Abstract
Hepatic adverse drug reactions (ADRs) to certain drugs may differ within each country, reflecting different patterns of prescription, socioeconomic status, and culture. The purpose of this study was to assess the suspected cause of hepatic ADRs using the spontaneously reported pharmacovigilance data from Korea. A total of 9,360 spontaneously reported adverse drug events (ADEs) from nine Pharmacovigilance Centers were analyzed. Risk of hepatic ADEs was assessed by calculating the reporting odds ratio (ROR). Of the 9,360 cases, 567 hepatic ADEs were reported. The most frequently prescribed drug classes inducing hepatic ADEs were anti-tuberculotics, cephalosporins, valproic acids, penicillins, quinolones, non-steroidal anti-inflammatory drugs (NSAIDs), anti-viral agents, and statins. ROR values were especially high in anti-tuberculosis drugs, systemic antifungal drugs for systemic use, anti-epileptics, propylthiouracil, and herbal medicines. Underlying diseases such as tuberculosis (6.9% vs 0.9%), pneumonia (4.9% vs 1.7%), intracranial injury including skull fracture (4.5% vs 0.9%), HIV (3.4% vs 0.4%), subarachnoid hemorrhage (2.8% vs 0.5%), and osteoporosis (2.4% vs 1.4%) were significantly more common in hepatic ADE group. In conclusion, anti-infective drugs, anti-epileptics, NSAIDs and statins are the most common suspects of the spontaneously reported hepatic ADEs, in Korea. Careful monitoring for such reactions is needed for the prescription of these drugs.
Keywords: Drug-Induced Hepatitis, Etiology, Spontaneous Pharmacovigilance
INTRODUCTION
Drug-induced liver diseases are important adverse drug reactions (ADRs) and impart significant medical burdens to patients, doctors, and pharmaceutical companies. Drugs are important causes of acute hepatitis, an extreme ADR, and are responsible for approximately 15.6% of liver transplantation for acute liver failure (1). The true incidence of hepatic ADRs is difficult to determine, but is reported to be between 1 in 10,000 and 1 in 100,000 patients (2). Drug-induced ADRs to the liver is the single most important reason for drug withdrawal from the market and can halt drug development (3, 4). Post-market surveillance using a reporting system is therefore especially important for hepatic ADRs.
Clinical studies have shown marked differences in such causative drugs. In Korea (5-7) and East Asian countries (8), herbal medicines, antibiotics, and non-steroidal anti-inflammatory drugs (NSAIDs) have been reported as major causative agents of hepatic ADRs. In Western countries and the United States, antibiotics, acetaminophen, NSAIDs, anti-tuberculosis agents, and central nervous system agents were recognized as major culprits (1, 9, 10). These studies were primarily performed by experienced hepatologists with hospitalized patients suffering from severe drug-induced liver disease. These studies may have limitations due to selection biases, including more serious hepatic ADRs, and focused on the general features of drug-induced liver diseases.
Since 2006, spontaneous reporting in pharmacovigilance systems has been established successfully in Korea (11), and the collected data were analyzed. Spontaneous ADR reporting systems have weak points such as inaccuracy of ADR diagnosis, underreporting, and an inability to obtain ADR incidences (12, 13), but is useful for signal detections of hepatic ADRs, and the reporting odds ratio has been recognized as a useful tool for detection of disproportionally reported drugs for a ADEs (14, 15). However, data from spontaneous reporting pharmacovigilance programs may allow evaluation of the general aspects of hepatic ADRs. In this study, we evaluated the drugs suspected as the causative drugs of hepatic ADRs and measured the reporting odds ratio (ROR) values using the spontaneous reporting pharmacovigilance data from nine Regional Pharmacovigilance Centers in Korea.
