Abstract
Non-technical summary
The human body controls its temperature through coordinated physiological processes. Prior to the current study, it remained unknown if differences between males and females existed in these processes. The results from the current study show that females have a lower whole-body sweat response during exercise in the heat compared to males, which results in a greater increase in body temperature. The physiological process responsible for the lower whole-body sweat rate was a lower thermosensitivity of the response, meaning a lower increase in sweat production for a given increase in body temperature. Knowledge of sex-related differences in the physiology of temperature regulation may lead to better improvements in heat exposure guidelines for industrial, military and athletic settings.
Abstract
It is unclear whether true physiological differences exist in temperature regulation between males and females during exercise, independently of differences in physical characteristics and metabolic heat production. Therefore, we examined differences in the onset threshold and thermosensitivity of whole-body sudomotor activity and cutaneous vascular conductance between males and females matched for body mass and surface area. Nine males and nine females performed 90 min of exercise at each of the following intensities in a warm/dry environment: 50% of maximum oxygen consumption (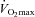 ) and at a fixed rate of metabolic heat production equal to 500 W. Evaporative heat loss (EHL, direct calorimetry) and cutaneous vascular conductance (CVC, laser-Doppler) were measured continuously. Mean body temperature was calculated from the measurements of oesophageal and mean skin temperatures. During exercise at 50%
) and at a fixed rate of metabolic heat production equal to 500 W. Evaporative heat loss (EHL, direct calorimetry) and cutaneous vascular conductance (CVC, laser-Doppler) were measured continuously. Mean body temperature was calculated from the measurements of oesophageal and mean skin temperatures. During exercise at 50% 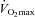 , a lower rate of sudomotor activity was observed in females (385 ± 12 vs. 512 ± 24 W, P < 0.001). However, irrespective of sex, individual EHL values were strongly associated with metabolic heat production (R2 = 0.82, P < 0.001). Nonetheless, a lower rate of EHL was observed in females when exercise was performed at 500 W of metabolic heat production (419 ± 7 vs. 454 ± 11 W, P = 0.032). Furthermore, a lower increase in EHL per increase in mean body temperature was observed in females (553 ± 77 vs. 795 ± 85 W °C−1, P = 0.051), with no differences in the onset threshold (36.77 ± 0.06 vs. 36.61 ± 0.11°C, P = 0.242). In contrast, no differences were observed in CVC. Collectively, these findings demonstrate that females have a lower thermosensitivity of the whole-body sudomotor response compared to males during exercise in the heat performed at a fixed rate of metabolic heat production.
, a lower rate of sudomotor activity was observed in females (385 ± 12 vs. 512 ± 24 W, P < 0.001). However, irrespective of sex, individual EHL values were strongly associated with metabolic heat production (R2 = 0.82, P < 0.001). Nonetheless, a lower rate of EHL was observed in females when exercise was performed at 500 W of metabolic heat production (419 ± 7 vs. 454 ± 11 W, P = 0.032). Furthermore, a lower increase in EHL per increase in mean body temperature was observed in females (553 ± 77 vs. 795 ± 85 W °C−1, P = 0.051), with no differences in the onset threshold (36.77 ± 0.06 vs. 36.61 ± 0.11°C, P = 0.242). In contrast, no differences were observed in CVC. Collectively, these findings demonstrate that females have a lower thermosensitivity of the whole-body sudomotor response compared to males during exercise in the heat performed at a fixed rate of metabolic heat production.
Introduction
The physiological variables of temperature regulation, consisting of the body temperature at onset of effector responses (onset threshold) and the increase in effector output per unit increase in body temperature (thermosensitivity), dictate the capacity of the human body to regulate its temperature. When comparing these variables between populations, however, all other environmental (e.g. air temperature and humidity, heat production) and physical (e.g. body mass/surface area) factors must remain constant. This is particularly relevant when comparing temperature regulation between males and females, since both have unique physical characteristics which make it difficult to discern whether differences in temperature regulation are attributed to either physiological or physical/environmental variables (Nunneley, 1978; Burse, 1979; Kenney, 1985).
Most studies examining differences in temperature regulation between males and females have focused on core temperature responses (Wyndham et al. 1965; Morimoto et al. 1967; Weinman et al. 1967; Shapiro et al. 1980, 1981; McLellan, 1998). Although sex differences in core temperature might intuitively suggest differences in the physiology of temperature regulation, core temperature alone does not provide an accurate assessment of thermoregulatory function when differences in physical characteristics between sexes are not taken into account (Gagnon et al. 2009). Furthermore, studies that have examined sex differences in heat loss responses during exercise have exclusively done so during weight-bearing exercise (i.e. treadmill) at a fixed external workload (Davies, 1979; Avellini et al. 1980a,b; Moran et al. 1999), or during exercise at a fixed percentage of maximum oxygen consumption (Paolone et al. 1978; Frye & Kamon, 1981; Horstman & Christensen, 1982; Keatisuwan et al. 1996; Ichinose-Kuwahara et al. 2010). Although a consistent finding from these studies is a lower sweat rate in females, this observation is proportional to the variations in metabolic heat production elicited by such experimental protocols (Havenith, 2001a; Gagnon et al. 2008). Since previous studies have not fully accounted for differences in physical and/or environmental variables, differential core temperature and/or sweating responses between sexes during exercise may not necessarily indicate a true sex-related difference in temperature regulation, but may rather be attributed to simple differences in physical characteristics and rate of metabolic heat production. As such, conclusions about whether sex can modulate the physiology of temperature regulation remain limited, a matter which has recently gained interest within this field of research (Schwiening et al. 2011).
Determining whether sex can modulate the physiology of temperature regulation has important mechanistic and practical implications. Sex has traditionally not been considered an independent modulator of temperature regulation (Sawka et al. 1996). It therefore often remains unknown if and/or how changes in thermoregulatory function differ between males and females, as a function of age, chronic disease and various physiological states. Such findings could lead to sex-specific improvements in public health, particularly in the improvement of heat exposure guidelines for industrial, military and sport settings which currently do not consider sex as a potential factor affecting heat stress and strain during work in the heat (United States Army Center for Health Promotion and Preventive Medicine, 2003; American Conference of Industrial Hygienists, 2007).
Therefore, the purpose of this study was to examine whether sex can modulate the physiological variables of human temperature regulation independently of physical and environmental factors. To achieve this objective, males and females were matched for body mass and surface area, while exercise at a fixed percentage of maximum oxygen consumption was compared to a condition of fixed metabolic heat production. The physiological variables of temperature regulation consisted of the onset threshold and thermosensitivity of the evaporative heat loss and cutaneous vascular conductance responses. We hypothesised that no sex-related differences in the onset threshold and thermosensitvity of the evaporative heat loss and cutaneous vascular conductance responses would be observed during exercise at a fixed rate of metabolic heat production. A secondary objective was to contrast the results from this condition with exercise performed at a fixed percentage of maximum oxygen consumption, which we hypothesised would result in a lower evaporative heat loss response in females, proportional to a lower rate of metabolic heat production.
Methods
Ethical approval
The current experimental protocol was approved by the University of Ottawa Health Sciences and Science Research Ethics Board, in accordance with the Declaration of Helsinki. Written informed consent was obtained from all volunteers prior to their participation in the study.
