Abstract
Background/Aims
When undergoing endoscopic submucosal dissection (ESD), patients with liver cirrhosis (LC) may suffer from a high risk of bleeding, bacteremia and tissue vulnerability. There have been few reports evaluating the efficacy and safety of ESD in patients with LC.
Methods
From January 2004 to March 2010, 23 patients with LC (cirrhosis group) underwent ESD for superficial gastric neoplastic lesions. The number of patients with a liver function in the Child-Pugh classes A and B were 20 and 3, respectively. The clinical outcomes and complications were compared with 69 patients without LC (control group) that were matched for age and sex.
Results
The en bloc resection, R0 resection and en bloc plus R0 resection rates of the cirrhosis group were 82.6%, 91.3%, and 82.6%, respectively, and did not show significant differences from the rates of the control group. No local recurrence was found in either group during the follow-up period. The procedure length of time (41.0 vs 39.0 minutes), rate of bleeding (4.3% vs 7.2%) and rate of perforation (0.0% vs 1.4%) in the cirrhosis group were also comparable to the results from the control group.
Conclusions
ESD was safely performed in patients with LC, and satisfactory outcomes were achieved with high en bloc and R0 resection rates for superficial gastric neoplastic lesions.
Keywords: Superficial gastric neoplastic lesion, Endoscopic submucosal dissection, Liver cirrhosis
INTRODUCTION
Endoscopic mucosal resection (EMR) has become a standard treatment for superficial gastric neoplastic lesion including gastric dysplasia and early gastric cancer (EGC) because of its minimal invasiveness and excellent long-term survival comparable to surgical resection.1-3 To achieve an accurate and reliable histopathological evaluation after EMR, en bloc resection is desirable.4 As EMR method uses snare, however, a trial of resecting lesions larger than 2.0 cm may result in piecemeal resections.5,6
To overcome the size limitation of EMR and consequently to improve en bloc resection rate, endoscopic submucosal dissection (ESD) method was recently developed and has been widely accepted these days in Far Eastern countries.7-11 As electrosurgical knife is used instead of snare and the submucosa beneath the lesion is directly dissected in ESD, there is theoretically no limitation in lesion size that can be resected in en bloc fashion. However, as ESD is highly advanced technique, it requires longer procedure time and can cause higher rate of complications such as bleeding and perforation compared to conventional EMR.4,7,8 Bleeding is the most common complication occurring in ESD and the rate is reported to be up to 15%.12
Patients with liver cirrhosis (LC) are exposed to high risk of bleeding in case of invasive treatments such as ESD because of low platelet count and coagulopathy accompanying LC.13 In addition, high rate of procedure-associated bacteremia is reported14-16 and vulnerability of tissue in portal hypertensive gastropathy also make them poor candidates for ESD. Therefore, patients with LC might be regarded as a distinct entity with high complication risk for gastric ESD and the clinical outcomes and complication of ESD in these patients need to be evaluated. To date, however, there have been few reports evaluating the efficacy and safety of ESD in patients with LC.17
In the present study, we compared the outcomes and complications of ESD in patients with and without LC and aimed to evaluate the feasibility and safety of ESD for superficial gastric neoplastic lesions in patients with LC.
