Abstract
The global obesity epidemic has resulted in significant morbidity and mortality. However, the medical treatment of obesity is limited. Gastric bypass is an effective surgical treatment but carries significant perioperative risks. The gut hormones, peptide tyrosine tyrosine (PYY) and glucagon-like peptide 1 (GLP-1), are elevated following gastric bypass and have been shown to reduce food intake. They may provide new therapeutic targets. This review article provides an overview of the central control of food intake and the role of PYY and GLP-1 in appetite control. Key translational animal and human studies are reviewed.
Keywords: Gut hormones, Appetite, Peptide tyrosine tyrosine, Glucagon-like peptide 1, Obesity
INTRODUCTION
It is currently estimated that nearly 2 billion adults worldwide are overweight (defined by a body mass index [BMI] ≥25 kg/m2) and a further estimated 500 million are obese (BMI ≥30 kg/m2). In 2008, The Health Survey of England classified 24% of adults and 16% of children as obese. These figures are rising sharply due to readily available high-calorie food and sedentary lifestyle. On a national level, extrapolations reveal that 40% of adults could be obese by 2025. It is well known that being overweight or obese carries an increased risk of type 2 diabetes, vascular disease, osteoarthritis, sleep apnoea and malignancy. It has even been predicted that the coming decades could see a reversal of twentieth century gains in life expectancy, due to detrimental health consequences arising from the obesity epidemic.1
Effective treatment of obesity has been shown worthwhile; a 92% reduction in diabetes deaths, 60% reduction in cancer deaths, 56% reduction in coronary artery disease deaths and 40% reduction in all-cause mortality was demonstrated in a retrospective matched cohort study of obese individuals undergoing bariatric surgery.2 Bariatric surgery, in particular Rouxen-Y gastric bypass (which involves the formation of a small stomach pouch and bypass of the proximal small bowel), is the most effective current treatment for obesity, leading to sustained weight loss of approximately 30%.3 However, the procedure is not without risk and carries a mortality rate of 0.5%.4
Lifestyle modification strategies (diet and exercise) currently form the main treatments for obesity. However, results are generally disappointing and the majority of people who attempt lifestyle modification regain any lost weight within 5 years.5 Drug treatments sibutramine and rimonabant were recently withdrawn due to cardiovascular and neuropsychiatric side effects respectively. The only licensed pharmacological treatment for obesity in the UK is orlistat, an intestinal lipase inhibitor. This produces modest weight loss (in a systematic review of randomized clinical trials, 60% of patients on orlistat achieved >5% weight loss after 1 year of treatment).6 Furthermore, as a consequence of its mechanism of action, orlistat often leads to unacceptable gastrointestinal side effects when fat is consumed. In 2010, the U.S. Food and Drug Administration (FDA) committee recommended approval for Contrave (Orexin Therapeutics Inc. and Takeda Pharmaceutical Co.) for the treatment of obesity. Contrave is a combination of naltrexone and bupropion, which act on central pathways to inhibit appetite. A multicentre, randomised, double-blind, placebo-controlled phase 3 trial of Contrave had demonstrated that just under half of patients achieved >5% weight loss after 1 year of treatment.7 However, despite the professional committee recommendation, lack of data made it difficult to assess the safety of Contrave in patients at risk for heart disease and stroke, so the FDA has not licensed the agent without further trial. Thus cardiovascular side effects remains a concern, given that such conditions are themselves inherently linked to obesity.
Accumulation of excess body fat (and hence development of obesity) occurs when energy intake exceeds energy expenditure in the long term. The intricacies underpinning this fine-tuned regulation of energy balance form part of a complex field that has been the focus of intense research over the past decade, bridging the disciplines of endocrinology, gastroenterology and neurobiology. Our progressive understanding of the physiological mechanisms of appetite regulation should help with the development of future pharmacological approaches to combat obesity. In particular, identification of how hormones that are secreted post-prandially by the gut interact with brain regions that modulate appetite may eventually allow for their manipulation as therapeutic strategies in obese patients. These anorectic gut hormones, amongst others, include peptide tyrosine-tyrosine (PYY) and glucagon-like peptide 1 (GLP-1). Although these peptide hormones will form the focus of this review article, in order to appreciate their roles in the control of appetite (and thus potential for development as anti-obesity agents), a brief prior overview of the neural control of feeding is necessary.
CO-ORDINATION OF APPETITE
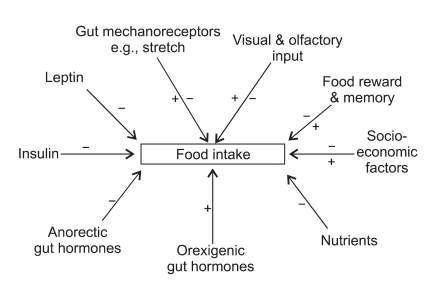
Post-prandially, activation of gut mechanoreceptors, changes in circulating nutrient concentration, and release of anorectic gut hormones all lead to a reduction in subsequent feeding.8 Longer term adiposity signals of energy balance such as leptin also interact with central nervous system (CNS) circuits to regulate food intake.9,10 However, apart from traditional homeostatic feedback regulation of energy balance, a variety of other factors influence food intake. These include food appearance, flavour and availability in addition to social, cultural, and economic influences. Importantly, there is also modulation of food intake by hedonic and mnemonic neuronal circuits.11 The modern consensus is therefore that there is interaction between homeostatic and non-homeostatic inputs, which together lead to co-ordination in terms of inducing either an orexigenic or anorectic response. Fig. 1 summarises the major determinants of appetite control.
Fig. 1.
The major determinants of appetite control.
