Abstract
Passive exposure to tobacco smoke significantly contributes to morbidity and mortality in children. Children, in particular, seem to be the most susceptible population to the harmful effects of environmental tobacco smoke (ETS). Paternal smoking inside the home leads to significant maternal and fetal exposure to ETS and may subsequently affect fetal health. ETS has been associated with adverse effects on pediatric health, including preterm birth, intrauterine growth retardation, perinatal mortality, respiratory illness, neurobehavioral problems, and decreased performance in school. A valid estimation of the risks associated with tobacco exposure depends on accurate measurement. Nicotine and its major metabolite, cotinine, are commonly used as smoking biomarkers, and their levels can be determined in various biological specimens such as blood, saliva, and urine. Recently, hair analysis was found to be a convenient, noninvasive technique for detecting the presence of nicotine exposure. Because nicotine/cotinine accumulates in hair during hair growth, it is a unique measure of long-term, cumulative exposure to tobacco smoke. Although smoking ban policies result in considerable reductions in ETS exposure, children are still exposed significantly to tobacco smoke not only in their homes but also in schools, restaurants, child-care settings, cars, buses, and other public places. Therefore, more effective strategies and public policies to protect preschool children from ETS should be consolidated.
Keywords: Tobacco smoke pollution, Nicotine, Cotinine, Child, Hair
Introduction
Tobacco smoke contains approximately 4,000 toxic chemicals, including oxidative gases, heavy metals, cyanide, and at least 50 carcinogens. Tobacco use is the most preventable cause of death and the most serious risk factor for cancer. Currently, 1.3 billion people smoke or use tobacco, and nearly 5 million worldwide die of diseases associated with tobacco smoke each year1). Environmental tobacco smoke (ETS) consists of particles much smaller than those in mainstream smoke, and therefore has greater penetrability to the airways of children.
Passive exposure to tobacco smoke significantly contributes to morbidity and mortality in children. Children, in particular, seem to be the most susceptible population to the harmful effects of ETS2). Children are exposed to tobacco smoke not only in their homes but also in schools, restaurants, child-care settings, cars, buses, and other public places. The home is the greatest single source of ETS for children. Paternal smoking inside the home leads to significant maternal and fetal exposure to ETS and may subsequently affect fetal health3).
ETS is defined as tobacco smoke produced by an active smoker both from the exhalation of smoked tobacco and by the burning end of the cigarette, which is inhaled by nonsmokers4). Exposure to ETS among children in their homes have been reported to vary from 27.6% in Africa, 34.3% in Southeast Asia, 50.6% in Western Pacific, and up to 77.8% in Europe5). ETS have been associated with adverse effects on pediatric health, including preterm birth, intrauterine growth retardation, perinatal mortality, respiratory illness, neurobehavioral problems, and decreased performance in school.
In this review, we discuss the effects of ETS on pediatric health and the technical issues concerning methods used for estimating ETS.
Pathophysiology of ETS
Fetuses and children are more vulnerable to the harmful effects of ETS because of their unique manner of exposure and their dynamic developmental physiology. Exposure to ETS during pregnancy can be fatal to growing embryos. Smoke condensates may induce the remodeling of embryonic vasculature, leading to various pathologies6). Exposure of the fetus to toxicants, which enter through the umbilical cord of the mother exposed to tobacco smoke, is associated with altered alveolar and respiratory bronchiole growth and development7).
Children are far more sensitive than adults to toxic chemicals in the environment. Proportional tobody weight, children drink more water, eat more food, and breathe more air than adults8). The physical attributes of children also cause them to live closer to the ground than adults do, which increases their exposure to toxins in dust, soil, and carpets. Children's ability to detoxify differ from that of adults owing to their physiological status and the immaturity of their enzyme systems and clearance mechanisms9). Children not only have higher metabolic rates but also inhale much greater volumes of air per kilogram body weight than adults (inhalation of 0.53 m3·kg-1·day-1 of air vs. inhalation of 0.2 m3·kg-1·day-1, respectively)2). The additional factor that increases children's exposure to ETS is their tendency to often sit closer to their parents, family members, or caregivers, which brings them closer to the source of pollutants than other passive smokers. Thus, the harmful effects of ETS on health are more severe in children than in adults.
ETS and respiratory health in infants and children
Prenatal maternal smoking and postnatal ETS lead to a dose-dependent decrease in lung function and respiratory morbidity in infants and children. Exposure of children to ETS in the home increases the incidence of middle ear disease10), asthma, wheeze, cough, bronchitis, bronchiolitis, pneumonia, and impaired pulmonary function.
