Abstract
Autoimmune pancreatitis (AIP) is a benign disorder and a unique form of chronic pancreatitis with several characteristic features. A cystic formation that mimics a pseudocyst is a rare finding. There have been a few reports of AIP complicated by pancreatic cysts. We present a case of AIP with multiple pseudocysts and obstructive jaundice caused by IgG4-associated cholangitis. We initially missed the diagnosis due to the pseudocyst. Based on the computed tomography images, laboratory findings and the therapeutic response to steroids, the case was diagnosed as AIP with pseudocysts and associated cholangiopathy.
Keywords: Autoimmune pancreatitis, Pseudocyst, Corticosteroid, IgG4 associated cholangitis
INTRODUCTION
It is well known that causes of chronic pancreatitis include alcohol, genetic or metabolic disorders, pancreato-biliary abnormalities and drugs. Since Sarles et al.1 reported chronic pancreatitis associated with hypergammaglobulinemia and lymphocytic infiltration with fibrosis of the pancreas in 1961, autoimmune mechanisms have come to the forefront as additional important causes of chronic pancreatitis.
Autoimmune pancreatitis (AIP) is a benign disease and a unique form of chronic pancreatitis characterized by the presence of auto-antibodies, high serum IgG4 level, diffuse pancreatic swelling, irregular narrowing of the main pancreatic duct and stenosis of the intrahepatic and extrahepatic bile ducts. Lymphoplasmocytic infiltration and fibrosis of the pancreas are typical histologic findings of AIP.2 IgG4-associated cholangitis, described below, is commonly manifested with AIP and its diagnostic criteria include: stricture of intrahepatic, proximal extrahepatic or intrapancreatic ducts; clinical imaging findings of AIP; and elevated serum IgG4.3
We now report a case of AIP with accompanying multiple pseudocysts and IgG4-associated cholangitis. We think that this is a unique case of AIP, with clinical characteristics and course very similar to those of idiopathic chronic pancreatitis as well as other cases of AIP associated pseudocyst.4
CASE REPORT
A 47-year-old male patient was referred to our hospital with pancreatic swelling, multiple cystic lesions of the pancreas on ultrasonography (USG) and a history of upper abdominal pain. Intermittent upper abdominal discomfort and dyspepsia had been developing for 1 year, but there were no specific abnormal findings on esophagogastroduodenoscopy and USG. Four months earlier, subsequent to an episode of abdominal pain, abdominal computed tomography (CT) had revealed pancreatic pseudocyst. Post-referral abdominal CT showed diffuse pancreatic swelling and variably sized multiple pseudocysts without pancreatic parenchymal calcification (Fig. 1). For follow-up periods in outpatient clinic, he had complained intermittent vague abdominal discomfort without a history of aggravation of abdominal symptoms. A yellowish facial color developed and became more severe, and the patient was admitted for evaluation. He had been treated for pulmonary tuberculosis 20 years previously, but there was no other significant past medical or family history, including cholelithiasis. He had a 15 pack year smoking history but had no history of excessive alcohol consumption. In physical examination and systemic review, blood pressure, pulse rate, body temperature, and respiratory rate were stable and body mass index was 19.6 kg/m2. The abdomen was soft and there was no palpable mass. There were no abnormal physical findings except systemic jaundice. Laboratory data were as follows (values in parentheses indicate normal range): white blood cell (WBC) count 6,440/mm3 (4,800 to 10,800/mm3); hemoglobin 11.9 g/dL (13 to 18 g/dL); platelet count 266,000/mm3 (130,000 to 400,000/mm3); aspartate aminotransferase (AST) 317 IU/L (0 to 37 IU/L); alanine aminotransferase (ALT) 484 IU/L (0 to 41 IU/L); alkaline phosphatase (ALP) 517 IU/L (35 to 129 IU/L); gammaglutamyl transpeptidase (GGT) 489 IU/L (8 to 61 IU/L); total bilirubin 12.01 mg/dL (0.01 to 1.1 mg/dL); direct bilirubin 10.48 mg/dL (0.01 to 0.3 mg/dL); amylase 82 IU/L (28 to 110 IU/L), lipase 675 IU/L (23 to 300 IU/L); fasting blood sugar 155 mg/dL (7 to 100 mg/dL); hemoglobin A1c 5.5% (4.0% to 6.5 %); CEA 5.36 ng/mL (0 to 4.3 ng/mL); CA 19-9 111.1 U/mL (0 to 37 U/mL).