MATERIALS AND METHODS
1. Patients
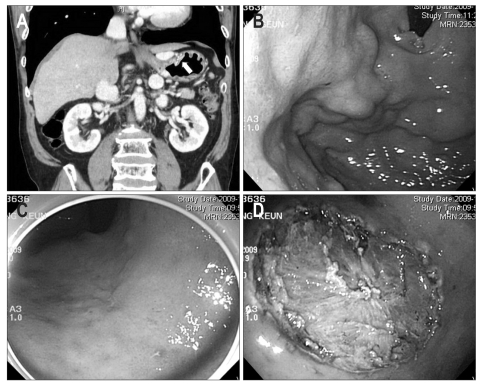
From January 2004 to March 2010, a total of 2,568 gastric ESD procedures were performed at Samsung Medical Center to remove superficial gastric neoplastic lesions (1,415 ESDs for EGCs and 1,153 ESDs for gastric dysplasias) and all these cases were consecutively collected in our database. Among them, 23 patients had LC and were enrolled in this study: 4 low grade gastric dysplasias, 4 high grade gastric dysplasias, and 15 EGCs. The grade of gastric dysplasia and carcinoma was determined according to the Vienna classification of gastrointestinal epithelial neoplasia: LGD in category 3, HGD in category 4, and intramucosal and submucosal carcinoma in category 5.18 Patients were diagnosed to have LC based on the radiologic findings, clinical data with laboratory investigation and medical history implying portal hypertension such as esophageal or gastric varix or ascites (Fig. 1).19,20 Patients' liver function was classified according to the Child-Pugh class: 20 patients in Child-Pugh class A and 3 patients in Child-Pugh class B. Table 1 summarizes the cirrhosis-associated features of these patients.
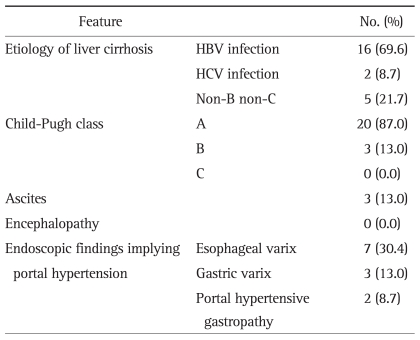
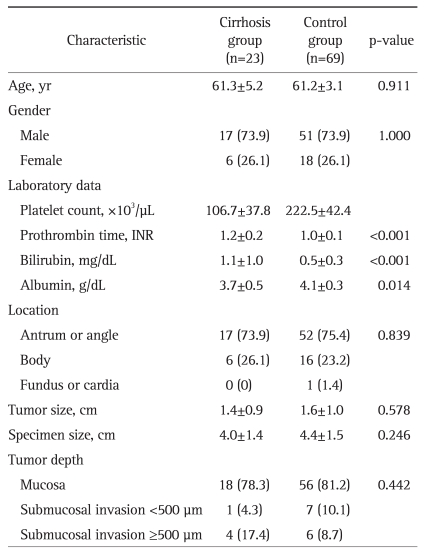
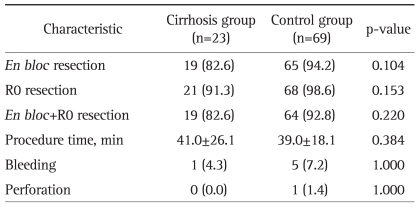
Fig. 1.
A case of a 60-year-old male patient with liver cirrhosis who underwent endoscopic submucosal dissection for early gastric cancer. His serum platelet count was 140×103/µL, and his Child-Pugh liver function classification was A (serum albumin, 3.5 g/dL; serum bilirubin, 1.7 mg/dL; prothrombin time in INR, 1.27). (A) Surface irregularity of the liver and marked gastric fundic varix (white arrow) is observed with abdominal computed tomography. (B) Gastric fundic varix is shown with upper endoscopy. (C) A 0.6-cm type 0-IIc lesion is observed at the lesser curvature side of the midbody. (D) A large, artificial, ulcer-induced after endoscopic submucosal dissection.
Table 1.
Cirrhosis-Associated Features of the 23 Patients with Liver Cirrhosis Who Underwent Endoscopic Submucosal Dissection for Superficial Gastric Neoplastic Lesions
HBV, hepatitis B virus; HCV, hepatitis C virus
Therapeutic efficacy and complication of ESD were compared between patients with LC (cirrhosis group) and without LC (control group). Control groups were matched for age and sex and randomly selected in the ratio of 1:3 from the patients without LC who underwent ESDs for superficial gastric neoplastic lesions from January 2004 to March 2010.
Informed consent was obtained from each patient included in the study. The study protocol conforms to the ethical guidelines of the Declaration of Helsinki in 1995 as reflected in a priori approval by the institution's human research committee.