A recent review by Stocks and Dezateux11) reveals that many studies have demonstrated a reduction in forced expiratory flows in infants exposed to parental smoking. Although several studies have shown reduced lung function in the early months of life in infants exposed passively to tobacco smoke during pregnancy12-15), it is difficult to separate the effects of prenatal and postnatal exposure on lung function during infancy. Current passive smoking is associated with effects ranging from -0.5% forced expiratory volume in 1 second to -2% maximal expiratory flow (MEF50)16). The effect of prenatal exposure to tobacco smoke can last at least up to adolescence17). Mannino et al.18), in the United States (US), also found a strong association between higher serum cotinine levels and worse lung function among children aged 8 to 16 years. A population-based cohort study (n=1,781) from 6 US sites in 2000 to 2006 found that childhood ETS exposure from 2 or more smokers compared with none is associated with early emphysema in adulthood after adjustment for demographic, anthropometric, parental, and participant characteristics19).
Many studies consistently demonstrated that parental smoking has an important impact on asthma and wheezing illnesses in infants and children. Exposure to ETS is associated with increased wheezing illnesses and increased symptoms in asthmatics20,21). A study conducted in 3-year-old children who were exposed both prenatally and postnatally to ETS reported increased prevalence of wheeze (odds ratio [OR], 1.14) when compared with children born to nonsmoking parents22). Maternal smoking increased the risk of asthma (adjusted OR, 1.35 for high exposure) during the first 7 years of life, in a dose-dependent manner according to the mothers' smoking rates23). Studies on the association of parental smoking with respiratory symptoms in school-aged children report an OR for asthma of 1.21, wheeze 1.40, and cough 1.35 for either parent smoking24,25).
In a review by Strachan and Cook26), increases in lower respiratory illness (LRI) were found to be associated with maternal smoking. Hospital admission with bronchiolitis was up to 3 times more likely with exposure to ETS. An OR of 1.69 for LRI was found for both parents smoking versus neither smoking.
Recently, gender-specific differences in the effects of ETS were noticed. Among children with allergic predisposition, more associations between ETS exposure and respiratory symptoms and diseases were detected in girls27).
ETS and infection in children
Exposure to ETS has been shown to be associated with increased prevalence of upper respiratory tract infections2). Several studies have also shown that parental smoking is associated with an increased incidence of both upper and lower respiratory tract infections (LRTI)28,29).
Gürkan et al.30) reported that children with respiratory syncytial virus bronchiolitis were found to have higher levels of cotinine when either the mother or both of the parents smoked, than do children with nonsmoking parents. This finding implies that the risk of acute respiratory infection can be elevated by heavy exposure to cigarette smoke. Passive smoking may play an important role in the development of respiratory infections and can cause airway inflammation in children with existing LRTI28).
A meta-analysis confirmed that ETS exposure at home has a major influence on the risk of LRI in infants, especially bronchiolitis31). Smoking by either parent or other household members significantly increased the risk of LRI (OR, 1.54 for any household member smoking).
A US National Cancer Institute report concluded that ETS exposure is strongly associated with otitis media, especially among children younger than 2 years32).
The mechanism by which ETS may be related to these infections is not entirely clear, but may be through suppression or modulation of the immune system, enhancement of bacterial adherence factors, or impairment of the mucociliary apparatus of the respiratory tract, or possibly through enhancement of toxicity of low levels of certain toxins that are not easily detected by conventional means29).
ETS and cardiovascular health in children
Environmental and genetic factors are the main determinants of cardiovascular disease risk factor clustering in families. ETS exposure may be associated with the progression of an index of atherosclerosis. Long-term exposure to ETS creates a state of permanent inflammation and an imbalance in the lipid profile that leads to lipid accumulation in the blood vessels of the heart and aorta33).
Children with long-term exposure to ETS may have an elevated risk for the development of premature coronary artery disease34). Permanent vascular damage is partly attributable to familial tobacco smoke exposure, an association that might be initiated in gestation. From a cohort of 732 young adults, birth data were collected and common carotid artery intima-media thickness (CIMT) was measured by ultrasound. The study showed that thicker CIMT in young adulthood is associated with ETS in pregnancy, especially paternal smoking35). However, longitudinal studies are necessary to determine the potential causal relevance of these associations.
Hypertension is the leading risk factor for cardiovascular disease36). Newborn infants of smoking parents show symptoms of cardiovascular stress hyperreactivity. Maternal smoking leads to long-lasting 'reprogramming' of infant blood pressure control mechanisms. The underlying dysfunction in a smoker's infant could plausibly be a precursor or an early marker of long-term susceptibility to complications, such as increased blood pressure37).
