Abstract
Diabetes is considered to be one of the most common chronic diseases worldwide. There is a growing scientific and public interest in connecting oxidative stress with a variety of pathological conditions including diabetes mellitus (DM) as well as other human diseases. Previous experimental and clinical studies report that oxidative stress plays a major role in the pathogenesis and development of complications of both types of DM. However, the exact mechanism by which oxidative stress could contribute to and accelerate the development of complications in diabetic mellitus is only partly known and remains to be clarified. On the one hand, hyperglycemia induces free radicals; on the other hand, it impairs the endogenous antioxidant defense system in patients with diabetes. Endogenous antioxidant defense mechanisms include both enzymatic and non-enzymatic pathways. Their functions in human cells are to counterbalance toxic reactive oxygen species (ROS). Common antioxidants include the vitamins A, C, and E, glutathione (GSH), and the enzymes superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GRx). This review describes the importance of endogenous antioxidant defense systems, their relationship to several pathophysiological processes and their possible therapeutic implications in vivo.
Keywords: Oxidative stress, Reactive oxygen species, Antioxidants, Diabetic mellitus, Free radicals, Cardiovascular diseases, Diabetic complications, Lipid peroxidation
Diabetes is widely recognised as one of the leading causes of death and disability worldwide.1 The prevalence of diabetes will rise from 6% to over 10% in the next decade.2 In 2000, the World Health Organization (WHO) recorded a total of 171 million people for all age groups worldwide (2.8% of the global population) who have diabetes, and the numbers are expected to rise to 366 million (4.4% of the global population) by 2030.3
Diabetes is a group of metabolic diseases characterised by high levels of blood sugar (hyperglycemia). It results from defects in insulin production and/or insulin action, and impaired function in the metabolism of carbohydrates, lipids and proteins which leads to long term health complications.4,5 In diabetic patients, long-term damage, dysfunction, and failure of different organs, especially the eyes (diabetic retinopathy), kidneys (diabetic nephropathy), nerves (diabetic neuropathy), heart (myocardial infarction), and blood vessels (atherosclerosis) are related to uncontrolled hyperglycemia.5–7
However, diabetic patients vary in their predisposition to the development of complications.8 The genetic hypothesis suggests that complications from diabetes are genetically predetermined as part of the diabetic syndrome, whereas the metabolic hypothesis suggests that complications such as cellular and vascular damage are the effects of long-term hyperglycemia.9 The Diabetes Control and Complications Trial (DCCT) convincingly showed that complications from diabetes can be delayed and reduced by maintaining tight glycemic control.9
Role of Oxidative Stress in Diabetic Complications
The balance between the rate of free radical generation and elimination is important. Excess cellular radical generation can be harmful;10 however, if there is a significant increase in radical generation, or a decrease in radical elimination from the cell, oxidative cellular stress ensues.11 There is convincing experimental and clinical evidence that the generation of reactive oxygen species (ROS) increases in both types of diabetes and that the onset of diabetes is closely associated with oxidative stress.2,12
Oxidative stress results from increased ROS and/or reactive nitrogen species (RNS).13 Examples of ROS include charged species such as superoxide and the hydroxyl radical, and uncharged species such as hydrogen peroxide and singlet oxygen.2 The possible sources of oxidative stress in diabetes might include auto-oxidation of glucose, shifts in redox balances, decreased tissue concentrations of low molecular weight antioxidants, such as reduced glutathione (GSH) and vitamin E, and impaired activities of antioxidant defence enzymes such as superoxide dismutase (SOD) and catalase (CAT).14 ROS generated by high glucose is causally linked to elevated glucose and other metabolic abnormalities important to the development of diabetic complications. However, the exact mechanism by which oxidative stress may contribute to the development of diabetic complications is undetermined.15
In the past few decades, increasing evidence has connected oxidative stress to a variety of pathological conditions, including cancer, cardiovascular diseases (CVDs), chronic inflammatory disease, post-ischaemic organ injury, diabetes mellitus, xenobiotic/drug toxicity, and rheumatoid arthritis.16,17 Over time, convincing evidence has established the role of free radicals and oxidative stress in the pathogenesis and development of complications from DM,9,18 including retinopathy, nephropathy, neuropathy, and accelerated coronary artery disease.19 Several studies have shown that elevated extra- and intra-cellular glucose concentrations result in oxidative stress20,21 which was reported both in experimental diabetes in animals and in diabetic patients.21–23 The source of oxidative stress is a cascade of ROS leaking from the mitochondria. This process has been associated with the onset of type 1 diabetes (T1DM) via the apoptosis of pancreatic beta-cells, and the onset of type 2 diabetes (T2DM) via insulin resistance.21 The underlying mechanisms in the onset of diabetes are complex because hyperglycemia could also be due to the cause-effect relationship of increased oxidative stress.21 Biomarkers of increased oxidative stress, as measured by indices of lipid peroxidation and protein oxidation, increase in both T1DM, and T2DM.24
The aetiology of oxidative stress in diabetes arises from a variety of mechanisms such as excessive oxygen radical production from auto-oxidation of glucose,25 glycated proteins, and glycation of antioxidative enzymes, which limit their capacity to detoxify oxygen radicals.20 In addition to these mechanisms, two others have been suggested as being responsible for the generation of oxygen radicals in diabetes. First, Jain demonstrated that high glucose levels could stimulate cytochrome P450-like activity by excessive nicotinamide adenine dinucleotide phosphate-oxidase (NADPH) produced by glucose metabolism.26 Second, ketosis, a hallmark of T1DM in particular, could increase oxygen radical production in diabetic patients.27
Nowadays, diabetic micro- and macroangiopathy are considered to be poly aetiological multifactorial diseases. A number of studies have evaluated the role of oxidative stress in the aetiology of microvascular and macrovascular complications of diabetes in the fasting state.28 Furthermore, there is growing evidence suggesting the role of hyperglycemia, hyperinsulinemia and dyslipidaemia in diabetic patients, all of which have been implicated in the development of macroangiopathies, which possibly act upon their ability to induce oxidative stress, leading to endothelial dysfunction and atherosclerosis.20 Many studies have suggested that oxidative stress is a common pathogenic factor for the dysfunction of beta and endothelial cells.29,30 Beta cell dysfunction results from prolonged exposure to high glucose, elevated free fatty acid (FFA) levels, or a combination of both.30 Beta cells are particularly sensitive to ROS because they are low in free-radical quenching (antioxidant) enzymes such as catalase (CAT), glutathione peroxidase (GPx) and superoxide dismutase (SOD).31 Therefore, the ability of oxidative stress to damage mitochondria and markedly blunt insulin secretion is not surprising.32,33 For example, it has been demonstrated that oxidative stress generated by short exposure of beta cell preparations to hydrogen peroxide (H2O2) increases the production of protein cyclin-dependent kinase inhibitor 1 (p21) and decreases insulin messenger ribonucleic acid (mRNA), cytosolic adenosine triphosphate (ATP), and calcium flux in cytosol and mitochondria.34 In other studies, much experimental evidence has been accumulated to show that various types of vascular cells are able to produce ROS under hyperglycemic conditions.20
