Abstract
Biomaterials that mimic the extracellular matrix in both modularity and crosslinking chemistry have the potential to recapitulate the instructive signals that ultimately control cell fate. Toward this goal, modular protein polymer-based hydrogels were created through genetic engineering and enzymatic crosslinking. Animal derived tissue transglutaminase (tTG) and recombinant human transglutaminase (hTG) enzymes were used for coupling two classes of protein polymers containing either lysine or glutamine, which have the recognition substrates for enzymatic crosslinking, evenly spaced along the protein backbone. Utilizing tTG under physiological conditions, crosslinking occurred within two minutes, as determined by particle tracking microrheology. Hydrogel composition impacted the elastic storage modulus of the gel over 4-fold and also influenced microstructure and degree of swelling, but did not appreciably effect degradation by plasmin. Mouse 3T3 and primary human fibroblasts were cultured in both 2- and 3-dimensions without a decrease in cell viability and displayed spreading in 2D. The properties of these gels, which are controlled through the specific nature of the protein polymer precursors, render these gels valuable for in situ therapies. Furthermore, the modular hydrogel composition allows tailoring of mechanical and physical properties for specific tissue engineering applications.
1. Introduction
In the last decade, biomedical hydrogel research has shifted from pre-formed implants to injectable materials that form a gel at the site of injection[1] and allow delivery by minimally invasive procedures [2]. Material precursors are delivered to the site prior to solidification, and gelation is triggered in vivo by chemical or physical changes [3]. For in situ cell-based therapies, biomaterials should be engineered to provide signals to promote tissue development and restore normal tissue function in vivo; to achieve this goal, the biomaterials must provide a three-dimensional support and interact with cells to control cellular function and guide the processes of tissue formation and regeneration [4]. Hydrogels currently used for in situ research are formed from natural or synthetic materials. While natural materials, such as collagen and fibrin, have built-in biological functionality and are nontoxic, there are several drawbacks including batch-to-batch variability, limited sources, the minimal ability to control material structure and properties, and possible pathogen transmission and immunogenicity [4]. Synthetic polymers, such as poly(ethylene glycol), poly(N-isopropylacrylamide-co-acrylic acid), and poly(lactic-co-glycolic acid), have been applied as biomaterials for tissue engineering because they address these shortcomings, most specifically allowing for design flexibility and control over material properties [5–7]. Chemically synthesized polymers, however, do not contain biological function, and may be toxic due to residual monomers and crosslinkers. In these materials, the lack of biomimicry requires the addition of bioactive peptides and degradable crosslinkers to allow for cell responsiveness. As an alternative, recombinant protein polymer-based materials may combine the advantages of both natural and synthetic materials while avoiding some of the limitations.
Genetic engineering of recombinant proteins can produce a monodisperse foundation for scaffold formation and allow facile incorporation of bioactivity. In addition, the protein monodispersity allows for an exact number of reaction sites for crosslinking. To date, the majority of purely protein polymer biomaterials have been designed to mimic the repetitive amino acid sequence of elastin [8–11] or silk [12, 13]. Although these mimics have unique properties, the dependence on secondary structure does not allow the structural flexibility of synthetic polymers. These mimetic protein polymers form physically crosslinked hydrogels that can have weak mechanical properties. Often additional chemical crosslinking schemes have been used to form more rigid and stable gels. For example, elastin-like protein polymers have been chemically crosslinked by tris-succinimidyl aminotriacetate [14], disuccinimidyl suberate [15], and hydroxymethylphosphine crosslinking chemistries [16], greatly improving gel mechanical properties. Chemical crosslinking, however, is not ideal for in situ gelation due to possible crosslinker toxicity in vivo.
Tissue transglutaminase has been investigated as an alternative to chemical crosslinking due to its mild and biocompatible reaction conditions. Tissue transglutaminase catalyzes the formation of covalent bonds between lysine and glutamine residues in a calcium dependent reaction. This crosslinking mechanism naturally occurs in vivo during the processes of wound healing and the stabilization and organization of the extracellular matrix [17, 18]. For these reasons, members of the transglutaminase family have been used in the synthesis of crosslinked hydrogels of poly(ethylene glycol) [19–23], and elastin-like protein polymers [24].
We present a new class of recombinant protein polymers to serve as a foundation for forming enzymatically crosslinked hydrogels. A family of high-molecular weight protein polymers was developed to act as substrates for enzymatic crosslinking into a hydrogel for tissue engineering applications. The recombinant proteins are macromolecular, water-soluble, and random coil. The protein polymers were designed to have evenly spaced TG substrates for crosslinking into a hydrogel with a range of viscoelastic properties. Both guinea pig and recombinant human transglutaminases were able to recognize and crosslink the protein polymers into self-supporting hydrogels. Recombinant proteins offer the ability to alter protein properties, such as molecular weight, reactive group spacing and bioactivity by redesigning the gene sequence; this allows for a modular hydrogel with tunable characteristics. Furthermore, the bottom up approach can facilitate customizable grafting of bioactive peptides on the hydrogel that can be chosen for the cell-signaling requirements of particular tissue types.
2. Materials and Methods
2.1 Protein polymer gene construction
Standard molecular biology techniques were used for gene synthesis, protein expression, and protein purification, unless otherwise noted [25]. Five monomeric genes were designed to encode amino acids for K4 (GKGASGKGA), K6 (GKGTGA), K8 (GKAGTGSA), where 4, 6, 8 referred to the frequency of lysine spacing in each sequence, and BQ a-block (GQQQLGGAGTGSA)2 (functional sequence) and BQ b-block (GAGQGEA)3 (soluble sequence) (Figure 1). The single-stranded DNA was purchased from Integrated DNA Technologies (Coralville, IA) and used to create multimers. Multimers of K4, K6, and K8 (denoted Kn) were created by self-ligation at the EarI restriction site as previously reported [26]. For BQ, blocks a and b were combined by digestion with EarI and subsequent T4 DNA ligation, and then concatermized by the controlled cloning method [27] to obtain (ab)n n=6, 9, 12, further denoted as BQn. The concatermized genes were cloned into the pET-19b (Novagen Inc., Madison, Wisconsin) as previously described [26]. Correct insertion was verified by electrophoresis of genes that were doubly digested with BamHI and NdeI restriction enzymes and through DNA sequencing (SeqWright, Houston, TX). The ligation into the expression vector pET-19 (Novagen Inc.) results in the gene of interest expressed as a fusion protein with an N-terminal histidine tag (tag amino acid sequence: GH10SSGHIDDDDKHM).
Figure 1.
DNA oligonucleotide sequences used to create repetitive genes, (A) K4 (B) K6 (C) K8 (D) BQ a-block and (E) BQ b-block. EarI restriction sites are underlined, and SapI restriction sites are in bold.