MATERIALS AND METHODS
For this study, 9,360 cases of adverse drug events (ADEs) were collected from nine Regional Pharmacovigilance Centers in Korea from January 2007 to December 2008. Each regional center collected ADEs from both general hospitals (90%) and general practitioners from private clinics or pharmacies (10%). The method of reporting ADEs was a voluntary system that relied on written reports submitted via fax, website, or electrical medical recording systems generated by physicians, pharmacists, and nurses. ADEs were coded using the World Health Organization (WHO) Adverse Reaction Terminology (ART) (16), which consists of four components: preferred terms, high-level terms, systemic organ class, and included terms. We evaluated the causality of these ADE cases according to WHO Uppsala Monitoring Center (UMC) criteria (17) at each regional center. WHO-UMC criteria classify ADEs as certain, probable, possible, unlikely, conditional and unassessable. The causality was assessed by the physicians majored in Internal Medicine. Once causality was assessed, the ADRs were characterized by severity as either serious or non-serious (17). The serious category included lifethreatening, disabling, or permanent disabilities or prolonged hospitalization. The ADRs were further classified by the type of reactions (17). Type A reactions were usually dose-related, predictable, preventable, and rarely life threatening, while Type B reactions were not dose-related and were idiosyncratic, unpreventable, and potentially life threatening. Type C reactions were both dose- and time-related. Hepatic ADRs were considered when the levels of serum alanine aminotransferase, alkaline phosphatase, or total bilirubin were increased to at least 2-fold higher than baseline without other plausible causes. All data of reported ADE are submitted to central office of the pharmacovigilance centers.
Descriptive statistics were performed to assess the ADR cases. An association between drugs and hepatic ADRs was assessed by comparing hepatic ADRs with non-hepatic ADRs and calculating the ROR as a measure of disproportion. Comparisons of these two groups were performed using an independent t test or chi-square test.
ROR values were calculated using the equation shown below. The basic assumption is that no relationship exists between the reported suspected ADR and the suspected drug, and the measure of disproportionality can be calculated by means of the Poisson probability (14, 15).
ROR = (a/c)/(b/d) = ad/bc
95% confidence interval of ROR = e ln(ROR) ± 1.96 √(1/a+1/b+1/c+1/d)
All calculations were performed using the statistical software package SPSS 15.0 (Statistical Package for the Social Sciences, Chicago, IL, USA).
Ethics statement
The study protocol was reviewed by the institutional review board of Severance Hospital Clinical Trials Center (IRB No. 4-2011-0372) and got permission for exemption.
RESULTS
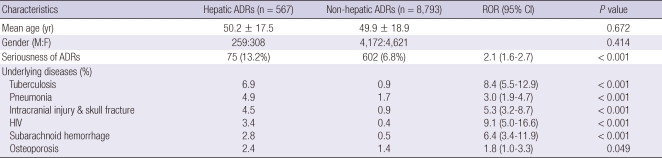
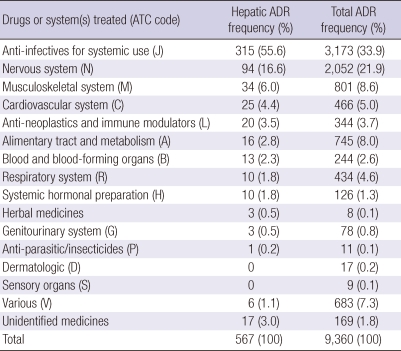
Causality assessment using WHO-UMC classified the 9,360 cases to certain (n = 942, 10.1%), probable (n = 3,444, 36.8%), possible (n = 4,252, 45.4%), unlikely (n = 661, 7.1%), and unaccessable (n = 61, 0.6%) groups. Hepatic ADRs comprised 6.1% (567 cases) of all enrolled ADRs (9,360 cases). Thirty-eight percent (n = 215) of hepatic ADE patients also had non-hepatic ADEs. WHO-UMC causality assessment of hepatic ADEs was as like; certain (6.9%), probable (22.6%), possible (60.7%), unlikely (9.5%) and unassessable (0.03%). There were no differences in age or gender of patients with hepatic or non-hepatic ADEs (Table 1). Enrolled subjects had an average of 1.7 underlying diseases that required treatment, and the patterns of these underlying diseases differed between hepatic and non-hepatic ADR groups. Patients with hepatic ADEs suffered more frequently with tuberculosis (6.9%), pneumonia (4.9%), intracranial injury including skull fracture (4.5%), HIV (3.4%), subarachnoid hemorrhage (2.8%), and osteoporosis (2.4%). Patients with hepatic ADEs experienced serious ADEs more frequently than patients in the other groups because of liver disease (13.2% vs 6.8%). ADEs were classified into WHO-ART categories and are shown in Table 2. The suspected drugs for hepatic ADEs were classified according to the WHO Anatomical Therapeutic Chemical (ATC) code. Anti-infectives (code-J, 55.6%), nervous system drugs (code-N, 16.6%) musculoskeletal system drugs (code-M, 6.0%) and cardiovascular system drugs (code-C, 4.4%) comprised the majority of causative drugs (Table 3).