The pathogenesis of diabetic nephropathy remains far from clear. An important role of oxidative stress for the development of nephropathy and neurological complications is suggested by experimental and clinical studies.35 These studies establish a causal relationship between oxidative stress and diabetic nephropathy by observations that 1) lipid peroxides and 8-hydroxydeoxyguanosine, indices of oxidative tissue injury, increase in the kidneys of diabetic rats with albuminuria; 2) high glucose directly increases oxidative stress in glomerular mesangial cells and target cells of diabetic nephropathy; 3) oxidative stress induces mRNA expression of transforming growth factor beta 1 (TGF-β1) and fibronectin, which are the genes implicated in diabetic glomerular injury, and 4) inhibition of oxidative stress ameliorates all the manifestations associated with diabetic nephropathy.36
Preview studies demonstrated that there is a close relationship between endothelial dysfunction and the development and progression of renal and cardiovascular pathology in patients with T1DM.37 The combined development of renal and cardiovascular complications is referred to as cardiorenal syndrome. The causes of the development of cardiorenal syndrome in T1DM are poorly understood. Previous studies suggest that endothelial dysfunction and the concomitant atherosclerotic process may lead to simultaneous development and progression of renal and cardiac pathology, since endothelial dysfunction is already present at the early stages of T1DM.38
Reactive Oxygen Species
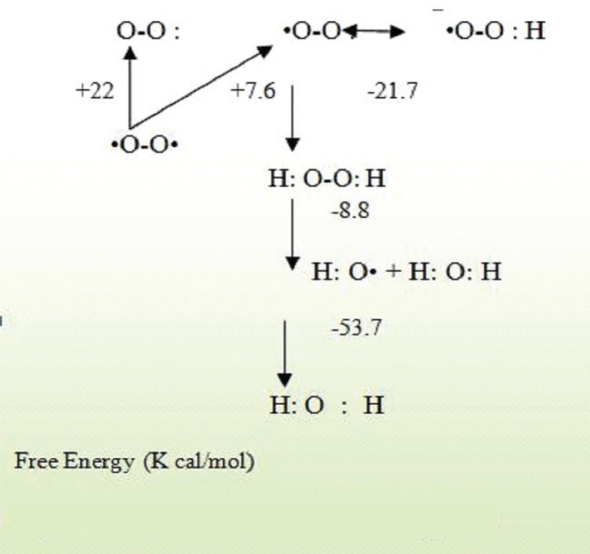
Although molecular oxygen is required to sustain life, it can be toxic through the formation of ROS.39 Indeed, the unusual triplet state of the oxygen molecule, due to the presence of two unpaired electrons, confers a remarkable chemical stability, based on the Pauli Exclusion Principle, which forbids reactions between a singlet and a triplet molecule. Due to electron spin constraints, the oxygen molecule cannot readily react with organic substrates.40 Approximately 1–3% of oxygen consumed by the body is converted into ROS.41 Activation of oxygen can occur through two different mechanisms. The first mechanism of activation is absorption of sufficient energy to reverse the spin on one of the unpaired electrons, called a monovalent reduction. The biradical form of oxygen is in a triplet ground state because the electrons have parallel spins. If triplet oxygen absorbs sufficient energy to reverse the spin of one of its unpaired electrons, it will become singlet oxygen, in which the two electrons have opposite spins [Figure 1]. This activation overcomes the spin restriction and singlet oxygen can consequently participate in reactions involving the simultaneous transfer of two electrons (divalent reduction). The second mechanism of activation is by the stepwise monovalent reduction of oxygen to form superoxide (O2), H2O2, hydroxyl radical (OH) and finally water according to the scheme shown in Figure 2. The first step in the reduction of oxygen forming superoxide is endothermic, but subsequent reductions are exothermic.42
Figure 1:
Nomenclature of the various forms of oxygen.42
Figure 2:
The activation states of oxygen.42
Humans are exposed to many carcinogens, but the most significant may be the reactive species derived from the metabolism of oxygen and nitrogen known as ROS and RNS. On the one hand, the formation of ROS and RNS in the human body can cause oxidative damage to biological macromolecules, especially the plasma membrane,43 which may contribute to the development of cancer, CVD, diabetes and other oxidative stress-mediated dysfunctions.44 On the other hand, ROS are known mediators of intracellular signalling cascades.45
Even though ROS are generated under physiological conditions and are involved to some extent as signalling molecules and defence mechanisms as seen in phagocytosis, neutrophil function, macrophages and other cells of immune system,46 ROS are a heterogeneous group of molecules that are generated by mature myeloid cells during innate immune responses, and are also implicated in normal intracellular signalling. When phagocytes are activated, they produce ROS in amounts high enough to kill intruding bacteria.47 Also, shear-stress induced vasorelaxation and excess production of ROS may, on the other hand, lead to oxidative stress, loss of cell function, and ultimately to apoptosis or necrosis.46 ROS are produced by oxidative phosphorylation, NADPH, xanthine oxidase, the uncoupling of lipoxygenases, cytochrome P450 monooxygenases, and glucose autoxidation.18 Once formed, ROS deplete antioxidant defenses, rendering the affected cells and tissues more susceptible to oxidative damage by reacting with lipids in cellular membranes, nucleotides in DNA,48 sulphydryl groups in proteins, and cross linking fragmentation of ribonucleoproteins,49 leading to changes in cellular structure and function.19
Levels of ROS are under tight control by the protective actions of antioxidant enzymes and nonenzymatic antioxidants in normal and healthy cells. However, in diabetes, excessive cellular levels of ROS are induced by hyperglycemia causing a major complication of DM.50 Furthermore, in the case of diabetes or insulin resistance, a higher oxidative glucose metabolism itself increases mitochondrial production of O2−• which will then be converted to OH• and H2O2.51 Beyond glucose, ROS formation is also increased by FFAs, through its direct effect on mitochondria.52 It has been proposed that over-expression and activity of mitochondrial inner membrane uncoupling proteins (UCPs) contribute to an increase in superoxide formation under diabetic conditions.53
The ROS52,54 listed in Table 1 include free radicals such as superoxide (O2−•) hydroxyl (OH•), and RNS52,54 include free radicals like nitric oxide (NO•). Of these reactive molecules, O2−•, NO• and ONOO− are the most widely studied species and play important roles in diabetic cardiovascular complications.12
Table 1:
Overview of different types of free reactive species2
| Reactive Oxygen Species | |
|---|---|
| RADICALS | NON RADICALS |
| Superoxide, O2−• | Hydrogen peroxide, H2O2 |
| Hydroxyl, OH• | Hypochlorous acid, HOCl− |
| Ozone, O3 | |
| Peroxyl, ROO• | Singlet oxygen,1O2 |
| Peroxynitrite, ONOO− | |
| Alkoxyl, RO• | |
| Hydroperoxyl, HO2 | |
| Reactive Nitrogen Species | |
| Nitric oxide, (nitrogen mono) NO• | Peroxynitrite, ONOO− |
| Alkyl peroxynitrites, ROONO | |
| Nitrogen dioxide, NO2• | Dinitrogen trioxide, N2O3 |
| Dinitrogen tetroxide, N2O4 | |
| Nitrous acid, HNO2 | |
| Nitronium anion, NO2+ | |
| Nitroxyl anion, NO− | |
| Nitrosyl cation, NO+ | |
| Nitryl chloride, NO2Cl | |
| Reactive Chlorine Species | |
| Atomic chlorine, Cl− | Hypochlorous acid, HOCl Chlorine, Cl2 |
| Nitronium (nitryl ) Chloride, NO2Cl | |
In diabetes, NADPH oxidase is a major source of the generation of ROS. NADPH oxidase is located in the plasma membrane of various renal cell types, including mesangial and proximal tubular cells, vascular smooth muscle cells, endothelial cells, and fibroblasts. NADPH oxidase-dependent overproduction of ROS plays a key role in promoting hyperglycemia-induced oxidative stress. The NADPH oxidase increase oxidative stress and finally this increases results in the development of diabetic nephropathy in rats.47
Oxidative Stress-Induced Cellular Damage
The targets of ROS damage include all major groups of biomolecules and can be described as follows:
A. PROTEINS
Since ROS can target almost all cellular compounds, several studies report that ROS can react with several amino acid residues in vitro, generating anything from modified and less active enzymes to denatured, non-functioning proteins.55 Fragmentation of the peptide chain and aggregation of cross-linked reaction products result in an altered electrical charge and increased susceptibility to proteolysis.42 The amino acids in a peptide differ in their susceptibility to attack, and the various forms of activated oxygen differ in their potential reactivity. Primary, secondary, and tertiary protein structures alter the relative susceptibility of certain amino acids.42 Experimental studies show that the decrease in the total protein concentration in the serum of diabetes-induced rats may be ascribed to: 1) decreased amino acids uptake;55 2) a greatly decreased concentration of a variety of essential amino acids;56 3) an increased conversion rate of glycogenic amino acids to CO2 and H2O, and 4) a reduction in protein synthesis secondary to a decreased amount and availability of mRNA.57