THE HYPOTHALAMUS
The hypothalamus, in particular the arcuate nucleus (ARC) within it, is thought to be pivotal in appetite regulation. By virtue of lying close to the median eminence (a circumventricular organ where an incomplete blood-brain barrier is thought to allow peripheral signals to gain access to the CNS), the ARC is ideally positioned to co-ordinate feeding. Lesions of the ARC result in hyperphagia and obesity in mice.12
Within the ARC, one discrete group of neurons contains neuropeptide Y (NPY) and agouti-related peptide (AgRP), activation of which enhances food intake.13 Another group comprises anorexigenic neurons containing pro-opiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART). POMC is the precursor of α-melanocyte stimulating hormone (α-MSH), which acts on melanocortin receptors (mainly MC4R) to reduce food intake.14 Furthermore, AgRP is a competitive antagonist at melanocortin receptors.15 CART is thought to have either orexigenic or anorexigenic effects depending on its site of action; central intracerebroventricular administration of CART reduces food intake in rats,16 whereas administration of CART directly into the ARC increases food intake.17
Axons from NPY/AgRP and POMC/CART neurons project from the ARC to other hypothalamic nuclei; one important example being the paraventricular nucleus (PVN). Destruction of the PVN leads to hyperphagia and obesity in rats.18
THE BRAINSTEM
The brainstem dorsal vagal complex (DVC) comprises the nucleus of the tractus solitarius (NTS), the area postrema (AP), and the dorsal motor nucleus of the vagus. The DVC is thought to be an important communication link between peripheral signals of food intake and hypothalamic nuclei.19 The absence of a complete blood-brain barrier in the AP may facilitate this. It is well established that there are neural projections from the brainstem to the hypothalamus20 and vice-versa.21 Additionally, vagal nerve afferents carry sensory information from the gut directly to the NTS. In support of this, transection of these gut sensory vagal nerve afferents results in increased meal size and duration.22
REWARD (HEDONIC) AND MNEMONIC PATHWAYS
Cortico-limbic 'reward centres' implicated in appetite regulation include the hippocampus, amygdala, nucleus accumbens, dorsal and ventral striatum, insula, anterior cingulate and prefrontal cortex. Communication between these regions and the brainstem or hypothalamus culminates in overall co-ordination of food consumption. In support of this, orexin neuron expression in the hypothalamus is increased by administration of an opioid µ-receptor agonist into the nucleus accumbens.23 Furthermore, in rats trained to associate the presentation of a food cup with the availability of food (resulting in conditioned food intake even in situations where they are satiated), the conditioning is abolished by disruption of amygdala-hypothalamus connections.24
With respect to linking reward to hedonic experience, recent evidence points to an important role for the orbitofrontal cortex (OFC), an area that receives converging sensory input.25 The OFC is in contact with other cortical reward areas, such as the prefrontal, insular, and anterior cingulate cortices. Furthermore, there is communication between the OFC, hippocampus and amygdala. The network of communication between these brain regions is thought to play a role in the formation and maintenance of a working memory for food experiences.26
THE ROLE OF GUT HORMONES IN THE CONTROL OF APPETITE
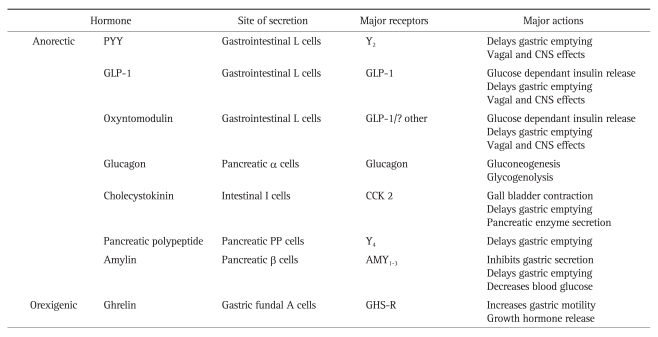
The gut is the largest endocrine organ in the body and secretes over 30 different regulatory peptide hormones. A number of these gut hormones are stimulated by gut nutrient content and interact with receptors at various points in the 'gut-brain axis' to affect short term and intermediate term feelings of hunger and satiety. The major gut hormones implicated in appetite control are outlined in Table 1. This review will focus on the role of the anorectic gut hormones PYY and GLP-1. Both hormones are released together following a meal to mediate postprandial satiety. As discussed previously, gastric bypass surgery is one of the most effective treatments for obese patients, where sustained weight loss results from diminished appetite. Notably, gastric bypass patients demonstrate increased levels of PYY and GLP-1, especially post-prandially.27-29 Significantly, inhibiting the PYY and GLP-1 responses results in return of appetite and increases food intake.29 Therefore, it is likely that elevated levels of PYY and GLP-1 play a key role in the sustained weight loss observed following gastric bypass surgery. It follows that investigation into the physiological interaction between these hormones and the CNS is of paramount importance in obesity research.
Table 1.
The Major Gut Hormones Involved in Appetite Regulation
PYY, peptide tyrosine tyrosine; CNS, central nervous system; GLP-1, glucagon-like peptide 1.
PYY
PYY is a 36-amino acid peptide which belongs to the pancreatic polypeptide (PP) family. This family of peptides, comprising PP, PYY, and NPY, share a common hair-pin-fold motif structure. PP, PYY, and NPY bind to G-protein coupled receptors Y1, Y2, Y4, Y5, and Y6, displaying promiscuity in their interactions with these receptors by virtue of their shared hair-pin-fold motif structure.
PYY is produced by the L cells of the gut, with highest concentrations found in the large bowel and the rectum.30 Two endogenous forms, PYY1-36 and PYY3-36, are released post-prandially into the circulation. PYY3-36 is further produced by cleavage of the Tyr-Pro amino terminal residues of PYY1-36 by the enzyme dipeptidyl peptidase IV (DPP-IV). PYY3-36 acts mainly via theY2 receptor.31 Indeed, PYY3-36 selectivity for the Y2 receptor is thought to be conferred by cleavage of the Tyr-Pro amino terminal residues of PYY1-36.31 In the fasted state, PYY1-36 predominates in the circulation, whereas post-prandially, PYY3-36 is the major circulating form.32 Following a meal, circulating levels of PYY3-36 rise within 15 minutes, peak at approximately 90 minutes and remain elevated for up to 6 hours.30 The magnitude of the rise in PYY3-36 is in proportion to the calories ingested.33 When exogenously administered intravenously, its circulating half-life is approximately 8 minutes.34
Given that PYY3-36 is released within 15 minutes of food intake, this must occur before ingested nutrients reach the distal small intestine and colon (where the greatest concentrations of PYY3-36 are found). Therefore, initial post-prandial release of PYY3-36 is likely to be under neural control. Further release of PYY3-36 is observed when the nutrients arrive in the distal gut and is particularly stimulated by a high fat diet.35 The protein content of the diet is thought to be influential for delayed PYY3-36 release approximately 2 hours post-prandially.36 PYY3-36 is likely to affect appetite via a direct central effect and also via its effects on gut motility - it acts as an 'ileal brake' and so leads to a sensation of fullness and satiety.37,38
Studies by Batterham et al.37,38 have shown that peripheral administration of PYY3-36 to rodents and humans leads to marked inhibition of food intake. In humans, ad libitum food intake at a buffet meal served 2 hours after the completion of a 90 minute intravenous infusion of PYY3-36 delivered at a dose of 0.8 pmol/kg/min, was reduced by 36% compared with an infusion of saline. The PYY3-36 infusion generated plasma levels of PYY3-36 similar to those achieved physiologically after a meal.37 Thus, it was suggested that PYY3-36 inhibits food intake physiologically. Energy intake measured over the subsequent 24 hours after the infusion of PYY3-36 was reduced by 33% compared with saline. This pointed towards a role for PYY3-36 in the intermediate control of food intake beyond the study test meal. Significantly, there was no reported nausea in subjects infused with PYY3-36.