2.2 Protein Expression and Purification
Protein synthesis was induced under T7 promoter control in the expression host BLR(DE3) cells (Novagen Inc.). Briefly, a 5-ml starter culture in 2×YT Broth (Fisher Scientific, Waltham, MA) was grown from a single colony for 8 hours. This was used to inoculate an overnight 100-ml LB broth culture, both supplemented with 200 μg/ml ampicillin (Sigma, St. Louis, MO) and 12.5 μg/ml tetracycline (Fisher Scientific). One-liter cultures of Difco Terrific Broth (Fisher Scientific) supplemented with 200 μg/ml ampicillin and 12.5 μg/ml tetracycline were inoculated with 25 ml from the overnight culture. The culture was induced with 0.5 mM isopropyl thiogalactoside (IPTG) (US Biologicals, Swampscott, MA) when the OD600 was between 0.6 and 0.8. The cells were harvested after 4 hours by centrifugation (3,700 g) and the cell pellet was resuspended in 6M guanidine hydrochloride, 20 mM sodium phosphate, 500 mM NaCl, pH 7.8 buffer. The cells were lysed by three successive freeze (−80°C)-thaw (37°C) cycles followed by sonication. Cellular debris was removed by centrifugation for 40 minutes at 10,000 g at 4°C. The protein was purified with Chelating Sepharose Fast Flow nickel-charged resin (GE Healthcare, Piscataway, NJ) under denaturing conditions with imidazole-based competitive elution. The proteins eluted in the fraction with 250 mM imidazole. The fractions containing purified protein, as determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis, were dialyzed against deionized water and then lyophilized.
2.3 General Characterization
Protein molar mass was determined by matrix assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF MS) (Perseptive Biosystems Voyager Pro DE) using sinapinic acid matrix at Northwestern University’s Integrated Molecular Structure Education and Research Center. Samples were dissolved in a 50% acetonitrile solution containing 0.1% trifluoroacetic acid. Amino acid analysis was conducted by the Yale Keck Facility (Yale University, New Haven, CT).
2.4 Recombinant hTG synthesis
The pET-28 encoding His tagged human transglutaminase 2 [28] was transformed into BLR(DE3) cells. 10 ml starter cultures that were grown overnight at 37°C in LB broth, supplemented with 50 μg/ml Kanamycin and 12.5 μg/ml Tetracycline, were used to inoculate 1L flasks of Terrific broth, also supplemented with 50 μg/ml Kanamycin and 12.5 μg/ml Tetracycline. The cultures were grown at 37°C until reaching an OD of 0.6 and then induced at 25°C with 25 μM IPTG. Cultures were grown overnight and then harvested by centrifugation at 4°C. The cell pellets were resuspended in approximately 3 times their volume in lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM Imidazole, pH 8.0). Cells were lysed by sonication and the supernatant was collected by centrifugation at 34,000 g. The lysates were incubated with Ni-NTA (Valencia, CA) slurry for 1 hour at 4°C and loaded into a column where the resin was washed with approximately 20X resin volume of lysis buffer and eluted with 15 ml of elution buffer (50 mM NaH2PO4, 300 mM NaCl, 250 mM imidazole, pH 8.0).
The eluent was diluted in FPLC exchange buffer (20 mM Tris, 1 mM DTT, 1 mM EDTA, pH 7.2 with DTT freshly added), and loaded onto an AKTA FPLC (GE Healthcare). A 5ml HiTrap Q anion exchange column (GE Healthcare) was used with a gradient of 2.5 ml/minute to reach 500mM NaCl in 1 hour. 5ml fractions of the elution peak were collected and concentrated to 250 μl using an Amicon 30kD cutoff filter (Millipore, Billerica, MA). A Bradford assay was used to determine sample concentration using BSA as a standard. The concentrate was diluted for a final concentration of 100 μM enzyme in exchange buffer with 20% glycerol, flash frozen in liquid N2 and stored at −80°C.
2.5 Hydrogel Formation with tTG and hTG
The general procedure for protein polymer hydrogel formation was as follows: Solution 1: the lysine containing protein Kn was resuspended in 200 mM MOPS, 20 mM CaCl2, pH 7.65 at 10 wt% unless otherwise indicated; Solution 2: the glutamine containing protein BQn was resuspended in 2 mM EDTA pH 7.3 at 10 wt% unless otherwise indicated; Solution 3: 20 units/ml of tTG from guinea pig liver (Sigma) was dissolved in 2 mM EDTA, 20mM DTT pH 7.7. Solutions were mixed and allowed to gel by incubation at 37°C for 15 min in a humid environment. Hydrogels were also formed with recombinant human transglutaminase (hTG). hTG was diluted to 20μM in exchange buffer with 20% glycerol, and a gel was formed by the incubation of 40% Solution 1, 40% Solution 2, and 20% hTG solution at 37°C for 5 min in a humid environment.
For swelling, degradation, toxicity, and SEM experiments, the final gelation volume was composed of 37.5% Solution 1, 37.5% Solution 2, and 25% Solution 3 (tTG) with the molar ratio of reactive sites Kn:BQn of 1:1.2. For rheological experiments Solution 3 was prepared so tTG was at the appropriate molar ratio to the number of K reactive sites, depending on test conditions. For all rheological experiments, prior to the addition of solution 3, the appropriate amounts of solution 1 & 2 were vortexed together; solution 3 was added in place and mixed with the protein precursor solution by pipette.
2.6 Viscoelastic Properties
Time for gelation on the microscale was determined by particle tracking microrheology. Enzymatic gelation time was assessed with passive particle tracking microrheology by tracing the Brownian motion of tracer particles during gelation. The particle tracking system was composed of a lab-build epifluorescent videomicroscope with a Nikon TE200 inverted microscope using a 0.5″ CCD TM-6710-CL camera. Videos were collected at 10–120 frames/sec and analyzed with IDL software (Research Systems Inc., Boulder, Co) and trajectories of the particles were extracted by algorithms modified from Crocker and Weeks[29] kindly provided by Victor Breedveld from the Georgia Institute of Technology. Samples composed of 1.5 wt% K830, 1.5 wt% BQ6 and 8.5 units/ml of tTG were suspended in 15 μl gelation buffer with neutral ~ 0.5 μm fluorescent tracer particles. The mean square displacement for the particles was calculated at each time interval prior to and post enzyme addition.
Hydrogel bulk material properties were determined by oscillatory rheology. Rheological experiments were performed with a Paar Physica MCR300 Rheometer with peltier temperature control. A stainless steel cone and plate (25mm diameter, 2° angle) device was used with 250 μl final sample volume. Approximately 93.75 μl of the Kn protein and 93.75 μl of the BQn protein were mixed by vortexing before pipetting onto the device. Enzyme, in the appropriate concentration to maintain a constant molar ratio of units to lysine reactive sites, in a volume of 62.5 μl was then added directly to the solution on the rheometer platform. Sample mixing was achieved by carefully pipetting up and down to ensure no air bubble formation. A humid chamber was achieved by placing a layer of wet kimwipes around the platform and placing a chamber cover on top. For frequency and strain sweep experiments, the cone and plate was lowered to the measuring height and held for 1 h with a temperature of 37°C using a temperature controlled plate. For time measurements, oscillatory tests began directly after sample mixing. Storage and loss modulus measurements during frequency sweeps were completed in the oscillator mode at 1 % strain, and strain sweep experiments were performed with a strain ranging from 1% to 100% at an angular frequency of 10 s−1.
