleus and gastrocnemius muscles, where the afferent nerve endings reside, did not differ between WKY and SHR. These results suggest that either (i) the increased amount of TRPv1 produced in the DRG is not transported to the afferent nerve endings or (ii) the TRPv1 being produced is going to other tissues subserved by the DRG (e.g. skin, joint). In addition, yet another factor could be at play. It is quite feasible that, although the expression of TRPv1 is not different between WKY and SHR in skeletal muscle, the number of active phosphorylated proteins is enhanced in hypertensive animals. For example, capsaicin-activated inward currents elicited via activation of TRPv1 receptors is largely modulated by protein kinase C-mediated phosphorylation (Mandadi et al. 2006). In some instances, even when TRPv1 protein expression is reduced (e.g. diabetic rats with peripheral neuropathy), the phosphorylation of TRPv1 is enhanced leading to increased functional activity (Hong & Wiley, 2005). Whether a similar situation exists within the skeletal muscle of hypertensive rats remains to be investigated.
Alternatively, it is plausible that metaboreflex overactivity manifests, in conjunction with or independent from the aforementioned possibilities, as a result of changes in the mechanisms by which afferent signals are processed within the central nervous system (CNS). In the current study, bypassing the skeletal muscle afferent nerve endings by directly stimulating sensory fibres within the sciatic nerve elicited a markedly enhanced pressor response in SHR. This opens the possibility that the same level of muscle afferent nerve traffic in WKY and SHR is interpreted differently within the CNS and may contribute to the disparity in cardiovascular responsiveness between the two groups of animals. Speculatively, metaboreflex overactivity in hypertension is not likely to be mediated solely by one mechanism, but a combination of several.
Analytical and methodological considerations
Several analytical and methodological issues should be considered when interpreting the findings of the current study. To begin with, both the peak (Fig. 2B) and integrated (Fig. 2C) pressor response to ‘supra-stimulation’ of the muscle metaboreflex during ischaemic muscle contraction were markedly augmented in hypertensive compared to normotensive animals. In contrast, the peak HR and MSNA responses were not significantly augmented by ‘supra-stimulation’ of the reflex. We recently demonstrated that selective activation of the muscle mechanoreflex elicits greater increases in RSNA in hypertensive compared to normotensive rats (Mizuno et al. 2011). Further, the abnormally large reflex-induced augmentations in RSNA were shown to be attenuated by blockade of skeletal muscle mechanoreceptors (Mizuno et al. 2011). These results suggest that the accentuated sympathetic responsiveness is evoked, in part, by the muscle mechanoreflex. Since the peak RSNA response occurred at the onset of muscle contraction (less than 3 s) in most animals within this study, consistent with mechanoreflex activation, ‘supra-stimulation’ of the muscle metaboreflex may not have affected the peak response (Fig. 2H). However, ‘supra-stimulation’ of the metaboreflex did exhibit an affect when the full time course of the RSNA response was considered (Fig. 2G), consistent with the known activation properties of metabolically sensitive afferent fibres (onset delayed 5–20 s after initiation of contraction). In fact, the integrated, but not the peak, RSNA response during ischaemic muscle contraction was significantly attenuated by local capsazepine administration (Fig. 4I). This strongly suggests that activation of the TRPv1 receptor contributes to the elicitation of the exaggerated sympathetic response to ischaemic muscle contraction in hypertensive rats.
With regard to the metabolites that potentially stimulate the TRPv1 receptor, intracellular pH dramatically decreases during exercise both in humans (Pan et al. 1988) and in rats (Giannesini et al. 2007) implicating protons as a viable candidate. Capsazepine inhibits proton-induced channel activation (Fox et al. 1995; Tominaga et al. 1998; Liu et al. 2004; Dang et al. 2005). However, there are conflicting reports that capsazepine does not fully antagonize the pH site on the TRPv1 receptor (Vyklicky et al. 1998; Habelt et al. 2000; Ugawa et al. 2002). This may explain, in part, why capsazepine was only partially effective in blocking the cardiovascular response to ischaemic exercise. Alternatively, it is also possible that the TRPv1 receptor was activated by a ligand other than protons. For example, we previously reported that capsazepine partially attenuates the pressor response to local hindlimb intra-arterial injection of anandamide, an endogenously produced cannabinoid, in normal rats (Williams et al. 2008). Further, lipooxygenase products are known to activate TRPv1 receptors (Hwang et al. 2000) and may play a role in modulating muscle reflex-mediated sympathetic responses (Rotto et al. 1990; Gao et al. 2008). Conversely, since the temperature threshold for TRPv1 is approximately 43°C (Caterina et al. 1997), heat production in skeletal muscle at the onset of intense exercise (∼0.2°C at 30 s) (Gonzalez-Alonso et al. 2000) does not seem a likely candidate for receptor activation. It should be noted that the dose of capsazepine used in this study was chosen as it has been shown to effectively attenuate, but not abolish, the cardiovascular response to activation of the EPR (Smith et al. 2010). Therefore, it is possible that TRPv1 receptors were not completely blocked in these experiments and thus their contribution to metaboreflex activation in hypertension underestimated.