Some studies have reported that obese individuals have lower basal fasting levels of PYY3-36 and have a smaller rise in postprandial levels.38 Obesity does not appear to be associated with resistance to PYY3-36, as in obese subjects, there was a 30% in a reduction in ad libitum food intake at a buffet meal served 2 hours after completion of a 90 minute infusion of PYY3-36 delivered at an unspecified dose based on body surface area.38 This compared with a 31% reduction in food intake in a group of lean subjects who also received a peripheral infusion of PYY3-36. Additionally, there was also a comparable reduction in 24-hour energy intake in both lean and obese subjects following PYY3-36 infusion.
A similar study administered a 90 minute intravenous infusion of PYY3-36 to lean and overweight human subjects at a dose of 0.8 pmol/kg/min, with 19% reduction in ad libitum food intake at a buffet meal served 2 hours after completion of the infusion.39 However, in contrast to the studies by Batterham et al., significant nausea was experienced by subjects, culminating in only 4 of the first 9 being able to complete their infusion. This study also found that there was increased thermogenesis, lipolysis, post-prandial insulin and glucose responses in those receiving PYY3-36, suggestive of increased sympathoadrenal activity and increased energy expenditure. However, the effect of nausea on these latter findings is a potential confounder.
Batterham et al.37 performed a series of experiments in rodents which shed considerable light on the action of PYY3-36 on CNS appetite circuits. Firstly, it was shown that chronic peripheral administration of PYY3-36 resulted in a decrease in food intake and body weight. Furthermore, a single peripheral injection of PYY3-36 resulted in induction of expression of the immediateearly gene c-fos (a marker of neuronal activation) in the hypothalamic ARC. This suggests that the ARC is an important site of action of the peptide. Furthermore, a single peripheral injection of PYY3-36 caused a decrease in expression of hypothalamic NPY mRNA 6 hours later. A subsequent single injection of PYY3-36 directly into the ARC inhibited food intake. Inhibition of food intake was also observed with intra-arcuate administration of aY2 receptor specific agonist. Significantly, this effect was absent in Y2 receptor knock-out mice. Addition of PYY3-36 to ex vivo hypothalamic explants inhibited release of NPY and stimulated release of α-MSH. This finding was further validated by electrophysiological studies, which demonstrated that POMC neurons showed disinhibition when exposed to PYY3-36. It therefore appears that circulating PYY3-36 inhibits appetite by acting directly on the ARC via the Y2 receptor, increasing the activity of anorexigenic POMC/α-MSH neurons, whilst suppressing orexigenic NPY neurons.
The Y2 receptor is abundantly distributed within the hypothalamic ARC, preoptic nucleus and dorsomedial nucleus.40 Given the presence of an incomplete blood-brain-barrier in the hypothalamic median eminence, which lies close to the ARC, it is plausible that circulating PYY3-36 accesses the CNS at this level. Furthermore, the Y2 receptor is found in the NTS of the brainstem.40 In keeping with this, peripheral injection of PYY3-36 activates c-fos expression in the AP and the NTS.41 Therefore, circulating PYY3-36 may access the brainstem via the incomplete blood brain barrier at the AP. Taking into account the presence of ascending and descending projections between the brainstem and hypothalamus (as discussed earlier), it is possible that there is communication between these areas with regard to PYY3-36 action.
Other forebrain areas that express the Y2 receptor include the posterior hypothalamic nuclei, medial nucleus of the amygdala, substantia nigra, and parabrachial area.42 Action on the limbic system (including the amygdala) may be of significance when considering modulation of hedonic pathways by PYY3-36.
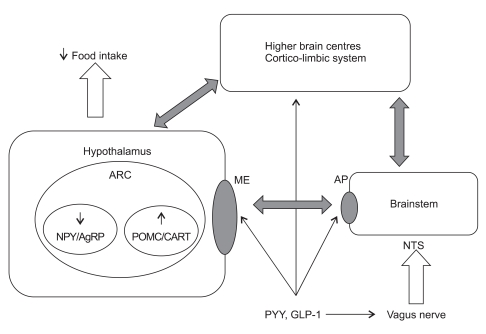
PYY3-36 may also act via the vagus-brainstem-hypothalamic pathway, as evidenced by a series of experiments by Koda et al .43 This group showed using vagal ligation studies that Y2 receptors are transported to the peripheral terminals of vagal afferent neurons. Furthermore, peripheral administration of PYY3-36 led to afferent vagal discharges, while disruption of the vagus-brainstem-hypothalamus pathway abolished its anorexigenic effect. A summary of the interactions of PYY with the brainstem, hypothalamus and higher brain centres is shown in Fig. 2.
Fig. 2.
The role of PYY and GLP-1 in appetite control.
PYY, peptide tyrosine tyrosine; GLP-1, glucagon-like peptide 1; ARC, arcuate nucleus; NPY, neuropeptide Y; AgRP, agouti-related peptide; POMC, pro-opiomelanocortin; CART, cocaine- and amphetamine-regulated transcript; ME, median eminence; AP, area postrema; NTS, nucleus of the tractus solitaries.
Batterham et al.44 demonstrated that intravenous infusion of PYY3-36 to humans not only resulted in reduced food intake, but modulated activity in the hypothalamus and OFC, as assessed by functional magnetic resonance imaging. During saline infusion visits, subjects' caloric intake correlated with signal change in the hypothalamus, whereas this switched to the OFC on study visits when PYY3-36 was infused. It was postulated that the presence of PYY3-36 switches regulation of food intake from a homeostatic brain region (hypothalamus) to a hedonic region (OFC).
DEVELOPING PYY BASED TREATMENTS FOR OBESITY
The translational research on PYY3-36 thus far leads to the prospect that analogues of the peptide, delivered subcutaneously, may be used to treat obesity. This work is already underway, focussing on making changes to the peptide sequence to confer greater Y2 receptor selectivity and prolonged action in vivo. Conferring Y2 receptor selectivity is especially important, given that in contrast to peripheral and intra-ARC injection, administration of PYY3-36 into the 3rd ventricle or the PVN is orexigenic in mice.45,46 This is thought to be due to action on Y1 and Y5 receptors in these regions. In contrast to the anorectic effect produced by Y2 receptor activation, Y1 and Y5 receptor activation promotes feeding.47
During future drug development, monitoring the effect of PYY analogues in reducing food intake without inducing nausea will be a priority. Administration of intra-nasal PYY3-36 was tested in obese human subjects as a weight-loss treatment, but encountered significant problems with nausea and vomiting.48 In this study, placebo or intra-nasal PYY3-36 was administered at a dose of 200 mcg 3 times daily or 600 mcg 3 times daily for 12 weeks. Only 70% of subjects in the low-dose group completed the study and this figure was even lower (26%) in the highdose group. The mean weight loss from baseline over the 12 weeks was 2.8 kg in the placebo group, 3.7 kg in the 200 mcg PYY3-36 group, and only 1.4 kg in the 600 mcg PYY3-36 group.48 Pharmacokinetics revealed rapid (Cmax20 min) absorption of PYY3-36 to pharmacological plasma levels. This is likely to have been a major contributor to nausea.