2.7 Scanning electron microscopy
Hydrogel morphology was determined by variable pressure scanning electron microscopy (VP-SEM) and traditional scanning electron microscopy (SEM). The gels were composed of 10 μl of both protein solutions (Solution 1 & 2) and 6.8 μl of Solution 3. The solution was mixed by vortexing and then dropped onto a slide treated with SigmaCote (Sigma) that created a hydrophobic glass slide surface to prevent solution spreading. After gelation at 37°C for 30 min, hydrogels were placed in ddH2O for 24 h to allow complete swelling. For traditional SEM, swollen gels were dehydrated at room temperature in ethanol solutions of increasing concentrations (10, 20, 40, 60, 80, 90, 100% ethanol). For each ethanol concentration, gels were incubated in the solution two times for 10 min each. The samples were critical point dried, mounted, and sputter-coated with 9 nm of Pb/Au. VP-SEM samples were stored in ddH2O prior to imaging. All SEM imaging was done using a Hitachi S3400-N VP-SEM with an accelerating voltage of 10–20 kV, working distance of 5 mm, SE or BSE detector, and pressure of 50 Pa in VP mode.
2.8 Swelling and Degradation
Hydrogels were cast into disks for swelling and degradation experiments. Approximately, 20 μl of both protein solutions (Solution 1 &2) were mixed by vortexing and then dropped onto a hydrophobic slide. Enzyme solution was added and mixed (13.3 μl Solution 3) by pipetting solution up and down. Spacers of 2 mm thickness were used to cast gels of uniform depth. A second hydrophobic glass slide was placed on top of the spacers and the top and bottom slide were clamped with clips. The precursor solution spread to form a disk of uniform thickness. Disks were approximately 2mm think with a diameter of ca. 5.4 mm. The solution was then allowed to gel in a humid environment for 30 minutes. After gelation, the hydrogel was gently removed from the slide and placed into a 1.5 ml eppendorf with buffer for subsequent experiments.
Swelling experiments were conducted by incubating the gels in 1.5 ml of ddH2O or PBS for up to 48 h. Swollen gels were removed from solution and the surface was slightly blotted dry before weighing to determine wet mass, MW. After salt removal no observable change in wet mass was detected in PBS vs. ddH2O (data not shown), for this reason, swelling is reported in water. The gels were weighed after 6, 24, and 48 h; after 24 h, no change in mass was observed, thus all swelling data is reported after 24 h incubations in water. Hydrogel dry weight, MD, was determined by gel lyophilization and then weighing. All mass determinations were done in triplicate on a high precision balance. The degree of swelling was calculated by (MW−MD)/MD.
The recombinant protein polymer hydrogels were characterized in terms of their biochemical degradability by plasmin (Sigma). Hydrogels were prepared as disks described above. After gelation for 30 min at 37°C, hydrogels were allowed to swell in 20 mM Tris, 150 mM NaCl, pH 7.6 for 24 hours. Initial wet weight was determined after rinsing the gel 3 times in ddH2O to remove residual salt. The hydrogel initial dry weight, MD0, was measured by lyophilizing the gel. The lyophilized hydrogels were allowed to swell and equilibrate in buffer for 24 h. The buffer was removed and 1 ml of plasmin solution (0.3 units/ml 20 mM Tris, 150 mM NaCl pH 7.6) was added to the samples. At each time point, plasmin solution was replaced with ddH2O and washed 3 times, and hydrogel wet mass was determined by weighing the gel (surfaces were blotted with a kimwipe to remove excess water). Two additional changes in water were done to remove residual salt, plasmin, and degraded protein polymer. Samples remained in water for 6 hours before being lyophilized. After lyophilization, sample dry weight was determined by measuring, MD. The mass loss was determined by MD−MD0/MD0.
2.9 Cell Culture and Viability Assays
NIH3T3 fibroblasts (ATCC, Manassas, VA) were cultured at 37 °C and 5% CO2 in DMEM (Gibco) supplemented with 1 % sodium pyruvate, 1% penicillin-streptomycin, 1.5 g/L NaHCO3, and 10% fetal bovine serum (Gibco). Cells were grown to approximately 75% confluence and removed with 0.05% Trypsin-EDTA in Hanks’ Balanced Salt Solution with phenol red (Fisher Scientific). Normal primary human dermal fibroblasts (kindly provided by Lifeline Cell Technology, Walkersville, MD) were cultured in Fibrolife S2 cell culture medium (Lifeline Cell Technology) supplemented 1% penicillin-streptomycin. At approximately 75% confluence, cells were removed with 0.05% Trypsin, 0.02% EDTA (Lifeline Cell Technology) and an equal volume of Trypsin Neutralizing solution (Lifeline Cell Technology) was added once cells lifted from the culture dish. Both fibroblast cell lines were grown on the surface of the protein polymer hydrogels, formed with 15 μl of Solution 1 and 15 μl of Solution 2 and 10 μl of Solution 3, and placed on a cell culture dish with a coverslip bottom. The gel was incubated at 37°C for 15 min; cells were then seeded on top at a density 1 × 105 cells/ml. Primary human dermal and NIH3T3 fibroblasts were encapsulated in the hydrogel as follows: primary dermal cells or NIH3T3 cells were trypsinized and gently centrifuged at 2,000 rpm for 3 min, media was removed from the cell pellet and the cells were resuspended in 15 μl of Solution 1 and 15 μl of Solution 2, resulting in approximately 1 × 105 cells/ml. After gentle mixing by pipetting, the suspension was added to the well of a cell culture dish with a coverslip bottom. Approximately 10 μl of Solution 3 with tTG was added and mixed by pipetting; the solution was incubated at 10 min at 37°C for gelation. Cell culture media was added and cells were incubated for 24 h before the assay. The viability of cells both on and encapsulated in the hydrogel, were determined using a LIVE/DEAD Viability/Cytotoxicity Kit (Molecular Probes, Invitrogen, Carlsbad, CA) which determines membrane integrity of the cells. Approximately, 20 μl of LIVE/DEAD reagent (4μM EthD-1, 2 μM calcein AM) was added directly to the cell culture media and allowed to react in a dark environment for 1 h. Confocal microscopy was used to visualize the fluorescently stained cells to characterize cell viability and morphology.