It should also be noted that, in addition to the TRPv1 receptor, it is likely that other skeletal muscle receptors and/or ion channels localized to chemically sensitive afferent fibres mediate, in part, metaboreflex overactivity in hypertension. For example, acid-sensing ion channels are known to contribute importantly to the metaboreflex in healthy cats (Hayes et al. 2007; McCord et al. 2009). Evidence to support the potential involvement of other receptors in the activation of the metaboreflex has also been reported including, but not limited to, the ATP receptors P2X2/3 and P2X3, the bradykinin receptor B2 and the cannabinoid receptor CB1 (Pan et al. 1993; Williams et al. 2008; McCord et al. 2010). Clearly, more research is necessary to definitively determine the skeletal muscle receptors and ligands mediating altered metaboreflex function in this disease.
Finally, during exercise the autonomic nervous system is regulated by integrating neural input from not only the EPR but also central command (Goodwin et al. 1972) and the arterial baroreflex (Potts et al. 1993). The decerebration procedure used in the present study removes the areas of the cerebral cortex from which central command signals arise. In addition, muscle contraction was electrically and involuntarily evoked; a manoeuvre that does not engage central command. As a result, central command was unlikely to contribute to the pressor and sympathetic responses elucidated in the present study. In hypertension, baroreflex sensitivity is attenuated (Moreira et al. 1992; Lanfranchi & Somers, 2002; Minami et al. 2003). It is possible that the exaggerated metaboreflex function demonstrated in hypertensive animals is due to a decrease in the buffering capacity of the baroreflex. However, we have previously demonstrated that baroreflex impairment in hypertension contributes minimally to altered skeletal muscle reflex function in SHR (Smith et al. 2006).
Clinical significance
It has recently been demonstrated that normotensive individuals displaying an exaggerated blood pressure response to exercise are more likely to develop future hypertension and are at a greater risk for cardiovascular death (Weiss et al. 2010). Early detection of this abnormal response to physical activity could potentially lead to the early treatment and prevention of hypertension in these individuals. Clearly, a better understanding of the pathophysiology generating the abnormal cardiovascular response to exercise would help facilitate this endeavour. In individuals with established hypertension, exercise has been shown to improve cardiovascular health and is a viable non-pharmacological treatment for chronic high blood pressure (Dengel et al. 1998). However, there are risks associated with physical activity in hypertension that must be taken into account before its prescription (Hoberg et al. 1990; Mittleman et al. 1993; Mittleman & Siscovick, 1996). Again, dissection of the mechanisms underlying the aberrant circulatory response to exercise in hypertension may prove beneficial to the development of novel therapeutic strategies targeted at reducing the risks associated with physical activity in this disease. To this end, the current study has identified the skeletal muscle metaboreflex, specifically the TRPv1 receptor, as a potential target for the treatment of cardiovascular hyper-excitability during exercise in hypertension.
Acknowledgments
This research was supported by grants from the National Institutes of Health Heart, Lung and Blood Institute (HL-088422 to S.A.S.) and the Lawson & Rogers Lacy Research Fund in Cardiovascular Disease (to J.H.M.). M.M. was supported by a research fellowship from the Japan Society for the Promotion of Science for young scientists. The authors thank Martha Romero and Julius Lamar Jr for their expert technical assistance.
Glossary
Abbreviations
- CHF
chronic heart failure
- DRG
dorsal root ganglia
- ECG
electrocardiograph
- EPR
exercise pressor reflex
- HR
heart rate
- MAP
mean arterial pressure
- RSNA
renal sympathetic nerve activity
- SHR
spontaneously hypertensive rat
- SNA
sympathetic nerve activity
- TRPv1
transient receptor potential vanilloid 1
- TTI
tension–time index
- WKY
Wistar–Kyoto rat
Author contributions
The experiments described in this article were performed in laboratories at UT Southwestern Medical Center at Dallas, TX, USA. All authors contributed to the conception and design of the experiments as well as the interpretation of data. M.M. and S.A.S. drafted the article and revised it with input from all authors. All authors approved the final version submitted.