Oral delivery of peptide hormones has been unsuccessful on the whole, due to the harsh environment of the gastrointestinal tract and thus failure of absorption across the intestinal wall. The subcutaneous route is therefore the established route for peptide hormone delivery; insulin being the classical example. However, it could be argued that oral administration of gut hormones better mimics the physiological post-prandial state (given that these peptides are naturally secreted from the gut, where they may act on local vagal afferents, in addition to being absorbed into the portal circulation before acting systemically). Subcutaneous administration does not allow for these potentially important local physiological gastrointestinal effects. Encouragingly, a recent study of oral delivery of PYY3-36 with sodium N-caprylate (Emisphere Technologies, Cedar Knolls, NJ, USA) to 12 healthy human subjects showed that there was effective absorption of the peptide from the gut.49 Supraphysiological plasma levels were achieved, but despite this, no nausea was encountered with administration of oral PYY3-36 alone. Energy intake at a buffet meal served 15 minutes later was reduced by 12% compared with placebo, although this was not found to be statistically significant. 24-hour energy intake was unchanged by oral PYY3-36 administration. The results are nevertheless promising for the future oral administration of PYY3-36 (and other gut hormones) as treatments for obesity. Emisphere's sodium N-caprylate technology uses hydrophobic moieties on carrier molecules, which when in association with a peptide such as PYY3-36, allows for the creation of a more lipophilic complex. This facilitates absorption of the peptide across the intestinal wall.
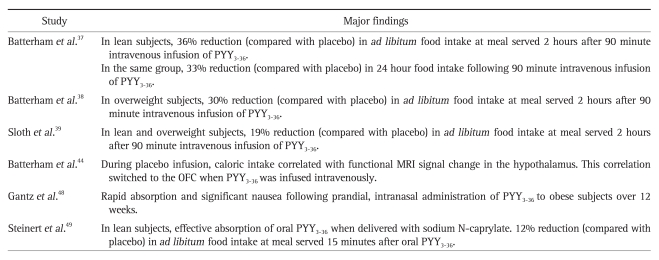
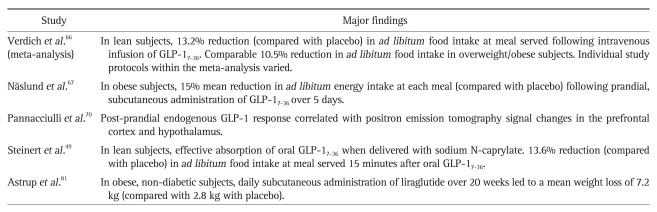
A summary of important translational human studies investigating the role of PYY3-36 in appetite control and as a potential treatment for obesity is shown in Table 2.
Table 2.
Important Translational Human Studies Involving PYY3-36
PYY, peptide tyrosine tyrosine; MRI, magnetic resonance imaging; OFC, orbitofrontal cortex.
GLP-1
Proglucagon is a 160-amino acid prohormone that is produced in the α-cells of the pancreatic islets, the L cells of the distal gut and within the CNS. Selective post-translational proteolysis of proglucagon by prohormone convertases 1 and 2 results in the tissue-specific production of a number of biologically-active fragments. In the gut and CNS, with respect to the regulation of appetite and food intake, the cleavage product GLP-1 will be the focus of this section.
GLP-1 is released post-prandially into the circulation from the gastrointestinal L cells in proportion to the calories ingested.50,51 It has been shown that GLP-1 shows a biphasic response after a meal. As with PYY, the first peak occurs before nutrients enter the distal gut8 and has been shown to be augmented by a high-carbohydrate meal.52 This is presumably via the effect of carbohydrates absorbed in the proximal gut. It is thought that the second peak of secretion of GLP-1 is triggered by free fatty acids in the intestinal lumen, via activation of the G-protein coupled receptors GPR 40,53 GPR 119,54,55 and GPR 120.56 The two forms synthesized are GLP-11-37 and GLP-11-36 amide. Further cleavage produces the bioactive fragments GLP-11-37 and GLP-17-36 amide . The major circulating bioactive species of GLP-1 is the truncated form GLP-17-36 amide.51 It is rapidly broken down by DPP-IV and has a half-life of 2 minutes.57
GLP-1 acts by binding to the G-protein coupled GLP-1 receptor (GLP-1R). GLP-11-37 and GLP-17-36 amide are equally potent at the GLP-1R.58 The GLP-1R is expressed in pancreatic islets, where GLP-1 functions as an incretin hormone. This allows for enhanced glucose-dependent insulin release post-prandially.59 GLP-1 also inhibits glucagon secretion and delays gastric emptying.60,61 The GLP-1R is also present in a number of CNS areas implicated in appetite control. These include the ARC and PVN in the hypothalamus and the AP in the brainstem.62,63 Neurons containing GLP-1 are located in the AP and NTS. Furthermore, there are projections to the dorsomedial hypothalamus.64
It is well established that administration of GLP-17-36 amide peripherally or centrally leads to reduced food intake in rodents, whereas co-administration of an antagonist of GLP-1 (exendin9-39) abolishes this effect.65 In the same study, single administration of exendin9-39 doubled food intake in satiated rats in addition to potentiating the feeding response to NPY administration, suggesting that GLP-1 regulates appetite physiologically.
