Abstract
Hepatocellular carcinoma (HCC) is a highly malignant tumor with limited treatment options in its advanced state. The molecular mechanisms underlying HCC remain unclear because of the complexity of its multi-step development process. Cancer stem cells (CSCs) are defined as a small population of cells within a tumor that possess the capability for self-renewal and the generation of heterogeneous lineages of cancer cells. To date, there have been two theories concerning the mechanism of carcinogenesis, i.e., the stochastic (clonal evolution) model and the hierarchical (cancer stem cell-driven) model. The concept of the CSC has been established over the past decade, and the roles of CSCs in the carcinogenic processes of various cancers, including HCC, have been emphasized. Previous experimental and clinical evidence indicated the existence of liver CSCs; however, the potential mechanistic links between liver CSCs and the development of HCC in humans are not fully understood. Although definitive cell surface markers for liver CSCs have not yet been found, several putative markers have been identified, which allow the prospective isolation of CSCs from HCC. The identification and characterization of CSCs in HCC is essential for a better understanding of tumor initiation or progression in relation to signaling pathways. These markers could be used along with clinical parameters for the prediction of chemoresistance, radioresistance, metastasis and survival and may represent potential targets for the development of new molecular therapies against HCC. This review describes the current evidence for the existence and function of liver CSCs and discuss the clinical implications of CSCs in patients demonstrating resistance to conventional anti-cancer therapies, as well as clinical outcomes. Such data may provide a future perspective for targeted therapy in HCC.
Keywords: Hepatocellular carcinoma, Stem cell, Cancer stem cell
INTRODUCTION
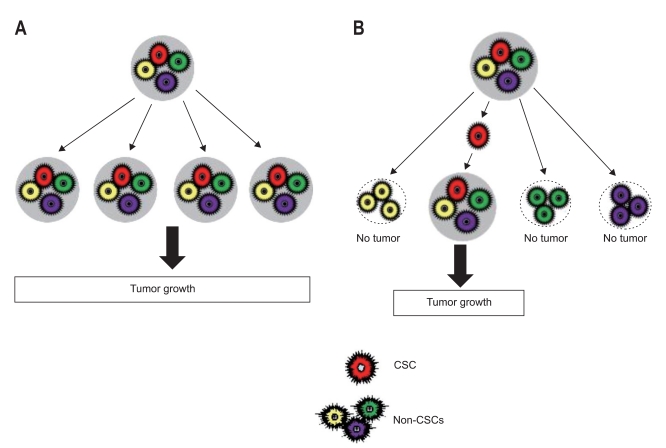
Normal cell turnover occurring continuously in various tissues of a healthy adult human being plays an essential role in maintenance of normal tissue function and architecture over time through tightly regulated biological processes. Under physiological conditions, this dynamic process is sustained by a long-lived, small population of cells known as "stem cells," which have three main properties, self-renewal, differentiation, and homeostatic control.1 Malignant tumors, on the other hand, are comprised of morphologically diverse cells and phenotypically heterogenous populations that possess high clonogenic and tumorigenic activity, and a varying degree of ability for self-renewal and differentiation into multiple cell types.2-6 Of those cells, certain minor cell populations have played an essential role in tumorigenesis; this phenomenon led to the new concept of tumor-initiating cells (T-ICs) or cancer stem cells (CSCs). CSCs, as defined by the American Association for Cancer Research Workshop, denotes cells within a tumor that possess the capacity for self-renewal and generation of heterogenous lineages of cancer cells that comprise the tumor.7 In an attempt to explain this phenomenon, two different theories have been proposed: one is "the stochastic model," which claims that every cancer cell in a tumor can ultimately acquire a capacity for self-renewal and multilineage potency, and therefore repopulate an entire tumor;8 the other is "the hierarchy model," which claims that every cancer cell in a tumor is heterogenous and that only a minority of cells serve as CSC, giving rise to tumors (Fig. 1).
Fig. 1.
Two general models for tumorigenesis. The stochastic model claims that every cancer cell in a tumor can ultimately acquire a capacity for self-renewal and multilineage potency, thereby forming new tumors (A), whereas the hierarchy model claims that cancer cells in a tumor are heterogeneous and that only a minority of these cells serves as cancer stem cells (CSCs), thus giving rise to tumors (B).
The first evidence for the existence of CSC was revealed in a study of acute myeloid leukemia by Bonnet and Dick.9 They found that a subpopulation of leukemia cells expressing a specific surface marker, CD34, but lacking the CD38 marker (CD34+/CD38-), was capable of initiation and progression of cancer in their experimental model; hence, the authors defined these cancer cells as CSCs because they possess biological properties unique to normal stem cells. However, this hierarchy model argued against the traditional stochastic theory, suggesting that all cells in a tumor have an equally malignant potential for propagation and malignancy.7,10 Since this hierachial or CSC model was first proven in solid tumors of breast cancer by Al-Hajj et al.,11 verifiable evidence for the existence of CSC has been reported in numerous solid tumors, including brain,12 colon,13 pancreatic,14 lung,15 prostate,16 liver,17 melanoma,18 and ovarian cancers.19 However, cancers do not always follow the hierachial manner in development of tumors.20 In addition, recent works have described several factors influencing the two models of carcinogenesis, including etiologic mutagens, microenvironment, and type of cancer;21 hence, further clarification might be required in order to understand the role of CSC in tumorigenesis of solid tumors.
This review will focus on the role of CSCs in hepatocarcinogenesis and its clinical implications, including biomarkers, chemo/radioresistance, and development of new targeted therapies for treatment of hepatocellular carcinoma (HCC).
MARKERS FOR IDENTIFICATION OF LIVER CSCs
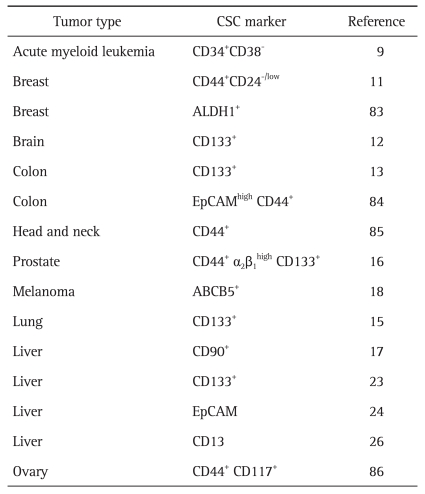
Identification of tumor specific biomarkers is essential for early detection of cancers or development of targeted therapy for treatment of cancers. In general, prospective isolation and characterization of CSCs are based on their immunogenicity and functional properties. Immunogenic isolation is performed using cell surface markers, while functional isolation is dependent on the surrogate characteristics of CSCs, including clonogenic growth, formation of tumor spheres, or resistance to chemo/radiotherapy. Extensive research has been conducted in recent years for identification of putative cancer stem cell markers. Since identification of the marker for leukemic stem cells (LSCs), candidate markers of stem cells for solid tumors have been explored. Currently, to be identified as a CSC marker for any type of tumor, the ability of a candidate marker to initiate the same tumor type should be proven in a xenograft model. Although it is not yet known whether all cancers possess subpopulations of CSCs, several putative CSC markers that have been reported to date are summarized in Table 1. However, various cell surface markers for CSCs from the same tumor type or the same marker in different types of cancers have been reported; therefore, it is difficult to define a single marker for CSCs in any tumor.
