Abstract
Background/Aims
We aimed to evaluate the efficacy and safety of peginterferon plus ribavirin for chronic hepatitis C (CHC) patients under real life setting in Korea.
Methods
We retrospectively analyzed the medical records of 758 CHC patients treated with peginterferon plus ribavirin between 2000 and 2008 from 14 university hospitals in the Gyeonggi-Incheon area in Korea.
Results
Hepatitis C virus (HCV) genotype 1 was detected in 61.2% of patients, while genotype 2 was detected in 35.5%. Baseline HCV RNA level was ≥6×105 IU/mL in 51.6% of patients. The sustained virological response (SVR) rate was 59.6% regardless of genotype; 53.6% in genotype 1 and 71.4% in genotype 2/3. On multivariate analysis, male gender (p=0.011), early virological response (p<0.001), genotype 2/3 (p<0.001), HCV RNA <6×105 IU/mL (p=0.005) and adherence to the drug >80% of the planned dose (p<0.001) were associated with SVR. The rate of premature discontinuation was 35.7%. The main reason for withdrawal was intolerance to the drug due to common adverse events or cytopenia (48.2%).
Conclusions
Our data suggest that the efficacy of peginterferon and ribavirin therapy in Koreans is better in Koreans than in Caucasians for the treatment of CHC, corroborating previous studies that have shown the superior therapeutic efficacy of this regimen in Asians.
Keywords: Chronic hepatitis C, Pegylated interferon alpha, Ribavirin, Korean
INTRODUCTION
Chronic hepatitis C virus (HCV) infection is a leading cause of chronic liver disease worldwide.1 Although the prevalence of anti-HCV has remained stable around 1% since 1991,2 chronic hepatitis C (CHC) is the third most common etiology of chronic liver disease and hepatocellular carcinoma (HCC) in Korea.3
After publication of three pivotal, randomized clinical trials,4-6 the combination of pegylated interferon alpha (peginterferon) and ribavirin is currently recommended as the standard of care for treatment of CHC.1,7 Recent well-designed clinical trials demonstrated variable rates of sustained virological response (SVR) between 39.8% and 66%, regardless of the genotype, and suggested several predictive variables for successful treatment and rates of common adverse events.8,9 However, the study subjects in clinical trials are usually highly selected individuals meeting complicated inclusion and exclusion criteria, so they may not reflect the general population of CHC patients encountered in routine clinical practice.1 Moreover, special attention may be given to the patients enrolled in clinical trials and this can be a factor influencing compliance or notification of adverse events.
There have been several Korean studies that evaluated the treatment efficacy of the peginterferon plus ribavirin regimen in CHC patients. These studies reported overall SVR rates of 63% to 81%, a range that seems to be somewhat higher than in Western countries.10-13 However, because there are a smaller proportion of CHC patients compared to chronic hepatitis B patients in Korea, it is difficult to perform a well-designed study to survey treatment efficacy for CHC in a single institution. Previous studies have limitations in that they were conducted in single institutions and do not have sufficiently large study populations to accurately reflect the Korean CHC population.
K(G)yeonggi-Incheon Peginterferon Alpha and Ribavirin Effect in CHC Treatment (KIPECT) is a multicenter study group from 14 university hospitals in the Gyeonggi and Incheon areas (a large province and a city surrounding Seoul) in Korea. The aims of this study were to evaluate the efficacy and safety of peginterferon plus ribavirin for the treatment of Korean CHC patients in routine clinical practice and to confirm that the treatment efficacy of this regimen in Korean CHC patients is superior to that reported in Western countries.
MATERIALS AND METHODS
1. Subjects
The study subjects were retrospectively included from 14 large-volume university hospitals in Gyeonggi and Incheon. CHC patients 18 years or older with detectable serum HCV RNA and/or elevated serum alanine aminotransferase (ALT) levels for more than 6 months, who had been treated with peginterferon plus ribavirin from January 2000 to September 2008 were included. Exclusion criteria were acute hepatitis C, history of prior exposure to interferon or peginterferon, and no available data on HCV genotype. Patients with normal serum ALT levels, patients co-infected with human immunodeficiency virus (HIV) or hepatitis B virus (HBV), patients with chronic renal disease, and intravenous drug users were all included. Baseline clinical and virologic characteristics were obtained by retrospective review of medical records, and when available, pre-treatment histologic data were also recorded. HCV RNA levels measured in copies/mL were converted into IU/mL using a conversion factor according to the particular assay used at each hospital.1 Hepatic steatosis was categorized as present or absent, and the degree of hepatic fibrosis was classified as recommended by the Korean Study Group for the Pathology of Digestive Disease: grade 0, no fibrosis; grade 1, portal fibrosis; grade 2, periportal fibrosis; grade 3, septal fibrosis; and grade 4, cirrhosis.14 Data collection was performed with an Excel-based case report form by physicians at each individual hospital from April 2009 to August 2009. The study protocol was approved by the Institutional Review Boards at each hospital and was conducted in accordance with the principles of the Declaration of Helsinki.