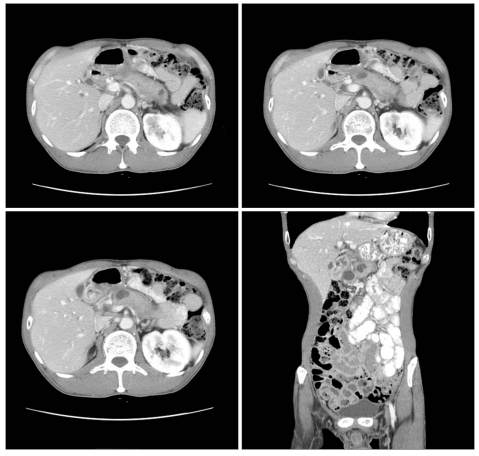
Fig. 1.
The computed tomography findings on the first visit of the patient to our hospital in May 2008. There is a diffuse enlargement of the pancreas with multiple pseudocysts without parenchymal calcification.
Initial dynamic CT demonstrated diffuse pancreatic swelling and multiple pseudocysts, new intrahepatic and extrahepatic bile duct dilatation, and irregular wall thickening and a 2 cm length stenosis of the proximal common bile duct (CBD) (Fig. 2). Endoscopic retrograde cholangiopancreatography (ERCP) showed stenosis of the proximal CBD without demonstrating malignancy (Fig. 2).
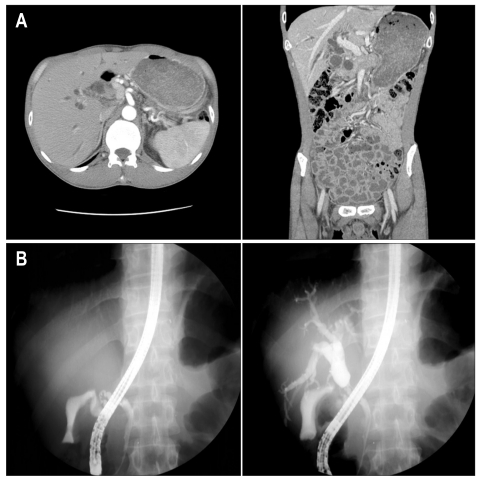
Fig. 2.
Computed tomography (CT) (A) and endoscopic retrograde cholangiopancreatography (ERCP) (B) findings at the time of admission for the evaluation of jaundice in July 2008. CT shows diffuse intra-hepatic duct dilatation, focal luminal wall thickening and a narrowing lesion at the proximal common bile duct (CBD). ERCP demonstrates the stenosis of the proximal CBD with upstream dilation.
We made the provisional diagnosis of chronic pancreatitis with biliary stricture and placed a biliary stent into stenotic bile duct. During outpatient follow-up, the jaundice resolved and tumor marker and biochemical laboratory data normalized, but vague abdominal discomfort, nausea and intermittent vomiting persisted. While follow-up abdominal dynamic CT revealed no bile duct dilatation and similar pancreato-biliary abnormalities to those on the initial CT, enhanced-phase CT showed diffuse pancreatic swelling without focal enhancing mass lesion at pancreas and low attenuation of peripancreatic area which findings were compatible with AIP.5
We performed additional laboratory tests for autoimmune disorders as follows (values in parentheses indicate normal range): antinuclear antibody was negative; antimitochondrial antibody (AMA) was negative; rheumatoid factor was negative; IgG 1,845 mg/dL (700 to 1,600 mg/dL); IgA 201 mg/dL (70 to 400 mg/dL); IgM 55 mg/dL (40 to 230 mg/dL); IgG subtype IV 2.28 g/L (0.06 to 1.21 g/L). Based on the findings of the two imaging criteria and a serologic criterion of the Asian Diagnostic criteria for AIP, we diagnosed AIP.4,6
Outpatient oral prednisolone therapy was started at a dose of 30 mg/day. After 3 months, the symptoms had completely resolved without any complications. Abdominal dynamic CT showed partial resolution of the pseudocysts, in size and numbers, and improvement of the diffuse pancreatic swelling (Fig. 3). ERCP revealed normal intra- and extrahepatic bile ducts, including the CBD and main pancreatic duct, and the plastic stent previously placed at the distal CBD was removed. Small stones were detected in the CBD and removed using basket. Laboratory data concerning liver function, and levels of pancreatic enzymes and IgG4 were normalized or improving (AST, 18 IU/L; ALT, 13 IU/L; ALP, 55 IU/L; GGT, 13.6 IU/L; total bilirubin, 0.74 mg/dL; direct bilirubin, 0.06 mg/dL; amylase, 30 IU/L, lipase, 10 IU/L; CEA, 2.92 ng/mL; CA 19-9, 5.4 U/mL; IgG4, 1.63 g/L). The dose of oral prednisolone was subsequently tapered in phases to 10 mg as the patient's vague abdominal discomfort, nausea and vomiting resolved. At present, the patient is asymptomatic on 10 mg oral prednisolone per day.