Participants
An effect size of 10% and standard deviation of 5%, estimated from previous publications (Gagnon et al. 2008, 2009), resulted in a minimum calculated (β = 0.9, α = 0.05) sample size of six participants in each group. Eighteen participants, nine males and nine females, were recruited within the University community and volunteered for the study. To eliminate the confounding influence of differences in physical characteristics between sexes, males and females were matched in pairs for body mass. Furthermore, to eliminate the influence of differences in hormonal status across the menstrual cycle, female participants performed each experimental session within the first and tenth day after the onset of their self-reported menses. Female participants taking oral contraceptives (n = 6) performed the experimental sessions during the no pill/placebo phase of oral contraceptive use. Hormonal status was confirmed by taking a venous blood sample on the day of each experimental session. None of the experimental sessions for female participants had to be withdrawn or repeated based on blood sample results. Participants were healthy, non-smoking and free of any known cardiovascular, metabolic or respiratory diseases. Participant characteristics are presented in Table 1.
Table 1.
Participant characteristics
| Sex | Age (years) | Body mass (kg) | Height (cm) | AD (m2) | Fat mass (kg) | Lean mass (kg) | Bone mass (kg) |
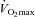 (l min−1) (l min−1) |
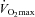 (ml kg−1 min−1) (ml kg−1 min−1) |
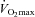 (ml kgLBM−1 min−1) (ml kgLBM−1 min−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| Males | 24 ± 4 | 66.4 ± 4.6 | 173 ± 6 | 1.79 ± 0.08 | 6.1 ± 2.2* | 57.2 ± 4.6* | 3.1 ± 0.3* | 3.83 ± 0.48* | 57.8 ± 6.3* | 66.9 ± 6.1 |
| Females | 27 ± 4 | 66.8 ± 5.0 | 168 ± 5 | 1.76 ± 0.08 | 17.1 ± 6.5 | 46.9 ± 4.8 | 2.7 ± 0.3 | 3.09 ± 0.31 | 46.7 ± 6.4 | 66.0 ± 6.5 |
AD, body surface area; 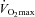 , maximum oxygen consumption; LBM, lean body mass.
, maximum oxygen consumption; LBM, lean body mass.
Significantly different from females (P≤ 0.05).
Values are mean ± standard deviation.
Experimental design
Participants volunteered for one screening visit and two experimental sessions. During the screening visit, measurements of body height and mass, as well as maximum oxygen uptake were determined. Body height was determined using a stadiometer (Detecto, model 2391, Webb City, MO, USA), while body mass was calculated as the sum of fat tissue mass, lean tissue mass and bone mass as determined using dual-energy X-ray absorpsiometry. Body surface area was calculated from the measurements of body height and mass (DuBois & DuBois, 1916). Maximum oxygen uptake was determined by an automated indirect calorimetry system (MOXUS system, Applied Electrochemistry, Pittsburgh, PA, USA) during a progressive incremental cycling protocol performed on an upright seated constant-load cycle ergometer (Corival, Lode B.V., Groningen, the Netherlands). The participants were asked to cycle continuously at 80 rpm, at a starting work rate of 80 W for 2 min. The work rate was then increased by 20 W increments every minute thereafter until the subject could not maintain a pedaling cadence of at least 60 rpm (Canadian Society for Exercise Physiology, 1986).
For each experimental session, participants reported to the laboratory between 07.00 and 09.00, after eating a small breakfast (i.e. toast and juice), but consuming no tea or coffee that morning. The participants were also asked to drink 500 ml of water the night prior to, as well as the morning of, each experimental session and to refrain from alcohol and exercise for 24 h prior to experimentation. Upon arrival at the laboratory, the participants voided their bladder and provided a urine sample before weighing themselves nude. Subsequently, the participants changed into shorts and sandals (as well as a sports bra for female participants), and sat quietly for a 30 min instrumentation period at an ambient room temperature of 24°C. Following instrumentation, the participant entered the calorimeter regulated to an ambient air temperature of 35.21 ± 0.24°C, a specific humidity of 4.27 ± 1.51 g kg−1 (∼12% relative humidity) and an air mass flow of 5.75 ± 0.28 kg air min−1. The participant, seated in the upright position, rested for a 45 min habituation period. Subsequently, the participant performed 90 min of continuous cycling exercise at either 50% of maximum oxygen consumption, or a rate of metabolic heat production equal to 500 W. A fixed percentage of maximum oxygen consumption (50%) was selected since it is a widely used approach to study sex-related differences in thermoregulation during exercise. In contrast, a fixed rate of absolute heat production (500 W) was chosen to provide the same requirement for heat loss for males and females. At the end of the exercise period, all instrumentation was removed except for the laser-Doppler probe, and participants remained seated for a 45 min local heating period to determine maximum skin blood flow (see details below). At the end of the local heating period, the participants exited the calorimeter and a final nude body mass measurement was obtained.
The two experimental sessions were performed on separate days, separated by a minimum of 48 h. For all experimentation, clothing was standardized to cotton underwear, running shorts, sandals and sports bra for female participants. Possible differences in acclimation status between males and females were not taken into account. However, since all experimentation occurred between the months of October and May, it was assumed that all participants were not heat acclimated.
Measurements
The main thermoeffector responses during exercise in the heat consist of whole-body sudomotor activity and cutaneous vasodilatation. Therefore, the current study focused on differences between sexes in evaporative heat loss and local skin blood flow.
Whole-body sudomotor activity was estimated from measurements of evaporative heat loss using the modified Snellen direct air calorimeter (Reardon et al. 2006). In order for evaporative heat loss to be a valid measure of whole-body sudomotor activity, we ensured that the environmental conditions provided a high vapour pressure gradient between the skin surface and the surrounding air. Furthermore, we continually maintained this vapour pressure gradient by continuously providing a high air mass flow of dry air through the calorimeter. Calorimeter outflow to inflow differences in absolute humidity were collected at 8 s intervals throughout the trials. The real-time data were displayed and recorded on a personal computer with LabVIEW software (Version 7.0, National Instruments, TX, USA). Evaporative heat loss was subsequently calculated using the following equation:
| (1) |
where mass flow is the rate of flow of air mass (kg air s−1); (Humidityout– Humidityin) is the calorimeter inflow–outflow difference in absolute humidity (g of water (kg of air)−1); and 2426 is the latent heat of vaporization of sweat (J (g of sweat)−1) at 30°C (Wenger, 1972).
Cutaneous vasodilatation was estimated using laser-Doppler velocimetry (PeriFlux System 5000, Perimed AB, Stockholm, Sweden). Prior to the start of the experimental trial, the laser-Doppler flow probe (PR 401 Angled Probe, Perimed AB) was affixed with an adhesive ring to the upper back in a site without superficial veins that demonstrated pulsatile activity. Upper back skin blood flow responses have been shown to be similar to those observed on the forearm and chest (Ichinose et al. 2009). The probe was not moved from its location throughout the experimental trial. To determine maximum skin blood flow, a local heating period to 42°C for 30 min and then to 44°C for an additional 15 min was performed at the end of each experimental trial. Cutaneous vascular conductance was subsequently calculated as laser-Doppler velocimetry output in arbitrary perfusion units (PU) divided by mean arterial pressure and expressed as a percentage of maximum.