A range of studies has demonstrated that peripheral administration of GLP-17-36 amide to humans leads to a dose-dependent reduction in appetite and ad libitum energy intake. A metaanalysis of major studies by Verdich et al.66 revealed that intravenous infusion of GLP-17-36 amide reduces energy intake dosedependently in both lean and overweight subjects. The protocols in the individual studies within the meta-analysis varied considerably, in addition to including a mixture of lean, overweight and obese subjects in the studies analysed. The duration of the infusions ranged between 0 and 240 minutes (spanning 60 minutes in the majority of studies). Furthermore, the timing of the ad libitum buffet meal with regard to the infusion varied between studies. Finally, individual protocols varied such that the dose of infused GLP-17-36 amide in individual studies ranged between 0.375 pmol/kg/min and 1.5 pmol/kg/min. Amalgamating the individual study data in the meta-analysis revealed that the mean infusion rate was 0.89 pmol/kg/min across all subjects. In lean subjects, infusion of GLP-17-36 amide led to a 13.2% reduction in energy intake at the ad libitum buffet meal compared with saline infusion. The reduction in ad libitum energy intake for overweight/obese subjects by GLP-17-36 amide was comparable (10.5%) with that of lean subjects.66 Data on nausea and other well-being scores were only included in 2 of the studies in the meta-analysis. Nausea was not encountered in either of these studies. In addition to the intravenous infusion studies described above, it was found that daily prandial subcutaneous injections of GLP-17-36 amide (76 nmol 4 times daily) to obese human subjects over 5 days led to a 15% mean reduction in energy intake at each meal compared with placebo. Over 5 days, this translated to a mean weight loss of 0.55 kg.67 However, feelings of sickness were reported in 8 out of 19 subjects receiving prandial subcutaneous injections of GLP-17-36 amide, compared with 2 out of 7 subjects receiving placebo injections.
The brainstem is thought to be an important site of action of peripheral GLP-1. In rodents, c-fos expression occurs in the brainstem only after the peripheral and not central injection of GLP-17-36 amide.65 In contrast, both peripheral and central injection of GLP-17-36 amide induce c-fos in the PVN.68 Furthermore, vagotomy or lesions of projections from the brainstem to the hypothalamus reduce the appetite suppression mediated by peripheral GLP-17-36 amide.69 In humans, a positron emission tomography (PET) study revealed that the post-prandial GLP-1 response is positively associated with changes in neuronal activity in the prefrontal cortex and hypothalamus.70 Therefore, as seems to be the case with PYY3-36, peripheral GLP-17-36 amide may either act via the vagus, with projections to the brainstem and then hypothalamus, or alternatively may act directly on the brainstem (gaining access from the circulation at the AP) or hypothalamus (gaining access from the circulation at the median eminence). Furthermore, action on brain food reward regions (such as the pre-frontal cortex) is likely, either directly or through communication via homeostatic centres. A summary of the interactions of GLP-1 with the brainstem, hypothalamus and higher brain centres is shown in Fig. 2.
DEVELOPING GLP-1 BASED TREATMENTS FOR OBESITY
The fact that circulating GLP-1 is rapidly broken down by DPPIV has been a challenge to developing its use clinically. Exenatide (exendin-4) is a currently licensed DPP-IV resistant GLP-1R agonist that has enjoyed success in producing improved glycaemic control and promoting weight loss when administered subcutaneously to overweight patients with type 2 diabetes.71,72 Long acting analogues of GLP-1 such as liraglutide are also licensed for subcutaneous use in type 2 diabetes as adjunctive therapy, following favourable efficacy and safety data.73-76 The half-life of liraglutide is prolonged by albumin binding. The fact that exenatide and liraglutide produce an incretin effect is favourable in enhancing endogenous insulin secretion in response to a post-prandial glucose load. Furthermore, their effects of suppressing food intake and causing weight loss (through reduction in gastric emptying and central effects on the CNS as discussed above) makes this line of treatment particularly attractive in treating overweight patients with type 2 diabetes. However, one major limitation surrounds their well-recognised side effect of nausea. Although it occurs commonly, the incidence of nausea declines with duration of treatment.71,72
In order to avoid the need for injections, nonpeptidic GLP-1R agonists in oral form have been developed and tested in rodents.77,78 Recently, delivery of oral GLP-17-36 with sodium Ncaprylate to healthy human subjects reduced energy intake by 13.6% during a meal served 15 minutes later, with marked effects on glucose homeostasis.49 However, in this study, 24-hour energy intake was unaffected by GLP-17-36.
In addition to GLP-1 receptor agonism, oral DPP-IV inhibitors such as sitagliptin and vildagliptin are also currently licensed as adjunctive therapy in type 2 diabetes. Although they confer the advantage of being available in oral form, unlike exenatide and liraglutide, they tend to be weight-neutral.79 This may well be due to the fact that DPP-IV has a role in activating other anorectic hormones such as PYY3-36, hence DPP-IV inhibition may be counter-productive in this regard. Finally, although currently not licensed, GPR 119 agonists (i.e., GLP-1 secretagogues) are under development for use in type 2 diabetes.80
Outside of the context of type 2 diabetes, liraglutide has proven successful in causing significant weight loss when administered over 20 weeks to obese, non-diabetic patients.81 Administration of liraglutide subcutaneously at a dose of 3 mg once daily led to a mean weight loss of 7.2 kg, compared with 2.8 kg with placebo and 4.1 kg with orlistat 120 mg orally 3 times daily. 76% of participants on liraglutide 3 mg once daily lost >5% body weight.81 Nausea and vomiting occurred more frequently in the liraglutide group than in the placebo group, but this rarely led to discontinuation of treatment. The results of this randomised, double-blind, placebo-controlled study hold promise for the licensing of GLP-1 analogues in obesity alone.
A summary of important translational human studies investigating the role of GLP-1 in appetite control and as a potential treatment for obesity is shown in Table 3.
Table 3.
Important Translational Human Studies Involving GLP-1
GLP-1, glucagon-like peptide 1.
DEVELOPING COMBINATION THERAPY BASED ON PYY AND GLP-1
Preliminary research has focused on deciphering the role and mode of action of individual gut hormones in appetite regulation. However, more recently, there has been an interest in investigating how these satiety factors act in concert to regulate appetite. This is particularly relevant when considering that in the physiological fed state, a multitude of gut hormones are released into the circulation. More effectively mimicking the physiological fed state using combination gut hormone analogues may prove promising for the future treatment of obesity.
In the case of PYY3-36 and GLP-17-36 amide, Neary et al.82 showed that in 10 healthy, lean human subjects, co-administration of PYY3-36 and GLP-17-36 amide (each at 0.4 pmol/kg/min over 120 minutes) produced a 27% reduction in ad libitum energy intake (compared with saline) during a buffet meal served 90 minutes into the infusion (the infusion was continued during the meal). Individually, single infusion of PYY3-36 and GLP-17-36 amide at the above doses did not produce a statistically significant reduction in energy intake compared with saline. Importantly, no nausea was experienced during any of the infusions, including the combined PYY3-36 and GLP-17-36 amide infusion.
In the same paper,82 rodent studies were carried out and findings strengthened those of the human study; combination of peripheral PYY3-36 and GLP-17-36 amide led to reduction in food intake that was greater than that observed when either hormone was infused individually at twice the dose. In the hypothalamic ARC, no changes were observed after low-dose PYY3-36 or GLP-17-36 amideindividually, but co-administration led to c-fos expression.