2. ESD
All ESD procedures were carried out under conscious sedation with the intravenous administration of midazolam combined with pethidine. Doses of drugs were adjusted according to the patients' condition. The average amounts of midazolam used were 3.6±1.0 mg and 3.8±1.3 mg in LC and control groups, respectively (p=0.494). The average amounts of pethidine used were 43.5±13.5 mg and 46.0±10.2 mg in LC and control groups, respectively (p=0.427). Before procedure, three patients with LC received platelet transfusion and one patient received fresh frozen plasma transfusion. All three patients receiving platelet transfusion had liver function of Child-Pugh class A. Before transfusion, platelet counts of these patients were 54×103/µL, 56×103/µL, and 66×103/µL, respectively. After transfusion, platelet counts increased up to 107×103/µL, 66×103/µL, and 105×103/µL, respectively. One patient receiving fresh frozen plasma had liver function of Child-Pugh class B. Before transfusion, prothrombin time of this patient was 2.2 in international normalized ratio (INR) and it was corrected to 1.6 after fresh frozen plasma transfusion. Taking aspirin, warfarin, and nonsteroidal anti-inflammatory drug were prohibited at least 1 week before procedure. Prophylactic antibiotics were not prescribed before procedure.
ESD was performed as previously described in detail.9 In brief, marking dots were made circumferentially at approximately 5 mm lateral to the margin of the lesion using a needle knife (KD-1L-1, Olympus, Tokyo, Japan; Needle papillotome, MTW Endoscopy, Wesel, Germany). After marking, a submucosal injection of saline or glycerin solution mixed with epinephrine and indigocarmine was performed around the lesion to lift it off the muscle layer. Then, an initial incision of mucosa was made with the needle knife to allow insertion of the tip of the knife into the submucosa. After the initial incision, a circumferential mucosal incision was performed outside the marking dots to separate the lesion from the surrounding non-neoplastic mucosa. This step was done using the electrosurgical knife such as needle, Flex (KD-630L; Olympus) or insulated-tipped (IT) knife (KD-610L; Olympus) with a high-frequency generator (Erbotom ICC 200; ERBE Elektromedizin Ltd., Tubingen, Germany). After the circumferential incision, an additional submucosal injection was performed beneath the lesion. Finally, the submucosal connective tissue just beneath the lesion was directly dissected using an electrosurgical knife such as needle, Flex or IT knife.
Hemostatic forceps (FD-410LR; Olympus) or hemoclips were used to control bleedings during and after resection. During and until 1 day after procedure, ranitidine was given via parental route and then standard dose of proton pump inhibitor was prescribed for 4 weeks.
3. Assessment of the therapeutic efficacy and complication
En bloc resection was defined as resection in one piece without fragmentation. R0 resection was defined as resection with tumor-free lateral and vertical resection margins.9
The procedure time was defined as the required time for marking, precutting, submucosal dissection and hemostasis.
Bleeding was defined as 1) intraoperative massive bleeding that required blood transfusion, 2) postoperative bleeding that required blood transfusion or endoscopic or surgical intervention because of hematemesis or melena, or 3) a decrease of the hemoglobin level more than 2 g/dL after the procedure.9
Perforation was diagnosed when mesenteric fat or intra-abdominal space was directly observed during the procedure (frank perforation) or free air was found on a plain chest X-ray after the procedure without a visible gastric wall defect during the procedure (microperforation).9,21
4. Follow-up after ESD
The patients were followed up with an upper endoscopy with a biopsy 2 months after ESD to confirm healing of the artificial ulcer and to exclude the presence of any residual tumor. Then, upper endoscopy was performed every 6 months for 3 years to check for local or metachronous recurrence. From the fourth year, upper endoscopy was performed annually. For patient with EGC, an abdominal computed tomography was also performed every 6 months for 3 years and then performed annually to detect extragastric recurrence.
5. Statistical analysis
Continuous data were analyzed using the Student's t-test. Categorical data analysis was done by χ2 test or Fisher's exact test. All p-values were 2-tailed and p-values less than 0.05 were considered statistically significant.