2. Treatment of CHC
Patients were treated with either pegylated interferon alfa-2a or pegylated interferon alfa-2b plus ribavirin. The starting dosage and dose modification of peginterferon and ribavirin were determined based on the current guidelines suggested by the Korean Association for the Study of the Liver.7 The duration of treatment was planned to be 24 weeks for genotype 2/3 and 48 weeks for non-genotype 2/3.7 However, according to the nature of this retrospective study, selection and discontinuation as well as dosing and treatment duration of peginterferon and ribavirin were not controlled, but reflected the clinical practice of the attending physicians.
3. Definition and evaluation of the treatment response
Virological responses during therapy were defined and evaluated according to the current guidelines.1,7 Quantitative HCV RNA values at baseline, 12 weeks after treatment, and at the end of treatment for HCV genotype 1/4 were recorded. The achievement of early virological response (EVR) was judged to be either complete (HCV RNA negative) or partial (more than 2 log10 reduction in HCV RNA level compared to baseline) based on the HCV RNA level at 12 weeks. For CHC patients infected with genotype 2/3 HCV, quantitative HCV RNA levels at baseline and at the end of treatment were recorded. Regardless of genotype, end-of-treatment response (ETR) was defined as HCV RNA negative status at the end of treatment. SVR was defined as HCV RNA negativity measured at 24 weeks after treatment cessation for all genotypes. HCV RNA negativity after 4 weeks of treatment was considered a rapid virological response (RVR), if tested. The same algorithm used for genotype 1/4 was applied in patients with genotype 5/6 HCV infection.
All analyses were made on an intention-to-treat basis.
4. Adverse events
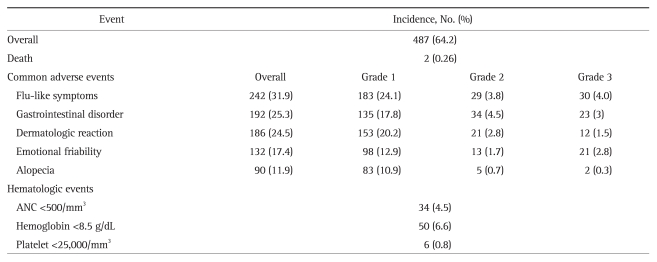
Adverse events were categorized as flu-like symptoms, emotional friability including depression or insomnia, alopecia, dermatologic reactions such as pruritis or rash, gastrointestinal disorders including nausea, vomiting, or diarrhea, and thyroid dysfunction. They were graded based on the severity: Grade I, mild symptoms that require no dose reduction; Grade II, dose reduction required due to an adverse event; Grade III, treatment discontinuation due to an adverse event. The incidence of hematologic events in terms of serum absolute neutrophil count <500/mm3, hemoglobin level <8.5 g/dL, or platelet count <25,000/mm3 were recorded. Serious adverse events were defined as death or problems requiring hospitalization.
5. Statistical analysis
Baseline clinical characteristics are presented as means±standard deviations (SDs). Binary logistic regression analysis was employed to identify clinical and virological factors associated with treatment response, and categorical variables were compared the chi-square test. All values were considered statistically significant when reaching a two-tailed p-value <0.05. Analyses were conducted using SPSS for Windows version 12.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
1. Baseline characteristics
A total of 758 CHC patients were included in the analysis. Detailed baseline demographics of the study subjects are presented in Table 1. Males comprised 60.2% of the sample, and the mean age was 50.2±12.0 years. Based on a cut-off body mass index of 25 kg/m2, 37.4% of the patients were classified as obese, and 14.1% of subjects had a history of diabetes mellitus. Eighty patients (10.6%) had a history of transfusion and 38 (5%) patients had a history of intravenous drug use. Eighteen patients (2.4%) were also positive for hepatitis B surface antigen and 6 patients (0.8%) were co-infected with HIV. Peginterferon alfa-2a was used in 61.9% of patients and peginterferon alfa-2b was used in 38.1%. HCV genotype 1 was the most common (61.2%) and genotype 2 was the second most common (35.5%). Genotype 1b, genotype 2a/c, genotype 2 of unclassified subtype, and genotype 1a constituted 54.1%, 26.5%, 4%, and 4% of patients, respectively. The mean HCV RNA level was 3.2±11.63×106 IU/mL and 51.6% had a high viral load when classified according to a cut-off value of 6×105 IU/mL. Histologic data on the degree of hepatic fibrosis was available for 244 subjects and 11.1% of them had cirrhosis. Histologic data on hepatic steatosis was available for 229 subjects, and steatosis was present in 30.2% of them.