Indirect calorimetry was used for the concurrent measurement of metabolic energy expenditure (Nishi, 1981). Expired gas was analysed for O2 (error of ±0.01%) and CO2 (error of ±0.02%) concentrations using electrochemical gas analysers located outside of the calorimeter chamber (AMETEK model S-3A/1 and CD 3A, Applied Electrochemistry). Expired air was recycled back into the calorimeter chamber in order to account for respiratory heat exchange. Prior to each session, gas mixtures of 4% CO2, 17% O2 and balance nitrogen were used to calibrate the gas analysers and a 3 l syringe was used to calibrate the turbine ventilometer (error ± 3%, typically <1%).
Mean arterial pressure was measured using a Finometer (Finapres Medical Systems, Amsterdam, the Netherlands) from the beat-to-beat recording of the right middle finger arterial pressure waveform with the volume-clamp method (Penaz, 1973) and physical criteria (Wesseling et al. 1995). Prior to beginning the measurement period, a level calibration was performed and brachial artery pressure reconstruction (Gizdulich et al. 1996, 1997) was calibrated with an upper arm return-to-flow systolic pressure detection (Bos et al. 1996).
Heart rate was monitored using a Polar coded transmitter, recorded continuously and stored with a Polar Advantage interface and Polar Precision Performance software (Polar Electro, Oy, Finland).
Oesophageal and rectal temperatures were measured with general purpose thermocouple temperature probes (Mallinckrodt Medical Inc., St Louis, MO, USA). The oesophageal probe was inserted 40 cm past the entrance of the nostril while the participants sipped water (250–500 ml) through a straw. The rectal probe was inserted to a depth of 15 cm past the anal sphincter. Skin temperature was measured at 10 sites using thermocouples (Concept Engineering, Old Saybrook, CT, USA) attached to the skin with surgical tape. Mean skin temperature was subsequently calculated using a 10-point weighting of the regional proportions determined by Hardy & DuBois (1938). Temperature data were collected using a HP Agilent data acquisition module (model 3497A) at a rate of one sample every 15 s and simultaneously displayed and recorded in spreadsheet format on a personal computer with LabVIEW software (Version 7.0, National Instruments, TX, USA).
Whole-body sweat production was calculated as the difference in pre- to post-measurements of body mass to the nearest 0.01 kg using a digital high-performance weighing terminal (model CBU150X, Mettler Toledo Inc., Mississauga, ON, Canada). Urine specific gravity was determined in duplicate using a handheld total solids refractometer (model TS400, Reichter Inc., Depew, NY, USA).
On the day of each experimental session, a venous blood sample (10 ml) was obtained from female participants to confirm that the session occurred in the follicular/low hormone phase of the menstrual cycle. The blood samples were collected with a SST vacutainer (BD Vacutainer, Franklin Lakes, NJ, USA) for the determination of plasma 17β-oestradiol and progesterone. Plasma concentrations of 17β-oestradiol and progesterone were quantified using automated chemiluminescent microparticle immunoassays (ARCHITECT system; Abbott Diagnostics, Abbott Park, IL, USA) by an independent external laboratory (Gamma-Dynacare Medical Laboratories, Ottawa, ON, Canada) using appropriate monoclonal antibody-coated microparticles and acridium-labelled conjugates. The plasma concentrations representative of the follicular phase of the menstrual cycle for 17β-oestradiol and progesterone are 46–604 pmol l−1 and 0.6–4.7 nmol l−1, respectively.
Statistical analyses
For all variables, minute averages were performed to carry out the statistical analyses. To examine sex-related differences in the thermal control of the evaporative heat loss and cutaneous vascular conductance responses, the visually determined linear portion of each response against mean body temperature was analysed using a simple linear regression. The onset threshold was defined as the intercept of the regression line with the evaporative heat loss and cutaneous vascular conductance values at rest, while the thermosensitivity was defined as the slope of the regression line (Cheuvront et al. 2009). To account for the relative influence of core and skin temperatures on the activation of heat loss responses (Hertzman et al. 1952; Nadel et al. 1971a,b), mean body temperature was calculated as: 0.9 × oesophageal temperature + 0.1 × mean skin temperature (Shibasaki et al. 2006).
All dependent variables were compared between groups (males vs. females) within each experimental condition (50% and 500 W). Independent samples t tests were used for single comparisons between groups, while a two-way mixed model analysis of variance was used for multiple comparisons using the repeated factor of time and the non-repeated factor of group. When a significant main effect was observed, post hoc comparisons were carried out with independent samples t tests. Furthermore, a simple linear correlation was performed to assess the relationship between end-exercise evaporative heat loss and rate of metabolic heat production values during exercise at a fixed percentage of maximum oxygen consumption. The level of significance for all analyses was set at an alpha level of P≤ 0.05, corrected for multiple comparisons using the Holm–Bonferonni approach. Analyses were performed using commercially available statistical software (SPSS 18.0 for Windows, SPSS Inc., Chicago, IL, USA). All values for sample parameters are reported as mean ± standard deviation, while those for variables are reported as mean ± standard error of the mean.
Results
Participant characteristics
By design, there were no differences in body mass between groups (P = 0.873). Furthermore, there were no differences in height between groups (P = 0.104), such that both sexes had a similar body surface area (P = 0.504). In contrast, males had a lower absolute fat mass (P < 0.001), as well as a greater absolute lean (P < 0.001) and bone (P = 0.021) mass compared to females. Finally, males had a higher maximum oxygen consumption both in absolute values (P = 0.001) and relative to body mass (P = 0.002). However, there were no sex differences in maximum oxygen consumption expressed as a function of lean body mass (P = 0.767).
Exercise at a fixed percentage (50%) of maximum oxygen consumption
On the day of the experimental session, plasma concentrations of 17β-oestradiol and progesterone for the female participants averaged 139 ± 28 pmol l−1 and 1.2 ± 0.2 nmol l−1, respectively. Baseline urine specific gravity did not differ between groups (males: 1.015 ± 0.002 vs. females: 1.015 ± 0.003, P = 0.816). Furthermore, there were no differences between groups in baseline oesophageal (P = 0.786, Table 2), rectal (P = 0.555, Table 2), mean skin (males: 34.72 ± 0.11°C vs. females: 34.54 ± 0.19°C, P = 0.432) and mean body (males: 36.68 ± 0.08°C vs. females: 36.67 ± 0.10°C, P = 0.936) temperatures. Similarly, heart rate (P = 0.409), metabolic heat production (P = 0.328), whole-body evaporative heat loss (P = 0.110) and cutaneous vascular conductance (P = 0.469) did not differ between males and females prior to the beginning of exercise.
Table 2.
Baseline and end-exercise oesophageal and rectal temperatures for males and females during exercise at a fixed percentage of 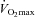 (50%) and a fixed rate of absolute heat production (500 W)
(50%) and a fixed rate of absolute heat production (500 W)
| Condition | Sex | Baseline Toes (°C) | End-ex Toes (°C) | Baseline Tre (°C) | End-ex Tre (°C) |
|---|---|---|---|---|---|
| 50% | Males | 36.90 ± 0.07 | 37.81 ± 0.08 | 36.90 ± 0.07 | 38.10 ± 0.09 |
| Females | 36.93 ± 0.09 | 37.80 ± 0.11 | 36.97 ± 0.08 | 38.19 ± 0.07 | |
| 500 W | Males | 36.65 ± 0.11 | 37.54 ± 0.08* | 36.78 ± 0.10 | 37.89 ± 0.07* |
| Females | 36.83 ± 0.09 | 38.13 ± 0.19 | 36.86 ± 0.08 | 38.35 ± 0.17 |
End-ex, end-exercise, Toes, oesophageal temperature; Tre, rectal temperature.