References
- Aoki K, Sato K, Kondo S, Pyon CB, Yamamoto M. Increased response of blood pressure to rest and handgrip in subjects with essential hypertension. Jpn Circ J. 1983;47:802–809. doi: 10.1253/jcj.47.802. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Dang K, Bielefeldt K, Gebhart GF. Differential responses of bladder lumbosacral and thoracolumbar dorsal root ganglion neurons to purinergic agonists, protons, and capsaicin. J Neurosci. 2005;25:3973–3984. doi: 10.1523/JNEUROSCI.5239-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PJ, Farquhar WB. Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: Role of the muscle metaboreflex. Am J Physiol Heart Circ Physiol. 2010;299:H1318–1327. doi: 10.1152/ajpheart.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengel D, Hagberg J, Pratley R, Rogus E, Goldberg A. Improvements in blood pressure, glucose metabolism and lipoprotein lipids after aerobic exercise plus weight loss in obese hypertensive middle-aged men. Metabolism. 1998;47:1075–1082. doi: 10.1016/s0026-0495(98)90281-5. [DOI] [PubMed] [Google Scholar]
- Fox AJ, Urban L, Barnes PJ, Dray A. Effects of capsazepine against capsaicin- and proton-evoked excitation of single airway C-fibres and vagus nerve from the guinea-pig. Neuroscience. 1995;67:741–752. doi: 10.1016/0306-4522(95)00115-y. [DOI] [PubMed] [Google Scholar]
- Gao Z, Koba S, Sinoway L, Li J. 20-HETE increases renal sympathetic nerve activity via activation of chemically and mechanically sensitive muscle afferents. J Physiol. 2008;586:2581–2591. doi: 10.1113/jphysiol.2008.150730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannesini B, Izquierdo M, Dalmasso C, Le Fur Y, Cozzone PJ, Verleye M, Le Guern ME, Gillardin JM, Bendahan D. Endotoxemia causes a paradoxical intracellular pH recovery in exercising rat skeletal muscle. Muscle Nerve. 2007;36:505–514. doi: 10.1002/mus.20843. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Quistorff B, Krustrup P, Bangsbo J, Saltin B. Heat production in human skeletal muscle at the onset of intense dynamic exercise. J Physiol. 2000;524:603–615. doi: 10.1111/j.1469-7793.2000.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol. 1972;226:173–190. doi: 10.1113/jphysiol.1972.sp009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habelt C, Kessler F, Distler C, Kress M, Reeh PW. Interactions of inflammatory mediators and low pH not influenced by capsazepine in rat cutaneous nociceptors. Neuroreport. 2000;11:973–976. doi: 10.1097/00001756-200004070-00015. [DOI] [PubMed] [Google Scholar]
- Hansen AH, Nielsen JJ, Saltin B, Hellsten Y. Exercise training normalizes skeletal muscle vascular endothelial growth factor levels in patients with essential hypertension. J Hypertens. 2010;28:1176–1185. doi: 10.1097/HJH.0b013e3283379120. [DOI] [PubMed] [Google Scholar]
- Hayes SG, Kindig AE, Kaufman MP. Blockade of acid sensing ion channels attenuates the exercise pressor reflex in cats. J Physiol. 2007;581:1271–1282. doi: 10.1113/jphysiol.2007.129197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoberg E, Schuler G, Kunze B, Obermoser AL, Hauer K, Mautner HP, Schlierf G, Kubler W. Silent myocardial ischemia as a potential link between lack of premonitoring symptoms and increased risk of cardiac arrest during physical stress. Am J Cardiol. 1990;65:583–589. doi: 10.1016/0002-9149(90)91034-4. [DOI] [PubMed] [Google Scholar]
- Hoheisel U, Reinohl J, Unger T, Mense S. Acidic pH and capsaicin activate mechanosensitive group IV muscle receptors in the rat. Pain. 2004;110:149–157. doi: 10.1016/j.pain.2004.03.043. [DOI] [PubMed] [Google Scholar]
- Hong S, Wiley JW. Early painful diabetic neuropathy is associated with differential changes in the expression and function of vanilloid receptor 1. J Biol Chem. 2005;280:618–627. doi: 10.1074/jbc.M408500200. [DOI] [PubMed] [Google Scholar]
- Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci U S A. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansco G, Kiraly E, Jansco-Gabor A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature. 1977;270:741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Iwamoto GA, Longhurst JC, Mitchell JH. Effects of capsaicin and bradykinin on afferent fibers with endings in skeletal muscle. Circ Res. 1982;50:133–139. doi: 10.1161/01.res.50.1.133. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Waldrop TG, Rybicki KJ, Ordway GA, Mitchell JH. Effects of static and rhythmic twitch contractions on the discharge of group III and IV muscle afferents. Cardiovasc Res. 1984;18:663–668. doi: 10.1093/cvr/18.11.663. [DOI] [PubMed] [Google Scholar]
- Lanfranchi PA, Somers VK. Arterial baroreflex function and cardiovascular variability: interactions and implications. Am J Physiol Regul Integr Comp Physiol. 2002;283:R815–R826. doi: 10.1152/ajpregu.00051.2002. [DOI] [PubMed] [Google Scholar]
- Leal AK, Williams MA, Garry MG, Mitchell JH, Smith SA. Evidence for functional alterations in the skeletal muscle mechanoreflex and metaboreflex in hypertensive rats. Am J Physiol Heart Circ Physiol. 2008;295:H1429–H1438. doi: 10.1152/ajpheart.01365.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Sinoway AN, Gau Z, Maile MD, Pu M, Sinoway LI. Muscle mechanoreflex and metaboreflex responses after myocardial infarction in rats. Circulation. 2004;110:3049–3054. doi: 10.1161/01.CIR.0000147188.46287.1B. [DOI] [PubMed] [Google Scholar]
- Liu M, Willmott NJ, Michael GJ, Priestley JV. Differential pH and capsaicin responses of Griffonia simplicifolia IB4 (IB4)-positive and IB4-negative small sensory neurons. Neuroscience. 2004;127:659–672. doi: 10.1016/j.neuroscience.2004.05.041. [DOI] [PubMed] [Google Scholar]
- Mandadi S, Tominaga T, Numazaki M, Murayama N, Saito N, Armati PJ, Roufogalis BD, Tominaga M. Increased sensitivity of desensitized TRPV1 by PMA occurs through PKCɛ-mediated phosphorylation at S800. Pain. 2006;123:106–116. doi: 10.1016/j.pain.2006.02.016. [DOI] [PubMed] [Google Scholar]
- McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JL, Tsuchimochi H, Kaufman MP. Acid-sensing ion channels contribute to the metaboreceptor component of the exercise pressor reflex. Am J Physiol Heart Circ Physiol. 2009;297:H443–H449. doi: 10.1152/ajpheart.00328.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JL, Tsuchimochi H, Kaufman MP. P2X2/3 and P2X3 receptors contribute to the metaboreceptor component of the exercise pressor reflex. J Appl Physiol. 2010;109:1416–1423. doi: 10.1152/japplphysiol.00774.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami N, Yoshikawa T, Kataoka H, Mori N, Nagasaka M, Kurosawa H, Kanazawa M, Kohzuki M. Effects of exercise and beta-blocker on blood pressure and baroreflexes in spontaneously hypertensive rats. Am J Hypertens. 2003;16:966–972. doi: 10.1016/s0895-7061(03)01010-0. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol. 1983;45:229–242. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ, Muller JE. Triggering of acute myocardial infarction by heavy physical exertion. Protection against triggering by regular exertion. Determinants of Myocardial Infarction Onset Study Investigators. N Engl J Med. 1993;329:1677–1683. doi: 10.1056/NEJM199312023292301. [DOI] [PubMed] [Google Scholar]
- Mittleman MA, Siscovick DS. Physical exertion as a trigger of myocardial infarction and sudden cardiac death. Cardiol Clin. 1996;14:263–270. doi: 10.1016/s0733-8651(05)70279-4. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Murphy MN, Mitchell JH, Smith SA. Skeletal muscle reflex-mediated changes in sympathetic nerve activity are abnormal in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2011;300:H968–H977. doi: 10.1152/ajpheart.01145.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira ED, Ida F, Oliveira VL, Krieger EM. Early depression of the baroreceptor sensitivity during onset of hypertension. Hypertension. 1992;19:II198–II201. doi: 10.1161/01.hyp.19.2_suppl.ii198. [DOI] [PubMed] [Google Scholar]
- Pan HL, Stebbins CL, Longhurst JC. Bradykinin contributes to the exercise pressor reflex: mechanism of action. J Appl Physiol. 1993;75:2061–2068. doi: 10.1152/jappl.1993.75.5.2061. [DOI] [PubMed] [Google Scholar]
- Pan JW, Hamm JR, Rothman DL, Shulman RG. Intracellular pH in human skeletal muscle by 1H NMR. Proc Natl Acad Sci U S A. 1988;85:7836–7839. doi: 10.1073/pnas.85.21.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering TG. Pathophysiology of exercise hypertension. Herz. 1987;12:119–124. [PubMed] [Google Scholar]