In vitro studies show that myeloperoxidase, a heme protein secreted by neutrophils and monocytes, catalyses a very specific reaction, leading to the conversion of L-tyrosine to 3-3-dityrosine.58 In addition, the oxidative formation of dityrosine in proteins may serve as a crosslink between adjacent polypeptide chains of the same or different proteins. Dityrosine is a very unusual molecule linked not by a peptide, but by a covalent bond resistant to protolytic degradation and acid hydrolysis.59 This property of dityrosine makes it a convenient marker for protein oxidation. Oxidatively induced cross-linking of proteins with this modified amino acid was suggested to be a signal for rapid and selective in vivo degradation by intracellular protease.60 However, accumulation of oxidatively modified proteins with age was thought to be a result of their increased resistance to degradation.61
B. LIPIDS
In recent years, much attention has been focused on the role of oxidation stress, and it has been reported that the imbalance between oxidative stress measures and antioxidant levels in diabetes is present because of the generation of ROS during glycation, and glucose and lipid oxidation.62,25 Lipid hydroperoxides (LHP), produced from a variety of long-chain polyunsaturated fatty acid precursors via intermediate radical reactions, involve oxygen and metal cations (iron and copper). The net result of these combined reactions is the generation of highly reactive and cytotoxic lipid radicals, which generate new LHP because of their close proximity in biomembranes to other lipids.63 In addition, diabetes produces disturbances of lipid profiles, especially an increased susceptibility to lipid peroxidation,64 which is responsible for an increased incidence of atherosclerosis,65 a major complication of DM.66 Lipid peroxidation is a critical biomarker of free radical-mediated oxidative stress, and it is probably the most explored area of research when it comes to ROS.67 It is also an important pathological indicator in many diseases.56 Consequently, mechanisms in the formation of lipid hydroperoxides and biologically active metabolites, together with their effect on cellular structure and function, are of increasing importance to the study of diabetogenesis.68
There is much evidence from experimental studies that polyunsaturated fatty acids (PUFA) in the plasma membrane, because of their multiple double bonds, are extremely susceptible to attack by free radicals.69 Hydroxyl radicals initiate a free radical chain reaction and remove a hydrogen atom from one of the carbon atoms in the PUFA and lipoproteins, causing lipid peroxidation characterised by membrane protein damage through subsequent free radical attacks.70 One major hypothesis as to why this occurs is that oxidised lipoprotein (oxLDL) contributes to the cardiovascular complications of diabetes. Low-density lipoprotein (LDL) is the major cholesterol carrier in plasma, and elevated levels of circulating LDL are associated with increased risk for atherosclerosis; notably, increased levels of oxLDL have been associated with hypertension in men.71
Several studies have demonstrated increased LDL oxidation in diabetic patients when compared to their corresponding controls.72 Oxidised lipids can affect cell function by accumulating in the cell membrane causing leakage of the plasma lemma and interfering with the function of membrane-bound receptors.73 In addition, the by-products of lipid peroxidation, such as unsaturated aldehydes and other metabolites, have cytotoxic and mutagenic properties, and oxLDL itself has a specific role in the pathogenesis of atherosclerosis.74 The effects of oxLDL on the vessel walls themselves include stimulation of cytokine and growth factor production and generation of endothelial dysfunction, including inhibition of endothelial cell vasodilator function, all of which contribute to atherosclerosis.41 Lipid peroxidation-mediated membrane defects have also been implicated in the decreased reactivity of thiol group membrane proteins.75 As a consequence, enriching the membranes with PUFA could restore oxidative membrane damage and subsequently normalise decreased membrane fluidity and function. Antioxidants (i.e., dietary supplements) compensating for loss of PUFA may enhance the activity of transmembrane enzymes, membrane-embedded receptors and membrane transport systems.52
Role of Antioxidant Defense System and Protection Mechanism
In the late 19th and early 20th centuries, chemists studied antioxidants, a loosely defined group of compounds characterised by their ability to be oxidised in place of other compounds present. At first, their uses ranged from food storage to the vulcanisation of rubber, but it was only later that biologists realised the importance of antioxidants in health with the 1960s publications of vitamins and flavanoids.39 In the 1970s, Cameron and Pauling, followed by later research, found that ascorbic acid (vitamin C) is a potential human cancer protective agent.76 With many well-known scientists actively researching antioxidants as protecting agents,77 explanations for the effects of antioxidants on cancer susceptibility and overall health expanded rapidly in subsequent decades with research into mechanisms, molecular targets, and molecular interactions.78 In recent years, many conferences and reviews testify to the increasing interest in the roles of the body's antioxidants system working together in human cells against toxic reactive oxygen species, their relationship with several pathophysiologic processes and their possible therapeutic implications.79
Antioxidant defense mechanisms involve both enzymatic and nonenzymatic strategies. Common non-enzymatic antioxidants include the vitamins A, C, and E, glutathione, α-lipoic acid, mixed carotenoids, coenzyme Q10 (CoQ10), several bioflavonoids, antioxidant minerals (copper, zinc, manganese and selenium), and cofactors like folic acid, uric acid, albumin, and vitamins B1, B2, B6, and B12. Enzymatic antioxidants include superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase.80 Through normal physiological processes, antioxidants affect signal transduction and regulation of proliferation and the immune response. While ROS have been linked to cancer, diabetic complications and CVD, antioxidants have shown promise as a possible therapy for the prevention and treatment of these diseases, especially given the tantalising links observed between diets high in fruits and vegetables (and presumably antioxidants) and decreased risks of cancer.41 Evidence from experimental, epidemiological and clinical studies have proven the utility of antioxidants which might therefore be helpful for treating diabetes and its complication.
Evidence from Experimental Studies
Worldwide, several animal studies on antioxidants as protective agents have been conducted. The most important studies have been performed utilising antioxidants in experimental diabetic models. The effects of antioxidants on oxidative stress are measured through certain observable biomarkers. These markers include the enzymatic activities of CAT, SOD, GSH-Px, and glutathione reductase (GSH-Rx), as well as thiobarbituric acid reacting substances (TBARS) levels, an indirect measurement of free radical production that has been shown to be consistently elevated in diabetes.12
Kunisaki investigated the effects of treatments with classical antioxidants such as vitamin E, vitamin C and lipoic acid. Specifically, vitamin E normalises retinal blood flow and protein kinase C (PKC) activity in the vascular tissue of diabetic rats.81 One short-term experimental study showed that high doses of vitamin C can improve some aspects of endothelial dysfunction in diabetes.82 Another experimental study has demonstrated that intraperitoneal administration of α-lipoic acid to streptozotocin (STZ) diabetic Wistar rats normalises TBARS levels in plasma, and the retina, liver, and pancreas.83 Furthermore, it has been reported that α-lipoic acid leads to a decrease in the severity of diabetic neuropathy by maintaining GSH levels and/or by its direct antioxidant properties.83 However, lipoic acid administration improved endothelial function in subjects with metabolic syndrome.