3. Results
3.1 Precursor Design
Recently, we reported on a modular class of unstructured protein polymers (Kn) that could serve as a lysine substrate for tissue transglutaminase to create multivalent scaffolds [26]. In the present study we combine these protein polymers with a class of random coil glutamine containing proteins for crosslinking into hydrogels by transglutaminase. Tissue transglutaminase catalyzes an acyl-transfer reaction between the γ-carboxamide group of protein-bound glutaminyl residues and the ε-amino group of lysine residues, resulting in the formation of ε-(γ-glutamyl)lysine isopeptide side-chain bridges [30]. Previous results indicated that although Kn does not contain the preferred lysine substrate for tTG (FKG) [20], it still serves as an amine group for enzymatic bond formation [26]. A completely protein polymer-based hydrogel was created using the lysine and glutamine containing proteins. The glutamine protein sequence [(GQQQLGGAGTGSA)2(GAGQGEA)3]nG contained a block with a glutamine substrate for tTG based on previous work by Hu and Messersmith on optimized transglutaminase substrates[20] and a second block included for water solubility. We refer to this block copolymer as BQn with n indicating the number of repeats. Although the water soluble block also contained a glutamine residue, it was hypothesized that it would have marginal tTG recognition due to the enzyme’s higher specificity to the glutamine substrate [20].
3.2 Hydrogel Formation
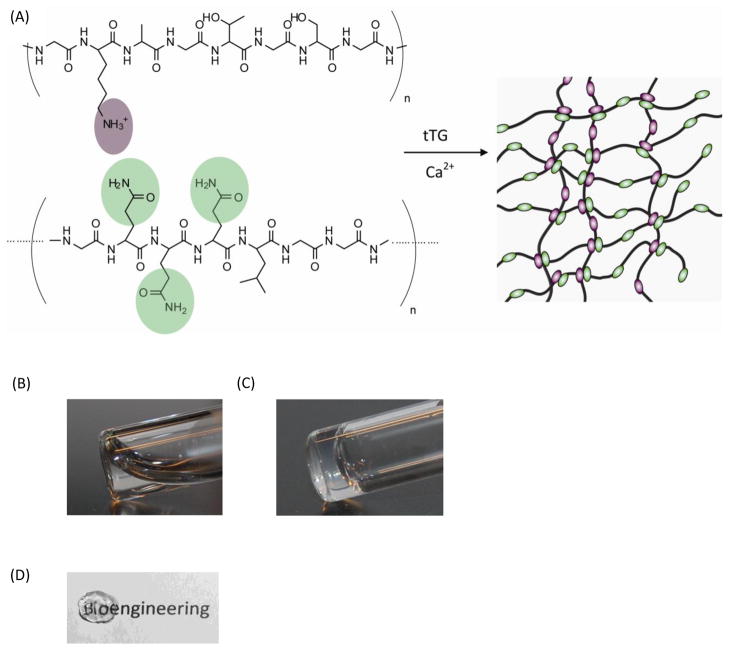
The optimal gelation conditions of substrate and enzyme concentrations were determined by visual observation and sample inversion. Precursor solutions of protein polymer were slightly turbid due to limited protein polymer solubility. However, the proteins appeared uniformly suspended at the maximum concentration investigated, 10 wt% Kn and 10 wt% BQn in the precursor solutions. After enzyme addition and incubation at 37°C, a solid gel was formed and was stable at room temperature in aqueous buffer (Figure 2A). Furthermore, recombinant human transglutaminase was used as an alternative to commercially available guinea pig tTG to form a protein polymer hydrogel. For clinical applications, allogeneic hTG is an ideal alternative to xenogeneic derived enzymes for a potentially decreased immune response in vivo. At the same substrate concentrations, the addition of hTG resulted in hydrogels that were similar in stability to those formed by tTG (Figure 2B).
Figure 2.
(A) Schematic of the protein polymer hydrogel formation by tissue transglutaminase enzymatic crosslinking. (B&C) Photograph of the precursor solution before and after enzymatic gelation with tTG. (D) Photograph of a transparent hydrogel crosslinked by hTG. Gels were composed of K830/BQ6 (8 wt% protein polymers and 0.26 units of tTG or 20μM hTG).
3.3 Viscoelastic Properties of Crosslinked Hydrogels
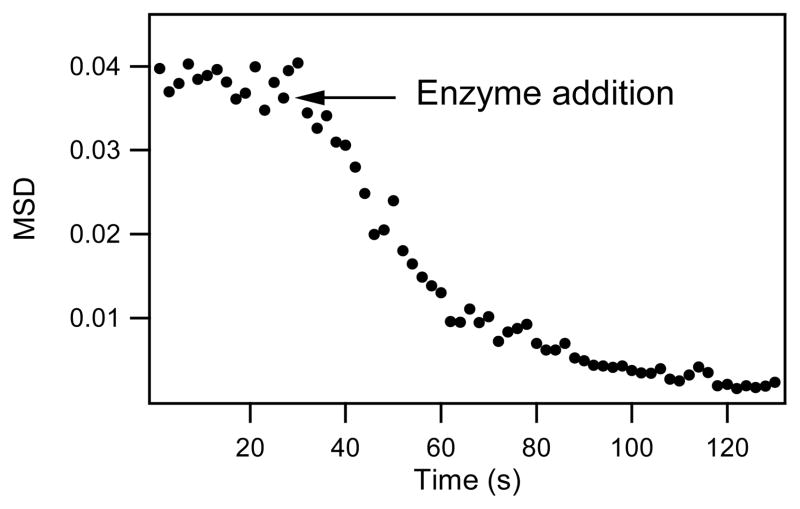
Microrheology, using videomicroscopy [29] and the multiple-particle tracking technique,[31, 32] was used to obtain the MSD-average of the tracer particles as a function of time from the particles’ trajectories. In viscous liquids, particles diffuse freely and the MSD versus time has a slope of 1 in a log-log plot [31]. The particle MSD in an elastic gel, however, is limited, resulting in a slope of zero. It was found that protein polymers in solution have tracer particles with a diffusive behavior (MSD of ca. 0.04 μm2); upon the addition of enzyme, the displacement rapidly decreased and approached zero, indicating the formation of a hydrogel on the micron scale (Figure 3). At low concentration of protein polymer (3 wt% total), roughly 90% of gelation occurred within the first 30 s after enzyme addition. After 1.5 minutes, the MSD of the tracer particles reached steady state at a MSD of ca. 0.0025 μm2, indicative of an elastic solid. Experiments conducted with higher protein concentration rapidly gelled and did not allow sufficient time for data collection.
Figure 3.
Mean-square-displacement as a function of lag time for tracer particles in protein polymer solution (1.5 wt% K830, 1.5 wt% BQ6) during gelation. tTG enzyme (8.5 units/ml) was added at 30 s.