Table 1.
Cancer Stem Cells Markers Identified in Various Cancers
CSC, cancer stem cell; EpCAM, epithelial cell adhesion molecule; ABCB5, adenosine triphosphate-binding cassette sub-family B member 5.
The first evidence for the existence of a CSC population in the liver was reported by Haraguchi et al.22 They examined a subset of stem cells identified as "side population" (SP) cells using the DNA-binding dye Hoechst 33342 in three hepatoma cell lines (HepG2, Hep3B, and Huh-7 cell), and found that Huh-7 and Hep3B cell lines contain SP cells; however, HepG2 cells did not show a SP. Subsequent studies have revealed candidate markers of liver CSC, including CD90,17 CD133,23 epithelial cell adhesion molecule (EpCAM),24 CD44,25 and CD13.26 On the other hand, CD133 identifies CSCs in certain specific tumors, including brain, prostate, colon, pancreas, lung, and ovarian cancer.27 CD90 (Thy-1), a surface marker for many types of stem cells, was expressed in HCC cell lines and human liver cancer specimens, and CD90+ HCC cells displayed tumorigenicity in a xenograft mice model.28 In addition, they demonstrated that CD90+CD44+ cells displayed a more aggressive phenotype than CD90+CD44-cells, leading to formation of metastatic lesions in the lung of immunodeficient mice.17 CD133, known as a human homologue of mouse prominin-1, is a pentaspan transmembrane cell surface glychoprotein.29 Since identification of CD133 antigen as a hematopoietic stem cell marker, its expression in various human embryonic epithelia has been demonstrated. Existence of CD133 in HCC cells was first reported by Suetsugu et al.,30 who found CD133 expression in a Huh-7 cell line and demonstrated that CD133+ Huh-7 cells have a higher proliferative property in vitro and tumorigenic potential in vivo. Characterization of EpCAM, the cell surface hepatic stem cell marker, in HCC cells and human HCC samples was initially revealed by Yamashita et al.,24 who demonstrated that EpCAM+ HCC cells possessed the hepatic cancer stem cell-like capabilities for self-renewal and differentiation. These cells also displayed a potential for initiation of highly aggressive and invasive HCC in an immunodeficient experimental mice model. Most recently, Haraguchi et al.26 reported that CD13 is a marker for semiquiescent CSCs in human liver cancer cell lines and clinical samples. Although several markers for identification and characterization of hepatic CSCs have been reported, a single cell surface marker has a limitation in its ability to define fully specific CSCs in any tumor. For this reason, many researchers have attempted to find other cell surface molecules in order to more precisely define the CSC subpopulation for a better understanding of hepatocarcinogenesis. Zhu et al.25 showed that co-expression of CD44 antigen in addition to CD133 in HCC cells represented more precise stem cell properties of CSCs, including self-renewal, differentiation, and aggressive proliferation. Taken together, various approaches to isolation and characterization of CSCs in HCC that allow for enrichment of hepatic CSCs have been explored, and these markers could be used along with clinical parameters for prediction of prognosis as well as providing a better understanding of tumor initiation or progression in HCC.
ROLE OF CSCs IN HEPATOCARCINOGENESIS
HCC is one of the most common malignancies worldwide and the third leading cause of cancer-related death. Common risk factors for development of HCC, including presence of liver cirrhosis irrespective of cause, chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections, chronic ethanol consumption, nonalcoholic steatohepatitis (NASH) and aflatoxin B1 (AFB1) have been well recognized.31
In general, hepatocyte necrosis followed by regeneration during chronic liver injury leads to liver cirrhosis. In the tissue of liver cirrhosis, stepwise hepatocyte transformation begins and then finally progresses to HCC via a series of hyperplastic and dysplastic stages. This concept of hepatocarcinogenesis has long been regarded as a multistep process associated with accumulation of genetic and epigenetic changes that pass through steps of initiation, promotion, and progression. However, occurrence of these genetic or molecular events accompanying alterations of cell cycle, differentiation, and apoptosis is dependent on the risk factors; therefore, a complete understanding of hepatocarcinogenesis is more difficult. Extensive research in recent decades has focused on identification of molecular biomarkers and cellular signaling pathways involved in development of HCC. In a number of previous studies, various signaling pathways, including p53, retinoblastoma protein (pRb), Wnt/β-catenin, Janus kinases (JAK)/signal transducers, mitogen-acivated protein kinase (MAPK), epidermal growth factor receptor transforming growth factor-β (EGF/TGF-β) pathways, stress response signaling, and activators of transcription (STAT), have been found to contribute to hepatocarcinogenesis.31 More recently, we demonstrated that SOX4 protein interacts with p53 and that this association in turn modulates p53-mediated transcription at the Bax promoter, leading to inhibition of apoptosis via suppression of Bax gene expression.32
So-called oval cells proposed in the past by several investigators are located in the Canals of Hering and have been recognized as putative hepatic stem/progenitor cells (HPCs) giving rise to hepatocytes and cholangiocytes.33 In general, hepatoytes and cholangiocytes contain cytoskeletal intermediate filaments, called cytokeratins (CK) which is differentially expressed depending on the organ or the type of differentiation. In normal liver, the combination of CK8 and CK18 was expressed in hepatocytes while that of CK7 and CK19 was expressed in cholangiocytes and bipotential (HPCs).34-36