Significantly different from females (P≤ 0.05). Values are mean ± standard error of the mean.
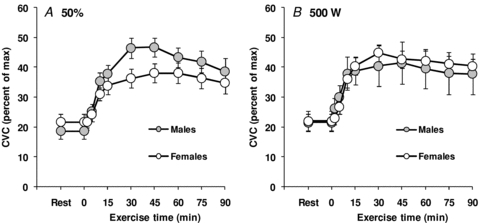
Based on the participants pre-determined absolute maximum oxygen consumption, the target oxygen consumption during exercise averaged 1917 ± 239 ml min−1 for males and 1544 ± 153 ml min−1 for females (P≤ 0.001). Consequently, rate of metabolic heat production was significantly greater in males during exercise (P < 0.001), which was paralleled by a greater rate of whole-body evaporative heat loss (P < 0.001, see Fig. 1A). However, the level of evaporative heat loss achieved during exercise significantly correlated (P < 0.001) with rate of metabolic heat production. In fact, differences in metabolic heat production between sexes explained more than 80% of the variance in evaporative heat loss (Fig. 2). The differences in whole-body evaporative heat loss were reflected by a greater whole-body sweat production in males (1.69 ± 0.06 kg vs. 1.29 ± 0.08 kg, P = 0.002). In contrast, there were no differences between sexes in cutaneous vascular conductance during exercise (P = 0.221, see Fig. 3A), as well as the maximum cutaneous vascular conductance values reached during the local heating period (males: 1.02 ± 0.18 PU mmHg−1 vs. females: 1.23 ± 0.25 PU mmHg−1, P = 0.500). Overall, the differences in whole-body evaporative heat loss between sexes were proportional to the differences in metabolic heat production such that no differences in both oesophageal (P = 0.953) and rectal (P = 0.433) temperatures were observed at the end of exercise (see Table 2). Furthermore, there were no significant differences in mean skin temperature throughout exercise (P = 0.117), which averaged 35.36 ± 0.08°C and 34.96 ± 0.16°C for males and females, respectively, at the end of exercise.
Figure 1. Sex-related differences in evaporative heat loss during exercise performed at either a fixed percentage of maximum oxygen consumption (50%, A) or a fixed rate of metabolic heat production (500 W, B).
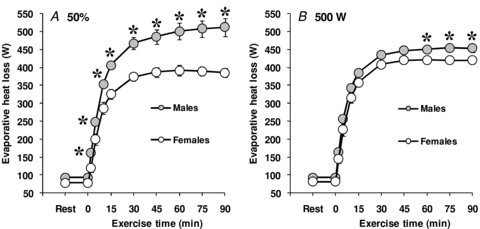
Values are mean ± standard error. *Significantly different from females (P ≤ 0.05).
Figure 2.
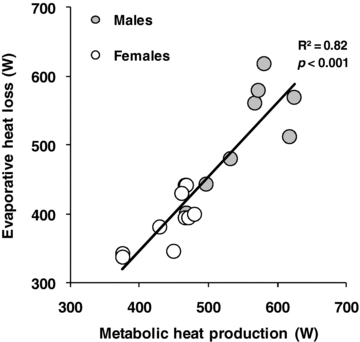
Relationship between evaporative heat loss and rate of metabolic heat production for males and females during exercise performed at a fixed percentage of maximum oxygen consumption
Figure 3. Sex-related differences in the cutaneous vascular response (CVC) during exercise performed at either a fixed percentage of maximum oxygen consumption (50%, A) or a fixed rate of metabolic heat production (500 W, B).
Values are mean ± standard error.
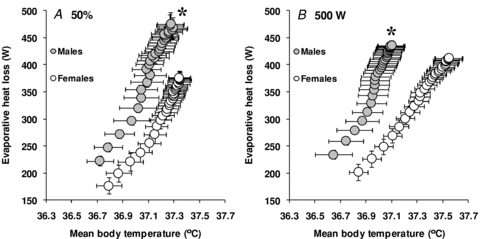
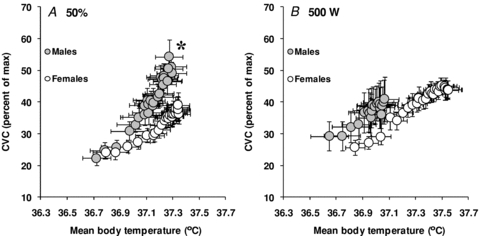
The mean body temperature onset threshold for evaporative heat loss (males: 36.67 ± 0.09°C vs. females: 36.76 ± 0.09°C, P = 0.518) and cutaneous vasodilatation (males: 36.70 ± 0.09°C vs. females: 36.76 ± 0.09°C, P = 0.617) did not differ between groups. However, the thermosensitivity of the whole-body evaporative heat loss (762 ± 56 W °C−1 vs. 559 ± 75 W °C−1, P = 0.049, see Fig. 4A) and cutaneous vascular conductance responses (52 ± 5% CVC °C−1 vs. 30 ± 4% CVC °C−1, P = 0.002, see Fig. 5A) were significantly greater in males compared to females.
Figure 4. Sex-related differences in the sensitivity of the evaporative heat loss response to changes in mean body temperature during exercise performed at either a fixed percentage of maximum oxygen consumption (50%, A) or a fixed rate of metabolic heat production (500 W, B).
The data represent the average slopes of the linear portion of the response. Values are mean ± standard error. *Significantly different slope from females (P ≤ 0.05).
Figure 5. Sex-related differences in the sensitivity of the cutaneous vascular conductance (CVC) response to changes in mean body temperature during exercise performed at either a fixed percentage of maximum oxygen consumption (50%, A) or a fixed rate of metabolic heat production (500 W, B).
The data represent the average slopes of the linear portion of the response. Values are mean ± standard error. *Significantly different slope from females (P ≤ 0.05).
Exercise at a fixed rate of absolute metabolic heat production (500 W)
On the day of the experimental session, plasma concentrations of 17β-oestradiol and progesterone for the female participants averaged 233 ± 66 pmol l−1 and 1.1 ± 0.3 nmol l−1, respectively. Baseline urine specific gravity did not differ between groups (males: 1.020 ± 0.003 vs. females: 1.014 ± 0.002, P = 0.249). Furthermore, there were no differences between groups in baseline oesophageal (P = 0.261, Table 2), rectal (P = 0.550, Table 2), mean skin (males: 34.83 ± 0.13°C vs. females: 34.83 ± 0.10°C, P = 0.962) and mean body (males: 36.45 ± 0.12°C vs. females: 36.60 ± 0.08°C, P = 0.353) temperatures. Similarly, heart rate (P = 0.226), metabolic heat production (P = 0.147), whole-body evaporative heat loss (P = 0.196) and cutaneous vascular conductance (P = 0.919) did not differ between males and females prior to the beginning of exercise.