Table 1.
Clinical characteristics of the patients with spontaneously reported adverse drug reactions (ADR)
ROR, reporting odds ratio; HIV, human immunodeficiency virus.
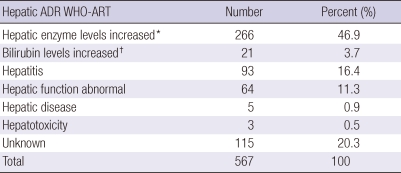
Table 2.
Preferred criteria of the WHO-Adverse Reaction Terminology for hepatic ADE reports
*Alanine aminotransferase level was increased at least more than two-fold compared to baseline; †total bilirubin level was increased at least more than two-fold compared to baseline. ADE, adverse drug events; WHO-ART, World Health Organization-Adverse Reaction Terminology.
Table 3.
WHO-Anatomical Therapeutic Chemical codes of drugs causing hepatic ADRs
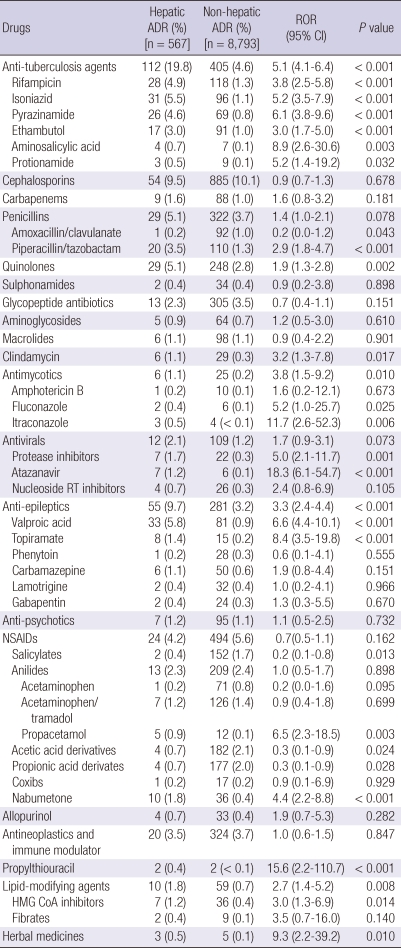
Each culprit drug was analyzed in detail (Table 4). The antituberculosis drugs were the most common triggers for hepatic toxicity: isoniazid (5.5%), rifampicin (4.9%), and pyrazinamide (4.6%). Their mean relative ROR for hepatic ADRs compared with non-hepatic ADRs was 5.1 (4.1-6.4, P < 0.001). Although the cephalosporins (9.5%), penicillins (5.1%), quinolones (5.1%), carbapenems (1.6%), and glycopeptide antibiotics (2.3%) also caused hepatic ADRs, their ROR values were between 0.7 and 1.9. Only one case of hepatic ADRs induced by amoxillin/clavulanic acid was reported in this study. NSAIDs were important causes of hepatic ADRs with different ROR values, comprising 4.2% of all drugs causing hepatic ADRs. Six cases of hepatic ADEs associated with systemic anti-fungal agents (1 case by amphotericin, 2 cases by fluconazole and 3 cases by itraconzole) were reported; but ROR values for fluconazole and itraconazole are especially higher (5.2-11.7). Antiviral agents were also emerging as the important causes of hepatic ADEs. Seven cases of hepatic ADE associated with atazanavir, protease inhibitor used for HIV infection, were reported (ROR 5.0, P < 0.001).
Table 4.