To date, the question about the cellular origin of HCC is still debated. There are two major hypotheses regarding the origination of HCC: 1) from dedifferentiation of mature hepatocytes or 2) from the block of stem cell differentiation. Bralet et al.37 demonstrated that some of mature hepatocytes gave rise to HCC in the chemically induced rat HCC model. In general, extracellular matrix (ECM) is known to important mediator in the process of liver fibrogenesis but it has two-edged roles of inhibitor and promoter in the carcinogenesis and progression of HCC. Fibroblast derived ECM inhibit tissue-specific gene expression in fetal and adult hepatocytes.38 ECM remodeling in cirrhosis inhibits the transactivation potential of liver-specific transcription factors in hepatocytes, resulting in dedifferentiation of hepatocytes leading to development of HCC.39 In addition, previous experimental data provided an evidence that small hepatocyte-like progenitor cells were activated and differentiated when the proliferation of hepatocytes was suppressed after partial hepatectomy in rats treated with pyrrolizidine alkaloid retrosine, suggesting that the dedifferentiation of mature hepatocytes occurs when the proliferation is suppressed.40 However, it is interesting to note the cellular origin of HCC in the case of human HCC samples showing the presence of cells expressing CK7 and CK19. It might be originated from dedifferentiation of mature hepatocytes to HPCs or from a maturation arrest of HPCs during carcinogenic process.41,42 Recently, evidence is growing that HPCs as well as mature hepatocytes can give rise to HCC. Recent studies have described involvement of HPCs in hepatocarcinogenesis via activation and proliferation in response to liver injury in a chemically-induced HCC rodent model.43,44 Furthermore, Theise et al.,45 confirmed the existence of HPCs in human liver cancers, suggesting that some liver cancers originate from HPCs. These results support the concept that human hepatocarcinogenesis can be based on transformation of HPCs. Therefore, a fundamental understanding of the characteristics of HPCs may provide insight into the carcinogenic process of the liver while it is occurring. The oval cells are activated and proliferate in premalignant hepatic lesions induced by chronic liver injury; therefore, it is possible to postulate that HPCs could serve as CSC in HCC. Proliferation of HPCs has also been referred to as a "ductular reaction," which was directly associated with the severity of the underlying premalignant liver disease.46,47 Regarding the fact that HCC is evolved from focal precusor lesions, recent studies have revealed that activation of HPCs produces a foci of small cell dysplasia, suggesting that they play a major role in histogenesis of HCC.48,49 Further mutation occurring during clonal expansion leads to progression to low grade dysplastic nodules and high grade dysplastic nodules, eventually resulting in HCC. In order to further demonstrate the hypothesis that HCC is originated from HPC, tumor cells should express markers that are specifically expressed on HPCs, including OV-6, CK7 and CK19, and chromogranin-A.50 Cells possessing the HPC phenotype are often found in hepatoblastoma, which is the most common childhood liver cancer.51 This tumor, as a fetal-like liver cancer comprising epithelial and mesenchymal components, is believed to originate from liver stem cells. On the other hand, the ductular reaction in human liver, which is comparable to the oval cell reaction in an experimental model of hepatocarcinogenesis, appears to be a response to acute or chronic liver injury, including viral hepatitis, massive hepatic necrosis, cholestatic liver disease, biliary obstruction, and alcoholic liver disease.52 Hepatocyte-like cells found in this reaction co-expressed hepatocytic and biliary specific antigen (HepPar1 and CK19, respectively), suggesting that ductular hepatocytes recapitulate the developmental stages of bipotential liver progenitor cells.53 Regarding origin of HCC, previous studies have demonstrated that markers specific to HPC were expressed in some hepatic malignancy, which was composed of two components: an HCC component and a cholangiocarcinoma component, indicating that this tumor may originate from a bipotential progenitor.45 However, because HBV can induce carcinogenesis via direct integration of the viral genome into host cell DNA in HBV-related HCC, CSCs in many different liver cancers cannot simply be regarded as being derived from HPCs.
Key signaling pathways that regulate the function of CSCs, such as Wnt/β-catenin, TGF-β, Hedgehog, Notch, and MYC, have been recently been revealed by recent advances in molecular techniques. Therefore, further study would be required in order to obtain convincing evidence for the origin of liver cancer.
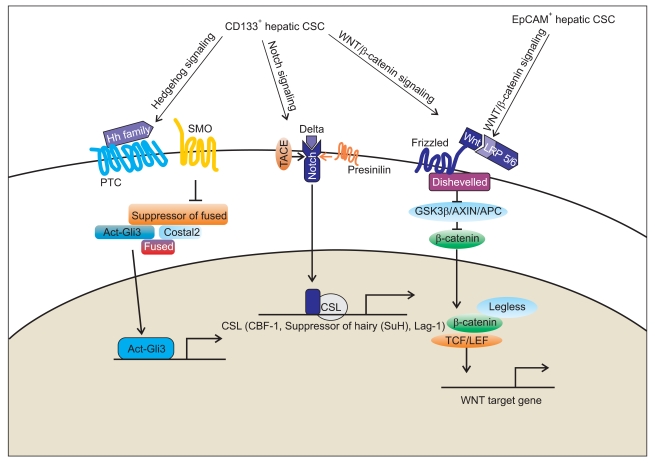
Among signaling pathways regulating CSCs, key signaling pathways have been implicated in development of HCC. Recently, Yamashita et al.24,54 demonstrated that activation of Wnt/β-catenin signaling enriched the EpCAM+ HCC cell population whereas inhibition of EpCAM using si RNA decreased the biologic properties of hepatic CSCs, indicating that EpCAM is a Wnt/β-catenin signaling target gene (Fig. 2). A study of the role TGF-β in HCC showed correlation of its late expression signature with more invasive tumor characteristics and poor clinical outcome, including metastasis and survival.55 In a recent work by Tang et al.,56 HCC was found to arise from interleukin (IL)-6 driven transformed stem cells with inactivated TGF-β signaling. They provided evidence to show that development of HCC may occur via dysregulated proliferation of HPCs under conditions in which the function of TGF-β was disrupted. The Notch and Hedgehog signaling pathways play an essential role in embryogenesis and regulation of cellular processes, including proliferation, angiogenesis, and self-renewal. Gao et al.57 recently examined expression of the Notch receptor in human HCC samples. They found that Notch1 and Notch4 were up-regulated; however, Notch2 was down-regulated, suggesting that Notch signaling might be involved in development of HCC. A recent study by Ma et al.23 showed preferential expression of specific genes closely linked with self-renewal, differentiation, and proliferation of stem cells, such as Wnt/β-catenin, Notch1, smoothened (SMO)/Hedgehog, Oct-3/4, and Bmi in CD133+ HCC cells (Fig. 2). These results suggest that targeting of these critical pathways in regulation of CSCs may be a promising tool for use in complete eradication of tumor cells.
Fig. 2.
The signaling pathways linked to hepatic cancer stem cells (CSCs) in hepatocarcinogenesis. Key signaling pathways that regulate the function of hepatic CSCs include Wnt/β-catenin, transforming growth factor (TGF-β), Hedgehog, Notch, and MYC. Epithelial cell adhesion molecule (EpCAM) is a Wnt/β-catenin signaling target gene, and activation of Wnt/β-catenin signaling regulates EpCAM expression in hepatocellular carcinoma (HCC) cell lines. In CD133+ HCC cells, Wnt/β-catenin, Notch1, and smoothened (SMO)/Hedgehog signaling pathways are linked with self-renewal, differentiation, and proliferation of hepatic CSCs. Targeting of these critical pathways in the regulation of hepatic CSCs may be a promising tool for the development of new therapies to eradicate tumor cells completely in HCC.
Gli3, glioma-associated oncogene family zinc finger 3; TACE, TNF-alpha converting enzyme; PTC, patched.