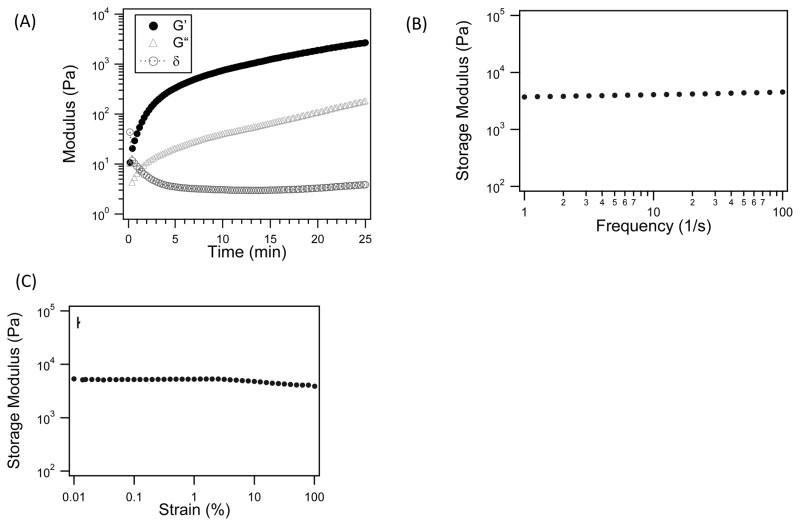
Moreover, the rapid gelation was confirmed by bulk rheological experiments using oscillatory rheometry. The precursor solution was placed on a rheometer plate at 37°C; the cone was lowered immediately after enzyme addition. Within 30 s, the hydrogel composed of K830 and BQ6 had a storage modulus (G′) two-orders of magnitude above the loss modulus (G′), and the phase angle (δ) was near zero, indicating rapid gelation (Figure 4). After, both a frequency and a strain sweep were completed and both sweeps were essentially flat with respect to the storage modulus, indicating the hydrogel behaved as a robust elastic solid. The strain sweep exhibited a small decrease of storage modulus at strains above 10% strain, suggesting damage to the gel structure. Further rheological measurements were conducted within the linear viscoelastic region where the storage modulus was independent of the applied strain.
Figure 4.
Rheological data collected during hydrogel enzymatic gelation. (A) The phase angle (δ), storage (G′) and loss (G″) moduli are plotted as a function of time. The storage moduli collected during a frequency oscillation sweep (B) and a strain sweep (C). Hydrogel were composed of 4 wt% K830, 4 wt% BQ6, and 4 unit/ml of tTG
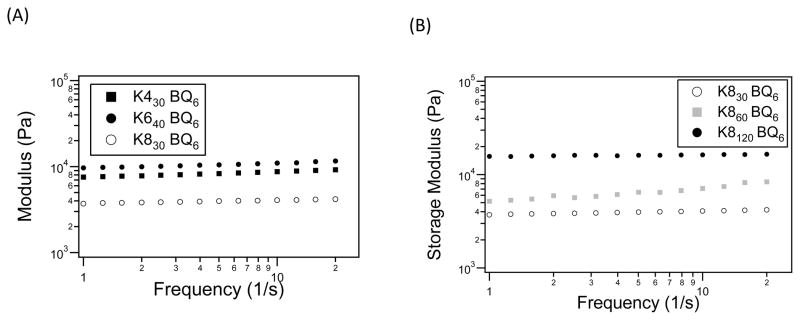
The storage modulus was investigated as a function of gel composition, where the effect of crosslink density was studied by varying the reactive group spacing in the lysine-containing substrate. At the same molar ratios and similar molecular weights, the storage modulus increased with closer spaced lysine groups (Figure 5A). Furthermore, changes in the molecular weight of the lysine precursors resulted in storage moduli that ranged from 4–16 kPa (Figure 5B). This data suggests that the magnitude of the viscoelastic behavior of the polymer network was determined by the molecular weight of the flexible protein polymers and substrate spacing. The nearly flat curves in Figure 5 indicate that the networks were very stable when exposed to a wide range of perturbation frequencies. The samples exhibited a plateau in the lower frequency range, 1–10 s−1, suggesting a stable crosslinked network. Furthermore, the storage modulus generally showed a slight increase with frequency, indicating that the hydrogels have a robust mechanical-damping ability.
Figure 5.
Hydrogel storage modulus after a 1 h gelation by tTG as a function of (A) lysine substrate spacing and (B) protein molecular weight.
3.4 Hydrogel Morphology
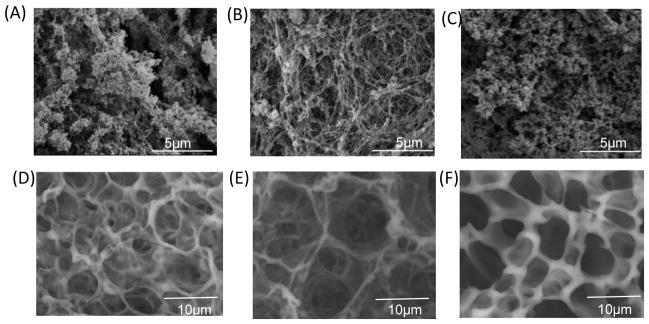
SEM micrographs of critical point dried gels suggested a morphology dependence on hydrogel composition (Figure 6). Dehydrated hydrogels composed of K830 and BQ6 had an irregular void space structure, with voids creating macropores ranging from less than 1 μm up to 5 μm. In contrast, the K860 and BQ6 hydrogel appeared as a woven network with more uniform macropores of an average of 2 μm diameter. The largest molecular weight protein gel, K8120 and BQ12 formed a dense structure composed of macropores less than 1 μm. During sample preparation, hydrogel volume decreased as ethanol concentration increased (data not shown), suggesting dehydration caused some collapse of the network. For this reason, variable pressure SEM was used to visualize unprocessed hydrated hydrogel networks. In variable pressure SEM, the sample is at higher-pressures than traditional SEM. The higher chamber pressures minimize outgassing from volatile samples, and charging is diminished on nonconductive samples due to a controlled amount of water vapor in the chamber. This permitted direct imaging of insulating materials without the need for a conductive surface coating. Variable pressure SEM gives an estimate of hydrogel structure in the natural hydrated state. Hydrated protein polymer hydrogels showed larger void spaces (Figure 6B). Both K830/BQ6 and K8120/BQ12 showed similar morphology; whereas, K860/BQ6 exhibited a highly interconnected porous woven network with macropores ranging from 3 to 7 μm. The increase in pore size of hydrated samples confirms the network collapse that occurs during ethanol dehydration used for traditional SEM.
Figure 6.
SEM microscopy images of hydrogels formed by (A) 4 wt% K830, 4 wt% BQ6, 4 unit/ml of tTG, (B) 4 wt% K860, 4 wt% BQ6, 4 unit/ml of tTG, (C) 4 wt% K8120, 4 wt% BQ12, 5.6 unit/ml of tTG. Environmental VP-SEM microscopy images of hydrogels formed by (D) 4 wt% K830, 4 wt% BQ6, 4 unit/ml of tTG, (E) 4 wt% K860, 4 wt% BQ6, 4 unit/ml of tTG, (F) 4 wt% K8120, 4 wt% BQ12, 5.6 unit/ml of tTG.