Causative drugs and their reporting odd ratios (RORs) for hepatic ADRs
Anti-epileptics, such as valproic acid (5.8%, ROR 6.6, P < 0.001), were also major culprits. The mean overall ROR of NSAIDs was 0.7 (0.5-1.1 ADRs by anilides [acetaminophen, a mixture including acetaminophen, and propacetamol], which included 2.3% of hepatic ADRs with low RORs [1.0]). Nabumetone comprised 1.8% of hepatic ADRs and had the highest ROR values (4.4, P < 0.001) among all the NSAIDs. In this study, only two cases of hepatic ADEs associated with propylthiouracil were reported, but their ROR value is especially high to 15.6 (P < 0.001). Lipid-modifying agents, such as statins (ROR 3.0 P = 0.014), were also frequently associated with hepatic ADEs. Herbal medicines have been reported as the important causes of hepatic ADRs in Korea and other Asian countries. But in this study, herbal medicines comprised only 0.5% of hepatic ADRs with high mean ROR values (9.3, P = 0.010). However, we could not identify the details of the herbal medicines prescribed.
DISCUSSION
This is the first study of hepatic ADRs based on the spontaneous reporting program in Korea. Our spontaneous reporting system collected data from doctors, nurses, and pharmacists working in general hospitals or private clinics. Spontaneous reporting systems are important for the surveillance hepatic ADRs but have several weak points. However, the incidence of ADRs cannot be estimated from the data of spontaneous ADR monitoring systems. Underreporting is a well-known problem in spontaneous reporting (13), and as denominators were unknown, our data cannot be used for estimations of hepatic ADR incidence. Our program for pharmacovigilance is mainly designed for the monitoring the ADEs inevitably occurred by adequately prescribed in hospitals and private clinics. The program did not include medication errors or drug abuse, and professionals for oriental medicine are not participating to the program. These features imperatively affected the results. The hepatotoxicity of drugs may be related to overdose of medication. In this study, the patients were prescribed to the therapeutic dosages. The major suspected drugs for hepatic ADRs in this study are antimicrobials, central nervous system drugs, and NSAIDs. These results are similar to reports from the United States and Europe (10) but differ from previous studies done in Korea (5-7) and East Asia (8). We hypothesize that this may be the reason why the incidence of hepatic ADRs after treatment with herbal medicines is lower than the incidences found in other Asian studies (5-8). Herbal medicines comprise 0.5% of suspected hepatic ADRs with high ROR values for hepatic ADR in this study, suggesting that herbal medications could be an important cause of hepatic ADRs in Korea, where the oriental medicine is popular (18). WHO and the Uppsala Monitoring Centre have emphasized the monitoring of herbal products for coordinated regional pharmacovigilance centers (19). We think that the activity is especially important in Korea, and now extend our activities to cover the herbal medicines prescribed by oriental medicine professionals and herbal foods purchased from market. There is another point that should be considered. This pharmacovigilance program started in 2006 from the educating general hospitals hosting the Regional Pharmacovigilance Centers and is now spreading to general practitioners in private clinics or pharmacies. The number of reported ADEs from private clinics or pharmacies is increasing, but the portion is still minor. About 90% of the reported ADE cases came from the 9 educating general hospitals, and this feature may explain the low incidence of ADEs in suspect of amoxicillin/clavulanic acid. Contrary to other reports (9), only one case of hepatic ADR induced by amoxicillin/clavulanic acid and another case due to phenytoin were reported in this study. Amoxicillin/clavulanic acid is still the first line antibiotics for acute sinusitis and other infectious diseases for general practitioners in Korea. However multi-drugs resistant Streptococcus pneumoniae or methicillin resistance Staphylococcus aureus are big problems for the community acquired infections in Korea and prescriptions of anti-streptococcal quinolones or other classes of antibiotics are increasing instead of amoxicillin/clavulanic acid (20). Phenytoin is a well known culprit for hepatic ADRs. In Korea, phenytoin rarely prescribed due to various ADRs and drug interactions, in addition to hepatic ADRs. Instead of phenytoin, other newly developed safe and effective anti-epileptics are popularly used.