CLINICAL IMPLICATIONS OF LIVER CSCs
1. Role of liver CSCs in chemoresistance
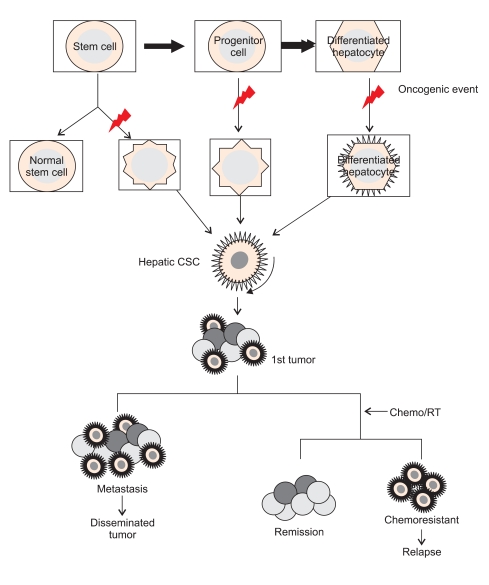
To date, the precise mechanism underlying chemoresistance, radioresistance, and tumor recurrence in different cancers is not yet clear. Furthermore, the exact reason for why the response to anticancer therapies was different even in the same type of cancer from different patients remains unclear. However, the biological and molecular features of CSCs within tumors, including diverse regulatory mechanisms and signaling pathways, may contribute to cancer cell survival. Although not all types of malignancies follow the hierarchical theory (CSC hypothesis), it might explain the reason why conventional anti-cancer therapies have shown such resistance. In general, conventional external beam radiotherapy or anticancer chemotherapy target proliferating cells and require cycling of cells to induce apoptosis. Therefore, it is plausible that CSCs containing a quiescent nature have inherent resistance to conventional therapies. In other words, rapidly proliferating cells can be killed with anti-cancer drugs while a quiescent slow-cycling stem cell population can escape therapeutic targeting, leading to recurrence of tumors.58 Furthermore, once chemotherapy-resistant CSCs re-enter the cell cycle, they generate rapidly dividing progenitor cells with the ability to reestablish the tumor. In addition, recent works have suggested several mechanisms for chemoresistance by CSCs. Adenosine triphosphate (ATP)-binding cassette (ABC) transporters commonly expressed on cellular membranes of both normal stem cells and CSCs include multidrug resistance transporter 1 (MDR1) and breast cancer resistance protein (BCRP) drug transport pumps and play an essential role in expelling anticancer drugs from cells, leading to chemoresistance.59 Another study reported on involvement of CSCs expressing aldehyde dehydrogenase 1 (ALDH1), which facilitates metabolism, in chemoresistance.60 SP cells, identified by Haraguchi et al.,22 have some characteristics of stem cells, and were found to express higher chemoresistance when compared with non-SP cells. In particular, Huh-7 SP cells expressing ABC transporters, such as MDR1 and BCRP1, showed chemoresistance to doxorubicin. In addition, Huh-7 SP cells were found to overexpress CEACAM6, which was known to be associated with chemoresistance to gemcitabine.61 Regarding the role of CD133 in development of HCC, CD133-expressing cancer cells are not only responsible for tumor initiation or progression, but also have stem cell-like properties, like colony-forming ability and differentiation potential.23 Moreover, Ma et al.62 recently demonstrated that CD133+ HCC cells contribute to chemoresistance through preferential activation of Akt/PKB and Bcl-2 cell survival response. More recently, Haraguchi et al.26 demonstrated that CD13 is a marker for semiquiescent CSCs in human liver cancer cell lines and that CD13 predominated in the G0 phase of the cell cycle, decreased reactive oxygen species (ROS)-induced DNA damage after genotoxic chemo/radiation stress, and protected cells from apoptosis. Taken together, these results suggest that last surviving CSCs during anti-cancer chemotherapy can lead to tumor regrowth or recurrence via their enhanced chemoresistance (Fig. 3).
Fig. 3.
The roles of hepatic cancer stem cells (CSCs) in tumorigenesis and treatment resistance in hepatocellular carcinoma. Normal stem cells self-renew and can differentiate into progenitor cells, which eventually differentiate into fully differentiated hepatocytes. CSCs can arise from normal stem cells, progenitor cells and even differentiated hepatocytes when oncogenic events occur during cellular processes. The expansion of hepatic CSCs results in the formation of the primary tumor, which is composed of a heterogeneous mass of cancer cells. The unlimited proliferation of hepatic CSCs promotes tumor growth and can give rise to distant metastases that occur in conjunction with the angiogenic process. When chemotherapy or radiotherapy is used, hepatic CSCs play an essential role in treatment resistance and can produce tumor recurrence.
2. Role of liver CSCs in radioresistance
Radiotherapy is aimed at destruction of cancer cells, which prevents tumor growth without damaging normal tissues around the tumor. Although radiation therapy has been widely used for curative or palliative therapy in various cancers, tumor recurrence is inevitable in such cases due to radioresistance. In this regard, extensive research has been conducted for investigation of the mechanisms of radioresistance in different cancers; however, to date, the mechanisms underlying development of radioresistance remain elusive. Recent evidence has accumulated to suggest correlation of the CSC-population with higher radioresistance compared with the non-CSC population.63,64 A study by Bao et al.63 focused on the role of putative CSCs in radioresistance in malignant gliomas. Glioma cells expressing CD133 were much more radioresistant than glioma cells lacking CD133 and survived ionizing radiation in increased proportions. In general, check point kinases, like Chk1 and Chk2, become activated during genotoxic stress in initiation of cell cycle arrest. They demonstrated that DNA damage check point response was more predominantly activated in CD133+ glioma cells, compared with CD133- glioma cells, suggesting that CD133-expressing cells contributed to radioresistance through activation of checkpoint response and repair mechanisms in response to DNA damage by radiation. Thus, inhibition of Chk1/2 kinases may disrupt the radioresistance of CSC-enriched cells, thereby providing a therapeutic advantage to reducing raioresistance of CSC. In another study using a breast cancer model, Phillips et al.,64 demonstrated that CD24-/low/CD44+ cancer-initiating cells isolated from breast cancer cell lines (MCF-7 and MDA-MB-231) are relatively radioresistant and increase in numbers after short courses of fractionated irradiation. Also, levels of reactive oxygen species and phosporylation of histone H2AX were decreased in a mammosphere culture. Furthermore, recent work by Woodward et al.65 suggests that the Wnt/β-catenin signaling pathway may be considered as one mechanism of radioresistance in breast cancer. They showed that progenitor cells in the mammary gland were more resistant to clinically relevant doses of radiation than nonprogenitor cells and that overexpression of the Wnt/β-catenin pathway enhanced the radioresistance of progenitor cells. In addition, radiation induced enrichment of SP progenitors in the human breast cancer cell line. Thus, these results suggest involvement of the Wnt/β-catenin SP in radioresistance in mammary progenitor cells as well as cells expressing CSC markers in breast cancer. In addition, several pathways implicated in radioresistance of CSC populations in various cancers have been proposed by recent works: Notch pathway,64 Hedgehog-Gli1 pathway,66 a pathway downstream of EGFR,67 and loss of the tumor suppressor PTEN.68
In general, hypoxia in tumors has been reported to have an association with chemoresistance and radioresistance.69 Transcription factor hypoxia inducible factor-1 (HIF-1) is activated in response to hypoxic stress on cells. HIF-1 activated by hypoxic damage in tumor cells following radiotherapy promotes expression of genes that allow tumor cells to survive, leading to development of radioresistance.70 However, studies addressing radioresistance in hypoxic CSCs after radiotherapy have been limited. Although it is not as yet entirely clear how hypoxia affects stemness of CSC, it might affect stem cell generation and maintenance in tumors through activation of HIFs.71 In addition, radioresistant hypoxic cells have the opportunity to reoxygenate before the next fraction, allowing CSCs to undergo differentiation.72
To date, there have been few reports regarding the underlying mechanism of radioresistance of CSC in HCC. However, recent work by Haraguchi et al.26 showed that CD13 reduced ROS-induced DNA damage after radiation therapy and protected cells from apoptosis in HCC cell lines, suggesting that a combination of a CD13 inhibitor with ROS-inducing radiation therapy may result in improved treatment of liver cancer.