By experimental design, rate of metabolic heat production during exercise did not differ between sexes (males: 508 ± 5 W vs. females: 504 ± 6 W, P = 0.573). Nonetheless, a greater rate of whole-body evaporative heat loss was observed in males (P = 0.028, see Fig. 1B). The greater rate of whole-body evaporative heat loss was paralleled by a greater whole-body sweat production in males (1.63 ± 0.07 kg vs. 1.38 ± 0.06 kg, P = 0.029). In contrast, there were no differences in cutaneous vascular conductance during exercise (P = 0.899, see Fig. 3B), as well as the maximum cutaneous vascular conductance values reached during local heating (males: 1.24 ± 0.34 PU mmHg−1 vs. females: 0.97 ± 0.20 PU mmHg−1, P = 0.362). The net consequence of the lower evaporative heat loss in females was a greater end-exercise oesophageal (P = 0.019) and rectal (P = 0.026) temperature (Table 2). In contrast, there were no significant differences between groups in mean skin temperature during exercise (P = 0.486), which averaged 35.38 ± 0.07°C and 35.58 ± 0.21°C for males and females, respectively, at the end of exercise.
The mean body temperature onset threshold for whole-body evaporative heat loss (males: 36.61 ± 0.11°C vs. females: 36.77 ± 0.06°C, P = 0.242) and cutaneous vasodilatation (males: 36.65 ± 0.11°C vs. females: 36.77 ± 0.08°C, P = 0.363) did not differ between groups. However, the thermosensitivity of the whole-body evaporative heat loss response was significantly greater in males compared to females (795 ± 85 W °C−1 vs. 553 ± 77 W °C−1, P = 0.051, see Fig. 4B). In contrast, there were no significant differences in the thermosensitivity of the cutaneous vascular conductance response (48 ± 13% CVC °C−1 vs. 38 ± 5% CVC °C−1, P = 0.447, see Fig. 5B).
Discussion
This is the first study to examine the independent effect of sex on the thermoeffector responses of whole-body sudomotor activity and cutaneous vasodilatation during exercise. The findings confirm previous observations that differences in sudomotor activity between males and females during exercise at a fixed percentage of maximum oxygen consumption are proportional to variations in metabolic heat production. However, contrary to our hypothesis, females demonstrate a lower whole-body sudomotor activity during exercise at a fixed rate of metabolic heat production (500 W). The physiological variable responsible for the lower sudomotor activity is a lower thermosensitivity of the response to changes in mean body temperature. In contrast, no differences in cutaneous vascular conductance were observed during exercise at a fixed rate of metabolic heat production. Collectively, these findings demonstrate that sex modulates the thermosensitvity of whole-body sudomotor activity during exercise, independently of differences in body mass, surface area and metabolic heat production.
Similar to previous studies which have examined sweating responses between males and females during exercise (Paolone et al. 1978; Frye & Kamon, 1981; Horstman & Christensen, 1982; Keatisuwan et al. 1996; Ichinose et al. 2009; Ichinose-Kuwahara et al. 2010), a lower rate of whole-body evaporative heat loss was observed in females when exercise was performed at a fixed percentage of maximum oxygen consumption. However, this response was paralleled by a lower rate of metabolic heat production (Fig. 2). These findings confirm previous observations that a lower sudomotor response in females during exercise at a fixed percentage of maximum oxygen consumption is proportional to a lower rate of metabolic heat production, and not necessarily due to physiological differences in temperature regulation (Gagnon et al. 2008). Nonetheless, the lower thermosensitivity of both the evaporative heat loss and cutaneous vascular conductance responses in females may suggest a physiological difference in the control of heat loss responses. However, greater exercise intensities (and therefore rates of metabolic heat production) are generally paralleled by a greater thermosensitivity of the sweating response (Montain et al. 1995; Kondo et al. 1998). In contrast, the thermosensitivity of the cutaneous vascular conductance response has generally been found to be unaffected by exercise intensity (Kondo et al. 2010). Yet, no differences between males and females were observed in the onset threshold and thermosensitivity of the cutaneous vascular response when exercise was performed at a fixed rate of metabolic heat production. Together, these observations suggest that the greater thermosensitivities observed in males during exercise at a fixed percentage of maximum oxygen consumption were due to a greater rate of metabolic heat production.
In order to isolate the physiological variables of temperature regulation, consisting of the onset threshold and themosensitivity of thermoeffector responses, males and females were matched for body mass and surface area to account for the main physical characteristics which determine the individual heat stress response (Havenith et al. 1995, 1998; del Coso et al. 2011). As such, the use of a fixed absolute heat production in constant environmental conditions provided a similar requirement for heat loss in both groups. Yet, females nonetheless exhibited a lower whole-body evaporative heat loss response compared to males, due to a lower thermosensitivity of the response, with no significant differences in the onset threshold. Consequently, these observations provide conclusive and novel evidence that sex can independently modulate whole-body sudomotor activity during exercise. A lower whole-body evaporative heat loss response for a given increase in mean body temperature suggests either: (1) altered afferent neural activity from peripheral (i.e. skin) and central (i.e. core) thermoreceptors causing a different integration of thermal information; (2) altered efferent neural activity for a given level of afferent input; and/or (3) an altered effector response (i.e. sweat production) for a given level of efferent neural activity.
Ichinose-Kuwahara et al. (2010) recently suggested that sex differences in sweat gland function improvements elicited by exercise training are intensity dependent. They reported a greater thermosensitivity of the sweating response in trained males compared to trained females. In contrast, no differences in thermosensitivity of the sweating response were observed between untrained males and untrained females. It should be noted, however, that these observations were made during exercise at fixed percentages (i.e. 35%, 50% and 65%) of maximum oxygen consumption. Since males and females of various training status were compared, both sexes exercised at different external workloads (and therefore rates of metabolic heat production), which may have confounded their results (Gagnon et al. 2008; Schwiening et al. 2011). Nonetheless, their observations, combined with the results of the current study, may provide important insight into the mechanisms responsible for the lower evaporative heat loss and thermosensitivity of the response observed in females at a fixed rate of metabolic heat production.
The study by Ichinose-Kuwahara et al. (2010) suggests that sex can modulate human temperature regulation in an intensity-dependent manner, such that differences are only observed above a certain requirement for heat loss. If sex modulates the level of thermal afferent and/or efferent neural activity during exercise, it would be expected that the differences in thermosensitivity of the sweating response reported by Ichinose-Kuwahara et al. (2010) would not only be limited to trained males and females, but would also be seen between the untrained males and females. Since they only observed differences in thermosensitivity of the sweating response between the males and females who exercised at the highest external workloads (and therefore metabolic heat production), there must be a point at which the requirement for heat loss exceeds the capacity of the sweat gland to contribute to temperature regulation which, as evidenced from the current study, is lower in females compared to males. Consequently, it can be hypothesized that thermal integration and subsequent efferent neural activity is similar between sexes during exercise in the heat, with sex modulating the effector organ (i.e. sweat gland) and its response (i.e. sweat rate). This modulation may not be seen at lower rates of metabolic heat production (or external workloads), since the requirement for heat loss would not exceed the ability of the sweat gland to contribute to temperature regulation. This would explain the lack of differences in sweat rate and thermosensitivity of the sweating response between untrained males and females, while a difference was observed between trained males and females in the study by Ichinose-Kuwahara et al. (2010).