Table 1.
Baseline Characteristics of the Study Subjects (n=758)
*Data are presented as mean±SD or number (%).
2. Treatment efficacy
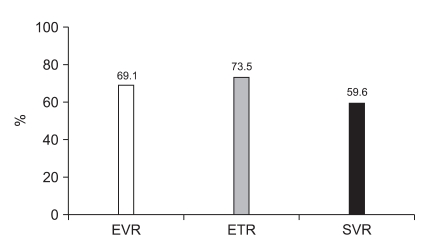
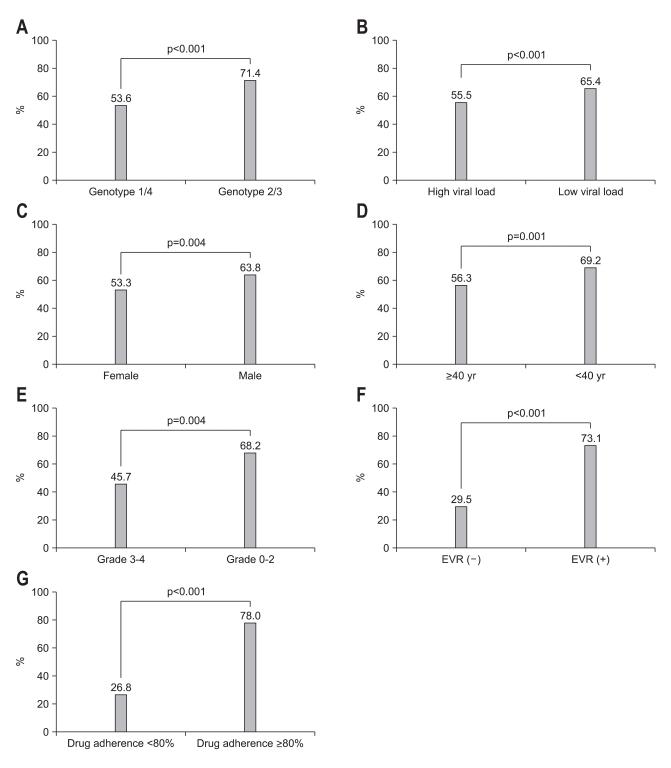
Regardless of the genotype, the overall SVR rate was 59.6%, and the rates of EVR and ETR were 69.1% and 73.5%, respectively (Fig. 1). SVR rate was compared according to various baseline factors (Fig. 2A-E) and on-treatment factors (Fig. 2F-G). The SVR rate of genotype 2/3 was higher than that of genotype 1/4 (71.4% vs 53.6%, p<0.001; Fig. 2A). When compared according to the baseline RNA level, patients with a low viral load (HCV RNA <6×105 IU/mL) had a higher SVR rate than those with a high viral load (65.4% vs 55.5%, p<0.001; Fig. 2B). Male patients had a higher SVR rate than females (63.8% vs 53.3%, p=0.004; Fig. 2C) and patients <40 years old had a higher SVR rate than patients ≥40 years old (69.2% vs 56.3%, p=0.001; Fig. 2D). SVR rate was also compared according to the grade of hepatic fibrosis in 244 subjects with available data. The patients with low grade (grade 0 to 2) fibrosis had a higher SVR rate than those with high grade (grade 3 to 4) fibrosis (68.2% vs 45.7%, p=0.004; Fig. 2E). There was no significant difference in SVR rate according to obesity, history of diabetes mellitus, baseline ALT levels, type of peginterferon, or presence of hepatic steatosis on histology. The patients with EVR (partial or complete) had a higher SVR rate than those without (73.1% vs 29.5%, p<0.001; Fig. 2F). Adherence to the drug regimen, defined as a cumulative drug dose greater than 80% of the planned dose, was associated with a higher SVR rate (78% vs 26.8%, p<0.001; Fig. 2G).
Fig. 1.
Overall treatment response irrespective of hepatitis C virus genotype. The rates of early virological response (EVR), end-of-treatment response (ETR), and sustained virological response (SVR) were 69.1%, 73.5%, and 59.6%, respectively.
Fig. 2.
Comparison of sustained virological response (SVR) rates according to baseline characteristics and on-treatment factors. The SVR rates were significantly better in chronic hepatitis C patients infected with genotype 2/3 (A), low pre-treatment viral load (B), male gender (C), age less than 40 years (D), and less advanced fibrosis on histology (E). During treatment, achievement of early virological response (EVR) (F) and adherence (a cumulative drug dose greater than 80% of the planned dose) (G) were associated with better SVR rate.