Simonetti et al.36) found that both systolic (+1.0 mmHg) and diastolic (+0.5 mmHg) blood pressure were higher in children of smoking parents. In healthy preschool children, parental smoking is an independent risk factor for higher blood pressure, adding to other familial and environmental risk factors36). However, blood pressure in children is not influenced by intrauterine effects38). Because of the high prevalence of risk factors in childhood, primary prevention of cardiovascular diseases should start as soon as children start school39).
ETS and neurobehavioral health in children
Active maternal smoking during pregnancy has been associated with a higher risk of behavioral disorders in children. These disorders range from personality temperament, neuropsychiatric outcomes such as attention disorders (e.g., attention deficit hyperactivity disorder [ADHD]) or conduct disorder, to lowered cognitive abilities40). Maternal smoking habits were associated with lowered cognitive development of children at age 4 years41). The possible explanation for this association is that biological pathways for tobacco neurotoxicity and psychosocial characteristics such as parental education level, intelligence, and mental health may also be involved in the interrelationship between smoking and neurodevelopment. However, results from cohort studies regarding the postnatal effects of tobacco smoke on neurodevelopment are not conclusive.
Data from adoptive families suggest that exposure to parental smoking represents an environmental risk for substance use in the adolescent offspring. In biologically related families, the effect of exposure to parental smoking is larger and more diverse, including substance use, disruptive behavior disorders, delinquency, deviant peer affiliations, aggressive attitudes, and preference for risk taking42).
ETS is also associated with an increased risk of psychiatric morbidity. In a population-based study in Finland (n=175,869), 24.7% had psychiatric diagnoses among children of mothers who smoked more than 10 cigarettes a day (OR, 1.85 [95% confidence interval, 1.74 to 1.96] and 13.7% among unexposed children43). Paternal prenatal smoking seems to be associated with conduct/externalizing problems through a causal intrauterine mechanism44).
Several studies also found an association between behavioral problems and maternal smoking45). A recent meta-analysis found that nicotine studies indicated a greater risk of ADHD-related disorders among children whose mothers smoked during pregnancy46).
However, further studies are necessary to reach a conclusion, owing to inconsistencies in these studies.
ETS and smoking biomarkers in children
A valid estimation of the risks associated with tobacco exposure depends on accurate measurement. Nicotine and its major metabolite, cotinine, are commonly used as smoking biomarkers, and their levels can be determined in various biological specimens such as blood, saliva, and urine.
Nicotine is both the primary addictive component of tobacco smoke and a potential toxin47). It is a major constituent of cigarettes, and is highly specific to tobacco smoke48). It has a half-life of approximately 2 to 3 hours in the blood, and is excreted in urine49). About 80% of nicotine is transformed to cotinine in the C-oxidation pathway50).
Cotinine is the major proximate metabolite of nicotine51). Plasma cotinine level correlates better than self-report to various measures of biological effects of smoking52). Cotinine levels in blood can be accurately estimated by measuring cotinine in saliva or urine51). Cotinine in different body fluids (plasma, urine, and saliva) has a longer half-life (15 to 19 hours) than that in blood51).
The degree of variability in the conversion of nicotine to cotinine is not great, and, even with this source of imprecision, cotinine levels accurately reflect exposure to nicotine from ETS. Urine cotinine depends on renal function, flow rate, and urinary pH. However, cotinine collection and analysis from these sources has several limitations with relative unreliability.
Hair analysis is a convenient, noninvasive technique for detecting the presence of nicotine exposure. Reduced inter-individual variability in hair makes it easier to standardize measurements. Hair collection does not necessitate special handling and storage measures like those required with body fluid samples53). Because cotinine accumulates in hair during hair growth, it is a unique measure of long-term, cumulative exposure to tobacco smoke4). In a study comparing urine cotinine levels with hair nicotine levels to measure ETS in 94 children aged 1 to 3 years from Norway, the authors found that nicotine in children's hair correlated more closely with parental smoking history (r=0.64, P<0.0001) than did cotinine in urine (r=0.50, P<0.0001)54).
A significant reduction in hair nicotine in subjects who used bleaching or hair dying was observed by Jurado et al.55). Hair cotinine correlates well with other measures of nicotine exposure. In a recent meta-analysis, Florescu et al.56) identified cutoff values for validation of cotinine as a marker for ETS exposure.