3.5 Hydrogel swelling and protease degradation
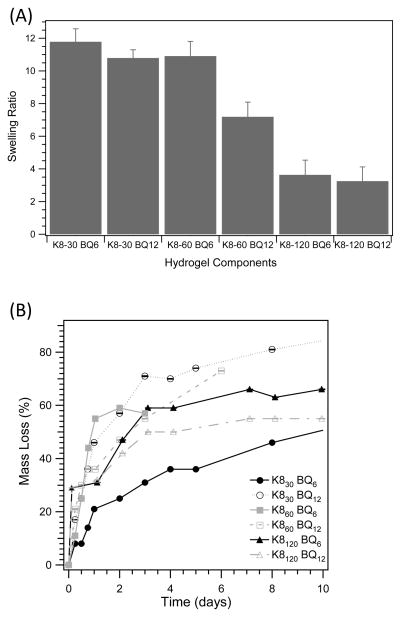
Equilibrium swelling studies suggested that swelling ratio was a function of hydrogel precursor components. Protein polymer substrates with smaller molecular weights had increased swelling over larger protein polymers at a similar substrate and enzyme ratio (Figure 7). With all Kn proteins, as the molecular weight of the BQn precursor increased the swelling ratio decreased; this trend was more pronounced for the hydrogel composed of K860. The largest Kn protein, K8120, had drastically decreased swelling in comparison to the other formulations, only approximately 3.5 times initial hydrogel mass, suggesting structural differences in the hydrogel due to crosslink density.
Figure 7.
(A) Swelling ratio as a function of hydrogel precursor components and (B) hydrogel mass loss by plasmin proteolytic degradation. Gel final composition was 4 wt% of each protein polymer and the tTG enzyme concentration was at a constant molar ratio to the number of K crosslinking sites.
The differences in swelling ratios were partially explained by hydrogel morphology as determined by traditional and variable pressure SEM images. The hydrogels with the highest swelling ratio, composed of K830/BQ6 and K860/BQ6, had the largest macropores as determined by traditional SEM. In agreement, the K8120 hydrogels that had the smallest macropores displayed the lowest swelling ratio. These data suggest solvation was related to the hydrogel pore size and morphology. Hydrated SEM partially supports these conclusions. For the K860-BQ6 hydrogel, VP-SEM indicated a morphology with larger void spaces consistent with increased swelling ability. However, both the lowest and highest molecular weight gels had similar morphology by hydrated SEM but a markedly different swelling ratio. This may indicate that morphology determined by VP-SEM is not a precise method to explain solvation effects.
The degradation of the protein polymer hydrogels was investigated by proteolytic remodeling by plasmin. The plasmin protease is a mode of matrix remodeling during cell migration and wound healing [33]. In solution, the Kn proteins were shown to be degraded by plasmin cleaving at the lysine residues within 24 h [26]. Protein polymer hydrogels, however, had much slower degradation that was dependent on gel composition (Figure 7B). All gel formulations showed a rapid initial mass loss of between 20 to 45% in the first 24 h. After 4 days, the rate of degradation significantly decreased to less than 15% mass loss for up to two weeks. The degradation of K860 samples was only conducted over 1 week due to difficulties with sample handling, since the gel lost structural integrity with increased mass loss. The K8120 samples were more manageable, possibly due to the increased crosslink density and lower swelling ratio. The swelling behavior of the protein polymer hydrogels was monitored during proteolytic degradation. Previous research suggested that as the network degrades, the hydrogel swells to a larger extent due to the decrease in crosslinking density [34]. This was supported for hydrogels created from K8120 proteins, indicating a predominance of bulk degradation. Interestingly, gels created by K830 and K860 proteins had an unexplained initial decrease in the swelling ratio during the first 12 h (data not shown).
3.6 Cell Viability and Morphology in two- and three-dimensions
Mouse 3T3 fibroblasts and primary human dermal fibroblasts were either cultured on top or encapsulated in the enzymatically crosslinked hydrogels. After 24 h, viability and membrane integrity was determined by the LIVE/DEAD viability/cytotoxicity assay, where viable cells fluoresce green through the reaction of calcein AM with intracellular esterases and non-viable cells fluoresce red due to the diffusion of ethidium homodimer into cells with damaged membranes. Fluorescent confocal microscopy images of cells stained with the LIVE/DEAD reagent show 3T3 and primary human dermal fibroblast cells tolerated both 2D culture and 3D encapsulation in the hydrogels shown by > 95% viability (Figure 8). In 2D, both cell types had elongated morphology indicating cell adhesion and spreading (Figure 8A, B). However, cells encapsulated within the hydrogels displayed a rounded morphology and no visible spreading. Furthermore, the cells appeared evenly distributed throughout the gel, indicating the encapsulation procedure and gelation time was adequate for creating a homogeneous cell suspension.
Figure 8.
Fluorescence micrographs of live (green) and dead (red) NIH3T3 fibroblasts cultured in (A) 2D and (C) 3D, and human primary dermal fibroblasts cultured in (B) 2D and (D) 3D. Hydrogel was composed of 4 wt% K830, 4 wt% BQ6, 4 unit/ml of tTG. Image magnification at 10x (A&B) or 20x (C&D).
4. Discussion
Although biomaterials can be designed to contain appropriate bioactive signals, there remains a need to make adaptable materials that can recapitulate natural cellular processes while maintaining control of physical and mechanical properties [4]. One approach is to use synthetic polymers, however they can have uncontrolled heterogeneity at differing length scales (molecular weights) that can effect mechanical properties and cellular functions. Protein polymer-based materials, however, can be synthesized with well defined function and structure.
Toward the development of a well-defined modular biomimetic material for tissue engineering, the goal of this study was to develop a biosynthetic unstructured recombinant protein polymer-based material that could be enzymatically crosslinked into a robust hydrogel. While the use of recombinant proteins as biomaterials has had some success, many of these systems were based on self-association by secondary structures which require specific amino acid sequences within the protein polymer main chain. Previous work on unstructured protein polymers has been limited; random coil proteins have predominately been used as either a flexible spacer sequence [35] or for the introduction of bioactivity [36, 37]. The advantages of unstructured protein polymers are that they better mimic the flexibility of synthetic polymers and can be designed with no sequence requirements. The challenges, however, are to design de novo protein sequences that are able to be expressed by E. coli, are soluble in aqueous solutions, maintain activity and have controllable material properties. To date, there has been no completely random coil-based hydrogel crosslinked either by chemically or enzymatically chemistries.
Tissue transgluatminase was employed as a crosslinking strategy to create proteolytic resistant bonds between protein polymers that contained evenly spaced reactive sites. Enzymatic crosslinking is a mild strategy that provides a safe environment for cell encapsulation and cell delivery. The enzymatically crosslinked protein polymer hydrogels displayed not only rapid gelation but also different material properties based on formulation The mechanical properties of a biomaterial can have a profound impact on cell and tissue behavior, affecting cell spreading, migration, proliferation, differentiation and the rate of tissue regeneration [38, 39]. The protein polymer hydrogels have controllable mechanical properties that can be tuned to control cellular fate. The viscoelastic properties ranged over a 4-fold increase in storage modulus dependent on protein gel precursors. Interestingly, the enzymatically crosslinked random coil protein polymers presented herein, have a greater modulus compared to recombinant elastin-like proteins that are self-assembled and further crosslinked by tTG [24].