In another study to determine the effects of vitamin E on oxidative stress and cell membrane fluidity in the brains of diabetes-induced experimental rats, Hong et al. reported that vitamin E was found to be effective for strengthening the antioxidant defense system. They noted a reduction of the accumulation of ROS such as superoxide radicals, a decrease in the generation of oxidative damaging substances such as the carbonyl value, a significantly improved lipid composition, and maintenance of membrane fluidity in the brains of the rats.84 Coleman suggested that triple antioxidant therapy (vitamin E, lipoic acid [LA] and vitamin C) in diabetic volunteers attenuates the experimental oxidative stress of methemoglobin formation in vitro and reduces haemoglobin glycation in vivo.85 Panjwani suggested that vitamin C administered alone or in combination with vitamin E reduced the fall in ulnar nerve conduction velocity.86
Sivan and Reece investigated whether dietary supplementation with vitamin E would reduce the incidence of diabetic embryopathy in an in vivo rat model.87 Fifty pregnant rats were designated as the control group and received a normal diet while the diabetic group received vitamin E supplements (400 mg/day). This experimental study found that supplementation with vitamin E reduces the incidence of neural tube defects by more than 75%.87 These findings suggest that vitamin E reduces this oxidative load, confers a protective effect against diabetic embryopathy, and thus may potentially serve as a dietary prophylaxis in the future.
These results are in line with the reports from Chang and others which showed that diabetic embryopathy of rat or mouse embryos is prevented by vitamin C, vitamin E, SOD, N-acetyl-cysteine, or glutathione ethyl ester.88 Another study by Otero et al. showed the effects of supplementation with vitamin E to diabetic mice. The beneficial effect of vitamin E observed in this model was shown to retard coronary atherosclerosis accelerated by DM, and was demonstrated to be due to a reduction in oxidative stress, and not secondary to a decrease in plasma glucose or cholesterol since their respective plasma concentrations remained unchanged in the diabetic mice supplemented with vitamin E.89
Furthermore, it has been recently reported that macrophages from diabetic mice are under excess oxidative stress, and that the antioxidant vitamin E can attenuate macrophage oxidative stress which exists in DM and leads to accelerated atherosclerosis development.90 In addition, there have been other experimental studies assessing the prophylactic effects of vitamin E against heart failure in type 1 diabetic cardiomyopathy. These studies have found that supplementation of streptozotocin-induced (STZ)-diabetic rats with 2000 IU of vitamin E/kg of feed beginning immediately after induction of DM and continuing for 8 weeks provided a significant protection against cardiac dysfunction induced by T1DM. This cardioprotective effect was simultaneously associated with the ability of vitamin E to blunt diabetes-induced amplification of myocardial infarction.91 These findings suggest the usefulness of vitamin E supplementation during the early phases of T1DM for the prophylaxis of cardiomyopathy and subsequent heart failure.
Ozkan et al. examined the protective effects of a triple antioxidant combination (vitamins E and C plus α-lipoic acid) on the total lipid and cholesterol levels and the fatty acid composition of brain tissues in experimental diabetic and non-diabetic rats. Vitamin E and α-lipoic acid were injected intraperitoneally (50 mg/kg) four times per week and vitamin C was provided as a supplement dissolved in the rats’ drinking water (50 mg/kg). The result of this study confirms that treatment with a triple combination of antioxidants protects the arachidonic acid level in the brains of diabetic and non-diabetic rats. In addition, both antioxidants and insulin treatment have beneficial effects on the D-6 desaturase system and unsaturated fatty acid levels. Antioxidants may also have protective effects on unsaturated fatty acids, and reduce oxidative stress, thus impairing the progression to LDL oxidation, cell membrane lipid peroxidation and decreased endoneurial blood flow, thereby reducing peripheral nerve and vascular dysfunction.92
Evidence from Clinical Studies
Early interest in the use of vitamin E supplementation in patients with CHD came from the enthusiastic reports of a Canadian physician, Evan Shute, who recommended vitamin E for all his patients with coronary heart disease (CHD) and presented a case series showing its significant benefit in reducing symptoms of angina pectoris.93 In a clinical trial study conducted by Bursell, et al., they evaluated 36 patients with T1DM. In their crossover trial, individuals were divided into two groups; one group received a placebo while the other group received high dose vitamin E supplementation (1,800 IU/day). The major aim of this study was to determine the effectiveness of vitamin E in normalising retinal blood flow and renal function in patients with T1DM. They found that oral vitamin E treatment appears to be effective in normalising retinal haemodynamic abnormalities and improving renal function in patients with T1DM, especially those with short disease duration, without inducing a significant change in glycemic control. This suggests that vitamin E supplementation may provide an additional benefit in reducing the risks for developing diabetic retinopathy or nephropathy.94
In terms of vitamin E supplementation in diabetes, Ross and others have demonstrated beneficial effects on cataract genesis in diabetic rats.95 Furthermore, in a study done over a four month period, Paolisso, et al. evaluated the efficacy of oral supplementation of vitamin E (900 mg\day) in T2DM patients. They found that supplementation of vitamin E decreased lowered insulin resistance and improved glucose uptake as shown by euglycemic glucose clamp assays performed both before and after the supplementation period.96 In addition, they also concluded that vitamin E may be a useful adjunct in reducing oxidative stress in T2DM. These findings confirmed an earlier study by Ceriello et al. in which glycosylated haemoglobin and other proteins were significantly decreased after two months’ treatment with either 600 or 1200 mg/day of vitamin E.97 The potential for vitamin E in pharmacological doses to be used as a means to delay or prevent secondary complications of diabetes seems well established.