The International Agency for Research on Cancer concluded that ETS causes lung cancer in humans. Since tobacco-specific N-nitrosamines are found only in tobacco products or related nicotine-containing materials, their adducts or metabolites should be specific biomarkers of tobacco exposure57). As known carcinogen-derived biomarkers of exposure to ETS, nicotine-derived nitrosoamines, such as 4-(methylnitrosoamino)-1-(3-pyridyl)-1-butanone (NNK), are specific for tobacco exposure and are metabolized to a butanol metabolite, (4-(methylnitrosoamino)-1-(3-pyridyl)-1-butanol (NNAL), and its glucuronide (NNAL-GLUC)57,58). Urine levels of NNAL+NNAL-GLUC are elevated in nonsmokers exposed to ETS. These biomarkers have a more direct relation to cancer risk than cotinine because NNK, but not nicotine, is carcinogenic57).
Several analytical procedures have been developed to quantify nicotine and cotinine in hair and other materials. The 4 broad techniques are colorimetry, chromatography, radioimmunoassay, and enzyme-linked immunosorbent assay. Colorimetry is the least desirable method because of its lack of specificity. Ryu et al.59) recently developed a method based on the highly sensitive liquid chromatography-tandem mass spectrometry technique that requires as little as 1 mg of hair to simultaneously measure nicotine and cotinine.
A meta-analysis reviewed the reference values for hair cotinine as a biomarker of ETS. Among unexposed nonsmokers, mean hair cotinine was 0.3 to 0.4 ng/mg in children. A cutoff value of 0.2 ng/mg was accurate in discriminating between exposed and unexposed children56).
ETS and health policy
From the various investigations carried out worldwide on the effects of smoking bans on ETS, it is clear that the policies result in considerable reductions in environmental smoking exposure. Several studies were performed to evaluate the impact of the smoke-free air policy indoors and outdoors. The US Center for Disease Control and Prevention carried out investigations on the level of serum cotinine in nonsmokers during the past decade, and found a reduction of approximately 70%, comparing pre- and post-ban levels60). Pearson et al.61) showed that the smoke-free indoor air policy was effective, dramatically reducing cotinine levels and almost eliminating reports of sensory symptoms due to ETS.
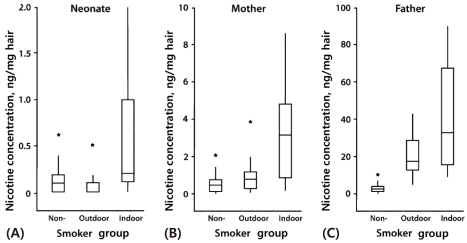
However, the smoking ban policy is not effective in protecting children and young adults from ETS exposure, because their primary source of ETS is typically the home. In Korea, we found that neonatal nicotine concentrations were significantly higher in indoor smokers than in outdoor smokers and nonsmokers3). These findings indicate that paternal smoking inside the home leads to significant fetal and maternal exposure to ETS and may subsequently affect fetal health (Fig. 1).
Fig. 1.
Comparative box plots of nicotine concentrations (ng/mg) in (A) neonatal, (B) maternal, and (C) paternal hair by smoker group, Korea, 2005 to 2007. Y-axes in each of the 3 parts are scaled differently depending on the distribution of nicotine concentration in each group. Asterisks denote outliers. The vertical line indicates the range of concentrations observed within each group; the box indicates the 25th and 75th percentiles; and the horizontal line in each box indicates the median concentration in each group (Reprinted from Seong MW, Hwang JH, Moon JS, Ryu HJ, Kong SY, Um TH, et al. Am J Epidemiol 2008;168:1140-4, with permission of Elsevier) [3].
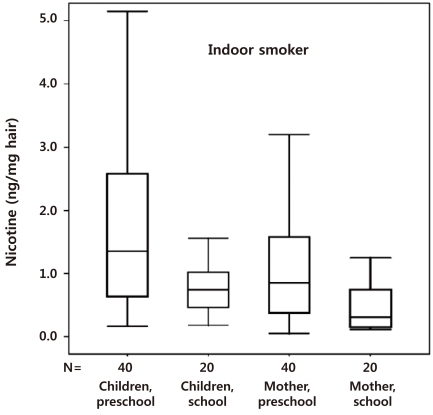
We also reported that the hair nicotine level of women whose spouses only smoked outside the home was still significantly higher than the level of those with nonsmoking spouses62). Thus, ETS is not completely prevented by smoking outdoors. Preschool children are more susceptible to secondhand smoke exposure than nonsmoking mothers and school children (Fig. 2). Thus, a strategy based on the separation of preschool children and pregnant women from the smoking activity of spouses might be inadequate to protect preschool children from ETS at home63), and public policies to reduce ETS exposure should be revised.
Fig. 2.
Nicotine concentrations (ng/mg hair) of nonsmoking household members in the indoor-smoker group. The box plots from left to right denote preschool children, school children, mothers of preschool children, and mothers of school children, respectively (Reprinted from Seong MW, Moon JS, Hwang JH, Ryu HJ, Kang SJ, Kong SY, et al. Clin Chim Acta 2010;411:72-6, with permission of Elsevier) [62].