Additional physical properties of hydrogels can also influence cell behavior. The morphology within the hydrogel microenvironment is important for controlling cell migration. Hydrogel pore size directly influences the strategy cells use to migrate through the material; when pore sizes are comparable to cell size, cells utilize amoeboid migration. In tighter gel networks, cells are required to use proteolytic strategies to first degrade the surrounding matrix to allow migration [4]. The protein polymer hydrogel morphology indicated a network with micron macropores, suggesting the need for cell protease secretion to migrate through the material.
The architecture and mechanical properties of the protein polymer hydrogels were closely related to each other and were functions of the molecular weight of the protein precursors. Hydrogels composed of the higher molecular weight protein polymers, with the greatest storage moduli and crosslinking density, had a limited ability to expand in aqueous solution. In addition, the macropore size of the gels influenced swelling ability, where gels with the largest pores had the highest swelling ratio. However, both gels made of K830 and K860 had similar swelling ratios and storage moduli, yet the morphology observed from VP-SEM suggested structural differences. These observations indicate that the mechanical properties are a function of the hydrogel microstructure which dictates material salvation. Further exploration in the ability to fine tune physical and mechanical properties in these systems is ongoing.
Cell material interaction was investigated by examining cell viability and spreading in both 2- and 3-dimensions. As anticipated due to the naturally derived material and mild crosslinking conditions, both primary dermal and murine fibroblasts displayed high viability both seeded onto and encapsulated into the protein polymer hydrogel. In 2 dimensions, both cell types displayed elongated expansion; however in 3D there was virtually no cell spreading. The ability for cells to form adhesions and elongate in 3D could be improved by the addition of cell adhesive sequences and increased proteolytic degradation sites.
The incorporation of secondary modules within the hydrogels is important to recapitulate the complexity of the natural extracellular matrix. The protein polymer hydrogels described herein, have the potential to create well-defined synthetic ECM by their modularity in both physical and bioactivity properties. Using the biosynthetic strategy presented herein, recombinant proteins can be engineered to have functional groups within the protein sequence to direct cell behavior by controlling cell adhesion, spreading, and the degradation of the network. Future research is ongoing to place functional groups within the protein polymer hydrogels and explore the impact on cell behavior for a variety of applications. An additional advantage of this system is that biofunctional moieties can be covalently attached as pendant groups by either chemical or enzymatic conjugation. Previously, as a proof-of-concept, we have shown the ability to conjugate a pedant cell adhesion RGD sequence onto the protein polymer backbone to introduce bioactivity [26]. For RGD, ligand presentation is important for activity and function [40], [41] where the placement of biofunctional groups as projections from a polymeric backbone can increase activity over the amino acid sequence included in the main chain. The ability to precisely design the location (pendant vs. main chain), number, and type of bioactive sequences within the protein polymer hydrogels is an essential factor in creating a versatile, well-defined synthetic ECM material.
5. Conclusions
We have created a class of protein polymer hydrogels that have properties easily modifiable by altering the polymer amino acid sequences and molecular weights. The unstructured proteins have the ability to form gels exclusively by enzymatic crosslinking. These hydrogels have increased design flexibility due to the independence of amino acid sequence, unlike self-assembling protein polymer-based hydrogels. Furthermore, the enzymatically crosslinked protein polymer hydrogels can be used to incorporate bioactivity, by the inclusion of biological sequences within the polymer or as attached pendant groups. The ability to create a well-defined synthetic extracellular niche with controlled material properties and functionality can be advantageous to create adaptive materials based on application requirements.
Acknowledgments
We thankfully acknowledge Victor Breedveld for modified code to analyze transient mircrorheology data. We thank Karen Havenstrite and Helen Blau at Stanford University for the use of their microscopy equipment. In addition, we thank Lydia Joubert at the Cell Sciences Imagining Facility at Stanford University, for assistance with electron microscopy. This work was funded by NIH/NIBIB Grant 1 R01EB003806.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Van Tomme SR, Storm G, Hennink WE. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int J Pharm. 2008;355:1–18. doi: 10.1016/j.ijpharm.2008.01.057. [DOI] [PubMed] [Google Scholar]
- 2.Hubbell JA. In situ material transformations in tissue engineering. MRS Bull. 1996;21:33–5. [Google Scholar]
- 3.Ruel-Gariepy E, Leroux JC. In situ-forming hydrogels - review of temperature-sensitive systems. Eur J Pharm Biopharm. 2004;58:409–26. doi: 10.1016/j.ejpb.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 5.Ifkovits JL, Burdick JA. Review: Photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. 2007;13:2369–85. doi: 10.1089/ten.2007.0093. [DOI] [PubMed] [Google Scholar]
- 6.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–51. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 7.Ratner BD, Bryant SJ. Biomaterials: Where we have been and where we are going. Annu Rev Biomed Eng. 2004;6:41–75. doi: 10.1146/annurev.bioeng.6.040803.140027. [DOI] [PubMed] [Google Scholar]
- 8.Wright ER, Conticello VP. Self-assembly of block copolymers derived from elastin-mimetic polypeptide sequences. Adv Drug Deliver Rev. 2002;54:1057–73. doi: 10.1016/s0169-409x(02)00059-5. [DOI] [PubMed] [Google Scholar]
- 9.Panitch A, Yamaoka T, Fournier MJ, Mason TL, Tirrell DA. Design and biosynthesis of elastin-like artificial extracellular matrix proteins containing periodically spaced fibronectin CS5 domains. Macromolecules. 1999;32:1701–3. [Google Scholar]
- 10.Meyer DE, Chilkoti A. Genetically encoded synthesis of protein-based polymers with precisely specified molecular weight and sequence by recursive directional ligation: examples from the elastin-like polypeptide system. Biomacromolecules. 2002;3:357–67. doi: 10.1021/bm015630n. [DOI] [PubMed] [Google Scholar]