In contrast, the rate of metabolic heat production chosen in the current study (500 W) evidently exceeded the capacity of the sweat gland to contribute to temperature regulation in females, since the greater end-exercise oesophageal and rectal temperatures could only be ascribed to differences in whole-body evaporative heat loss. Furthermore, a given level of thermal afferent and/or efferent activity would only be expected to result in a lower thermosensitivity of the effector response if a maximal effector output could not offset the overall requirement for heat loss. In this situation, the combination of a lower maximal effector output and a greater increase in body temperature would result in a lower thermosensitivity of the response, as observed for evaporative heat loss in females during the 500 W condition of the current study. This hypothesis is further supported by previous observations of a lower sweat output per gland, despite a greater number of active sweat glands in females during passive heating (Bar-Or et al. 1968; Inoue et al. 2005), as well as a lower eccrine sweat gland response to given doses of acetylcholine (Kahn & Rothman, 1942; Gibson & Shelley, 1948) and pilocarpine (Madeira et al. 2010). However, since these observations were made during passive heat stress or in the absence of heat stress, future studies are needed to directly assess the specific mechanism mediating the lower whole-body evaporative heat loss response observed during exercise in the current study. Furthermore, we are unable to determine at which rate of metabolic heat production this modulation occurs since we did not compare responses between males and females at progressively increasing rates of metabolic heat production. Future studies are warranted to address this hypothesis.
While whole-body sudomotor activity represents the main thermoeffector response, particularly during exercise in the heat, increases in skin blood flow also represent an important avenue for heat exchange. In contrast to the observed differences in sudomotor activity, no sex differences in cutaneous vascular conductance were observed. To our knowledge, only Inoue et al. (2005) have specifically examined sex-related differences in skin blood flow during heat stress. Although their observations were made during passive heating, they observed similar skin blood flow responses between males and females on the forehead, chest, back and forearm. As such, the observations in the current study support these findings and extend them to exercise in the heat. However, Inoue et al. (2005) did note a greater skin blood flow response on the thigh in females. Furthermore, Hodges et al. (2010) have recently reported lower peak blood flow responses in females following a period of forearm occlusion. As such, future studies should consider examining potential sex differences in regional skin blood flow during exercise at greater combinations of exercise intensity and/or environmental temperatures.
Perspectives
The net outcome of the lower whole-body sudomotor thermosensitivity in females was shown as a greater oesophageal and rectal temperature at the end of exercise performed at a fixed rate of metabolic heat production. Prior to the current study, it was generally thought that sex differences in temperature regulation were associated with differences in physical characteristics and not with physiological differences in heat loss responses (Sawka et al. 1996; Havenith, 2001b). The findings of the current study therefore have important implications. They warrant considering sex as an independent factor in modulating human temperature regulation, particularly when examining the effects of age, chronic disease and various physiological states (e.g. dehydration, orthostatic stress, etc.). Since the majority of human temperature regulation studies focus on male participants only, or do not include enough female participants to analyse potential sex differences, it is often unknown if and/or how changes in temperature regulation differ between males and females as a function of such factors. Considering sex as a modulator of human temperature regulation could lead to improvements in public health, particularly for heat exposure guidelines which do not currently provide sex-specific exposure limits for safe work in the heat (United States Army Center for Health Promotion and Preventive Medicine, 2003; American Conference of Industrial Hygienists, 2007).
Considerations
The current study employed whole-body direct calorimetry to assess sex-related differences in sudomotor activity, the main thermoeffector during exercise in the heat. The design of the calorimeter (high air mass flow), as well as the environmental conditions employed (low specific humidity) ensure that our measurements of whole-body evaporative heat loss reflect local changes in sweat rate. In fact, the lower whole-body evaporative heat loss observed in females was paralleled by a lower whole-body sweat production. It is also interesting to note that the onset thresholds for evaporative heat loss, expressed as a change from baseline rest, were generally lower than those reported for local measurements of sweat rate, which may be due to the sensitivity of whole-body calorimetry. Nonetheless, we acknowledge that measurements of local sweat rate and/or the number of active sweat glands would provide insight into the specific mechanisms (number of glands vs. sweat output per gland) responsible for the lower whole-body evaporative heat loss response observed in females. It is possible that differences in absolute maximum oxygen consumption (‘fitness’) between males and females may explain the observed results. Several observations argue against this reasoning, however. First, the differences in absolute maximum oxygen consumption between sexes in the current study are due to differences in the amount of metabolically active tissue and not necessarily to differences in training status, since no differences were observed when maximum oxygen consumption was expressed as a function of lean body mass. Second, resting heart rates and core temperatures, a strong indication of training status, did not differ between groups. Third, recent evidence suggests that maximum oxygen consumption itself does not modulate sweat production, nor the thermosensitivity of the response (Jay et al. 2011). Finally, female participants performed the experimental trials between the first and tenth day after the onset of menses, or during the no pill/placebo period of oral contraceptive use. These time periods were primarily chosen to ensure low levels of progesterone, which has been associated with an elevated resting core temperature and parallel increases in thermoeffector onset thresholds (Stephenson & Kolka, 1985). However, it is possible that elevations in oestrogen, which have been shown to decrease resting core temperature and onset thresholds of thermoeffector responses (Stephenson & Kolka, 1999), may have occurred by the tenth day after the onset of menses. It is equally possible that progestin exposure may not have completely subsided during the no pill/placebo period. Nonetheless, baseline oesophageal temperature and onset thresholds of thermoeffector responses did not differ between sexes.
Conclusion
The current study examined the effect of sex on whole-body sudomotor activity and cutaneous vascular conductance during exercise in the heat. When exercise was performed at a fixed rate of absolute metabolic heat production, females demonstrated a lower evaporative heat loss, and lower thermosensitivity of the response, despite a similar requirement for heat loss compared to males. Importantly, these results were not due to differences in physical characteristics, as both sexes were matched for body mass and surface area. In contrast, no differences in cutaneous vascular conductance were observed. These results demonstrate that sex modulates whole-body sudomotor activity during exercise in the heat, independently of differences in body mass, surface area and rate of metabolic heat production.
Acknowledgments
The current study was performed by D.G., in partial fulfillment for the degree of Doctor of Philosophy from the University of Ottawa. D.G. is supported by an Alexander Graham Bell Canadian Graduate Scholarship from the Natural Sciences and Engineering Research Council. The current work was supported by the Natural Sciences and Engineering Research Council (RGPIN-298159-2004 and RGPIN-298159-2009) and Leaders Opportunity Fund from the Canada Foundation for Innovation (22529). Dr Kenny is supported by a University of Ottawa Research Chair in Environmental Physiology. The authors wish to thank Dr Ollie Jay and Dr Craig Crandall for providing critical input into the development of this work, as well as all the participants who volunteered for the present study.
Author contributions
All authors contributed to the conception and design of the experiments, to the collection, analysis and interpretation of data, as well as to the drafting and critical revising of the manuscript. All authors have approved the final version of the manuscript. All experiments were performed at the University of Ottawa.