Conclusion
ETS exposure significantly contributes to morbidity and mortality in children. Children, in particular, seem to be the most susceptible population to the harmful effects of ETS. Sufficient evidence indicates a significant association between ETS and health problems in children, including respiratory diseases, infection, and neurobehavioral problems. Pediatricians need to be concerned about these adverse effects of ETS exposure.
Children are exposed to tobacco smoke not only in their homes but also in schools, restaurants, child-care settings, cars, buses, and other public places in many countries, including Korea where smoking is culturally and socially allowable. More effective strategies and public policies to protect preschool children from ETS should be consolidated.
References
- 1.Brower V. World Health Organization focuses on antitobacco efforts in developing nations through treaty. J Natl Cancer Inst. 2006;98:667–668. doi: 10.1093/jnci/djj221. [DOI] [PubMed] [Google Scholar]
- 2.Cheraghi M, Salvi S. Environmental tobacco smoke (ETS) and respiratory health in children. Eur J Pediatr. 2009;168:897–905. doi: 10.1007/s00431-009-0967-3. [DOI] [PubMed] [Google Scholar]
- 3.Seong MW, Hwang JH, Moon JS, Ryu HJ, Kong SY, Um TH, et al. Neonatal hair nicotine levels and fetal exposure to paternal smoking at home. Am J Epidemiol. 2008;168:1140–1144. doi: 10.1093/aje/kwn231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Florescu A, Ferrence R, Einarson T, Selby P, Soldin O, Koren G. Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: focus on developmental toxicology. Ther Drug Monit. 2009;31:14–30. doi: 10.1097/FTD.0b013e3181957a3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warren CW, Jones NR, Peruga A, Chauvin J, Baptiste JP, Costa de Silva V, et al. Global youth tobacco surveillance, 2000-2007. MMWR Surveill Summ. 2008;57:1–28. [PubMed] [Google Scholar]
- 6.Ejaz S, Ejaz A, Sohail A, Lim CW. Vascular and morphogenetic abnormalities associated with exposure of cigarette smoke condensate during chicken and murine embryogenesis. Biomed Environ Sci. 2010;23:305–311. doi: 10.1016/S0895-3988(10)60068-2. [DOI] [PubMed] [Google Scholar]
- 7.Avdalovic M, Putney L, Tyler N, Finkbeiner W, Pinkerton K, Hyde D. In utero and postnatal exposure to environmental tobacco smoke (ETS) alters alveolar and respiratory bronchiole (RB) growth and development in infant monkeys. Toxicol Pathol. 2009;37:256–263. doi: 10.1177/0192623308330788. [DOI] [PubMed] [Google Scholar]
- 8.Landrigan PJ, Carlson JE. Environmental policy and children's health. Future Child. 1995;5:34–52. [PubMed] [Google Scholar]
- 9.Ginsberg G, Hattis D, Sonawane B, Russ A, Banati P, Kozlak M, et al. Evaluation of child/adult pharmacokinetic differences from a database derived from the therapeutic drug literature. Toxicol Sci. 2002;66:185–200. doi: 10.1093/toxsci/66.2.185. [DOI] [PubMed] [Google Scholar]
- 10.Kitchens GG. Relationship of environmental tobacco smoke to otitis media in young children. Laryngoscope. 1995;105(5 Pt 2) Suppl 69:1–13. doi: 10.1288/00005537-199505001-00001. [DOI] [PubMed] [Google Scholar]
- 11.Stocks J, Dezateux C. The effect of parental smoking on lung function and development during infancy. Respirology. 2003;8:266–285. doi: 10.1046/j.1440-1843.2003.00478.x. [DOI] [PubMed] [Google Scholar]
- 12.Martinez FD, Morgan WJ, Wright AL, Holberg CJ, Taussig LM. Diminished lung function as a predisposing factor for wheezing respiratory illness in infants. N Engl J Med. 1988;319:1112–1117. doi: 10.1056/NEJM198810273191702. [DOI] [PubMed] [Google Scholar]
- 13.Hanrahan JP, Tager IB, Segal MR, Tosteson TD, Castile RG, Van Vunakis H, et al. The effect of maternal smoking during pregnancy on early infant lung function. Am Rev Respir Dis. 1992;145:1129–1135. doi: 10.1164/ajrccm/145.5.1129. [DOI] [PubMed] [Google Scholar]
- 14.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ The Group Health Medical Associates. Asthma and wheezing in the first six years of life. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 15.Young S, Le Souëf PN, Geelhoed GC, Stick SM, Turner KJ, Landau LI. The influence of a family history of asthma and parental smoking on airway responsiveness in early infancy. N Engl J Med. 1991;324:1168–1173. doi: 10.1056/NEJM199104253241704. [DOI] [PubMed] [Google Scholar]