In Western countries and the United States, acetaminophen is a common cause of dose related drug-induced hepatic injury: it is the most common cause of liver transplantation for acute liver failure in the United States (1, 21). Acetaminophen overdose is often used for suicide attempts in Western societies, which is not a familiar concept in Korea. While acetaminophen use is popular in Korea, only a few cases of acetaminophen-induced hepatitis were reported in this study. This result may be due to the characteristics of the enrolled cases, which are primarily from the general hospital with adequately regulated prescriptions and a pharmacovigilance program that does not include patient drug abuse.
There is another limitation to this study. Aithal et al. (22) reported that approximately 50% of spontaneously reported hepatic ADRs are misdiagnosed. We enrolled nine Regional Pharmacovigilance Centers, but their activities, experiences, capabilities and organization may differ among them. For diagnosis of hepatic ADRs, underlying causes of hepatitis such as viral hepatitis, autoimmune hepatitis, alcoholic liver disease, steatohepatitis, and other metabolic diseases should be excluded (23). The enrolled cases were from spontaneous reports of both nonhepatologist and hepatologist physicians, pharmacists, and nurses. Clinical data were not detailed in the reports, and we could not evaluate the clinical courses and features of enrolled patients. Hepatic ADRs are usually classified as hepatocellular, mixed, and cholestatic (3). We could not classify hepatic ADRs with this classification scheme, but merely indicated the registered WHO-ART coding, which is the official coding system for the pharmacovigilance program for hepatic ADRs in this study (16). We also used WHO-UMC criteria for causality assessment. The criteria classify ADEs as certain, probable, possible, unlikely, conditional and unassessable. In this study, only 6.9% and 22.6% of hepatic ADRs are certain or probable. This means that significant portion of the hepatic ADRs in this study may not be actually the cause of hepatitis. Some antibiotics with very low risk of hepatotoxicities, such as glycopeptides antibiotics and aminoglycosides were reported as the suspects, but their ROR values are 0.7 and 1.2, respectively, and possibility of misdiagnosis could not be excluded.
Many investigators have used the Russel Uclaf Causality Assessment Model (RUCAM) for assessing hepatic ADRs (24, 25), but there are limitations in clinical use as it has lower reliability (26). WHO-UMC criteria are globally accepted causality assessment criteria for ADRs in general. Compared to RUCAM, WHO-UMC criteria may have some weak points in subjectivity and limited reproducibility. Many criteria for causality assessment are currently available, but they share key components for assessment, such as chronological relationship between administration of drug and onset of ADRs, exclusion of other possible causes not involving drugs, previous information on similar events attributed to the suspected drugs, improvement after discontinuation of causative drugs, and aggravation or reappearance of ADR by re-administration of causative drugs. However, our data may have advantages for obtaining the general aspects of drugs that cause hepatic ADRs in Korea compared with previous studies done by hepatologists with patients admitted due to hepatic ADRs. This study included cases of hepatic ADRs with various severities.
Anti-infective drugs were the most common cause of hepatic ADRs in this study. However, marked differences in ROR values were found among the anti-infectives. As is known, anti-tuberculosis and anti-fungal agents have high ROR values. In this study, anti-viral agents were also emerging as the important causes of hepatic ADRs. In Korea, the prevalence of tuberculosis is very high and the prevalence of HIV infection is soaring, and our data might reflect the situation. Cephalosporins are also important causes of hepatic ADRs, as they are commonly prescribed in Korea. However, their ROR values were not elevated compared with the incidence of non-hepatic ADRs due to cephalosporins.
Marked differences in underlying diseases requiring treatment were found in our study. Patients with hepatic ADRs more frequently suffered from tuberculosis, pneumonia, brain injury or subarachnoid hemorrhage, and HIV infection. The differences may reflect the drugs that cause hepatic ADRs. Infection with HIV is generally considered a predisposition to hepatic ADRs. HIV infection is a well-known risk factor for drug allergy; and the relationship between HIV and hepatic ADRs is therefore apparent (27). It is unclear whether HIV infection is related to the development of hepatic ADRs, but it is not surprising that HIV-infected patients are susceptible to hepatic ADRs, as they commonly are treated with drugs well known to cause hepatic ADRs such as isoniazid, rifampicin, trimethoprim-sulfamethoxazole, NSAIDs, and antiviral agents (28).