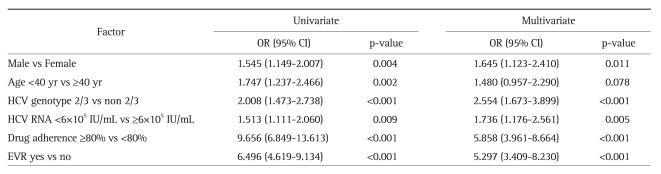
3. Factors associated with SVR
To identify predictors of SVR, univariate and multivariate analysis was performed using a binary logistic regression model (Table 2). Male gender, age <40 years, genotype 2/3, low baseline viral load, achievement of EVR, and cumulative drug dose ≥80% of the planned dose were all statistically significant on univariate analysis and were therefore included in the multivariate model. On multivariate analysis, cumulative drug dose ≥80% of the planned dose (odds ratio [OR], 5.858; 95% confidence interval [CI], 3.961 to 8.664; p<0.001), achievement of EVR (OR, 5.297; 95% CI, 3.409 to 8.230; p<0.001), HCV genotype 2/3 (OR, 2.554; 95% CI, 1.673 to 3.899; p<0.001), HCV RNA <6×105 IU/mL (OR, 1.736; 95% CI, 1.176 to 2.561; p=0.005), and male gender (OR, 1.645; 95% CI, 1.123 to 2.410; p=0.011) were all associated with SVR.
Table 2.
Univariate and Multivariate Analysis of Factors Associated with Sustained Virological Response
OR, odds ratio; CI, confidence interval; HCV, hepatitis C virus; EVR, early virological response.
4. Adverse events
Four hundred eighty-seven subjects (64.2%) experienced one or more common adverse events (Table 3). Flu-like symptoms were the most common adverse event and occurred in 31.9% of patients. Gastrointestinal disorders occurred in 25.3% and dermatologic reactions were observed in 24.5%. Emotional friability developed in 17.4% and alopecia occurred in 11.9% of the patients. Four cases of thyroid dysfunction after treatment occurred, all of which presented as hyperthyroidism. One patient complained of tremor and one case of third cranial nerve palsy was observed during the treatment period. As shown in Table 3, most of the common adverse events were grade 1 in severity, however, some led to the discontinuation of treatment. Cytopenia during the treatment period was also noted. Neutropenia with an absolute neutrophil count <500/mm3, anemia based on serum hemoglobin <8.5 g/dL, and thrombocytopenia according to a platelet count <25,000/mm3 occurred in 4.5%, 6.6%, and 0.8% of patients, respectively. Two deaths occurred during the treatment period; one was an unexpected sudden death and the other was caused by severe sepsis secondary to neutropenia. Invasive infections developed in 4 subjects, which included 2 cases of urinary tract infection leading to sepsis, one case of tuberculosis peritonitis, and one case of severe sepsis originating from acute gastroenteritis. Eight adverse events were considered treatment-related serious adverse events, including invasive infections and cases of cytopenia requiring hospital admission.
Table 3.
Summary of Adverse Events
ANC, absolute neutrophil count.
5. Discontinuation of peginterferon plus ribavirin
Premature discontinuation of peginterferon plus ribavirin occurred in 35.7% (271 subjects) of all patients. When the 271 cases were classified according to the cause of discontinuation, intolerance to the treatment drug (common adverse events or cytopenia) was the most common (48.2%) and loss to follow-up was the second (26.5%). Fifty-one patients (18.8%) requested to stop treatment due to economic problems, and treatment was terminated in 20 patients (2.7%) due to the lack of a virologic response.
6. Additional analysis of RVR
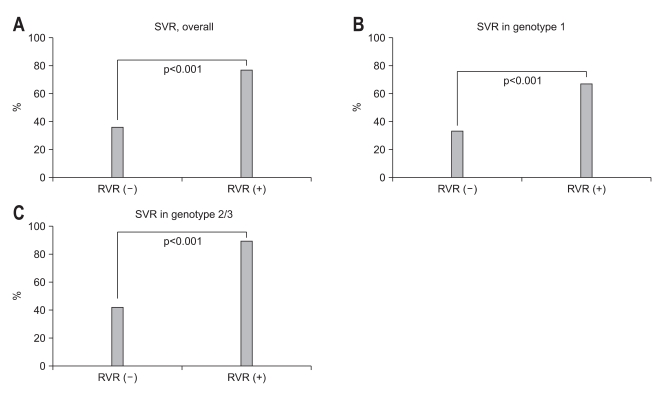
Because the concept of RVR appeared relatively recently, only 193 patients (25.5%) had available data on RVR. Subgroup analysis was performed with these patients. This subgroup showed male predominance (55.4%), and 72.5% of patients in this group were 40 years old or older. One hundred eighteen (61.1%) had HCV genotype 1/4 and 52.8% of the patients had HCV RNA level ≥6×105 IU/mL. The overall RVR rate in these 191 subjects was 57% regardless of the genotype; 51.7% in patients infected with genotype 1/4 and 65.3% in patients with genotype 2/3. The patients with RVR had a significantly higher SVR than those without (77.3% vs 36.1%; OR, 6.007; 95% CI, 3.193 to 11.300; p<0.001) irrespective of genotype (Fig. 3A). When analyzed by genotype, the achievement of RVR was associated with SVR both in genotype 1/4 (67.2% vs 33.3%; OR, 4.100; 95% CI, 1.903 to 8.833; p<0.001; Fig. 3B) and genotype 2/3 (89.8% vs 42.3%; OR, 12.000; 95% CI, 3.582 to 40.189; p<0.001; Fig. 3C).