In a recent study of 12 healthy human subjects, co-administration of oral PYY3-36 and GLP-17-36 amide in conjunction with sodium N-caprylate lead to a 21.5% reduction in energy intake during a meal served 15 minutes later.49 Admittedly, 2 subjects experienced significant gastrointestinal side effects of nausea, abdominal discomfort and vomiting. Furthermore, 24-hour energy intake was no different compared with placebo.
CONCLUSION
The global obesity epidemic has escalated the need to discover new therapeutic strategies to counteract it. Lifestyle modification alone is largely ineffective in modern Western society, owing to the abundance of high-calorie food available at little cost, coupled with a generally sedentary lifestyle. The development of bariatric surgical techniques over the past decade is a major breakthrough in treatment for the very obese, although the procedure is itself not without risk, cannot be tailored easily to individual need and does not address the needs of hundreds of millions of people who do not meet the requirements for the procedure, yet who remain at significant risk from the complications of being overweight or obese.
The role of gut hormones in appetite control has been studied for over 30 years, with clear demonstration that they have a role in mediating post-prandial satiety. Despite this there has been no contribution from gut hormones to the pharmacological market for obesity alone. Following gastric bypass surgery anorectic gut hormones, such as PYY and GLP-1, are elevated. They play a crucial role in the subsequent reduction in food intake. There has been renewed interest in the potential of these peptides to treat obesity. The concept of targeting a physiological system and deceiving the brain into falsely believing that feeding has occurred would seem to hold more promise than targeting ubiquitous central neurotransmitter systems. The latter blunderbuss approach has led to the downfall of several previous pharmacological approaches to tackling obesity. Within the next few years, it is likely that we can look forward to the licensing of GLP-1 and PYY based treatments for obesity alone. Pharmacokinetic and pharmacodynamic hurdles will need to be overcome. Additionally, it is likely that in order to mimic a co-ordinated post-prandial satiety response, several anorectic gut hormone-based treatments may have to be administered in combination, perhaps along with antagonists of orexigenic hormonal signals. The overall goal will be to reproduce the physiological consequences of a gastric bypass procedure solely by means of gut hormone based therapy, thereby effectively creating a 'medical bypass.'
Footnotes
Akila De Silva has no competing interests to declare. Stephen R. Bloom declares an association with Thiakis, now a subsidiary of Pfizer.
References
- 1.Olshansky SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 2.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 3.Maggard MA, Shugarman LR, Suttorp M, et al. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005;142:547–559. doi: 10.7326/0003-4819-142-7-200504050-00013. [DOI] [PubMed] [Google Scholar]
- 4.Marsk R, Freedman J, Tynelius P, Rasmussen F, Näslund E. Antiobesity surgery in Sweden from 1980 to 2005: a population-based study with a focus on mortality. Ann Surg. 2008;248:777–781. doi: 10.1097/SLA.0b013e318189b0cf. [DOI] [PubMed] [Google Scholar]
- 5.Weiss EC, Galuska DA, Kettel Khan L, Gillespie C, Serdula MK. Weight regain in U.S. adults who experienced substantial weight loss. Am J Prev Med. 2007;33:34–40. doi: 10.1016/j.amepre.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 6.Hutton B, Fergusson D. Changes in body weight and serum lipid profile in obese patients treated with orlistat in addition to a hypocaloric diet: a systematic review of randomized clinical trials. Am J Clin Nutr. 2004;80:1461–1468. doi: 10.1093/ajcn/80.6.1461. [DOI] [PubMed] [Google Scholar]
- 7.Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebocontrolled, phase 3 trial. Lancet. 2010;376:595–605. doi: 10.1016/S0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- 8.Delzenne N, Blundell J, Brouns F, et al. Gastrointestinal targets of appetite regulation in humans. Obes Rev. 2010;11:234–250. doi: 10.1111/j.1467-789X.2009.00707.x. [DOI] [PubMed] [Google Scholar]
- 9.Farooqi IS, Bullmore E, Keogh J, Gillard J, O'Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007;317:1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab. 2009;297:E1247–E1259. doi: 10.1152/ajpendo.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng H, Berthoud HR. Eating for pleasure or calories. Curr Opin Pharmacol. 2007;7:607–612. doi: 10.1016/j.coph.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969;164:719–721. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- 13.Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 15.Rossi M, Kim MS, Morgan DG, et al. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139:4428–4431. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- 16.Kristensen P, Judge ME, Thim L, et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393:72–76. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- 17.Abbott CR, Rossi M, Wren AM, et al. Evidence of an orexigenic role for cocaine- and amphetamine-regulated transcript after administration into discrete hypothalamic nuclei. Endocrinology. 2001;142:3457–3463. doi: 10.1210/endo.142.8.8304. [DOI] [PubMed] [Google Scholar]
- 18.Leibowitz SF, Hammer NJ, Chang K. Hypothalamic paraventricular nucleus lesions produce overeating and obesity in the rat. Physiol Behav. 1981;27:1031–1040. doi: 10.1016/0031-9384(81)90366-8. [DOI] [PubMed] [Google Scholar]
- 19.Bailey EF. A tasty morsel: the role of the dorsal vagal complex in the regulation of food intake and swallowing. Focus on "BDNF/TrkB signaling interacts with GABAergic system to inhibit rhythmic swallowing in the rat," by Bariohay et al. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1048–R1049. doi: 10.1152/ajpregu.90701.2008. [DOI] [PubMed] [Google Scholar]
- 20.Ter Horst GJ, de Boer P, Luiten PG, van Willigen JD. Ascending projections from the solitary tract nucleus to the hypothalamus. A Phaseolus vulgaris lectin tracing study in the rat. Neuroscience. 1989;31:785–797. doi: 10.1016/0306-4522(89)90441-7. [DOI] [PubMed] [Google Scholar]