In this study, there were no differences in ages or gender ratios between hepatic and non-hepatic ADR groups. Age and gender have been previously regarded as risk factors for hepatic ADRs. Traditionally, patients at advanced ages and women were assumed to be susceptible groups (7, 21), but this assumption becomes now controversial. There was no difference in age or gender between hepatic or non-hepatic ADR groups in this study. Our result was consistent with that of Shapiro and Lewis (29), which reviewed published data and did not find any evidence that patients at advanced ages or women were more susceptible to hepatic ADRs caused by any drug.
Our study showed that anti-infectives, anti-epileptics, and NSAIDs are the most commonly prescribed drugs for the spontaneously reported hepatic ADRs in Korea. Careful monitoring for drug-induced ADRs is needed for tuberculosis, pneumonia, intracranial injuries, and HIV infections. As this study is based on the spontaneous reporting program, it may contribute to the recognition of the general aspects of hepatic ADRs occurred in hospitals of Korea.
Footnotes
This study was supported by a grant from Korean Food and Drug Administration (09182KFDA842) in 2010.
References
- 1.Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug induced liver injury in the United States. Liver Transpl. 2004;10:1018–1023. doi: 10.1002/lt.20204. [DOI] [PubMed] [Google Scholar]
- 2.Larrey D. Epidemiology and individual susceptibility to adverse drug reactions affecting the liver. Semin Liver Dis. 2002;22:145–155. doi: 10.1055/s-2002-30105. [DOI] [PubMed] [Google Scholar]
- 3.Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006;354:731–739. doi: 10.1056/NEJMra052270. [DOI] [PubMed] [Google Scholar]
- 4.Meadows M. Serious liver injury. Leading reason for drug removals, restrictions. FDA Consum. 2001;35:8–9. [PubMed] [Google Scholar]
- 5.Kang SH, Kim JI, Jeong KH, Ko KH, Ko PG, Hwang SW, Kim EM, Kim SH, Lee HY, Lee BS. Clinical characteristics of 159 cases of acute toxic hepatitis. Korean J Hepatol. 2008;14:483–492. doi: 10.3350/kjhep.2008.14.4.483. [DOI] [PubMed] [Google Scholar]
- 6.Kim JB, Sohn JH, Lee HL, Kim JP, Han DS, Hahm JS, Lee DH, Kee CS. Clinical characteristics of acute toxic liver injury. Korean J Hepatol. 2004;10:125–134. [PubMed] [Google Scholar]
- 7.Seo JC, Jeon WJ, Park SS, Kim SH, Lee KM, Chae HB, Park SM, Youn SJ. Clinical expeience of 48 acute toxic hepatitis patients. Korean J Hepatol. 2006;12:74–81. [PubMed] [Google Scholar]
- 8.Wai CT, Tan BH, Chan CL, Sutedja DS, Lee YM, Khor C, Lim SG. Drug-induced liver injury at an Asian center: a prospective study. Liver Int. 2007;27:465–474. doi: 10.1111/j.1478-3231.2007.01461.x. [DOI] [PubMed] [Google Scholar]
- 9.Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, García-Ruiz E, García-Muñoz B, González-Grande R, Pizarro A, Durán JA, Jiménez M, Rodrigo L, Romero-Gomez M, Navaro JM, Planas R, Costa J, Borras A, Soler A, Salmerón J, Martin-Vivaldi R Spanish Group for the Study of Drug-induced Liver Disease. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129:512–521. doi: 10.1016/j.gastro.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Daven T, Serano J, Yang H, Rochon J Drug Induced Liver Injury Network (DILIN) DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–1934. doi: 10.1053/j.gastro.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin YS, Lee YW, Choi YH, Park BJ, Jee YK, Choi SK, Kim EG, Park JW, Hong CS. Spontaneous reporting of adverse drug events by Korean regional pharmacovigilance centers. Pharmacoepidemiol Drug Saf. 2009;18:910–915. doi: 10.1002/pds.1796. [DOI] [PubMed] [Google Scholar]