- Potts JT, Shi XR, Raven PB. Carotid baroreflex responsiveness during dynamic exercise in humans. Am J Physiol Heart Circ Physiol. 1993;265:H1928–H1938. doi: 10.1152/ajpheart.1993.265.6.H1928. [DOI] [PubMed] [Google Scholar]
- Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res. 1962;11:370–380. doi: 10.1161/01.res.11.3.370. [DOI] [PubMed] [Google Scholar]
- Rondon MU, Laterza MC, de Matos LD, Trombetta IC, Braga AM, Roveda F, Alves MJ, Krieger EM, Negrao CE. Abnormal muscle metaboreflex control of sympathetic activity in never-treated hypertensive subjects. Am J Hypertens. 2006;19:951–957. doi: 10.1016/j.amjhyper.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Rotto DM, Schultz HD, Longhurst JC, Kaufman MP. Sensitization of group III muscle afferents to static contraction by arachidonic acid. J Appl Physiol. 1990;68:861–867. doi: 10.1152/jappl.1990.68.3.861. [DOI] [PubMed] [Google Scholar]
- Sausen MT, Delaney EP, Stillabower ME, Farquhar WB. Enhanced metaboreflex sensitivity in hypertensive humans. Eur J Appl Physiol. 2009;105:351–356. doi: 10.1007/s00421-008-0910-8. [DOI] [PubMed] [Google Scholar]
- Seguro C, Sau F, Zedda N, Scano G, Cherchi A. Arterial blood pressure behavior during progressive muscular exercise in subjects with stable arterial hypertension. Cardiologia. 1991;36:867–877. [PubMed] [Google Scholar]
- Smith SA, Leal AK, Williams MA, Murphy MN, Mitchell JH, Garry MG. The TRPv1 receptor is a mediator of the exercise pressor reflex in rats. J Physiol. 2010;588:1179–1189. doi: 10.1113/jphysiol.2009.184952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol. 2001;537:961–970. doi: 10.1111/j.1469-7793.2001.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Williams MA, Leal AK, Mitchell JH, Garry MG. Exercise pressor reflex function is altered in spontaneously hypertensive rats. J Physiol. 2006;577:1009–1020. doi: 10.1113/jphysiol.2006.121558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Williams MA, Mitchell JH, Mammen PP, Garry MG. The capsaicin-sensitive afferent neuron in skeletal muscle is abnormal in heart failure. Circulation. 2005;111:2056–2065. doi: 10.1161/01.CIR.0000162473.10951.0A. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Tsuchimochi H, McCord JL, Leal AK, Kaufman MP. Dorsal root tetrodotoxin-resistant sodium channels do not contribute to the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. Am J Physiol Heart Circ Physiol. 2011;300:H652–663. doi: 10.1152/ajpheart.00859.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa S, Ueda T, Ishida Y, Nishigaki M, Shibata Y, Shimada S. Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human nociceptors. J Clin Invest. 2002;110:1185–1190. doi: 10.1172/JCI15709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vongpatanasin W, Wang Z, Arbique D, Arbique G, Admas-Huet B, Mitchell J, Victor RG, Thomas GD. Functional sympatholysis is impaired in hypertensive humans. J Physiol. 2011;589:1209–1220. doi: 10.1113/jphysiol.2010.203026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyklicky L, Knotkova-Urbancova H, Vitaskova Z, Vlachova V, Kress M, Reeh PW. Inflammatory mediators at acidic pH activate capsaicin receptors in cultured sensory neurons from newborn rats. J Neurophysiol. 1998;79:670–676. doi: 10.1152/jn.1998.79.2.670. [DOI] [PubMed] [Google Scholar]
- Wang HJ, Li YL, Gao L, Zucker IH, Wang W. Alteration in skeletal muscle afferents in rats with chronic heart failure. J Physiol. 2010;588:5033–5047. doi: 10.1113/jphysiol.2010.199562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SA, Blumenthal RS, Sharrett AR, Redberg RF, Mora S. Exercise blood pressure and future cardiovascular death in asymptomatic individuals. Circulation. 2010;121:2109–2116. doi: 10.1161/CIRCULATIONAHA.109.895292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MA, Smith SA, O'Brien DE, Mitchell JH, Garry MG. The group IV afferent neuron expresses multiple receptor alterations in cardiomyopathyic rats: evidence at the cannabinoid CB1 receptor. J Physiol. 2008;586:835–845. doi: 10.1113/jphysiol.2007.140392. [DOI] [PMC free article] [PubMed] [Google Scholar]