Vitamin C supplementation is effective in reducing sorbitol accumulation in the red blood cells of diabetics. These findings have been confirmed by a clinical study carried out by Cunningham, et al., who investigated the effect of two different doses of vitamin C supplements (100 and 600 mg) during a 58 day trial on young adults with T1DM. The results of this study showed that within 30 days, vitamin C supplementation at either dose normalised sorbitol levels in those with diabetes. Furthermore, vitamin C also helps to reduce capillary fragility which also contributes to complications from DM. Moreover, several studies suggest that chronic vitamin C administration has beneficial effects on glucose and lipid metabolism in T2DM patients.98 In addition, vitamin C supplementation improved glycemic control, fasting blood glucose levels, cholesterol and triglycerides.99
In a placebo-controlled, randomised trial of diabetic patients, Reaven investigated the effect of 1600 IU of RRR-α-tocopherol supplementation daily for 10 weeks on yperglycemia-induced LDL modifications. The result of the study pointed to a reduction of approximately 60% of plasma LDL oxidation in diabetic patients, which was statistically significant when compared to healthy controls.100 These results are supported and confirmed by other similar clinical data. Salonen, et al. found that doses equal to or higher than 450 IU are sufficient to significantly ameliorate the susceptibility of LDL to oxidation, indicating that relatively high doses of RRR-α-tocopherol for supplementation are needed.101 Furthermore, Bellomo and others have shown that the effects of RRR-α-tocopherol supplementation on LDL oxidation are accompanied by a concomitant reduction in autoantibody levels against hyperglycemia-induced LDL modifications. Moreover, clinical studies have shown an inhibitory effect of RRR-α-tocopherol supplementation on the hyperglycemia-induced LDL modifications in T1DM and T2DM diabetic patients.100
Based on nutrition recommendations and interventions for diabetes, there is no clear evidence of benefits that can be derived from vitamin or mineral supplementation in people with diabetes. Routine supplementation with antioxidants, such as vitamins E and C and carotene, is not advised because of lack of evidence of efficacy, and concern related to long term safety, and therefore cannot be recommended.102 The majority of studies included in this review support a possible role of antioxidant supplementation in reducing the risk of CVD and diabetic complications. However, the results of the majority of the prospective randomised controlled antioxidant clinical trials have failed to demonstrate a significant benefit in antioxidant supplementation in the prevention of atherosclerotic events, as shown in Table 2. As also supported by more recent meta-analysis studies of antioxidant-intervention trials in humans, doses of vitamin E supplements (400 or 800 IU) given in many clinical trials are associated with adverse effects,103 or are associated with an increased risk of death.104 Furthermore, the results of the majority of the prospective randomised controlled antioxidant clinical trials have failed to demonstrate a significant benefit of antioxidant supplementation (vitamins C, E and β-carotene) in primary patients (i.e. those without clinical evidence of CVD) or secondary patients (i.e. those with clinical evidence of CVD) in the prevention of cardiovascular events.105 Moreover, in clinical trials the supplementation of vitamins C and E, and/or β-carotene was associated with an increased risk for all-cause mortality, a finding also supported by a recent meta-analysis.104
Table 2:
Selected controlled clinical trials of antioxidant supplements’ effect on preventing diabetic complications
| Study name with type of supplementation | Number | Sex | Age | Dose | Duration in years (Y) or months (M) | Study Outcome |
|---|---|---|---|---|---|---|
|
Dietary supplementation (GISSI). 1999106 Vitamin E |
11,324 | M, F | No age limits | 300 mg | 3.5 Y | No effect on MI + CVD death + stroke |
|
Yusuf, et al. 2000107 Vitamin E |
9541 | M, F | ≥55 | 400 IU | 4.5 Y | No effect on MI, CVD death, or stroke |
|
Pruthi, et al. 2001108 Vitamin E |
39,910 | M | No age limits | 100 IU/day | 2 Y | 37% lower risk of CHD |
|
Kritharides, et al. 2002109 Vitamins A and C, folic acid, niacin,β-carotene, selenium, zinc |
87,000 | M, F | No age limits | Not available | 10 Y | Significantly lower risk of CVD, mortality |
|
Deepak, et al. 2003105 Vitamins E and C, and β-carotene |
20,500 | M, F | 40–80 | VE 600 mg, VC 250 mg, β-carotene 20 mg | 5 Y | No significant difference in cardiovascular mortality and incidence of vascular events between vitamin and placebo groups |
|
Lee, et al. 2005110 Vitamin E |
40000 | F | ≥45 | 600 IU | 10 Y | No effect on cardiovascular events |
|
Costacou, et al. 2006111 Vitamin supplementation with multivitamin pills |
M, F | <17 | Multivitamin pills | 10 Y | Significant decreased risk of CAD incidence in T1DM | |
|
Milman, et al. 2008112 Vitamin E |
1434 | M, F | ≥55 | 400 U/day | 18 M | Reduced cardiovascular events in individuals with DM |
|
Hodis, et al. 2009113 Folic acid, Vitamin B12, and B6 |
506 | M, F | 40–89 | Folic acid 5 mg,VB12 0.4 mg, VB6 50 mg | 3.1Y | Significantly reduced progression of early-stage subclinical atherosclerosis and low risk for CVD |
|
Shargorodsky, et al. 2010114 Combined antioxidant (Vitamins E and C, coenzyme Q10) |
70 | M, F | No age limits | VE (400 IU/day),VC (1000 mg/d), Q10 (120mg\day) | 6 M | Positive effects on cardiovascular risk factors |
Conclusion
In conclusion, there is considerable evidence that induction of oxidative stress is a key process in the onset of diabetic complications. The precise mechanisms by which oxidative stress may accelerate the development of complications in diabetes are only partly known. Evidence for the protective effect of antioxidants has been presented in experimental, clinical, and epidemiological studies, which have demonstrated that antioxidants might be helpful in treating diabetes and its complications. In contrast, the results of the majority of the prospective randomised controlled antioxidant clinical trials have failed to demonstrate a significant benefit, in the prevention of cardiovascular events, of antioxidant supplementation (vitamins C, E and β-carotene) in primary patients without clinical evidence of CVD, or in secondary patients with clinical evidence of CVD.
References
- 1.American Diabetes Association Diagnosis and classification of diabetes mellitus. J Diabetes Care. 2010;33:S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosen P, Nawroth PP, King G, Moller W, Tritschler HJ, Packer L. The role of oxidative stress in the onset and progression of diabetes and its complications: A summary of a Congress Series sponsored by UNESCO MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev. 2001;17:189–912. doi: 10.1002/dmrr.196. [DOI] [PubMed] [Google Scholar]
- 3.Wild SH, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 4.Oktayoglu GS, Basaraner H, Yanardag R, Bolkent S. The effects of combined treatment of antioxidants on the liver injury in STZ diabetic rats. Diges Dis Sci. 2009;54:538–46. doi: 10.1007/s10620-008-0381-0. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tesfaye S, Gill G. Chronic diabetic complications in Africa. Afr J Diabetes Med. 2011;19:4–8. [Google Scholar]
- 7.Chintan AP, Nimish LP, Nayana B, Bhavna M, Mahendra G, Hardik T. Cardiovascular complication of diabetes mellitus. J Appl Pharm Sci. 2011;4:1–6. [Google Scholar]
- 8.Jain SK. The mechanism(s) of complications and benefits of vitamin E supplementation in diabetic patients. From: www.diabetologia.html. Accessed: Jul 2000.