- 12.Moride Y, Haramburu F, Requejo AA, Bégaud B. Under-reporting of adverse drug reactions in general practice. Br J Clin Pharmacol. 1997;43:177–181. doi: 10.1046/j.1365-2125.1997.05417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vallano A, Cereza G, PedróS C, Agusti A, Danés I, Aguilera C, Arnau JM. Obstacles and solutions for spontaneous reporting of adverse drug reactions in the hospital. Br J Clin Pharmacol. 2005;60:653–658. doi: 10.1111/j.1365-2125.2005.02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Puijenbroek EP, Bate A, Leufkens HG, Lindquist M, Orre R, Egberts AC. A comparison of measures of disporportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 2002;11:3–10. doi: 10.1002/pds.668. [DOI] [PubMed] [Google Scholar]
- 15.Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 2004;13:519–523. doi: 10.1002/pds.1001. [DOI] [PubMed] [Google Scholar]
- 16.The Uppsala Monitoring Centre. The WHO-ART Adverse Reaction Terminology. Uppsala: the Uppsala Monitoring Centre; 2005. [Google Scholar]
- 17.Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356:1255–1259. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- 18.Shin YS, Park JW, Lee YW, Kim CW, Dhong HJ, Park HS, Cho YJ, Cho SH, Pyun BY, Lee KH, Lee HR, Hong CS. Current status of oriental medicine in treating Korean allergy patients. Pharmacoepidemiol Drug Saf. 2011;20:99–104. doi: 10.1002/pds.1947. [DOI] [PubMed] [Google Scholar]
- 19.WHO and the Uppsala Monitoring Centre. Safety monitoring of medicinal products: Guidelines for setting up and running a pharmacovigilance centre. Uppsala: the Uppsala Monitoring Centre; 2000. [Google Scholar]
- 20.Song JH, Joo EJ. The crisis of antimicrobial resistance: current status and future strategies. J Korean Med Assoc. 2010;53:999–1005. [Google Scholar]
- 21.Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil O, Hay JE, Hynan L, Crippin JS, Blei AT, Samuel G, Reisch J, Lee WM U.S. Acute Liver Failure Study Group. Results of prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 22.Aithal GP, Rawlins MD, Day CP. Accuracy of hepatic adverse drug reaction reporting in one English health region. BMJ. 1999;319:1541. doi: 10.1136/bmj.319.7224.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agarwal VK, McHutchison JG, Hoofnagle JH Drug-induced Liver Injury Network. Important elements for the diagnosis of drug-induced liver injury. Clin Gastroenterol Hepatol. 2010;8:463–470. doi: 10.1016/j.cgh.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danan G, Benichou C. Causality assessment of adverse reactions to drugs: I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–1330. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 25.Benichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs: II. An original model for validation of drug causality assessment methods: Case reports with positive rechallenge. J Clin Epidemiol. 1993;46:1331–1336. doi: 10.1016/0895-4356(93)90102-7. [DOI] [PubMed] [Google Scholar]
- 26.Rochon J, Protiva P, Seeff LB, Fontana RJ, Liangpunsakul S, Watkins PB, Davern T, McHutchison JG Drug-induced liver injury network (DILIN) Reliability of Roussel Uclaf Causality Assessment Method for assessing causality in drug-induced liver injury. Hepatology. 2008;48:1175–1183. doi: 10.1002/hep.22442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akhtar AJ, Shaheen M. Jaundice in African-American and Hispanic patients with AIDS. J Natl Med Assoc. 2007;99:1381–1385. [PMC free article] [PubMed] [Google Scholar]
- 28.Ungo JR, Jones D, Ashkin D, Hollender ES, Bernstein D, Albanese AP, Pitchenik AE. Anti-tuberculosis drug-inuced hepatotoxicity. The role of hepatitis C virus and the human immunodeficiency virus. Am J Respir Crit Care Med. 1998;157:1871–1876. doi: 10.1164/ajrccm.157.6.9711039. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro MA, Lewis JH. Causality assessment of drug-induced hepatotoxicity: promises and pitfalls. Clin Liver Dis. 2007;11:477–505. doi: 10.1016/j.cld.2007.06.003. [DOI] [PubMed] [Google Scholar]