RESULTS
Table 2 summarizes the clinicopathologic characteristics of cirrhosis group and control group. No significant difference was found in age, gender, location, tumor size, specimen size, or tumor depth. However, cirrhosis group showed significant difference from control group in platelet count, prothrombin time, bilirubin, and albumin levels. Among patients enrolled, neither cirrhosis nor control group had ulcerative lesion.
Table 2.
The Comparison of the Clinicopathologic Characteristics between Patients with and without Cirrhosis Who Underwent Endoscopic Submucosal Dissection for Superficial Gastric Neoplastic Lesions
Data are presented as mean±SD or number (%).
Table 3 summarizes the clinical outcomes of ESD in cirrhosis and control group. En bloc resection rate, R0 resection rate and en bloc plus R0 resection rate of cirrhosis group were 82.6%, 91.3%, and 82.6%, respectively and did not show significant difference from those of control group. All four lesions not achieving en bloc plus R0 resection in cirrhosis group were EGCs and were resected in piecemeal fashion. The Child-Pugh classification was A in all four cases. Among these four cases two cases showed positive resection margins and underwent additional gastrectomy. Two cases with negative resection margin did not show residual cancer in follow-up upper endoscopy. Procedure time of cirrhosis group was also comparable to that of control group.
Table 3.
The Comparison of the Clinical Outcomes between Patients with and without Cirrhosis Who Underwent Endoscopic Submucosal Dissection for Superficial Gastric Neoplastic Lesions
Data are presented as mean±SD or number (%).
For ESD-associated complication, no significant difference was observed in bleeding rate or perforation rate between two groups. Only one case of bleeding was reported in cirrhosis group. This patient showed melena and hematemesis 5 days after ESD. On emergency endoscopy, active bleeding was found and hemostasis was successfully achieved using hemoclips. This patient's Child-Pugh class was B and platelet count was 105×103/µL. Before ESD, 6 units of fresh frozen plasma were transfused to correct prothrombin time. Prothrombin time in INR was 1.6 at the time of procedure. In this study, no patients in cirrhosis group underwent perforation during procedure. No increase in ascites, no development of hepatic encephalopathy, no worsening of liver function occurred after ESD. However, fever developed one day after ESD in two patients in cirrhosis group. In these two patients, fever was successfully controlled 2 days after ESD with treatment. Blood cultures showed negative result in both patients.
Among patients without undergoing additional treatment after ESD, median duration of follow up was 17.5 months (range, 2 to 72 months) for cirrhosis group and 26.0 months (range, 5 to 60 months) for control group, respectively. No local recurrence was found in either group during follow-up period.
DISCUSSION
In case of abdominal surgery, patients with LC often undergo severe complication such as hepatic failure, massive ascites, intra-abdominal bleeding, multi-organ failure, and sepsis.13,22 Some studies reported high mortality rate of up to 30% in these patients.23,24 Therefore, patients with LC are currently regarded as a distinct entity with high complication rate for abdominal open surgery. Although far less invasive compared to surgery, ESD also carries the potential risk of severe complication in patient with LC because of low platelet count, coagulopathy, high rate of bacteremia, and vulnerability of tissue due to portal hypertensive gastropathy. Therefore, it is required to elucidate the clinical outcomes and complication of ESD in patients with LC.
In the present study, en bloc resection rate, R0 resection rate and en bloc plus R0 resection rate of cirrhosis group were 82.6%, 91.3%, and 82.6%, respectively and did not show significant difference from those of control group without cirrhosis. No local recurrence was found in either group during follow-up period. In addition to these favorable outcomes, bleeding and perforation rates in cirrhosis group was also comparable to those of control group.