- 21.Ter Horst GJ, Luiten PG, Kuipers F. Descending pathways from hypothalamus to dorsal motor vagus and ambiguus nuclei in the rat. J Auton Nerv Syst. 1984;11:59–75. doi: 10.1016/0165-1838(84)90008-0. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz GJ. The role of gastrointestinal vagal afferents in the control of food intake: current prospects. Nutrition. 2000;16:866–873. doi: 10.1016/s0899-9007(00)00464-0. [DOI] [PubMed] [Google Scholar]
- 23.Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27:11075–11082. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrovich GD, Setlow B, Holland PC, Gallagher M. Amygdalohypothalamic circuit allows learned cues to override satiety and promote eating. J Neurosci. 2002;22:8748–8753. doi: 10.1523/JNEUROSCI.22-19-08748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 26.Verhagen JV. The neurocognitive bases of human multimodal food perception: consciousness. Brain Res Rev. 2007;53:271–286. doi: 10.1016/j.brainresrev.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG, Aylwin SJ. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg. 2006;93:210–215. doi: 10.1002/bjs.5227. [DOI] [PubMed] [Google Scholar]
- 28.le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.le Roux CW, Welbourn R, Werling M, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246:780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 30.Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985;89:1070–1077. doi: 10.1016/0016-5085(85)90211-2. [DOI] [PubMed] [Google Scholar]
- 31.Dumont Y, Fournier A, St-Pierre S, Quirion R. Characterization of neuropeptide Y binding sites in rat brain membrane preparations using [125I][Leu31,Pro34]peptide YY and [125I]peptide YY3-36 as selective Y1 and Y2 radioligands. J Pharmacol Exp Ther. 1995;272:673–680. [PubMed] [Google Scholar]
- 32.Grandt D, Schimiczek M, Beglinger C, et al. Two molecular forms of peptide YY (PYY) are abundant in human blood: characterization of a radioimmunoassay recognizing PYY 1-36 and PYY 3-36. Regul Pept. 1994;51:151–159. doi: 10.1016/0167-0115(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 33.Degen L, Oesch S, Casanova M, et al. Effect of peptide YY3-36 on food intake in humans. Gastroenterology. 2005;129:1430–1436. doi: 10.1053/j.gastro.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Lluis F, Fujimura M, Gómez G, Salvá JA, Greeley GH, Jr, Thompson JC. Cellular localization, half-life, and secretion of peptide YY. Rev Esp Fisiol. 1989;45:377–384. [PubMed] [Google Scholar]
- 35.Essah PA, Levy JR, Sistrun SN, Kelly SM, Nestler JE. Effect of macronutrient composition on postprandial peptide YY levels. J Clin Endocrinol Metab. 2007;92:4052–4055. doi: 10.1210/jc.2006-2273. [DOI] [PubMed] [Google Scholar]
- 36.Helou N, Obeid O, Azar ST, Hwalla N. Variation of postprandial PYY 3-36 response following ingestion of differing macronutrient meals in obese females. Ann Nutr Metab. 2008;52:188–195. doi: 10.1159/000138122. [DOI] [PubMed] [Google Scholar]
- 37.Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 38.Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 39.Sloth B, Holst JJ, Flint A, Gregersen NT, Astrup A. Effects of PYY1-36 and PYY3-36 on appetite, energy intake, energy expenditure, glucose and fat metabolism in obese and lean subjects. Am J Physiol Endocrinol Metab. 2007;292:E1062–E1068. doi: 10.1152/ajpendo.00450.2006. [DOI] [PubMed] [Google Scholar]
- 40.Gustafson EL, Smith KE, Durkin MM, et al. Distribution of the neuropeptide Y Y2 receptor mRNA in rat central nervous system. Brain Res Mol Brain Res. 1997;46:223–235. doi: 10.1016/s0169-328x(97)00017-x. [DOI] [PubMed] [Google Scholar]
- 41.Blevins JE, Chelikani PK, Haver AC, Reidelberger RD. PYY(3-36) induces Fos in the arcuate nucleus and in both catecholaminergic and non-catecholaminergic neurons in the nucleus tractus solitarius of rats. Peptides. 2008;29:112–119. doi: 10.1016/j.peptides.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dumont Y, Jacques D, Bouchard P, Quirion R. Species differences in the expression and distribution of the neuropeptide Y Y1, Y2, Y4, and Y5 receptors in rodents, guinea pig, and primates brains. J Comp Neurol. 1998;402:372–384. [PubMed] [Google Scholar]
- 43.Koda S, Date Y, Murakami N, et al. The role of the vagal nerve in peripheral PYY3-36-induced feeding reduction in rats. Endocrinology. 2005;146:2369–2375. doi: 10.1210/en.2004-1266. [DOI] [PubMed] [Google Scholar]
- 44.Batterham RL, ffytche DH, Rosenthal JM, et al. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450:106–109. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]
- 45.Morley JE, Levine AS, Grace M, Kneip J. Peptide YY (PYY), a potent orexigenic agent. Brain Res. 1985;341:200–203. doi: 10.1016/0006-8993(85)91490-8. [DOI] [PubMed] [Google Scholar]
- 46.Stanley BG, Daniel DR, Chin AS, Leibowitz SF. Paraventricular nucleus injections of peptide YY and neuropeptide Y preferentially enhance carbohydrate ingestion. Peptides. 1985;6:1205–1211. doi: 10.1016/0196-9781(85)90452-8. [DOI] [PubMed] [Google Scholar]
- 47.Kanatani A, Mashiko S, Murai N, et al. Role of the Y1 receptor in the regulation of neuropeptide Y-mediated feeding: comparison of wild-type, Y1 receptor-deficient, and Y5 receptor-deficient mice. Endocrinology. 2000;141:1011–1016. doi: 10.1210/endo.141.3.7387. [DOI] [PubMed] [Google Scholar]
- 48.Gantz I, Erondu N, Mallick M, et al. Efficacy and safety of intranasal peptide YY3-36 for weight reduction in obese adults. J Clin Endocrinol Metab. 2007;92:1754–1757. doi: 10.1210/jc.2006-1806. [DOI] [PubMed] [Google Scholar]
- 49.Steinert RE, Poller B, Castelli MC, Drewe J, Beglinger C. Oral administration of glucagon-like peptide 1 or peptide YY 3-36 affects food intake in healthy male subjects. Am J Clin Nutr. 2010;92:810–817. doi: 10.3945/ajcn.2010.29663. [DOI] [PubMed] [Google Scholar]
- 50.Ghatei MA, Uttenthal LO, Bryant MG, Christofides ND, Moody AJ, Bloom SR. Molecular forms of glucagon-like immunoreactivity in porcine intestine and pancreas. Endocrinology. 1983;112:917–923. doi: 10.1210/endo-112-3-917. [DOI] [PubMed] [Google Scholar]
- 51.Orskov C, Rabenhøj L, Wettergren A, Kofod H, Holst JJ. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes. 1994;43:535–539. doi: 10.2337/diab.43.4.535. [DOI] [PubMed] [Google Scholar]
- 52.Smeets AJ, Soenen S, Luscombe-Marsh ND, Ueland Ø, Westerterp-Plantenga MS. Energy expenditure, satiety, and plasma ghrelin, glucagon-like peptide 1, and peptide tyrosine-tyrosine concentrationsfollowing a single high-protein lunch. J Nutr. 2008;138:698–698. doi: 10.1093/jn/138.4.698. [DOI] [PubMed] [Google Scholar]