- 16.Moshammer H, Hoek G, Luttmann-Gibson H, Neuberger MA, Antova T, Gehring U, et al. Parental smoking and lung function in children: an international study. Am J Respir Crit Care Med. 2006;173:1255–1263. doi: 10.1164/rccm.200510-1552OC. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham J, Dockery DW, Speizer FE. Maternal smoking during pregnancy as a predictor of lung function in children. Am J Epidemiol. 1994;139:1139–1152. doi: 10.1093/oxfordjournals.aje.a116961. [DOI] [PubMed] [Google Scholar]
- 18.Mannino DM, Moorman JE, Kingsley B, Rose D, Repace J. Health effects related to environmental tobacco smoke exposure in children in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. 2001;155:36–41. doi: 10.1001/archpedi.155.1.36. [DOI] [PubMed] [Google Scholar]
- 19.Lovasi GS, Diez Roux AV, Hoffman EA, Kawut SM, Jacobs DR, Jr, Barr RG. Association of environmental tobacco smoke exposure in childhood with early emphysema in adulthood among nonsmokers: the MESA-lung study. Am J Epidemiol. 2010;171:54–62. doi: 10.1093/aje/kwp358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez FD, Morgan WJ, Wright AL, Holberg C, Taussig LM Group Health Medical Associates. Initial airway function is a risk factor for recurrent wheezing respiratory illnesses during the first three years of life. Am Rev Respir Dis. 1991;143:312–316. doi: 10.1164/ajrccm/143.2.312. [DOI] [PubMed] [Google Scholar]
- 21.Landau LI. Parental smoking: asthma and wheezing illnesses in infants and children. Paediatr Respir Rev. 2001;2:202–206. doi: 10.1053/prrv.2001.0141. [DOI] [PubMed] [Google Scholar]
- 22.Johansson A, Ludvigsson J, Hermansson G. Adverse health effects related to tobacco smoke exposure in a cohort of three-year olds. Acta Paediatr. 2008;97:354–357. doi: 10.1111/j.1651-2227.2007.00619.x. [DOI] [PubMed] [Google Scholar]
- 23.Jaakkola JJ, Gissler M. Maternal smoking in pregnancy, fetal development, and childhood asthma. Am J Public Health. 2004;94:136–140. doi: 10.2105/ajph.94.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook DG, Strachan DP. Health effects of passive smoking. 3. Parental smoking and prevalence of respiratory symptoms and asthma in school age children. Thorax. 1997;52:1081–1094. doi: 10.1136/thx.52.12.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strachan DP, Cook DG. Health effects of passive smoking. 6. Parental smoking and childhood asthma: longitudinal and case-control studies. Thorax. 1998;53:204–212. doi: 10.1136/thx.53.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strachan DP, Cook DG. Health effects of passive smoking. 1. Parental smoking and lower respiratory illness in infancy and early childhood. Thorax. 1997;52:905–914. doi: 10.1136/thx.52.10.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong GH, Wang D, Yang ZH, Zhang PF, Ren WH, Zhao YD, et al. Gender-specific differences in effects of prenatal and postnatal environmental tobacco smoke exposure on respiratory symptoms in 23,474 children with and without allergic predisposition: results from 25 districts of northeast China. Int J Environ Health Res. 2011;21:173–188. doi: 10.1080/09603123.2010.515673. [DOI] [PubMed] [Google Scholar]
- 28.Keskinoglu P, Cimrin D, Aksakoglu G. Relationships between cotinine, lower respiratory tract infection, and eosinophil cationic protein in children. Eur J Pediatr. 2007;166:455–459. doi: 10.1007/s00431-006-0263-4. [DOI] [PubMed] [Google Scholar]
- 29.Kum-Nji P, Meloy L, Herrod HG. Environmental tobacco smoke exposure: prevalence and mechanisms of causation of infections in children. Pediatrics. 2006;117:1745–1754. doi: 10.1542/peds.2005-1886. [DOI] [PubMed] [Google Scholar]
- 30.Gürkan F, Kiral A, Dağli E, Karakoç F. The effect of passive smoking on the development of respiratory syncytial virus bronchiolitis. Eur J Epidemiol. 2000;16:465–468. doi: 10.1023/a:1007658411953. [DOI] [PubMed] [Google Scholar]