Fig. 3.
The association between rapid virological response (RVR) and sustained virological response (SVR) was analyzed in 193 patients. The patients who showed RVR had significantly higher rates of SVR than those who did not, irrespective of genotype (A). When analyzed according to hepatitis C genotype, the association between RVR and SVR remained significant in genotype 1 (B) and genotype 2/3 (C).
DISCUSSION
In Korea, chronic HCV infection is one of the leading causes of chronic liver disease and HCC. In one study, 11% of 823 cases of liver cirrhosis were ascribed to HCV infection.15 Although HBV infection is still the most common cause of chronic liver disease and HCC, the overall prevalence of HBsAg positivity has decreased as a result of the nationwide vaccination program.16 However, according to the online disease statistics database of the Korean Centers for Disease Control and Prevention, the number of reported cases of CHC have increased annually. In 2003, 2,033 cases of CHC were reported, and this number had increased to 4,401 in 2006 and 6,406 in 2009.17
Studies of the natural history of CHC provide justification for the treatment of chronic HCV infection as up to 80% of individuals infected with HCV will remain chronically infected, and the risk of developing liver cirrhosis in these patients ranges from 5% to 25% over periods of 25 to 30 years.18,19 Patients with HCVrelated liver cirrhosis are at risk for the development of hepatic decompensation (30% over 10 years) as well as HCC (1% to 3% per year).20 Even though several management modalities such as endoscopic variceal ligation or surveillance programs for early detection of HCC have been developed, a remarkable improvement in long-term survival has not been observed. Thus, more efficient management strategies specific to the etiologic agent are required to improve survival.15 In one retrospective study including 2,145 CHC patients with a median follow-up period of 55 months, achieving SVR was associated with halted disease progression in patients with CHC. The hazard ratio for disease progression was 3.1 for patients without SVR compared to those with SVR.21 The results of this study explain why active application of antiviral therapy and efforts to improve treatment efficacy in CHC patients are required.
The treatment of CHC has evolved significantly in the past two decades in an attempt to stop viral replication and disease progression.22 The combination of peginterferon and ribavirin, which is the current standard of care for CHC, requires less frequent injection due to a prolonged half-life and resulted in improved SVR rates around 55% in several clinical trials.1 However, patients infected with genotype 1 HCV still show lower SVR rates, and adverse events and intolerance to the combination therapy remain a concern. Recent trials have achieved improved SVR rates of 61% to 68% and 67% to 75% in patients with HCV genotype 1 infection by combining new HCV-specific protease inhibitors, telaprevir or boceprevir.22 Although these new drugs look promising, they seems to be added to peginterferon plus ribavirin therapy, and the role of peginterferon plus ribavirin combination therapy is expected to last. Thus, a treatment strategy to improve treatment outcome and reduce adverse events is required. In this regard, pretreatment predictors of response are useful for estimating the likelihood of SVR, in identifying optimal candidates, and advising patients.
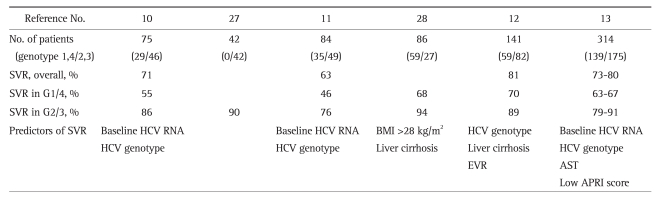
The treatment efficacy of peginterferon plus ribavirin combination therapy varies among different HCV genotypes. Several well-designed and controlled clinical trials reported SVR rates of 32% to 40.9% in genotype 1 and 65% to 96% in genotype 2/3.8,9,23 A retrospective multicenter study in the setting of routine clinical practice performed in the United States reported somewhat worse treatment outcomes; SVR rates of 20% in genotype 1, 52% in genotype 2, and 43% in genotype 3.24 An ethnic difference in treatment response to peginterferon plus ribavirin therapy has been suggested. One retrospective study of patients with CHC treated at 23 German gastroenterology practices in a routine clinical setting revealed a SVR rate of 46.5% in genotype 1- and 77.3% in genotype 2/3-infected patients.25 It has been suggested that Asian CHC patients are more likely to have SVR than Caucasians.26 In accordance with these findings, Korean CHC patients seem to have better treatment outcomes, with SVR rates of 46% to 70% in genotype 1 infection and 76% to 94% in genotype 2/3 infection.10-13,27-30 Treatment outcome from Korean population is summarized in Table 4 (data from only published article was included).