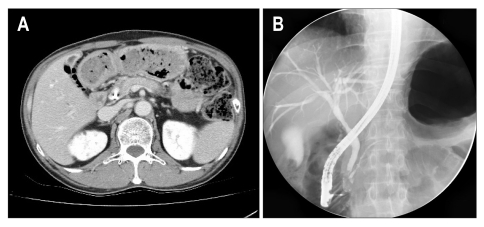
Fig. 3.
The computed tomography (CT) and endoscopic retrograde cholangiopancreatography (ERCP) findings after corticosteroid treatment for 3 months. (A) CT reveals an improvement in parenchymal swelling and the size and numbers of pseudocysts. (B) ERCP shows a complete resolution of the luminal narrowing and stricture of the proximal common bile duct.
DISCUSSION
In 1995, Yoshida et al.7 summarized the characteristics of AIP as follows: 1) Increase of serum gamma globulin or IgG levels; 2) Presence of auto-antibodies; 3) Diffuse enlargement of pancreas; 4) Irregular narrowing of the main pancreatic duct on ERCP; 5) Fibrotic changes and lymphocyte infiltration of the pancreas on histology; 6) Asymptomatic or mild symptoms, usually no history of acute pancreatitis; 7) Stenosis of the CBD in the pancreas, with dilatation of upstream bile duct and liver dysfunction; 8) No pancreatic calcification; 9) No pancreatic cyst; 10) Frequently associated with other autoimmune disorders; 11) Good response to steroid treatment.
There have only been a few reports of AIP complicated with pancreatic cyst and such cysts are rare, probably due to the absence of severe tissue necrosis and/or lack of stasis of the pancreatic juice in this condition.
In this case, when the patient visited our hospital initially, he had a history of an acute attack of pancreatitis and diffuse enlargement of the pancreas with multiple pseudocysts on imaging. Chronic pancreatitis or pancreatic cystic neoplasm were therefore deemed more likely than AIP. Also, pseudocysts located in the pancreatic head are particularly suggestive of pancreatic cancer, and we therefore performed ERCP to exclude pancreatic malignancy. Responsiveness of AIP to corticosteroid treatment corresponds to elevation of IgG/IgG4 or else the presence of other auto-antibodies such as ANA.8 In addition, pseudocyst associated with AIP might represent a highly active inflammatory process, and these lesions have been shown to predict responsiveness to corticosteroid treatment if associated with AIP.4
Though we were unable to determine the exact mechanism of the pathogenesis of the cyst in the present patient, the absence of both severe acute episodes of pancreatitis and a marked elevation of serum pancreatic enzymes prior to cyst formation suggests that severe tissue necrosis did not contribute to the pathogenesis. Cyst formation in AIP may be associated with a highly active state of the inflammatory process, as we found a high serum IgG4 concentration that closely correlated with the active state of this disease. Pseudocyst activity and progression is known to be associated with stenosis of the pancreatic duct. Steroids are thought to induce pseudocyst regression through inhibition of inflammation of the pancreatic duct, thus reducing the stenosis and improving the drainage of pancreatic juice.