- 9.DCCT The Diabetes Control and Complications Trial research group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 10.Rice EC, Miller N, Paganaga G. Antioxidant properties of phenolic compounds. Tre Pla Sci. 1997;2:152–9. [Google Scholar]
- 11.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabet. 2005;4:5. doi: 10.1186/1475-2840-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joseph LE, Ira DG, Betty AM, Gerold MG. Are oxidative stress activated signaling pathways mediators of insulin resistance and cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Haskins K, Bradley B, Powers K. Oxidative stress in type 1 diabetes. Ann N Y Acad Sci. 2003;1005:43–54. doi: 10.1196/annals.1288.006. [DOI] [PubMed] [Google Scholar]
- 15.Kowluru RA, Chan PS. Oxidative stress and diabetic retinopathy. Exp Diabetes Res. 2007;4:43603. doi: 10.1155/2007/43603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Faramawy SM, Rizk RA. Spectrophotometric studies on antioxidants-doped liposomes. J Am Sci. 2011;7:363–9. [Google Scholar]
- 17.Samanthi RPM, Rolf EA, Jelena AJ, Maria A, Paresh CD. Novel conjugates of 1,3-diacylglycerol and lipoic acid: synthesis, DPPH assay, and RP-LC-MS-APCI analysis. J Lipids. 2011;10:1–10. doi: 10.1155/2011/419809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niedowicz D, Daleke D. The role of oxidative stress in diabetic complications. Cell Biochem Biophys. 2005;43:289–330. doi: 10.1385/CBB:43:2:289. [DOI] [PubMed] [Google Scholar]
- 19.Phillips M, Cataneo RN, Cheema T, Greenberg J. Increased breath biomarkers of oxidative stress in diabetes mellitus. Clin Chim Acta. 2004;344:189–94. doi: 10.1016/j.cccn.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 20.Giugliano D, Ceriello A, Paolisso G. Diabetes mellitus, hypertension and cardiovascular diseases: which role for oxidative stress? Metabolism. 1995;44:363–8. doi: 10.1016/0026-0495(95)90167-1. [DOI] [PubMed] [Google Scholar]
- 21.West IC. Radicals and oxidative stress in diabetes. Diabetes Med. 2000;17:171–80. doi: 10.1046/j.1464-5491.2000.00259.x. [DOI] [PubMed] [Google Scholar]
- 22.Sayed MR, Iman MM, Dawlat AS. Biochemical changes in experimental diabetes before and after treatment with mangifera indica and psidium guava extracts. Int J Pharm Biomed Sci. 2011;2:29–41. [Google Scholar]
- 23.Agnieszka P, Dorota R, Iren A, Maciej J, Stefan A. High glucose concentration affects the oxidantantioxidant balance in cultured mouse podocytes. J Cell Biochem. 2011;112:1661–72. doi: 10.1002/jcb.23088. [DOI] [PubMed] [Google Scholar]
- 24.Cederberg J, Basu S, Eriksson UJ. Increased rate of lipid peroxidation and protein carbonylation in experimental diabetic pregnancy. Diabetologia. 2001;44:766–74. doi: 10.1007/s001250051686. [DOI] [PubMed] [Google Scholar]
- 25.Wolff SP. Diabetes mellitus and free radicals: free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications. Br Med Bull. 1993;49:642. doi: 10.1093/oxfordjournals.bmb.a072637. [DOI] [PubMed] [Google Scholar]
- 26.Jain SK. Hyperglycemia can cause membrane lipid peroxidation and osmotic fragility in human red blood cells. J Biol Chem. 1989;264:21340–5. [PubMed] [Google Scholar]
- 27.Jain SK, Kannan K, Lim G. Ketosis (acetoacetate) can generate oxygen radicals and cause increased lipid peroxidation and growth inhibition in human endothelial cells. Free Radic Biol Med. 1998;25:1083–8. doi: 10.1016/s0891-5849(98)00140-3. [DOI] [PubMed] [Google Scholar]
- 28.Zagar A, Wani AI, Masoodi SR, Laway BA, Bashir MI. Mortality in diabetes mellitus data from a developing region of the world. Diabetes Res Clin Pract. 1999;43:67–74. doi: 10.1016/s0168-8227(98)00112-0. [DOI] [PubMed] [Google Scholar]
- 29.Zourek M, Kyselova P, Mudra J, Krcma M, Jankoveci Z, Lacigova S, et al. The relationship between glycemia, insulin and oxidative stress in hereditary hypertriglyceridemic rats. Physiol. 2008;57:531–8. doi: 10.33549/physiolres.931255. [DOI] [PubMed] [Google Scholar]
- 30.Evans J, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress activated signaling pathways mediators of insulin resistance and beta cell dysfunction. Diabetes. 2003:52–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin producing cells. Diabetes. 1997;46:1733–42. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- 32.Lu HT, Chin S, Ya WC, Ching YY, Chin CW, Dong ZH, et al. Arsenic induces pancreatic cell apoptosis via the oxidative stress-regulated mitochondria-dependent and endoplasmic reticulum stress-triggered signaling pathways. Toxicol Lett. 2011;20:15–26. doi: 10.1016/j.toxlet.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 33.Gisela D, Peter KD, Martina D. Oxidative stress and beta-cell dysfunction. Eur J Physiol. 2010;460:703–18. doi: 10.1007/s00424-010-0862-9. [DOI] [PubMed] [Google Scholar]
- 34.Maechler P, Jornot L, Wolheim CB. Hydrogen peroxide alters mitochondrial activation and insulin secretion in pancreatic beta cells. J Biol Chem. 1999;274:27905–13. doi: 10.1074/jbc.274.39.27905. [DOI] [PubMed] [Google Scholar]
- 35.Sharma P, Joel S, Shuping S, Betty L, Ruc T. Diabetic nephropathy is associated with oxidative stress and decreased renal nitric oxide production. J Am Soc Nephrol. 2007;18:2945–52. doi: 10.1681/ASN.2006080895. [DOI] [PubMed] [Google Scholar]
- 36.Ha H, Kim KH. Pathogenesis of diabetic nephropathy: the role of oxidative stress and protein kinase C. Diabetes Res Clin Pract. 1999;45:147–51. doi: 10.1016/s0168-8227(99)00044-3. [DOI] [PubMed] [Google Scholar]
- 37.Shestakova MV, Jarek IR, Ivanishina NS, Kuharenko SS, Yadrihinskaya MN, Aleksandrov AA, et al. Role of endothelial dysfunction in the development of cardiorenal syndrome in patients with type 1 diabetes mellitus. Diabetes Res Clin Pract. 2005;68:S65–72. doi: 10.1016/j.diabres.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Rossing P, Huggard P, Borch-Johnsen K, Parving HH. Predictors of mortality in IDDM: 10 year observational follow-up study. BMJ. 1996;313:779–84. doi: 10.1136/bmj.313.7060.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rowe L. DNA damage-induced reactive oxygen species: A genotoxic stress response. PhD Thesis, 2009, Emory University, Georgia, USA.
- 40.Miinea CP. Antioxidant protection mechanisms and arachidonic acid metabolism in diabetic rat nerve and Schwann cells cultured in elevated glucose. PhD Thesis, 2002. University of Houston, Texas, USA. [Google Scholar]
- 41.Seifried HE, Anderson DE, Fisher EI, Milner JA. A review of the interaction among dietary antioxidants and reactive oxygen species. J Nutr Biochem. 2007;18:567–79. doi: 10.1016/j.jnutbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 42.Oxidative stress. From: http://www.plantstress.com/Articles. Accessed: Dec 1996.