References
- American Conference of Industrial Hygienists (ACGIH) Heat Stress and Strain: TLV Physical Agents Documentation. Cincinnati, OH: ACGIH; 2007. pp. 1–36. [Google Scholar]
- Avellini BA, Kamon E, Krajewski JT. Physiological responses of physically fit men and women to acclimation to humid heat. J Appl Physiol. 1980a;49:254–261. doi: 10.1152/jappl.1980.49.2.254. [DOI] [PubMed] [Google Scholar]
- Avellini BA, Shapiro Y, Pandolf KB, Pimental NA, Goldman RF. Physiological responses of men and women to prolonged dry heat exposure. Aviat Space Environ Med. 1980b;51:1081–1085. [PubMed] [Google Scholar]
- Bar-Or O, Lundegren HM, Magnusson LI, Buskirk ER. Distribution of heat-activated sweat glands in obese and lean men and women. Hum Biol. 1968;40:235–248. [PubMed] [Google Scholar]
- Bos WJW, Van Goudoever J, Van Montfrans GA, Van den Meiracker AH, Wesseling KH. Reconstruction of brachial artery pressure from noninvasive finger pressure measurement. Circulation. 1996;94:1870–1875. doi: 10.1161/01.cir.94.8.1870. [DOI] [PubMed] [Google Scholar]
- Burse RL. Sex differences in human thermoregulatory response to heat and cold stress. Hum Factors. 1979;21:687–699. doi: 10.1177/001872087912210606. [DOI] [PubMed] [Google Scholar]
- Canadian Society for Exercise Physiology. Certified Fitness Appraiser Resource Manual. Gloucester, Ontario: CSEP; 1986. Determination of aerobic power, Chapter II; pp. 1–32. [Google Scholar]
- Cheuvront SN, Bearden SE, Kenefick RW, Ely BR, Degroot DW, Sawka MN, Montain SJ. A simple and valid method to determine thermoregulatory sweating threshold and sensitivity. J Appl Physiol. 2009;107:69–75. doi: 10.1152/japplphysiol.00250.2009. [DOI] [PubMed] [Google Scholar]
- Davies CT. Thermoregulation during exercise in relation to sex and age. Eur J Appl Physiol. 1979;42:71–79. doi: 10.1007/BF00421907. [DOI] [PubMed] [Google Scholar]
- del Coso J, Hamouti N, Ortega JF, Fernandez-Elias VE, Mora-Rodriguez R. Relevance of individual characteristics for thermoregulation during exercise in a hot-dry environment. Eur J Appl Physiol. 2011;111:2173–2181. doi: 10.1007/s00421-011-1847-x. [DOI] [PubMed] [Google Scholar]
- DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–871. [Google Scholar]
- Frye AJ, Kamon E. Responses to dry heat of men and women with similar aerobic capacities. J Appl Physiol. 1981;50:65–70. doi: 10.1152/jappl.1981.50.1.65. [DOI] [PubMed] [Google Scholar]
- Gagnon D, Dorman LE, Jay O, Hardcastle SG, Kenny GP. Core temperature differences between sexes during intermittent exercise: physical considerations. Eur J Appl Physiol. 2009;105:453–461. doi: 10.1007/s00421-008-0923-3. [DOI] [PubMed] [Google Scholar]
- Gagnon D, Jay O, Lemire B, Kenny GP. Sex-related differences in evaporative heat loss: the importance of metabolic heat production. Eur J Appl Physiol. 2008;104:821–829. doi: 10.1007/s00421-008-0837-0. [DOI] [PubMed] [Google Scholar]
- Gibson TE, Shelley WB. Sexual and racial differences in the response of sweat glands to acetylcholine and pilocarpine. J Invest Dermatol. 1948;11:137–142. doi: 10.1038/jid.1948.78. [DOI] [PubMed] [Google Scholar]
- Gizdulich P, Imholz BPM, van den Meiracker AH, Parati G, Wesseling KH. Finapres tracking of systolic pressure and baroreflex sensitivity improved by waveform filtering. J Hypertens. 1996;14:243–250. doi: 10.1097/00004872-199602000-00014. [DOI] [PubMed] [Google Scholar]
- Gizdulich P, Prentza A, Wesseling KH. Models of brachial to finger pulse wave distortion and pressure decrement. Cardiovasc Res. 1997;33:698–705. doi: 10.1016/s0008-6363(97)00003-5. [DOI] [PubMed] [Google Scholar]
- Hardy J, DuBois E. The technique of measuring radiation and convection. J Nutr. 1938;15:461–475. [Google Scholar]
- Havenith G. Human surface to mass ratio and body core temperature in exercise heat stress – a concept revisited. J Therm Biol. 2001a;26:387–393. [Google Scholar]
- Havenith G. Individualized model of human thermoregulation for the simulation of heat stress response. J Appl Physiol. 2001b;90:1943–1954. doi: 10.1152/jappl.2001.90.5.1943. [DOI] [PubMed] [Google Scholar]
- Havenith G, Coenen JM, Kistemaker L, Kenney WL. Relevance of individual characteristics for human heat stress response is dependent on exercise intensity and climate type. Eur J Appl Physiol. 1998;77:231–241. doi: 10.1007/s004210050327. [DOI] [PubMed] [Google Scholar]
- Havenith G, Luttikholt VG, Vrijkotte TG. The relative influence of body characteristics on humid heat stress response. Eur J Appl Physiol. 1995;70:270–279. doi: 10.1007/BF00238575. [DOI] [PubMed] [Google Scholar]
- Hertzman AB, Randall WC, Peiss CN, Seckendorf R. Regional rates of evaporation from the skin at various environmental temperatures. J Appl Physiol. 1952;5:153–161. doi: 10.1152/jappl.1952.5.4.153. [DOI] [PubMed] [Google Scholar]
- Hodges GJ, Sharp L, Clements RE, Goldspink DF, George KP, Cable NT. Influence of age, sex, and aerobic capacity on forearm and skin blood flow and vascular conductance. Eur J Appl Physiol. 2010;109:1009–1015. doi: 10.1007/s00421-010-1441-7. [DOI] [PubMed] [Google Scholar]
- Horstman DH, Christensen E. Acclimatization to dry heat: active men vs. active women. J Appl Physiol. 1982;52:825–831. doi: 10.1152/jappl.1982.52.4.825. [DOI] [PubMed] [Google Scholar]
- Ichinose-Kuwahara T, Inoue Y, Iseki Y, Hara S, Ogura Y, Kondo N. Sex differences in the effects of physical training on sweat gland responses during a graded exercise. Exp Physiol. 2010;95:1026–1032. doi: 10.1113/expphysiol.2010.053710. [DOI] [PubMed] [Google Scholar]
- Ichinose TK, Inoue Y, Hirata M, Shamsuddin AK, Kondo N. Enhanced heat loss responses induced by short-term endurance training in exercise women. Exp Physiol. 2009;94:90–102. doi: 10.1113/expphysiol.2008.043810. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Tanaka Y, Omori K, Kuwahara T, Ogura Y, Ueda H. Sex- and menstrual cycle-related differences in sweating and cutaneous blood flow in response to passive heat exposure. Eur J Appl Physiol. 2005;94:323–332. doi: 10.1007/s00421-004-1303-2. [DOI] [PubMed] [Google Scholar]
- Jay O, Bain AR, Deren TM, Sacheli M, Cramer MN. Large differences in peak oxygen uptake do not independently alter changes in core temperature and sweating during exercise. Am J Physiol Regul Integr Comp Physiol. 2011;301:R832–R444. doi: 10.1152/ajpregu.00257.2011. [DOI] [PubMed] [Google Scholar]