Corticosteroid treatment is clinically, radiologically, and serologically effective and has become accepted as a standard treatment for AIP. There have been some studies on the shortterm effectiveness of corticosteroid treatment in AIP, and, more recently, on the long-term prognosis for patients with AIP, including those receiving corticosteroid treatment.9 It is difficult for all patients with AIP to be treated with corticosteroid, and there is currently no consensus on when to initiate corticosteroid treatment in patients with AIP. Hirano et al.10 reported that a growing pancreatic pseudocyst developed in an AIP patient who did not receive corticosteroid treatment, while Nakazawa et al.11 reported the spontaneous disappearance of a pancreatic cyst in an AIP patient without corticosteroid treatment. Kawakami et al.12 suggested that, in AIP patients, corticosteroid treatment should be started immediately after pseudocyst appearance because of the possibility of pseudocyst regression with treatment.
The risk of adverse effects of corticosteroid treatment (such as susceptibility to infection, impaired glucose tolerance and peptic ulcer) should also be taken into consideration.
The indications for corticosteroid therapy in AIP patients have included bile duct stenosis and various other symptoms and clinical findings.10 In this case, we started corticosteroid treatment after diagnosis of IgG4-associated cholangitis, which caused obstructive jaundice due to bile duct stenosis, and pseudocysts. After corticosteroid treatment, there were improvements in clinical symptoms, such as reduced abdominal discomfort and nausea. Radiologic regression of the diffuse pancreatic swelling, bile duct stenosis and pseudocysts was also seen and the patient has recovered well with no complications. More studies are required regarding the long-term prognosis and appropriate duration of corticosteroid treatment for AIP.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Sarles H, Sarles JC, Muratore R, Guien C. Chronic inflammatory sclerosis of the pancreas: an autonomous pancreatic disease? Am J Dig Dis. 1961;6:688–698. doi: 10.1007/BF02232341. [DOI] [PubMed] [Google Scholar]
- 2.Kawa S, Hamano H. Clinical features of autoimmune pancreatitis. J Gastroenterol. 2007;42(Suppl 18):9–14. doi: 10.1007/s00535-007-2044-x. [DOI] [PubMed] [Google Scholar]
- 3.Björnsson E. Immunoglobulin G4-associated cholangitis. Curr Opin Gastroenterol. 2008;24:389–394. doi: 10.1097/MOG.0b013e3282f6a7c5. [DOI] [PubMed] [Google Scholar]
- 4.Muraki T, Hamano H, Ochi Y, et al. Corticosteroid-responsive pancreatic cyst found in autoimmune pancreatitis. J Gastroenterol. 2005;40:761–766. doi: 10.1007/s00535-005-1622-z. [DOI] [PubMed] [Google Scholar]
- 5.Sohn JH, Byun JH, Yoon SE, et al. Abdominal extrapancreatic lesions associated with autoimmune pancreatitis: radiological findings and changes after therapy. Eur J Radiol. 2008;67:497–507. doi: 10.1016/j.ejrad.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Okazaki K, Kawa S, Kamisawa T, et al. Clinical diagnostic criteria of autoimmune pancreatitis: revised proposal. J Gastroenterol. 2006;41:626–631. doi: 10.1007/s00535-006-1868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshida K, Toki F, Takeuchi T, et al. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40:1561–1568. doi: 10.1007/BF02285209. [DOI] [PubMed] [Google Scholar]
- 8.Kim KP, Kim MH, Song MH, et al. Autoimmune chronic pancreatitis. Am J Gastroenterol. 2004;99:1605–1616. doi: 10.1111/j.1572-0241.2004.30336.x. [DOI] [PubMed] [Google Scholar]
- 9.Uchida K, Yazumi S, Nishio A, et al. Long-term outcome of autoimmune pancreatitis. J Gastroenterol. 2009;44:726–732. doi: 10.1007/s00535-009-0049-3. [DOI] [PubMed] [Google Scholar]
- 10.Hirano K, Tada M, Isayama H, et al. Long-term prognosis of autoimmune pancreatitis with and without corticosteroid treatment. Gut. 2007;56:1719–1724. doi: 10.1136/gut.2006.115246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakazawa T, Ohara H, Sano H, et al. Difficulty in diagnosing autoimmune pancreatitis by imaging findings. Gastrointest Endosc. 2007;65:99–108. doi: 10.1016/j.gie.2006.03.929. [DOI] [PubMed] [Google Scholar]
- 12.Kawakami H, Kuwatani M, Shinada K, et al. Autoimmune pancreatitis associated with hemorrhagic pseudocysts: a case report and literature review. Intern Med. 2008;47:603–608. doi: 10.2169/internalmedicine.47.0731. [DOI] [PubMed] [Google Scholar]