Table 4.
Summary of Previous Studies on the Treatment of Chronic Hepatitis C with Peginterferon Plus Ribavirin in Korea
SVR, sustained virological response; G1/4, genotype 1/4; G2/3, genotype 2/3; HCV, hepatitis C virus; BMI, body mass index; EVR, early virological response; AST, aspartate aminotransferase; APRI, aspartate aminotransferase platelet ratio index.
Although several studies of peginterferon plus ribavirin treatment have been conducted in Korean CHC patients, the data are still not sufficient to develop guidelines specific to Koreans. Thus, we and the KIPECT study group determined that a large confirmatory study was needed. The SVR rate in this study was 59.6% in all patients regardless of genotype, 53.6% in genotype 1/4, and 71.5% in genotype 2/3. These response rates are higher than those reported in Western CHC patients, and our findings provide evidence of better treatment outcome in Korean patients. The mechanism underlying the superior response rate in Asian patients remained unclear until recently. Recent studies have suggested that a genetic polymorphism might account for the ethnic differences in response rates. These studies reported a higher frequency of favorable interleukin 28B alleles in Asian patients with CHC, which may be a reason for better treatment outcomes observed in Asian CHC patients.31-33 A high frequency of favorable alleles and their association with SVR was also found in 118 Korean patients with CHC. The frequencies of major homozygotes (TT), heterozygotes (GT), and minor homozygotes (GG) of rs8099917 were 85%, 14%, and 1%, respectively.34 Of the 55 patients with HCV genotype 1 infection in that study, the SVR rate was 67% and 44% for the major allele and minor or hetero allele, respectively.
Prediction of SVR during treatment would allow clinicians to identify patients most likely to benefit from therapy. The viral genotype and pretreatment viral load have been consistently regarded as two major predictors of SVR. SVR rates were higher in patients infected with non-genotype 1 HCV and in those with a viral load less than 6×105 IU/mL. Other less consistently reported baseline characteristics associated with a favorable response include the dose of peginterferon and ribavirin, female gender, age younger than 40 years, non-African-American race, lower body weight, the absence of insulin resistance, elevated ALT levels, and the absence of advanced fibrosis on liver biopsy.8,9,23 In terms of viral kinetics, RVR35-37 and EVR5,38 are also helpful in predicting treatment response. Baseline HCV RNA level, HCV genotype, and liver cirrhosis also have been documented as predictors of treatment response in studies of Koran patients (Table 4). In this study, pretreatment baseline characteristics of HCV genotype 2/3 and low viral load (HCV RNA less than 6×105 IU/mL) were significant predictors of SVR and these findings were consistent with previous results. However, our finding that males had a higher SVR rate than females is some-what different from other studies, but no other studies of Korean patients with HCV infection have identified female gender as a predictor of SVR. Whether a difference in treatment efficacy of peginterferon plus ribavirin therapy between males and females actually exists is uncertain.
The achievement of EVR during treatment was strongly associated with SVR (OR, 5.297; p<0.001) in this study. Because many of the study subjects had been treated before the concept of RVR appeared, HCV RNA at 4 weeks after treatment was tested in only 193 patients (25.5%). When the analysis was performed in these subjects, the achievement of RVR was significantly associated with SVR in all patients (77.3% vs 36.1%, p<0.001) as well as in patients infected with genotype 1 HCV (67.2% vs 33.3%, p<0.001) and genotype 2/3 (89.8% vs 42.3%, p<0.001). However, because these results are based on univariate analysis, they require confirmation on multivariate analysis including other baseline clinical characteristics.
Treatment with peginterferon and ribavirin therapy was associated with many adverse events. In this study, 64.2% of subjects experienced at least one or more adverse events. As reported in other studies, flu-like symptoms were the most common (31.9%), and gastrointestinal symptoms and skin reactions were the second (25.3%) and the third (24.5%) most common. Most adverse reactions were mild in severity and easily manageable. However, 88 patients (11.6%) discontinued treatment due to common adverse events. Given that drug adherence ≥80% of the planned dose had the strongest association (OR, 5.858; 95% CI, 3.409 to 8.230; p<0.001) with SVR in this study, careful and thoughtful management of common adverse events and encouragement of patients to adhere to their treatment regimen are critical.
Our KIPECT study is the first and largest multicenter study to evaluate the efficacy and tolerability of peginterferon plus ribavirin therapy for the treatment of CHC patients in routine clinical practice in Korea. As reported in other studies, SVR rates in Korean CHC patients were higher than that in Western CHC patients. Other findings including predictors of SVR, adverse events, and discontinuation were not different from those reported in other studies.