- 53.Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57:2280–2287. doi: 10.2337/db08-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chu ZL, Carroll C, Alfonso J, et al. A role for intestinal endocrine cell-expressed g protein-coupled receptor 119 in glycemic control by enhancing glucagon-like peptide-1 and glucose-dependent insulinotropic peptide release. Endocrinology. 2008;149:2038–2047. doi: 10.1210/en.2007-0966. [DOI] [PubMed] [Google Scholar]
- 55.Lauffer LM, Iakoubov R, Brubaker PL. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes. 2009;58:1058–1066. doi: 10.2337/db08-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hirasawa A, Tsumaya K, Awaji T, et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11:90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 57.Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucosedependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136:3585–3596. doi: 10.1210/endo.136.8.7628397. [DOI] [PubMed] [Google Scholar]
- 58.Thorens B, Porret A, Bühler L, Deng SP, Morel P, Widmann C. Cloning and functional expression of the human islet GLP-1 receptor. Demonstration that exendin-4 is an agonist and exendin-(9-39) an antagonist of the receptor. Diabetes. 1993;42:1678–1678. doi: 10.2337/diab.42.11.1678. [DOI] [PubMed] [Google Scholar]
- 59.Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagonlike peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2:1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 60.Gutniak M, Orskov C, Holst JJ, Ahrén B, Efendic S. Antidiabetogenic effect of glucagon-like peptide-1 (7-36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med. 1992;326:1316–1322. doi: 10.1056/NEJM199205143262003. [DOI] [PubMed] [Google Scholar]
- 61.Willms B, Werner J, Holst JJ, Orskov C, Creutzfeldt W, Nauck MA. Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous glucagon-like peptide-1 (GLP-1)-(7-36) amide in type 2 (noninsulin-dependent) diabetic patients. J Clin Endocrinol Metab. 1996;81:327–332. doi: 10.1210/jcem.81.1.8550773. [DOI] [PubMed] [Google Scholar]
- 62.Wei Y, Mojsov S. Tissue-specific expression of the human receptor for glucagon-like peptide-I: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett. 1995;358:219–224. doi: 10.1016/0014-5793(94)01430-9. [DOI] [PubMed] [Google Scholar]
- 63.Shughrue PJ, Lane MV, Merchenthaler I. Glucagon-like peptide-1 receptor (GLP1-R) mRNA in the rat hypothalamus. Endocrinology. 1996;137:5159–5162. doi: 10.1210/endo.137.11.8895391. [DOI] [PubMed] [Google Scholar]
- 64.Larsen PJ, Tang-Christensen M, Jessop DS. Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 1997;138:4445–4455. doi: 10.1210/endo.138.10.5270. [DOI] [PubMed] [Google Scholar]
- 65.Turton MD, O'Shea D, Gunn I, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 66.Verdich C, Flint A, Gutzwiller JP, et al. A meta-analysis of the effect of glucagon-like peptide-1 (7-36) amide on ad libitum energy intake in humans. J Clin Endocrinol Metab. 2001;86:4382–4389. doi: 10.1210/jcem.86.9.7877. [DOI] [PubMed] [Google Scholar]
- 67.Näslund E, King N, Mansten S, et al. Prandial subcutaneous injections of glucagon-like peptide-1 cause weight loss in obese human subjects. Br J Nutr. 2004;91:439–446. doi: 10.1079/BJN20031064. [DOI] [PubMed] [Google Scholar]
- 68.Baggio LL, Huang Q, Brown TJ, Drucker DJ. Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology. 2004;127:546–558. doi: 10.1053/j.gastro.2004.04.063. [DOI] [PubMed] [Google Scholar]
- 69.Abbott CR, Monteiro M, Small CJ, et al. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagalbrainstem-hypothalamic pathway. Brain Res. 2005;1044:127–131. doi: 10.1016/j.brainres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 70.Pannacciulli N, Le DS, Salbe AD, et al. Postprandial glucagon-like peptide-1 (GLP-1) response is positively associated with changes in neuronal activity of brain areas implicated in satiety and food intake regulation in humans. Neuroimage. 2007;35:511–517. doi: 10.1016/j.neuroimage.2006.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ratner RE, Maggs D, Nielsen LL, et al. Long-term effects of exenatide therapy over 82 weeks on glycaemic control and weight in over-weight metformin-treated patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2006;8:419–428. doi: 10.1111/j.1463-1326.2006.00589.x. [DOI] [PubMed] [Google Scholar]
- 72.Riddle MC, Henry RR, Poon TH, et al. Exenatide elicits sustained glycaemic control and progressive reduction of body weight in patients with type 2 diabetes inadequately controlled by sulphonylureas with or without metformin. Diabetes Metab Res Rev. 2006;22:483–491. doi: 10.1002/dmrr.646. [DOI] [PubMed] [Google Scholar]
- 73.Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84–90. doi: 10.2337/dc08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zinman B, Gerich J, Buse JB, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD) Diabetes Care. 2009;32:1224–1230. doi: 10.2337/dc08-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–481. doi: 10.1016/S0140-6736(08)61246-5. [DOI] [PubMed] [Google Scholar]
- 76.Marre M, Shaw J, Brändle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU) Diabet Med. 2009;26:268–278. doi: 10.1111/j.1464-5491.2009.02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Knudsen LB, Kiel D, Teng M, et al. Small-molecule agonists for the glucagon-like peptide 1 receptor. Proc Natl Acad Sci U S A. 2007;104:937–942. doi: 10.1073/pnas.0605701104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen D, Liao J, Li N, et al. A nonpeptidic agonist of glucagon-like peptide 1 receptors with efficacy in diabetic db/db mice. Proc Natl Acad Sci U S A. 2007;104:943–948. doi: 10.1073/pnas.0610173104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Green BD, Flatt PR, Bailey CJ. Dipeptidyl peptidase IV (DPP IV) inhibitors: a newly emerging drug class for the treatment of type 2 diabetes. Diab Vasc Dis Res. 2006;3:159–165. doi: 10.3132/dvdr.2006.024. [DOI] [PubMed] [Google Scholar]
- 80.Lauffer L, Iakoubov R, Brubaker PL. GPR119: "double-dipping" for better glycemic control. Endocrinology. 2008;149:2035–2037. doi: 10.1210/en.2008-0182. [DOI] [PubMed] [Google Scholar]
- 81.Astrup A, Rössner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebocontrolled study. Lancet. 2009;374:1606–1616. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- 82.Neary NM, Small CJ, Druce MR, et al. Peptide YY3-36 and glucagon-like peptide-17-36 inhibit food intake additively. Endocrinology. 2005;146:5120–5127. doi: 10.1210/en.2005-0237. [DOI] [PubMed] [Google Scholar]