- 43.Vaughan M. Oxidative damage to macromolecules mini review series. J Biol Chem. 1997;272:18513. [Google Scholar]
- 44.Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82:291–5. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 45.Nordberg J, Arnér ESJ. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol & Medic. 2001;31:1287–312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 46.Halliwell B, Cross CE, Gutteridge JMC. Antioxidants in human health and disease. Annu Rev Nutr. 1996;16:33–50. doi: 10.1146/annurev.nu.16.070196.000341. [DOI] [PubMed] [Google Scholar]
- 47.Hole PS, Darley RL, Tonks A. Do reactive oxygen species play a role in myeloid leukemia? Blood. 2011;117:5816–26. doi: 10.1182/blood-2011-01-326025. [DOI] [PubMed] [Google Scholar]
- 48.Ahsan H, Ali A, Ali R. Oxygen free radicals and systemic autoimmunity. Clin Exp Immunol. 2003;31:398–404. doi: 10.1046/j.1365-2249.2003.02104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waris G, Alam K. Attenuated antigenicity of ribonucleoproteins modified by reactive oxygen species. Biochem Molec Biol Int. 1998;45:33–45. doi: 10.1080/15216549800202412. [DOI] [PubMed] [Google Scholar]
- 50.Lim J. Effects of low-level light therapy on 2,3,7,8-tetrachlorodibenzo-p-dioxin and diabetes-induced oxidative damage in chicken and rat kidneys. PhD Thesis, 2009, Indiana University USA. [Google Scholar]
- 51.Nishikawa T, Edelstein DD, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–90. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 52.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–22. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 53.Rolo AP, Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006;212:167–78. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 54.Turko IV, Marcondes S, Murad F. Diabetes-associated nitration of tyrosine and inactivation of succinyl-CoA:3-oxoacid CoA transferase. Am J Physiol Heart Circ Physiol. 2001;281:H2289–94. doi: 10.1152/ajpheart.2001.281.6.H2289. [DOI] [PubMed] [Google Scholar]
- 55.Butterfield DA, Howard B, Subramaniam R, Hall N, Hensley K, Yatin S, et al. Structural and functional changes in proteins induced by free radical-mediated oxidative stress and protective action of the antioxidants N-tert-butyl-alpha-phenylnitrone and vitamin E. Ann N Y Acad Sci. 1998;854:448–62. doi: 10.1111/j.1749-6632.1998.tb09924.x. [DOI] [PubMed] [Google Scholar]
- 56.Brosnan JT, Man KC, Hall HE, Clobourne SA, Brosnan ME. Inter organ metabolism of amino acids in streptozotocin-diabetic rats. Am J Physiol. 1984;244:E151–8. doi: 10.1152/ajpendo.1983.244.2.E151. [DOI] [PubMed] [Google Scholar]
- 57.Peavy DE, Taylor JM, Jefferson LS. Time course of changes in albumin synthesis and mRNA in diabetic and insulin treated diabetic rats. Am J Physiol. 1985;248:E656–63. doi: 10.1152/ajpendo.1985.248.6.E656. [DOI] [PubMed] [Google Scholar]
- 58.Leeuwen C, Rasmussen JE, Hsu FF, Mueller DM, Pennathur S, Heinecke JW. Mass spectrometric quantification of markers for protein oxidation by tyrocyle radicals, copper, and hydrocyle radicals in LDL isolated from human athersosclerotic plaques. J Biol Chem. 1997;227:3520–6. doi: 10.1074/jbc.272.6.3520. [DOI] [PubMed] [Google Scholar]
- 59.Lipinski B. Pathophysiology of oxidative stress in diabetes mellitus. J Diabetes Complications. 2001;15:203–10. doi: 10.1016/s1056-8727(01)00143-x. [DOI] [PubMed] [Google Scholar]
- 60.Davies KJ. Protein damage and degredation by oxygen radicals: IV. Degredation of denatured protein. J Biol Chem. 1987;262:9914–20. [PubMed] [Google Scholar]
- 61.Berlett BS, Stadtman ER. Protein oxidation in aging, disease and oxidative stress. J Biol Chem. 1997;272:20313–16. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 62.Rosen P, Du XL, Tschope D. Role of oxygen derived radicals for vascular dysfunction in the diabetic heart: prevention by a-tocopherol? Molec Cell Biochem. 1998;188:103–11. [PubMed] [Google Scholar]
- 63.Nishigaki I, Hagishara M, Tsumakawa H, Maseki M, Yagi K. Lipid peroxide levels of serum lipoprotein fractions of diabetic patients. Biochem Med. 1981;25:373–8. doi: 10.1016/0006-2944(81)90096-x. [DOI] [PubMed] [Google Scholar]
- 64.Lyons TJ. Oxidized low density lipoproteins: a role in the pathogenesis of atherosclerosis in diabetes? Diabet Med. 1991;8:411–19. doi: 10.1111/j.1464-5491.1991.tb01624.x. [DOI] [PubMed] [Google Scholar]
- 65.Giugliano D, Ceriollo A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–67. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- 66.Steiner G. Atherosclerosis, the major complication of diabetes. Adv Exp Med Biol. 1985;189:277–97. doi: 10.1007/978-1-4757-1850-8_15. [DOI] [PubMed] [Google Scholar]
- 67.Yla HS. Oxidized LDL and atherogenesis. Ann N Y Acad Sci. 1999;874:134–7. doi: 10.1111/j.1749-6632.1999.tb09231.x. [DOI] [PubMed] [Google Scholar]
- 68.Crabb M. Diabetic Complications: Scientific and clinical aspects. New York: Churchill Livingston Inc.; 1987. [Google Scholar]
- 69.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 70.Halliwell B. Oxidation of low-density lipoproteins: questions of initiation, propagation, and the effect of antioxidants. J Clin Nutr. 1995;61:670–7S. doi: 10.1093/ajcn/61.3.670S. [DOI] [PubMed] [Google Scholar]
- 71.Frostegard J, Wu R, Lemne C, Thulin T, Witztum JL, Faire U. Circulating oxidized low-density lipoprotein is increased in hypertension. Clin Sci (Lond) 2003;105:615–20. doi: 10.1042/CS20030152. [DOI] [PubMed] [Google Scholar]
- 72.Rabini RA, Fumelli P, Galassi R. Increased susceptibility to lipid oxidation of low-density lipoproteins and erythrocyte membranes from diabetic patients. Metab Clin Exp. 1994;43:1470–4. doi: 10.1016/0026-0495(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 73.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–4. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 74.Goldstein JL, Ho YK, Basu SK, Brown M. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci. 1979;76:333–7. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ohyashiki T, Sakata N, Matsui K. Changes in SH reactivity of the protein in porcine intestinal brush-border membranes associated with lipid peroxidation. J Biochem. 1994;115:224–9. doi: 10.1093/oxfordjournals.jbchem.a124322. [DOI] [PubMed] [Google Scholar]
- 76.Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: prolongation of survival times in terminal human cancer. Proc Natl Acad Sci. 1976;73:3685–9. doi: 10.1073/pnas.73.10.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Willett WC, MacMahon B. Diet and cancer - an overview. N Engl J Med. 1984;310:633–8. doi: 10.1056/NEJM198403083101006. [DOI] [PubMed] [Google Scholar]
- 78.World Cancer Research Fund / American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington, DC: AICR; 1997. [Google Scholar]
- 79.Mats J, Pérez G, Cristina D, Castro J. Antioxidant enzymes and human diseases. Clin Biochem. 1999;32:595–603. doi: 10.1016/s0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 80.Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Molec Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 81.Kunisaki MSE, Bursell AC, Clermont H, Ishii LM, Ballas MR, Jirousek F, et al. Vitamin E prevents diabetes-induced abnormal retinal blood flow via the diacylglycerol-protein kinase C pathway. Am J Physiol Endocrinol Metab. 1995;269:239–46. doi: 10.1152/ajpendo.1995.269.2.E239. [DOI] [PubMed] [Google Scholar]
- 82.Mohora M, Greabu M, Muscurel C, Duţă C, Totan A. The sources and the targets of oxidative stress in the etiology of diabetic complications. Romanian J Biophys. 2007;17:63–84. [Google Scholar]
- 83.Obrosova IFL, Greene D. Early changes in lipid peroxidation and antioxidative defense in rat retina. Eur J Pharm. 2000;398:139–46. doi: 10.1016/s0014-2999(00)00286-7. [DOI] [PubMed] [Google Scholar]