Our study is limited due to its retrospective and observational design; however, our findings are meaningful in that they reflect the current clinical practices in Korea. The fact that RVR was only available in some patients is a shortcoming of this study, and conclusions regarding the possibility of shortening the treatment period cannot be made. In order to create a treatment strategy tailored to Korean CHC patients, further research should focus on RVR in CHC as well as the influence of genetic polymorphisms of interleukin 28B on treatment outcome. The KIPECT study is a representative study of CHC treatment in Korea, and we expect that our findings will be helpful building informing revised guidelines for the treatment of CHC in Korea.
ACKNOWLEDGEMENTS
This study was supported by the Korean Association for the Study of the Liver in 2009.
The authors would like to thank Yu Jin Kim, MD and Eun Jung Kim, RN for their help with data collection and analysis.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Ghany MG, Strader DB, Thomas DL, Seeff LB American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suh DJ, Jeong SH. Current status of hepatitis C virus infection in Korea. Intervirology. 2006;49:70–75. doi: 10.1159/000087266. [DOI] [PubMed] [Google Scholar]
- 3.Lim YS. Current status of liver disease in Korea: hepatitis C. Korean J Hepatol. 2009;15(Suppl 6):S25–S28. doi: 10.3350/kjhep.2009.15.S6.S25. [DOI] [PubMed] [Google Scholar]
- 4.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 5.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 6.Hadziyannis SJ, Sette H, Jr, Morgan TR, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–355. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 7.The Korean Association for the Study of the Liver. Practice guideline for management of hepatitis C. Korean J Hepatol. 2004;10(Suppl):S101–S125. [Google Scholar]
- 8.Rumi MG, Aghemo A, Prati GM, et al. Randomized study of peginterferon-alpha2a plus ribavirin vs peginterferon-alpha2b plus ribavirin in chronic hepatitis C. Gastroenterology. 2010;138:108–115. doi: 10.1053/j.gastro.2009.08.071. [DOI] [PubMed] [Google Scholar]
- 9.Ascione A, De Luca M, Tartaglione MT, et al. Peginterferon alfa-2a plus ribavirin is more effective than peginterferon alfa-2b plus ribavirin for treating chronic hepatitis C virus infection. Gastroenterology. 2010;138:116–122. doi: 10.1053/j.gastro.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Lee H, Choi MS, Paik SW, et al. Peginterferon alfa-2a plus ribavirin for initial treatment of chronic hepatitis C in Korea. Korean J Hepatol. 2006;12:31–40. [PubMed] [Google Scholar]
- 11.Kim KT, Han SY, Kim JH, et al. Clinical outcome of pegylated interferon and ribavirin therapy for chronic hepatitis C. Korean J Hepatol. 2008;14:36–45. doi: 10.3350/kjhep.2008.14.1.36. [DOI] [PubMed] [Google Scholar]
- 12.Kang MJ, Jung EU, Park SW, et al. Effects of pegylated interferon and ribavirin in Korean patients with chronic hepatitis C virus infection. Korean J Hepatol. 2008;14:318–330. doi: 10.3350/kjhep.2008.14.3.318. [DOI] [PubMed] [Google Scholar]
- 13.Sinn DH, Shin SR, Kil JS, et al. Efficacy of peg-interferon-α-2a plus ribavirin for patients aged 60 years and older with chronic hepatitis C in Korea. J Gastroenterol Hepatol. 2011;26:469–476. doi: 10.1111/j.1440-1746.2010.06478.x. [DOI] [PubMed] [Google Scholar]
- 14.Yu E Korean Study Group for the Pathology of Digestive Diseases. Histologic grading and staging of chronic hepatitis: on the basis of standardized guideline proposed by the Korean Study Group for the Pathology of Digestive Diseases. Korean J Hepatol. 2003;9:42–46. [PubMed] [Google Scholar]
- 15.Kim YS, Um SH, Ryu HS, et al. The prognosis of liver cirrhosis in recent years in Korea. J Korean Med Sci. 2003;18:833–841. doi: 10.3346/jkms.2003.18.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chae HB, Kim JH, Kim JK, Yim HJ. Current status of liver diseases in Korea: hepatitis B. Korean J Hepatol. 2009;15(Suppl 6):S13–S24. doi: 10.3350/kjhep.2009.15.S6.S13. [DOI] [PubMed] [Google Scholar]
- 17.Disease web statistics system for chronic hepatitis C [Internet] Korea Centers for Disease Control & Prevention. c2011. [cited 2011 Sep 28]. Available from: http://stat.cdc.go.kr.