- 31.Jones LL, Hashim A, McKeever T, Cook DG, Britton J, Leonardi-Bee J. Parental and household smoking and the increased risk of bronchitis, bronchiolitis and other lower respiratory infections in infancy: systematic review and meta-analysis. Respir Res. 2011;12:5. doi: 10.1186/1465-9921-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.California Environmental Protection Agency. Health effects of exposure to environmental tobacco smoke. Tob Control. 1997;6:346–353. doi: 10.1136/tc.6.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan H, Wong LS, Bhattacharya M, Ma C, Zafarani M, Yao M, et al. The effects of second-hand smoke on biological processes important in atherogenesis. BMC Cardiovasc Disord. 2007;7:1. doi: 10.1186/1471-2261-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moskowitz WB, Schwartz PF, Schieken RM Medical College of Virginia. Childhood passive smoking, race, and coronary artery disease risk: the MCV Twin Study. Arch Pediatr Adolesc Med. 1999;153:446–453. doi: 10.1001/archpedi.153.5.446. [DOI] [PubMed] [Google Scholar]
- 35.Geerts CC, Bots ML, Grobbee DE, Uiterwaal CS. Parental smoking and vascular damage in young adult offspring: is early life exposure critical? The atherosclerosis risk in young adults study. Arterioscler Thromb Vasc Biol. 2008;28:2296–2302. doi: 10.1161/ATVBAHA.108.173229. [DOI] [PubMed] [Google Scholar]
- 36.Simonetti GD, Schwertz R, Klett M, Hoffmann GF, Schaefer F, Wühl E. Determinants of blood pressure in preschool children: the role of parental smoking. Circulation. 2011;123:292–298. doi: 10.1161/CIRCULATIONAHA.110.958769. [DOI] [PubMed] [Google Scholar]
- 37.Cohen G, Jeffery H, Lagercrantz H, Katz-Salamon M. Long-term reprogramming of cardiovascular function in infants of active smokers. Hypertension. 2010;55:722–728. doi: 10.1161/HYPERTENSIONAHA.109.142695. [DOI] [PubMed] [Google Scholar]
- 38.Brion MJ, Leary SD, Smith GD, Ness AR. Similar associations of parental prenatal smoking suggest child blood pressure is not influenced by intrauterine effects. Hypertension. 2007;49:1422–1428. doi: 10.1161/HYPERTENSIONAHA.106.085316. [DOI] [PubMed] [Google Scholar]
- 39.Schwandt P, Geiss HC, Haas GM. Global cardiovascular risk in children and their families: the Prevention Education Program (PEP), Nürnberg. Nutr Metab Cardiovasc Dis. 2001;11(Suppl 5):35–39. [PubMed] [Google Scholar]
- 40.Apostol GG, Jacobs DR, Jr, Tsai AW, Crow RS, Williams OD, Townsend MC, et al. Early life factors contribute to the decrease in lung function between ages 18 and 40: the Coronary Artery Risk Development in Young Adults study. Am J Respir Crit Care Med. 2002;166:166–172. doi: 10.1164/rccm.2007035. [DOI] [PubMed] [Google Scholar]
- 41.Julvez J, Ribas-Fitó N, Torrent M, Forns M, Garcia-Esteban R, Sunyer J. Maternal smoking habits and cognitive development of children at age 4 years in a population-based birth cohort. Int J Epidemiol. 2007;36:825–832. doi: 10.1093/ije/dym107. [DOI] [PubMed] [Google Scholar]
- 42.Keyes M, Legrand LN, Iacono WG, McGue M. Parental smoking and adolescent problem behavior: an adoption study of general and specific effects. Am J Psychiatry. 2008;165:1338–1344. doi: 10.1176/appi.ajp.2008.08010125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ekblad M, Gissler M, Lehtonen L, Korkeila J. Prenatal smoking exposure and the risk of psychiatric morbidity into young adulthood. Arch Gen Psychiatry. 2010;67:841–849. doi: 10.1001/archgenpsychiatry.2010.92. [DOI] [PubMed] [Google Scholar]
- 44.Brion MJ, Victora C, Matijasevich A, Horta B, Anselmi L, Steer C, et al. Maternal smoking and child psychological problems: disentangling causal and noncausal effects. Pediatrics. 2010;126:e57–e65. doi: 10.1542/peds.2009-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fergusson DM, Horwood LJ, Lynskey MT. Maternal smoking before and after pregnancy: effects on behavioral outcomes in middle childhood. Pediatrics. 1993;92:815–822. [PubMed] [Google Scholar]
- 46.Linnet KM, Dalsgaard S, Obel C, Wisborg K, Henriksen TB, Rodriguez A, et al. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. Am J Psychiatry. 2003;160:1028–1040. doi: 10.1176/appi.ajp.160.6.1028. [DOI] [PubMed] [Google Scholar]