- 84.Hong JH, Kim MJ, Park MR, Kwang AJ, Lee IS, Byun BH, et al. Effects of vitamin E on oxidative stress and membrane fluidity in brain of streptozotocin-induced diabetic rats. Clinic Chimic Acta. 2004;340:107–15. doi: 10.1016/j.cccn.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 85.Coleman MD, Fernandes S, Khanderia L. A preliminary evaluation of a novel method to monitor a triple antioxidant combination (vitamins E, C and [alpha]-lipoic acid) in diabetic volunteers using in vitro methaemoglobin formation. J Environ Toxicol Pharmacol. 2003;14:69–75. doi: 10.1016/S1382-6689(03)00027-9. [DOI] [PubMed] [Google Scholar]
- 86.Panjwani U, Yadav DK, Kumar A, Singh SB, Selvamurthy W. Effect of vitamin C and E supplementation in modulating the peripheral nerve conduction following cold exposure in humans. Int J Biometeorol. 2003;48:103–7. doi: 10.1007/s00484-003-0183-1. [DOI] [PubMed] [Google Scholar]
- 87.Sivan E, Reece EA, Wu YK, Homko CJ, Polansky M. Dietary vitamin E prophylaxis and diabetic embryopathy: Morphologic and biochemical analysis. Am J Obstet Gynecol. 1996;175:793–9. doi: 10.1016/s0002-9378(96)80001-9. [DOI] [PubMed] [Google Scholar]
- 88.Chang TM, Horal M, Jain S, Wang F, Patel R, Loeken MR. Oxidant regulation of gene expression and neural tube development: insights gained from diabetic pregnancy on molecular causes of neural tube defects. Diabetologia. 2003;46:538–45. doi: 10.1007/s00125-003-1063-2. [DOI] [PubMed] [Google Scholar]
- 89.Otero P, Bonet B, Herrera E, Rabano A. Development of atherosclerosis in the diabetic BALB/c mice prevention with vitamin E administration. Atheroscler. 2005;182:259–65. doi: 10.1016/j.atherosclerosis.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 90.Hayek T, Kaplan M, Kerry R, Aviram M. Macrophage NADPH oxidase activation, impaired cholesterol fluxes, and increased cholesterol biosynthesis in diabetic mice: a stimulatory role for D-glucose. Atheroscler. 2007;195:277–86. doi: 10.1016/j.atherosclerosis.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 91.Hamblin M, Smith HM, Hill MF. Dietary supplementation with vitamin E ameliorates cardiac failure in type 1 diabetic cardiomyopathy by suppressing myocardial generation of 8-isoprostaglandin F2 [alpha] and oxidized glutathione. J Card Fail. 2007;13:884–92. doi: 10.1016/j.cardfail.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ozkan Y, Yilmaz OK, Ihsan AO, Yasemin E. Effects of triple antioxidant combination (vitamin E, vitamin C and a-lipoic acid) with insulin on lipid and cholesterol levels and fatty acid composition of brain tissue in experimental diabetic and non-diabetic rats. Cell Biol Int. 2005;29:754–60. doi: 10.1016/j.cellbi.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 93.Shute EV. Proposed study of vitamin E therapy. CMAJ. 1972;106:1057. [PMC free article] [PubMed] [Google Scholar]
- 94.Bursell SE, Clermont AC, Aiello LP, Aiello LM, Schlossman DK, Feener EP, et al. High-dose vitamin E supplementation normalizes retinal blood flow and creatinine clearance in patients with type 1 diabetes. Diabetes Care. 1999;22:1245. doi: 10.2337/diacare.22.8.1245. [DOI] [PubMed] [Google Scholar]
- 95.Ross WM, Creighton MO, Trevithick JR, Stewart-DeHaan PJ, Sanwal M. Modelling cortical cataractogenesis: VI. Induction by glucose in vitro or in diabetic rats: prevention and reversal by glutathione. Exp Eye Res. 1983;37:559–73. doi: 10.1016/0014-4835(83)90132-x. [DOI] [PubMed] [Google Scholar]
- 96.Paolisso G, Amore A, Giugliano D, Ceriello A, Varricchio M, Onofrio F. Pharmacologic doses of vitamin E improve insulin action in healthy subjects and non-insulin-dependent diabetic patients. Am J Clin Nutr. 1993;57:650. doi: 10.1093/ajcn/57.5.650. [DOI] [PubMed] [Google Scholar]
- 97.Ceriello A, Giugliano D, Quatraro A, Russo PD, Lefebvre PJ. Metabolic control may influence the increased superoxide generation in diabetic serum. Diabet Med. 1991;8:540–2. doi: 10.1111/j.1464-5491.1991.tb01647.x. [DOI] [PubMed] [Google Scholar]
- 98.Cunningham JJ, Mearkle PL, Brown RG. Vitamin C: an aldose reductase inhibitor that normalizes erythrocyte sorbitol in insulin-dependent diabetes mellitus. J Am Coll Nutr. 1994;13:344. doi: 10.1080/07315724.1994.10718420. [DOI] [PubMed] [Google Scholar]
- 99.Eriksson J, Kohvakka A. Magnesium and ascorbic acid supplementation in diabetes mellitus. Ann Nutr Metab. 1995;39:217–23. doi: 10.1159/000177865. [DOI] [PubMed] [Google Scholar]
- 100.Reaven PD, Herold DA, Barnett J, Edelman S. Effects of vitamin E on susceptibility of low-density lipoprotein and low-density lipoprotein subfractions to oxidation and on protein glycation in NIDDM. Diabetes Care. 1995;18:807. doi: 10.2337/diacare.18.6.807. [DOI] [PubMed] [Google Scholar]
- 101.Salonen JT, Korpela H, Salonen R, Nyyssonen K, Yli-Kerttula S, Yamamoto R, et al. Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet. 1997;339:883–7. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- 102.American Diabetes Association Nutrition recommendations and interventions for diabetes. Diabetes Care. 2008;31:S61–78. doi: 10.2337/dc08-S061. [DOI] [PubMed] [Google Scholar]
- 103.Maret G, Traber AB, Jan F, Stevens AC. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic Biol Med. 2011;51:1000–13. doi: 10.1016/j.freeradbiomed.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–57. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 105.Deepak P, Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet. 2003;361:2017–23. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- 106.Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico (GISSI) Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–55. [PubMed] [Google Scholar]
- 107.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The heart outcomes prevention evaluation study investigators. N Engl J Med. 2000;342:154–60. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 108.Pruthi S, Allison TG, Hensrud DD. Vitamin E supplementation in the prevention of coronary heart disease. Mayo Clin Proc. 2001;76:1131–6. doi: 10.4065/76.11.1131. [DOI] [PubMed] [Google Scholar]
- 109.Kritharides L, Stocker R. The use of antioxidant supplements in coronary heart disease. Atheroscler. 2002;164:211–9. doi: 10.1016/s0021-9150(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 110.Lee IM, Cook N R, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the women’s health study: a randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 111.Costacou T, Zgibor JC, Evans RW, Tyurina YY, Kagan VE, Orchard TJ. Antioxidants and coronary artery disease among individuals with type 1 diabetes: findings from the Pittsburgh Epidemiology of Diabetes Complications Study. J Diabet Complications. 2006;20:387–94. doi: 10.1016/j.jdiacomp.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 112.Milman U, Blum S, Shapira C, Aronson D, Miller-Lotan R, Anbinder Y, et al. Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin 2-2 genotype: a prospective double-blinded clinical trial. Arterioscler Thromb Vasc Biol. 2008;28:341–7. doi: 10.1161/ATVBAHA.107.153965. [DOI] [PubMed] [Google Scholar]
- 113.Hodis HH, Mack WJ, Dustin L, Mahrer PR, Azen SP, Detrano R, et al. High-dose B vitamin supplementation and progression of subclinical atherosclerosis: a randomized controlled trial stroke. 2009;40:730–6. doi: 10.1161/STROKEAHA.108.526798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shargorodsky M, Debby O, Matas Z, Zimlichman R. Effect of long-term treatment with antioxidants (vitamin C, vitamin E, coenzyme Q10 and selenium) on arterial compliance, humoral factors and inflammatory markers in patients with multiple cardiovascular risk factors. Nutr Metab (Lond) 2010;7:55. doi: 10.1186/1743-7075-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]