3. Role of liver CSCs in clinical outcomes
In recent years, many clinical investigators have attempted to determine whether the existence of CSCs is associated with clinical outcomes in solid cancers. Although considerable evidence has not emerged, the clinical relevance of CSC remains a major challenge for current anti-cancer therapy. To date, numerous clinical and experimental studies have shown the association between expression of specific genes and clinical outcomes, including metastasis, survival, cancer relapse, and disease progression. However, few studies have shown a correlation with clinical outcomes dependent on the existence of CSCs in a given malignancy. Nonetheless, several important studies have provided evidence showing that hepatic stem/progenitor cell markers could predict the prognosis of HCC patients. Through comparative genomic investigations in both human and animal models, Lee et al.73 demonstrated that individuals with HCC who showed expression of markers for fetal progenitor cells had a poor prognosis. In another study, HCC expressing EpCAM and alpha-fetoprotein (AFP) characterized by Wnt/β-catenin signaling activation was associated with poor survival.24 In addition, a recent study by Song et al.74 showed correlation of CD133+ HCC, characterized by higher proliferating properties and greater ability to induce tumor formation, with advanced tumor stages, poor survival, and tumor recurrence. In addition, CK7 and CK19, known to be markers for early hepatoblasts and mature HPCs, have also been reported to have an association with postoperative tumor recurrence due to its aggressive tumor characteristics, like invasiveness.75
Taken together, although the significance of hepatic stem/progenitor signature on clinical outcome has been recognized in a clinical base, further extensive experimental and clinical research should be conducted in order to determine a future therapeutic plan after primary treatment for HCC.
THERAPEUTIC STRATEGIES TARGETING LIVER CSCs
Solid tumors, including HCC, are major health burdens worldwide and present a major therapeutic challenge. However, no traditional anti-cancer therapies for complete eradication of cancer cells have been developed to date. Furthermore, because this traditional anti-cancer therapy primarily targets rapidly dividing cells, it could not eradicate CSCs in tumors, and, as a drawback, it also affects rapidly dividing healthy cells. Therefore, exploration of new anti-cancer therapies that disrupt critical "stemness" pathways regulating self-renewal, pleuripotency, chemoresistance, radioresistance, and angiogenesis is essential.7 Nowadays, since introduction of the concept of CSCs in different tumors, there have been many advances in the unraveling of cancer pathophysiology, including tumor angiogenesis, metastasis, and resistance to chemotherapy/radiotherapy. In addition, accumulating evidence suggests that epigenetic regulation, microRNA, and tumor microenvironment play key roles in regulation of stem cell self-renewal.76 In addition, since candidate cell surface markers for CSCs have been identified and characterized in various cancers, the paradigm for cancer therapy may be changed to target therapy for CSCs. Furthermore, recent research has suggested that evolving strategies for targeted cancer therapy aim at effective targeting of CSCs, but not non-CSCs, because normal stem cells have been more sensitive than CSCs in response to chemotherapy.77 Thus, a highly qualified assay system for specific purification and characterization of CSCs should be developed for use in CSC-targeting drug discovery. Currently, the therapeutic strategies for targeting CSCs have been developed through suppressing tumor growth, inducing apoptosis and enhancing differentiation via blockade of CSC pathway, disrupting microenvironment and increasing the sensitivity to chemotherapy or radiotherapy. To this end, small molecule inhibitors, fusion proteins and viral-mediated gene therapy have been used. However, the clinical application of the small molecule inhibitors have remained limited for the reasons like lower specificity and serious adverse effect.78 On the other hand, virus-mediated gene therapy targeting CSC pathway has offered some potential therapeutic advantages over proteins- or small molecule inhibitors-based therapies in terms of long-term therapeutic efficacy without drug resistance.66 However, the immunogenicity of viral vectors is yet to be resolved.
To date, several surface markers for liver CSCs, including CD90, CD133, EpCAM, CD44, and CD13 have been reported. These markers may be powerful targets for use in development of targeted drugs for treatment of HCC. Among previous studies focusing on characterization of liver CSCs, Yang et al.,17 found that CD90+CD44+ HCC cells have a more aggressive tumor phenotype and stronger metastatic potential than CD90-CD44- HCC cells, and demonstrated that inhibition of CD44 with antibody induces the death of aggressive CD90+ HCC cells, resulting in prevention of local and metastatic tumor formation. These results suggest that CSCs-targeting therapeutic approaches involving destruction of CD90+CD44+ liver CSCs may offer promise for use in HCC treatment. In another study, EpCAM+ HCC cells showed Wnt/β-catenin signaling activation and EpCAM blockade with siRNA, resulting in significant attenuation of both tumorigenic and invasive potential of liver CSCs.24 These results suggest that targeting of Wnt/β-catenin signaling, which is downstream of EpCAM, may therefore provide an attractive therapy for treatment of HCC. In addition, a more recent study by Haraguchi et al.26 suggested CD13, a marker for semiquiescent CSCs in human HCC cell lines, as a therapeutic target for liver CSCs. In their study, CD13 reduced ROS-induced DNA damage following genotoxic stress, such as a chemotherapeutic agent or radiation treatment, resulting in protection of cells from apoptosis. Inhibition of CD13 suppresses self-renewal and proliferation of tumor-initiating liver CSCs, suggesting that a combination of ROS-inducing chemotherapy with CD13 inhibitor may be a promising approach to chemoresistant HCC. However, because the biological characteristics of liver CSCs, including their heterogeneity and hierarchy, comprise a complex nature, further characterization of liver CSCs function should be clarified for development of targeted drugs for treatment of HCC.