In this study, only one case of bleeding was reported in cirrhosis group. This patient showed melena and hematemesis 5 days after ESD and his liver function was classified as Child-Pugh class B. Ogura et al.17 reported the similar trend in their case report about ESD in patients with LC. In their case report, bleeding rate after ESD was 20% and all the patients undergoing bleeding showed severe liver dysfunction of Child-Pugh class B.17 It is well known that open gastrectomy is associated with high complication and mortality rates in patients with severe liver dysfunction. Jang et al.22 reported the clinical outcomes after curative surgery for gastric cancer in patients with LC. In their study, postoperative ascites (63.6% vs 13%) and hepatic encephalopathy (36.4% vs 4.3%) occurred significantly more frequently in patients with Child-Pugh class B or C than in those with class A. In addition, postoperative mortality in patients with Child-Pugh class B or C was significantly higher as compared with Child-Pugh class A (27.2% vs 4.3%).22 Although the present study showed favorable result of ESD in patients with LC, more caution and further studies are warranted for ESD in patients with severe liver dysfunction (Child-Pugh class B or C).
In our study, two patients in cirrhosis group showed fever after ESD. As risk for bacteremia associated with gastrointestinal bleeding is well established in patients with LC,25 prophylactic administration of antibiotics is currently recommended for all LC patient with acute bleeding regardless of endoscopic procedure.16 However, there has been no study on the frequency of bacteremia in patients with LC undergoing ESD. In addition, several studies reported the low rate of bacteremia after EMR or ESD.26,27 Blood culture results were negative in both patients with fever in the present study. Therefore, necessity for antibiotics prophylaxis is still unclear in patients with LC undergoing ESD and further study is required.
The present study had several limitations. It was a retrospective study performed in a single center and the number of patients with Child-Pugh Class B or C was small. Follow-up duration after ESD was relatively short to confirm long-term outcomes.
In conclusion, the result of the present study indicated that even in patients with LC, ESD could be safely performed and could achieve satisfactory outcomes with high en bloc and R0 resection rate for superficial gastric neoplastic lesions. Given the small number of patients with Child-Pugh class B or C included in this study, further large study is required to confirm the efficacy and safety of ESD in patients with severe liver dysfunction.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Kim JJ, Lee JH, Jung HY, et al. EMR for early gastric cancer in Korea: a multicenter retrospective study. Gastrointest Endosc. 2007;66:693–700. doi: 10.1016/j.gie.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Lee JH, Kim JJ. Endoscopic mucosal resection of early gastric cancer: experiences in Korea. World J Gastroenterol. 2007;13:3657–3661. doi: 10.3748/wjg.v13.i27.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soetikno R, Kaltenbach T, Yeh R, Gotoda T. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol. 2005;23:4490–4498. doi: 10.1200/JCO.2005.19.935. [DOI] [PubMed] [Google Scholar]
- 4.Gotoda T. Endoscopic resection of early gastric cancer: the Japanese perspective. Curr Opin Gastroenterol. 2006;22:561–569. doi: 10.1097/01.mog.0000239873.06243.00. [DOI] [PubMed] [Google Scholar]
- 5.Chiu PW. Endoscopic submucosal dissection-bigger piece, better outcome! Gastrointest Endosc. 2006;64:884–885. doi: 10.1016/j.gie.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Das A. Endoscopic submucosal dissection: cure in one piece. Endoscopy. 2006;38:1044–1046. doi: 10.1055/s-2006-944834. [DOI] [PubMed] [Google Scholar]
- 7.Fujishiro M. Endoscopic submucosal dissection for stomach neoplasms. World J Gastroenterol. 2006;12:5108–5112. doi: 10.3748/wjg.v12.i32.5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929–942. doi: 10.1007/s00535-006-1954-3. [DOI] [PubMed] [Google Scholar]