- 47.Hatsukami DK, Stead LF, Gupta PC. Tobacco addiction. Lancet. 2008;371:2027–2038. doi: 10.1016/S0140-6736(08)60871-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benowitz NL. Biomarkers of environmental tobacco smoke exposure. Environ Health Perspect. 1999;107(Suppl 2):349–355. doi: 10.1289/ehp.99107s2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benowitz NL, Jacob P., 3rd Nicotine and cotinine elimination pharmacokinetics in smokers and nonsmokers. Clin Pharmacol Ther. 1993;53:316–323. doi: 10.1038/clpt.1993.27. [DOI] [PubMed] [Google Scholar]
- 50.Tricker AR. Nicotine metabolism, human drug metabolism polymorphisms, and smoking behaviour. Toxicology. 2003;183:151–173. doi: 10.1016/s0300-483x(02)00513-9. [DOI] [PubMed] [Google Scholar]
- 51.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18:188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- 52.Pérez-Stable EJ, Benowitz NL, Marín G. Is serum cotinine a better measure of cigarette smoking than self-report? Prev Med. 1995;24:171–179. doi: 10.1006/pmed.1995.1031. [DOI] [PubMed] [Google Scholar]
- 53.Al-Delaimy WK. Hair as a biomarker for exposure to tobacco smoke. Tob Control. 2002;11:176–182. doi: 10.1136/tc.11.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nafstad P, Botten G, Hagen JA, Zahlsen K, Nilsen OG, Silsand T, et al. Comparison of three methods for estimating environmental tobacco smoke exposure among children aged between 12 and 36 months. Int J Epidemiol. 1995;24:88–94. doi: 10.1093/ije/24.1.88. [DOI] [PubMed] [Google Scholar]
- 55.Jurado D, Muñoz C, Luna Jde D, Fernández-Crehuet M. Environmental tobacco smoke exposure in children: parental perception of smokiness at home and other factors associated with urinary cotinine in preschool children. J Expo Anal Environ Epidemiol. 2004;14:330–336. doi: 10.1038/sj.jea.7500329. [DOI] [PubMed] [Google Scholar]
- 56.Florescu A, Ferrence R, Einarson TR, Selby P, Kramer M, Woodruff S, et al. Reference values for hair cotinine as a biomarker of active and passive smoking in women of reproductive age, pregnant women, children, and neonates: systematic review and meta-analysis. Ther Drug Monit. 2007;29:437–446. doi: 10.1097/FTD.0b013e318074df6e. [DOI] [PubMed] [Google Scholar]
- 57.Hecht SS. Carcinogen derived biomarkers: applications in studies of human exposure to secondhand tobacco smoke. Tob Control. 2004;13(Suppl 1):i48–i56. doi: 10.1136/tc.2002.002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hecht SS, Carmella SG, Murphy SE, Akerkar S, Brunnemann KD, Hoffmann D. A tobacco-specific lung carcinogen in the urine of men exposed to cigarette smoke. N Engl J Med. 1993;329:1543–1546. doi: 10.1056/NEJM199311183292105. [DOI] [PubMed] [Google Scholar]
- 59.Ryu HJ, Seong MW, Nam MH, Kong SY, Lee DH. Simultaneous and sensitive measurement of nicotine and cotinine in small amounts of human hair using liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:2781–2782. doi: 10.1002/rcm.2659. [DOI] [PubMed] [Google Scholar]
- 60.Pirkle JL, Bernert JT, Caudill SP, Sosnoff CS, Pechacek TF. Trends in the exposure of nonsmokers in the U.S. population to secondhand smoke: 1988-2002. Environ Health Perspect. 2006;114:853–858. doi: 10.1289/ehp.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pearson J, Windsor R, El-Mohandes A, Perry DC. Evaluation of the immediate impact of the Washington, D.C., smoke-free indoor air policy on bar employee environmental tobacco smoke exposure. Public Health Rep. 2009;124(Suppl 1):134–142. [PMC free article] [PubMed] [Google Scholar]
- 62.Seong MW, Moon JS, Hwang JH, Ryu HJ, Kang SJ, Kong SY, et al. Preschool children and their mothers are more exposed to paternal smoking at home than school children and their mothers. Clin Chim Acta. 2010;411:72–76. doi: 10.1016/j.cca.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 63.Yoo SH, Paek YJ, Kim SS, Lee DH, Seo DK, Seong MW, et al. Hair nicotine levels in non-smoking pregnant women whose spouses smoke outside of the home. Tob Control. 2010;19:318–324. doi: 10.1136/tc.2009.033134. [DOI] [PubMed] [Google Scholar]