CONCERNS ABOUT CANCER STEM CELL-TARGETED THERAPY
Although cancer stem cell-targeted therapy has strong potential to eliminate CSCs, some problems are yet to be resolved. It has been known that normal stem cells and CSCs share several common biological properties.8 In particular, because some signaling pathways for survival, such as Wnt/β-catenin, Notch, Hedgehog, Bmi-179 are shared in both cells, the cellular damages on normal stem cells would be expected to occur soon if CSC-target therapy centered on these pathways. Thus, in order to reduce the risk of such cellular damage, it is essential to select the signaling pathway which is exclusively expressed on CSCs for targeting sites. For instance, EpCAM known as CSC marker plays an essential role in cellular proliferation, migration and mitogenic signal transduction.80 However, EpCAM in normal cells is also predominantly expressed in intercellular spaces where epithelial cells form very tight junctions and function a homotypic calcium-independent cell adhesion molecule. In addition, Wnt signaling pathway which regulates cellular processes in tumor development, also plays a key role in maintenance of homeostasis in mature tissues.81 Therefore, the physiology of normal cells may be changed when trying to target them. Nonetheless, recent study by Yilmaz et al.82 demonstrated successful targeted therapy without damaging normal stem cell in leukemia. In the experiment, they used Rapamycin to suppress the activation of Akt-mTOR pathway resulting from PTEN deletion in leukemic stem cells, showing that Rapamycin depleted LSCs but restored normal hematopoietic stem cell function. However, to date, little information is only available about the natures of the biological properties regulating signaling pathways in both cancer and normal stem cells. The characteristic difference between both cells need to be elucidated to provide new therapeutic target.
CONCLUSIONS AND FUTURE PERSPECTIVES
Compared with other vital organs, the liver is a unique organ in terms of regeneration potential. Even though the liver is removed by partial hepatectomy or is injured by various noxious stimuli, such as virus toxins, the liver has the capacity for renewal of hepatocytes from HPCs, leading to restoration of its size and function. However, unlike benign liver diseases, HCC comprises a complex pathogenesis linked to many signaling pathways with or without involvement of liver CSCs. Although previous experimental and clinical works have indicated the existence of liver CSCs, potential mechanistic links between liver CSCs and development of HCC in human are not fully understood. Nonetheless, accumulating evidence supports the suggestion that generation of multiple lineage cells by liver CSCs in a hierarchical manner is responsible for tumor initiation, metastasis, and relapse after anti-cancer therapy in HCC. However, some of the surface markers for normal stem cells and CSCs in the liver are overlapped; thus, it is not easy to distinguish them by simple methods. Indeed, an inappropriate attack by the newly developed targeted drug for CSCs against normal stem cells could result in a serious life-threatening disaster. Thus, in order to distinguish liver CSCs from normal stem cells, development of a highly qualified assay system composed of both antigenic and functional approaches for isolation, purification, and characterization of CSCs should be required. In fact, conventional chemo/radiotherapy can reduce or destroy tumor bulk but cannot completely eradicate rare CSCs within a tumor. Thus, in order to develop more effective anti-cancer therapy for avoidance of chemo/radioresistance for HCC, further investigation based on biological properties of liver CSCs should be performed at the levels of cells, in vivo and human.
Although recent evidence has demonstrated that a certain type of HCC is hierarchically organized by CSCs, current research findings have not completely clarified the way in which liver CSCs initiate tumor growth. Nonetheless, it may help to explain the mechanism of chemo/radioresistance and tumor recurrence after treatment for HCC. Novel therapeutic approaches for treatment of HCC should be based on CSC-specific targeting using surface markers for liver CSCs, such as CD90, CD133, EpCAM, CD44, and CD13. Furthermore, combination therapy using conventional methods with specific CSCs-targeted therapy for suppression or destruction of CSCs may be a more integrated therapeutic approach for treatment of HCC. Further studies in the future are needed in order to clarify the exact nature of CSCs for unraveling the mechanism underlying HCC.
ACKNOWLEDGEMENTS
This work was funded by Nuclear R&D program of the Ministry of Science and Technology of the Republic of Korea (2010-0017595).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Dalerba P, Clarke MF. Cancer stem cells and tumor metastasis: first steps into uncharted territory. Cell Stem Cell. 2007;1:241–242. doi: 10.1016/j.stem.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Fidler IJ, Hart IR. Biological diversity in metastatic neoplasms: origins and implications. Science. 1982;217:998–1003. doi: 10.1126/science.7112116. [DOI] [PubMed] [Google Scholar]
- 3.Griffin JD, Löwenberg B. Clonogenic cells in acute myeloblastic leukemia. Blood. 1986;68:1185–1195. [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 5.Sabbath KD, Ball ED, Larcom P, Davis RB, Griffin JD. Heterogeneity of clonogenic cells in acute myeloblastic leukemia. J Clin Invest. 1985;75:746–753. doi: 10.1172/JCI111756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park CH, Bergsagel DE, McCulloch EA. Mouse myeloma tumor stem cells: a primary cell culture assay. J Natl Cancer Inst. 1971;46:411–422. [PubMed] [Google Scholar]
- 7.Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 8.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 9.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 10.Dick JE. Stem cells: self-renewal writ in blood. Nature. 2003;423:231–233. doi: 10.1038/423231a. [DOI] [PubMed] [Google Scholar]
- 11.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 13.Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 14.Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Eramo A, Lotti F, Sette G, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 16.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 17.Yang ZF, Ho DW, Ng MN, et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Schatton T, Murphy GF, Frank NY, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curley MD, Therrien VA, Cummings CL, et al. CD133 expression defines a tumor initiating cell population in primary human ovarian cancer. Stem Cells. 2009;27:2875–2883. doi: 10.1002/stem.236. [DOI] [PubMed] [Google Scholar]