- 18.Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36(5 Suppl 1):S35–S46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 19.Liang TJ, Rehermann B, Seeff LB, Hoofnagle JH. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med. 2000;132:296–305. doi: 10.7326/0003-4819-132-4-200002150-00008. [DOI] [PubMed] [Google Scholar]
- 20.Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463–472. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 21.Sinn DH, Paik SW, Kang P, et al. Disease progression and the risk factor analysis for chronic hepatitis C. Liver Int. 2008;28:1363–1369. doi: 10.1111/j.1478-3231.2008.01860.x. [DOI] [PubMed] [Google Scholar]
- 22.Jang JY, Chung RT. New treatments for chronic hepatitis C. Korean J Hepatol. 2010;16:263–277. doi: 10.3350/kjhep.2010.16.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McHutchison JG, Lawitz EJ, Shiffman ML, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361:580–593. doi: 10.1056/NEJMoa0808010. [DOI] [PubMed] [Google Scholar]
- 24.Backus LI, Boothroyd DB, Phillips BR, Mole LA. Predictors of response of US veterans to treatment for the hepatitis C virus. Hepatology. 2007;46:37–47. doi: 10.1002/hep.21662. [DOI] [PubMed] [Google Scholar]
- 25.Mauss S, Hueppe D, John C, et al. Estimating the likelihood of sustained virological response in chronic hepatitis C therapy. J Viral Hepat. 2011;18:e81–e90. doi: 10.1111/j.1365-2893.2010.01372.x. [DOI] [PubMed] [Google Scholar]
- 26.Missiha S, Heathcote J, Arenovich T, Khan K Canadian Pegasys Expanded Access Group. Impact of asian race on response to combination therapy with peginterferon alfa-2a and ribavirin in chronic hepatitis C. Am J Gastroenterol. 2007;102:2181–2188. doi: 10.1111/j.1572-0241.2007.01431.x. [DOI] [PubMed] [Google Scholar]
- 27.Jung EU, Park JH, Pae KI, et al. Short-term therapy with pegylated interferon plus ribavirin for the chronic hepatitis C genotype 2 patients. Korean J Hepatol. 2007;13:341–348. doi: 10.3350/kjhep.2007.13.3.341. [DOI] [PubMed] [Google Scholar]
- 28.Lee HJ, Eun JR, Choi JW, Kim KO, Moon HJ. Comparison of therapeutic results between combination therapy of peginterferon alpha-2a plus ribavirin and interferon alpha-2b plus ribavirin according to treatment duration in patients with chronic hepatitis C. Korean J Hepatol. 2008;14:46–57. doi: 10.3350/kjhep.2008.14.1.46. [DOI] [PubMed] [Google Scholar]
- 29.Lee DS, Park SY, Lee HS, et al. Outcome of combination therapy of pegylated inerferone with ribavirin in chronic hepatitis C: single center study. Korean J Hepatol. 2009;15(Suppl 3):S134. [Google Scholar]
- 30.Song YJ, Lee YJ, Choi BJ, et al. Tailored therapy for treatmentnaive chronic hepatitis C with pegylated interferon-alpha and ribavirin: real practice experience. Korean J Hepatol. 2010;16(Suppl 3):S57. [Google Scholar]
- 31.Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 32.Suppiah V, Moldovan M, Ahlenstiel G, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100–1104. doi: 10.1038/ng.447. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka Y, Nishida N, Sugiyama M, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105–1109. doi: 10.1038/ng.449. [DOI] [PubMed] [Google Scholar]
- 34.Sinn DH, Kim YJ, Lee ST, et al. Association of a single nucleotide polymorphism near the interleukin-28B gene with response to hepatitis C therapy in Asian patients. J Gastroenterol Hepatol. 2011;26:1374–1379. doi: 10.1111/j.1440-1746.2011.06744.x. [DOI] [PubMed] [Google Scholar]
- 35.Jensen DM, Morgan TR, Marcellin P, et al. Early identification of HCV genotype 1 patients responding to 24 weeks peginterferon alpha-2a (40 kd)/ribavirin therapy. Hepatology. 2006;43:954–960. doi: 10.1002/hep.21159. [DOI] [PubMed] [Google Scholar]
- 36.Ferenci P, Fried MW, Shiffman ML, et al. Predicting sustained virological responses in chronic hepatitis C patients treated with peginterferon alfa-2a (40 KD)/ribavirin. J Hepatol. 2005;43:425–433. doi: 10.1016/j.jhep.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Shiffman ML, Suter F, Bacon BR, et al. Peginterferon alfa-2a and ribavirin for 16 or 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2007;357:124–134. doi: 10.1056/NEJMoa066403. [DOI] [PubMed] [Google Scholar]
- 38.Davis GL, Wong JB, McHutchison JG, Manns MP, Harvey J, Albrecht J. Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology. 2003;38:645–652. doi: 10.1053/jhep.2003.50364. [DOI] [PubMed] [Google Scholar]