- 11.Nagapudi K, Brinkman WT, Leisen J, Thomas BS, Wright ER, Haller C, et al. Protein-based thermoplastic elastomers. Macromolecules. 2005;38:345–54. [Google Scholar]
- 12.Asakura T, Nitta K, Yang MY, Yao JM, Nakazawa Y, Kaplan DL. Synthesis and characterization of chimeric silkworm silk. Biomacromolecules. 2003;4:815–20. doi: 10.1021/bm034020f. [DOI] [PubMed] [Google Scholar]
- 13.Prince JT, McGrath KP, Digirolamo CM, Kaplan DL. Construction, cloning, and expression of synthetic genes encoding spider dragline silk. Biochemistry. 1995;34:10879–85. doi: 10.1021/bi00034a022. [DOI] [PubMed] [Google Scholar]
- 14.Trabbic-Carlson K, Setton LA, Chilkoti A. Swelling and mechanical behaviors of chemically cross-linked hydrogels of elastin-like polypeptides. Biomacromolecules. 2003;4:572–80. doi: 10.1021/bm025671z. [DOI] [PubMed] [Google Scholar]
- 15.Straley KS, Heilshorn SC. Independent tuning of multiple biomaterial properties using protein engineering. Soft Matter. 2009;5:114–24. [Google Scholar]
- 16.Nettles DL, Kitaoka K, Hanson NA, Flahiff CM, Mata BA, Hsu EW, et al. In situ crosslinking elastin-like polypeptide gels for application to articular cartilage repair in a goat osteochondral defect model. Tissue Eng Part A. 2008;14:1133–40. doi: 10.1089/ten.tea.2007.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenberg CS, Birckbichler PJ, Rice RH. Transglutaminases - multifunctional cross-linking enzymes that stabilize tissues. Faseb J. 1991;5:3071–7. doi: 10.1096/fasebj.5.15.1683845. [DOI] [PubMed] [Google Scholar]
- 18.Raghunath M, Hopfner B, Aeschlimann D, Luthi U, Meuli M, Altermatt S, et al. Cross-linking of the dermo-epidermal junction of skin regenerating from keratinocyte autografts - anchoring fibrils are a target for tissue transglutaminase. J Clin Invest. 1996;98:1174–84. doi: 10.1172/JCI118901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sperinde JJ, Griffith LG. Synthesis and characterization of enzymatically-cross-linked poly(ethylene glycol) hydrogels. Macromolecules. 1997;30:5255–64. [Google Scholar]
- 20.Hu BH, Messersmith PB. Rational design of transglutaminase substrate peptides for rapid enzymatic formation of hydrogels. J Am Chem Soc. 2003;125:14298–9. doi: 10.1021/ja038593b. [DOI] [PubMed] [Google Scholar]
- 21.Ehrbar M, Rizzi SC, Hlushchuk R, Djonov V, Zisch AH, Hubbell JA, et al. Enzymatic formation of modular cell-instructive fibrin analogs for tissue engineering. Biomaterials. 2007;28:3856–66. doi: 10.1016/j.biomaterials.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 22.Ehrbar M, Rizzi SC, Schoenmakers RG, San Miguel B, Hubbell JA, Weber FE, et al. Biomolecular hydrogels formed and degraded via site-specific enzymatic reactions. Biomacromolecules. 2007;8:3000–7. doi: 10.1021/bm070228f. [DOI] [PubMed] [Google Scholar]
- 23.Sanborn TJ, Messersmith PB, Barron AE. In situ crosslinking of a biomimetic peptide-PEG hydrogel via thermally triggered activation of factor XIII. Biomaterials. 2002;23:2703–10. doi: 10.1016/s0142-9612(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 24.McHale MK, Setton LA, Chilkoti A. Synthesis and in vitro evaluation of enzymatically cross-linked elastin-like polypeptide gels for cartilaginous tissue repair. Tissue Eng. 2005;11:1768–79. doi: 10.1089/ten.2005.11.1768. [DOI] [PubMed] [Google Scholar]
- 25.Current protocols in molecular biology. Hoboken, N.J: John Wiley & Sons; 1998. [Google Scholar]
- 26.Davis NE, Karfeld-Sulzer LS, Ding S, Barron AE. Synthesis and characterization of a new class of cationic protein polymers for multivalent display and biomaterial applications. Biomacromolecules. 2009;10:1125–34. doi: 10.1021/bm801348g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Won JI, Barron AE. A new cloning method for the preparation of long repetitive polypeptides without a sequence requirement. Macromolecules. 2002;35:8281–7. [Google Scholar]
- 28.Piper JL, Gray GM, Khosla C. High selectivity of human tissue transglutaminase for immunoactive gliadin peptides: Implications for Celiac Sprue. Biochemistry. 2002;41:386–93. doi: 10.1021/bi011715x. [DOI] [PubMed] [Google Scholar]
- 29.Crocker JC, Grier DG. Methods of digital video microscopy for colloidal studies. J Colloid Interface Sci. 1996;179:298–310. [Google Scholar]
- 30.Lorand L, Conrad SM. Transglutaminases. Mol Cell Biochem. 1984;58:9–35. doi: 10.1007/BF00240602. [DOI] [PubMed] [Google Scholar]
- 31.Mason TG, Weitz DA. Optical measurements of frequency-dependent linear viscoelastic moduli of complex fluids. Phys Rev Lett. 1995;74:1250–3. doi: 10.1103/PhysRevLett.74.1250. [DOI] [PubMed] [Google Scholar]
- 32.MacKintosh FC, Schmidt CF. Microrheology. Curr Opin Colloid Interface Sci. 1999;4:300–7. [Google Scholar]
- 33.Pittman RN, Ivins JK, Buettner HM. Neuronal plasminogen activators - cell-surface binding-sites and involvement in neurite outgrowth. J Neurosci. 1989;9:4269–86. doi: 10.1523/JNEUROSCI.09-12-04269.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anseth KS, Bowman CN, Brannon-Peppas L. Mechanical properties of hydrogels and their experimental determination. Biomaterials. 1996;17:1647–57. doi: 10.1016/0142-9612(96)87644-7. [DOI] [PubMed] [Google Scholar]
- 35.Petka WA, Harden JL, McGrath KP, Wirtz D, Tirrell DA. Reversible hydrogels from self-assembling artificial proteins. Science. 1998;281:389–92. doi: 10.1126/science.281.5375.389. [DOI] [PubMed] [Google Scholar]
- 36.Halstenberg S, Panitch A, Rizzi S, Hall H, Hubbell JA. Biologically engineered protein-graft-poly(ethylene glycol) hydrogels: A cell adhesive and plasm in-degradable biosynthetic material for tissue repair. Biomacromolecules. 2002;3:710–23. doi: 10.1021/bm015629o. [DOI] [PubMed] [Google Scholar]
- 37.Rizzi SC, Ehrbar M, Halstenberg S, Raeber GP, Schmoekel HG, Hagenmuller H, et al. Recombinant protein-co-PEG networks as cell-adhesive and proteolytically degradable hydrogel matrixes. Part II: biofunctional characteristics. Biomacromolecules. 2006;7:3019–29. doi: 10.1021/bm060504a. [DOI] [PubMed] [Google Scholar]
- 38.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–43. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 39.Mitragotri S, Lahann J. Physical approaches to biomaterial design. Nat Mater. 2009;8:15–23. doi: 10.1038/nmat2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saha K, Pollock JF, Schaffer DV, Healy KE. Designing synthetic materials to control stem cell phenotype. Curr Opin Chem Biol. 2007;11:381–7. doi: 10.1016/j.cbpa.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hern DL, Hubbell JA. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J Biomed Mater Res. 1998;39:266–76. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]