- 9.Min BH, Lee JH, Kim JJ, et al. Clinical outcomes of endoscopic submucosal dissection (ESD) for treating early gastric cancer: comparison with endoscopic mucosal resection after circumferential precutting (EMR-P) Dig Liver Dis. 2009;41:201–209. doi: 10.1016/j.dld.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Oka S, Tanaka S, Kaneko I, et al. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877–883. doi: 10.1016/j.gie.2006.03.932. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe K, Ogata S, Kawazoe S, et al. Clinical outcomes of EMR for gastric tumors: historical pilot evaluation between endoscopic submucosal dissection and conventional mucosal resection. Gastrointest Endosc. 2006;63:776–782. doi: 10.1016/j.gie.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 12.Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228–1235. doi: 10.1016/j.gie.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 13.del Olmo JA, Flor-Lorente B, Flor-Civera B, et al. Risk factors for nonhepatic surgery in patients with cirrhosis. World J Surg. 2003;27:647–652. doi: 10.1007/s00268-003-6794-1. [DOI] [PubMed] [Google Scholar]
- 14.Rerknimitr R, Chanyaswad J, Kongkam P, Kullavanijaya P. Risk of bacteremia in bleeding and nonbleeding gastric varices after endoscopic injection of cyanoacrylate. Endoscopy. 2008;40:644–649. doi: 10.1055/s-2008-1077294. [DOI] [PubMed] [Google Scholar]
- 15.Chen WC, Hou MC, Lin HC, et al. Bacteremia after endoscopic injection of N-butyl-2-cyanoacrylate for gastric variceal bleeding. Gastrointest Endosc. 2001;54:214–218. doi: 10.1067/mge.2001.116566. [DOI] [PubMed] [Google Scholar]
- 16.ASGE Standards of Practice Committee. Banerjee S, Shen B, et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2008;67:791–798. doi: 10.1016/j.gie.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 17.Ogura K, Okamoto M, Sugimoto T, et al. Efficacy and safety of endoscopic submucosal dissection for gastric cancer in patients with liver cirrhosis. Endoscopy. 2008;40:443–445. doi: 10.1055/s-2007-995650. [DOI] [PubMed] [Google Scholar]
- 18.Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–255. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Tsao G, Lim JK Members of Veterans Affairs Hepatitis C Resource Center Program. Management and treatment of patients with cirrhosis and portal hypertension: recommendations from the Department of Veterans Affairs Hepatitis C Resource Center Program and the National Hepatitis C Program. Am J Gastroenterol. 2009;104:1802–1829. doi: 10.1038/ajg.2009.191. [DOI] [PubMed] [Google Scholar]
- 20.Gore RM, Levine MS. Textbook of gastrointestinal radiology. 2nd ed. Philadelphia: W.B. Saunders; 2000. [Google Scholar]
- 21.Jeong G, Lee JH, Yu MK, et al. Non-surgical management of microperforation induced by EMR of the stomach. Dig Liver Dis. 2006;38:605–608. doi: 10.1016/j.dld.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Jang HJ, Kim JH, Song HH, et al. Clinical outcomes of patients with liver cirrhosis who underwent curative surgery for gastric cancer: a retrospective multi-center study. Dig Dis Sci. 2008;53:399–404. doi: 10.1007/s10620-007-9884-3. [DOI] [PubMed] [Google Scholar]
- 23.Liu CL, Fan ST, Lo CM, et al. Abdominal drainage after hepatic resection is contraindicated in patients with chronic liver diseases. Ann Surg. 2004;239:194–201. doi: 10.1097/01.sla.0000109153.71725.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mansour A, Watson W, Shayani V, Pickleman J. Abdominal operations in patients with cirrhosis: still a major surgical challenge. Surgery. 1997;122:730–735. doi: 10.1016/s0039-6060(97)90080-5. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez J, Navasa M, Gomez J, et al. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140–148. doi: 10.1053/jhep.2002.30082. [DOI] [PubMed] [Google Scholar]
- 26.Lee TH, Hsueh PR, Yeh WC, Wang HP, Wang TH, Lin JT. Low frequency of bacteremia after endoscopic mucosal resection. Gastrointest Endosc. 2000;52:223–225. doi: 10.1067/mge.2000.107718. [DOI] [PubMed] [Google Scholar]
- 27.Min BH, Chang DK, Kim DU, et al. Low frequency of bacteremia after an endoscopic resection for large colorectal tumors in spite of extensive submucosal exposure. Gastrointest Endosc. 2008;68:105–110. doi: 10.1016/j.gie.2007.11.051. [DOI] [PubMed] [Google Scholar]