- Kahn D, Rothman S. Sweat response to acetylcholine. J Invest Dermatol. 1942;5:431–444. [Google Scholar]
- Keatisuwan W, Ohnaka T, Tochihara Y. Physiological responses of men and women during exercise in hot environments with equivalent WBGT. Appl Hum Sci. 1996;15:249–258. doi: 10.2114/jpa.15.249. [DOI] [PubMed] [Google Scholar]
- Kenney WL. A review of comparative responses of men and women to heat stress. Environ Res. 1985;37:1–11. doi: 10.1016/0013-9351(85)90044-1. [DOI] [PubMed] [Google Scholar]
- Kondo N, Nishiyasu T, Inoue Y, Koga S. Non-thermal modification of heat-loss responses during exercise in humans. Eur J Appl Physiol. 2010;110:447–458. doi: 10.1007/s00421-010-1511-x. [DOI] [PubMed] [Google Scholar]
- Kondo N, Takano S, Aoki K, Shibasaki M, Tominaga H, Inoue Y. Regional differences in the effect of exercise intensity on thermoregulatory sweating and cutaneous vasodilation. Acta Physiol Scand. 1998;164:71–78. doi: 10.1046/j.1365-201X.1998.00407.x. [DOI] [PubMed] [Google Scholar]
- McLellan TM. Sex-related differences in thermoregulatory responses while wearing protective clothing. Eur J Appl Physiol. 1998;78:28–37. doi: 10.1007/s004210050383. [DOI] [PubMed] [Google Scholar]
- Madeira LG, da Fonseca MA, Fonseca IA, de Oliveira KP, Passos RL, Machado-Moreira CA, Rodrigues LO. Sex-related differences in sweat gland cholinergic sensitivity exist irrespective of differences in aerobic capacity. Eur J Appl Physiol. 2010;109:93–100. doi: 10.1007/s00421-009-1262-8. [DOI] [PubMed] [Google Scholar]
- Montain SJ, Latzka WA, Sawka MN. Control of thermoregulatory sweating is altered by hydration level and exercise intensity. J Appl Physiol. 1995;79:1434–1439. doi: 10.1152/jappl.1995.79.5.1434. [DOI] [PubMed] [Google Scholar]
- Moran DS, Shapiro Y, Laor A, Izraeli S, Pandolf KB. Can gender differences during exercise-heat stress be assessed by the physiological strain index? Am J Physiol Regul Physiol. 1999;276:R1798–R1804. doi: 10.1152/ajpregu.1999.276.6.R1798. [DOI] [PubMed] [Google Scholar]
- Morimoto T, Slabochova Z, Naman RK, Sargent F. Sex differences in physiological reactions to thermal stress. J Appl Physiol. 1967;22:526–532. doi: 10.1152/jappl.1967.22.3.526. [DOI] [PubMed] [Google Scholar]
- Nadel ER, Bullard RW, Stolwijk JA. Importance of skin temperature in the regulation of sweating. J Appl Physiol. 1971a;31:80–87. doi: 10.1152/jappl.1971.31.1.80. [DOI] [PubMed] [Google Scholar]
- Nadel ER, Mitchell JW, Saltin B, Stolwijk JA. Peripheral modifications to the central drive for sweating. J Appl Physiol. 1971b;31:828–833. doi: 10.1152/jappl.1971.31.6.828. [DOI] [PubMed] [Google Scholar]
- Nishi Y. Measurement of thermal balance in man. In: Cena K, Clark J, editors. Bioengineering, Thermal Physiology and Comfort. New York, NY: Elsevier; 1981. pp. 29–39. [Google Scholar]
- Nunneley SA. Physiological responses of women to thermal stress: a review. Med Sci Sports Exerc. 1978;10:250–255. [PubMed] [Google Scholar]
- Paolone AM, Wells CL, Kelly GT. Sexual variations in thermoregulation during heat stress. Aviat Space Environ Med. 1978;49:715–719. [PubMed] [Google Scholar]
- Penaz J. Digest of the 10th International Conference on Medical and Biological Engineering. Dresden: 1973. Photoelectric measurement of blood pressure, volume and flow in the finger; p. 104. [Google Scholar]
- Reardon FD, Leppik KE, Wegmann R, Webb P, Ducharme MB, Kenny GP. The Snellen human calorimeter revisited, re-engineered and upgraded: design and performance characteristics. Med Biol Eng Comput. 2006;44:721–728. doi: 10.1007/s11517-006-0086-5. [DOI] [PubMed] [Google Scholar]
- Sawka MN, Wenger CB, Pandolf KB. Thermoregulatory responses to acute exercise-heat stress and heat acclimation. In: Fregly MJ, Blatteis CM, editors. Handbook of Physiology, Section 4, Environmental Physiology. New York, NY: Oxford University Press; 1996. pp. 157–186. [Google Scholar]
- Schwiening CJ, Mason MJ, Thompson M. Absolute power, not sex, promotes perspiration. Exp Physiol. 2011;96:556–558. doi: 10.1113/expphysiol.2010.055996. [DOI] [PubMed] [Google Scholar]
- Shapiro Y, Pandolf KB, Avellini BA, Pimental NA, Goldman RF. Heat balance and transfer in men and women exercising in hot-dry and hot-wet conditions. Ergonomics. 1981;24:375–386. doi: 10.1080/00140138108924859. [DOI] [PubMed] [Google Scholar]
- Shapiro Y, Pandolf KB, Goldman RF. Sex differences in acclimation to a hot-dry environment. Ergonomics. 1980;23:635–642. doi: 10.1080/00140138008924778. [DOI] [PubMed] [Google Scholar]
- Shibasaki M, Wilson TE, Crandall CG. Neural control and mechanisms of eccrine sweating during heat stress and exercise. J Appl Physiol. 2006;100:1692–1701. doi: 10.1152/japplphysiol.01124.2005. [DOI] [PubMed] [Google Scholar]
- Stephenson LA, Kolka MA. Menstrual cycle phase and time of day alter reference signal controlling arm blood flow and sweating. Am J Physiol Regul Physiol. 1985;249:R186–R191. doi: 10.1152/ajpregu.1985.249.2.R186. [DOI] [PubMed] [Google Scholar]
- Stephenson LA, Kolka MA. Esophageal temperature threshold for sweating decreases before ovulation in premenopausal women. J Appl Physiol. 1999;86:22–28. doi: 10.1152/jappl.1999.86.1.22. [DOI] [PubMed] [Google Scholar]
- United States Army Center for Health Promotion and Preventive Medicine. Heat Stress Control and Heat Casualty Management, Technical Bulletin, Medical. Vol. 507. Washington, DC: Headquarters, Department of the Army and Airforce; 2003. pp. 1–72. [Google Scholar]
- Weinman KP, Slabochova Z, Bernauer EM, Morimoto T, Sargent F. Reactions of men and women to repeated exposure to humid heat. J Appl Physiol. 1967;22:533–538. doi: 10.1152/jappl.1967.22.3.533. [DOI] [PubMed] [Google Scholar]
- Wenger CB. Heat of evaporation of sweat: thermodynamic considerations. J Appl Physiol. 1972;32:456–459. doi: 10.1152/jappl.1972.32.4.456. [DOI] [PubMed] [Google Scholar]
- Wesseling KH, de Wit B, van der Hoeven GMA, van Goudoever J, Settels JJ. Physiocal, calibrating finger vascular physiology for Finapres. Homeostasis. 1995;36:67–82. [Google Scholar]
- Wyndham CH, Morrison JF, Williams CG. Heat reactions of male and female Caucasians. J Appl Pysiol. 1965;20:357–364. doi: 10.1152/jappl.1965.20.3.357. [DOI] [PubMed] [Google Scholar]