- 20.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 21.Alison MR. Liver stem cells: implications for hepatocarcinogenesis. Stem Cell Rev. 2005;1:253–260. doi: 10.1385/SCR:1:3:253. [DOI] [PubMed] [Google Scholar]
- 22.Haraguchi N, Utsunomiya T, Inoue H, et al. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells. 2006;24:506–513. doi: 10.1634/stemcells.2005-0282. [DOI] [PubMed] [Google Scholar]
- 23.Ma S, Chan KW, Hu L, et al. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542–2556. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 24.Yamashita T, Ji J, Budhu A, et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012–1024. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu Z, Hao X, Yan M, et al. Cancer stem/progenitor cells are highly enriched in CD133+CD44+ population in hepatocellular carcinoma. Int J Cancer. 2010;126:2067–2078. doi: 10.1002/ijc.24868. [DOI] [PubMed] [Google Scholar]
- 26.Haraguchi N, Ishii H, Mimori K, et al. CD13 is a therapeutic target in human liver cancer stem cells. J Clin Invest. 2010;120:3326–3339. doi: 10.1172/JCI42550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Brien CA, Kreso A, Jamieson CH. Cancer stem cells and self-renewal. Clin Cancer Res. 2010;16:3113–3120. doi: 10.1158/1078-0432.CCR-09-2824. [DOI] [PubMed] [Google Scholar]
- 28.Yang ZF, Ngai P, Ho DW, et al. Identification of local and circulating cancer stem cells in human liver cancer. Hepatology. 2008;47:919–928. doi: 10.1002/hep.22082. [DOI] [PubMed] [Google Scholar]
- 29.Weigmann A, Corbeil D, Hellwig A, Huttner WB. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc Natl Acad Sci U S A. 1997;94:12425–12430. doi: 10.1073/pnas.94.23.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suetsugu A, Nagaki M, Aoki H, Motohashi T, Kunisada T, Moriwaki H. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem Biophys Res Commun. 2006;351:820–824. doi: 10.1016/j.bbrc.2006.10.128. [DOI] [PubMed] [Google Scholar]
- 31.Aravalli RN, Steer CJ, Cressman EN. Molecular mechanisms of hepatocellular carcinoma. Hepatology. 2008;48:2047–2063. doi: 10.1002/hep.22580. [DOI] [PubMed] [Google Scholar]
- 32.Hur W, Rhim H, Jung CK, et al. SOX4 overexpression regulates the p53-mediated apoptosis in hepatocellular carcinoma: clinical implication and functional analysis in vitro. Carcinogenesis. 2010;31:1298–1307. doi: 10.1093/carcin/bgq072. [DOI] [PubMed] [Google Scholar]
- 33.Evarts RP, Nagy P, Marsden E, Thorgeirsson SS. A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis. 1987;8:1737–1740. doi: 10.1093/carcin/8.11.1737. [DOI] [PubMed] [Google Scholar]
- 34.van Eyken P, Sciot R, van Damme B, de Wolf-Peeters C, Desmet VJ. Keratin immunohistochemistry in normal human liver. Cytokeratin pattern of hepatocytes, bile ducts and acinar gradient. Virchows Arch A Pathol Anat Histopathol. 1987;412:63–72. doi: 10.1007/BF00750732. [DOI] [PubMed] [Google Scholar]
- 35.Lai YS, Thung SN, Gerber MA, Chen ML, Schaffner F. Expression of cytokeratins in normal and diseased livers and in primary liver carcinomas. Arch Pathol Lab Med. 1989;113:134–138. [PubMed] [Google Scholar]
- 36.Roskams T, De Vos R, Van Eyken P, Myazaki H, Van Damme B, Desmet V. Hepatic OV-6 expression in human liver disease and rat experiments: evidence for hepatic progenitor cells in man. J Hepatol. 1998;29:455–463. doi: 10.1016/s0168-8278(98)80065-2. [DOI] [PubMed] [Google Scholar]
- 37.Bralet MP, Pichard V, Ferry N. Demonstration of direct lineage between hepatocytes and hepatocellular carcinoma in diethylnitrosamine-treated rats. Hepatology. 2002;36:623–630. doi: 10.1053/jhep.2002.35540. [DOI] [PubMed] [Google Scholar]
- 38.Nagaki M, Shidoji Y, Yamada Y, et al. Regulation of hepatic genes and liver transcription factors in rat hepatocytes by extracellular matrix. Biochem Biophys Res Commun. 1995;210:38–43. doi: 10.1006/bbrc.1995.1624. [DOI] [PubMed] [Google Scholar]
- 39.Wu XZ, Chen D, Xie GR. Extracellular matrix remodeling in hepatocellular carcinoma: effects of soil on seed? Med Hypotheses. 2006;66:1115–1120. doi: 10.1016/j.mehy.2005.12.043. [DOI] [PubMed] [Google Scholar]
- 40.Avril A, Pichard V, Bralet MP, Ferry N. Mature hepatocytes are the source of small hepatocyte-like progenitor cells in the retrorsine model of liver injury. J Hepatol. 2004;41:737–743. doi: 10.1016/j.jhep.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 41.Roskams TA, Libbrecht L, Desmet VJ. Progenitor cells in diseased human liver. Semin Liver Dis. 2003;23:385–396. doi: 10.1055/s-2004-815564. [DOI] [PubMed] [Google Scholar]
- 42.Sell S. Cellular origin of cancer: dedifferentiation or stem cellmaturation arrest? Environ Health Perspect. 1993;101(Suppl 5):15–26. doi: 10.1289/ehp.93101s515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sell S, Dunsford HA. Evidence for the stem cell origin of hepatocellular carcinoma and cholangiocarcinoma. Am J Pathol. 1989;134:1347–1363. [PMC free article] [PubMed] [Google Scholar]
- 44.Sell S. Cellular origin of hepatocellular carcinomas. Semin Cell Dev Biol. 2002;13:419–424. doi: 10.1016/s1084952102001295. [DOI] [PubMed] [Google Scholar]
- 45.Theise ND, Yao JL, Harada K, et al. Hepatic 'stem cell' malignancies in adults: four cases. Histopathology. 2003;43:263–271. doi: 10.1046/j.1365-2559.2003.01707.x. [DOI] [PubMed] [Google Scholar]
- 46.Lowes KN, Brennan BA, Yeoh GC, Olynyk JK. Oval cell numbers in human chronic liver diseases are directly related to disease severity. Am J Pathol. 1999;154:537–541. doi: 10.1016/S0002-9440(10)65299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Libbrecht L, Desmet V, Van Damme B, Roskams T. The immunohistochemical phenotype of dysplastic foci in human liver: correlation with putative progenitor cells. J Hepatol. 2000;33:76–84. doi: 10.1016/s0168-8278(00)80162-2. [DOI] [PubMed] [Google Scholar]
- 48.Libbrecht L, Desmet V, Roskams T. Preneoplastic lesions in human hepatocarcinogenesis. Liver Int. 2005;25:16–27. doi: 10.1111/j.1478-3231.2005.01016.x. [DOI] [PubMed] [Google Scholar]
- 49.Marshall A, Rushbrook S, Davies SE, et al. Relation between hepatocyte G1 arrest, impaired hepatic regeneration, and fibrosis in chronic hepatitis C virus infection. Gastroenterology. 2005;128:33–42. doi: 10.1053/j.gastro.2004.09.076. [DOI] [PubMed] [Google Scholar]
- 50.Libbrecht L, Roskams T. Hepatic progenitor cells in human liver diseases. Semin Cell Dev Biol. 2002;13:389–396. doi: 10.1016/s1084952102001258. [DOI] [PubMed] [Google Scholar]
- 51.Ruck P, Xiao JC, Pietsch T, Von Schweinitz D, Kaiserling E. Hepatic stem-like cells in hepatoblastoma: expression of cytokeratin 7, albumin and oval cell associated antigens detected by OV-1 and OV-6. Histopathology. 1997;31:324–329. doi: 10.1046/j.1365-2559.1997.2750870.x. [DOI] [PubMed] [Google Scholar]
- 52.Desmet V, Roskams T, Van Eyken P. Ductular reaction in the liver. Pathol Res Pract. 1995;191:513–524. doi: 10.1016/s0344-0338(11)80870-8. [DOI] [PubMed] [Google Scholar]
- 53.Haque S, Haruna Y, Saito K, et al. Identification of bipotential progenitor cells in human liver regeneration. Lab Invest. 1996;75:699–705. [PubMed] [Google Scholar]
- 54.Yamashita T, Budhu A, Forgues M, Wang XW. Activation of hepatic stem cell marker EpCAM by Wnt-beta-catenin signaling in hepatocellular carcinoma. Cancer Res. 2007;67:10831–10839. doi: 10.1158/0008-5472.CAN-07-0908. [DOI] [PubMed] [Google Scholar]
- 55.Coulouarn C, Factor VM, Thorgeirsson SS. Transforming growth factor-beta gene expression signature in mouse hepatocytes predicts clinical outcome in human cancer. Hepatology. 2008;47:2059–2067. doi: 10.1002/hep.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang Y, Kitisin K, Jogunoori W, et al. Progenitor/stem cells give rise to liver cancer due to aberrant TGF-beta and IL-6 signaling. Proc Natl Acad Sci U S A. 2008;105:2445–2450. doi: 10.1073/pnas.0705395105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao J, Song Z, Chen Y, et al. Deregulated expression of Notch receptors in human hepatocellular carcinoma. Dig Liver Dis. 2008;40:114–121. doi: 10.1016/j.dld.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 58.Ricci-Vitiani L, Fabrizi E, Palio E, De Maria R. Colon cancer stem cells. J Mol Med (Berl) 2009;87:1097–1104. doi: 10.1007/s00109-009-0518-4. [DOI] [PubMed] [Google Scholar]
- 59.Wulf GG, Wang RY, Kuehnle I, et al. A leukemic stem cell with intrinsic drug efflux capacity in acute myeloid leukemia. Blood. 2001;98:1166–1173. doi: 10.1182/blood.v98.4.1166. [DOI] [PubMed] [Google Scholar]
- 60.Magni M, Shammah S, Schiró R, Mellado W, Dalla-Favera R, Gianni AM. Induction of cyclophosphamide-resistance by aldehyde-dehydrogenase gene transfer. Blood. 1996;87:1097–1103. [PubMed] [Google Scholar]
- 61.Duxbury MS, Ito H, Benoit E, Waseem T, Ashley SW, Whang EE. A novel role for carcinoembryonic antigen-related cell adhesion molecule 6 as a determinant of gemcitabine chemoresistance in pancreatic adenocarcinoma cells. Cancer Res. 2004;64:3987–3993. doi: 10.1158/0008-5472.CAN-04-0424. [DOI] [PubMed] [Google Scholar]
- 62.Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27:1749–1758. doi: 10.1038/sj.onc.1210811. [DOI] [PubMed] [Google Scholar]
- 63.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 64.Phillips TM, McBride WH, Pajonk F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 65.Woodward WA, Chen MS, Behbod F, Alfaro MP, Buchholz TA, Rosen JM. WNT/beta-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci U S A. 2007;104:618–623. doi: 10.1073/pnas.0606599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17:165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lammering G, Hewit TH, Holmes M, et al. Inhibition of the type III epidermal growth factor receptor variant mutant receptor by dominant-negative EGFR-CD533 enhances malignant glioma cell radiosensitivity. Clin Cancer Res. 2004;10:6732–6743. doi: 10.1158/1078-0432.CCR-04-0393. [DOI] [PubMed] [Google Scholar]
- 68.Puc J, Parsons R. PTEN loss inhibits CHK1 to cause double stranded-DNA breaks in cells. Cell Cycle. 2005;4:927–929. doi: 10.4161/cc.4.7.1795. [DOI] [PubMed] [Google Scholar]
- 69.Zhou J, Schmid T, Schnitzer S, Brüne B. Tumor hypoxia and cancer progression. Cancer Lett. 2006;237:10–21. doi: 10.1016/j.canlet.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 70.Williams KJ, Telfer BA, Xenaki D, et al. Enhanced response to radiotherapy in tumours deficient in the function of hypoxiainducible factor-1. Radiother Oncol. 2005;75:89–98. doi: 10.1016/j.radonc.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 71.Baumann M, Krause M, Hill R. Exploring the role of cancer stem cells in radioresistance. Nat Rev Cancer. 2008;8:545–554. doi: 10.1038/nrc2419. [DOI] [PubMed] [Google Scholar]
- 72.Bernier J, Hall EJ, Giaccia A. Radiation oncology: a century of achievements. Nat Rev Cancer. 2004;4:737–747. doi: 10.1038/nrc1451. [DOI] [PubMed] [Google Scholar]
- 73.Lee JS, Heo J, Libbrecht L, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12:410–416. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- 74.Song W, Li H, Tao K, et al. Expression and clinical significance of the stem cell marker CD133 in hepatocellular carcinoma. Int J Clin Pract. 2008;62:1212–1218. doi: 10.1111/j.1742-1241.2008.01777.x. [DOI] [PubMed] [Google Scholar]
- 75.Durnez A, Verslype C, Nevens F, et al. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology. 2006;49:138–151. doi: 10.1111/j.1365-2559.2006.02468.x. [DOI] [PubMed] [Google Scholar]
- 76.Ma S, Chan KW, Guan XY. In search of liver cancer stem cells. Stem Cell Rev. 2008;4:179–192. doi: 10.1007/s12015-008-9035-z. [DOI] [PubMed] [Google Scholar]
- 77.Cairns J. Somatic stem cells and the kinetics of mutagenesis and carcinogenesis. Proc Natl Acad Sci U S A. 2002;99:10567–10570. doi: 10.1073/pnas.162369899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Phatak P, Burger AM. Telomerase and its potential for therapeutic intervention. Br J Pharmacol. 2007;152:1003–1011. doi: 10.1038/sj.bjp.0707374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003;423:255–260. doi: 10.1038/nature01572. [DOI] [PubMed] [Google Scholar]
- 80.Münz M, Kieu C, Mack B, Schmitt B, Zeidler R, Gires O. The carcinoma-associated antigen EpCAM upregulates c-myc and induces cell proliferation. Oncogene. 2004;23:5748–5758. doi: 10.1038/sj.onc.1207610. [DOI] [PubMed] [Google Scholar]
- 81.Takahashi-Yanaga F, Kahn M. Targeting Wnt signaling: can we safely eradicate cancer stem cells? Clin Cancer Res. 2010;16:3153–3162. doi: 10.1158/1078-0432.CCR-09-2943. [DOI] [PubMed] [Google Scholar]
- 82.Yilmaz OH, Valdez R, Theisen BK, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 83.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dalerba P, Dylla SJ, Park IK, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prince ME, Sivanandan R, Kaczorowski A, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang S, Balch C, Chan MW, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]