Abstract
A variety of reactions of singlet oxygen with metal-bound thiolates are described, and contrasted with the photooxidation of organic sulfides. Superficially, these two processes appear to involve similar mechanisms, but there are important differences: unlike the photooxidation of organic sulfides, the rate of the initial reaction of metal-thiolates with singlet oxygen (kt) appears to be affected by protic solvents and acids. The nucleophilicity of the thiolate moiety is reduced by addition of acids or in protic solvents, leading to significantly lower kt values. The primary intermediate in the photooxidation of organic sulfides is a nucleophilic persulfoxide, which can be stabilized by protic solvents or by addition of acid. However, the primary intermediate in the photooxidation of metal thiolates cannot be trapped with phosphite, suggesting that it may be less nucleophilic than its organic counterpart. Support for this hypothesis is also derived from the rather modest (compared with organic sulfides) acceleration of the rate of product formation by addition of acid.
Keywords: photooxidation, persulfoxide, metal thiolate, singlet oxygen, peroxide
Introduction
The reaction of singlet oxygen with organic sulfides proceeds via at least two transient intermediates (1–4). A large amount of research has focused on the nature of these extremely unstable species, none of which has been directly observed. Indeed, a recent review of ‘exotic types of unusual peroxides’ by Sawwan and Greer noted that the reactivity of heteroatom-containing peroxides is still waiting to be uncovered (5). Exploring the chemistry of ‘unusual peroxides’ (5) is of interest, because such peroxides may be the primary intermediates in many oxidation reactions initiated by reactive oxygen species. We hypothesize that oxidative damage attributed to reactive oxygen species may at times be caused by secondary reactions from unusual peroxidic intermediates. In this paper, we will first discuss the chemistry of singlet dioxygen with organic sulfides and dithiolato complexes followed by presentation of our own research with Co complexes bearing only one thiolato ligand.
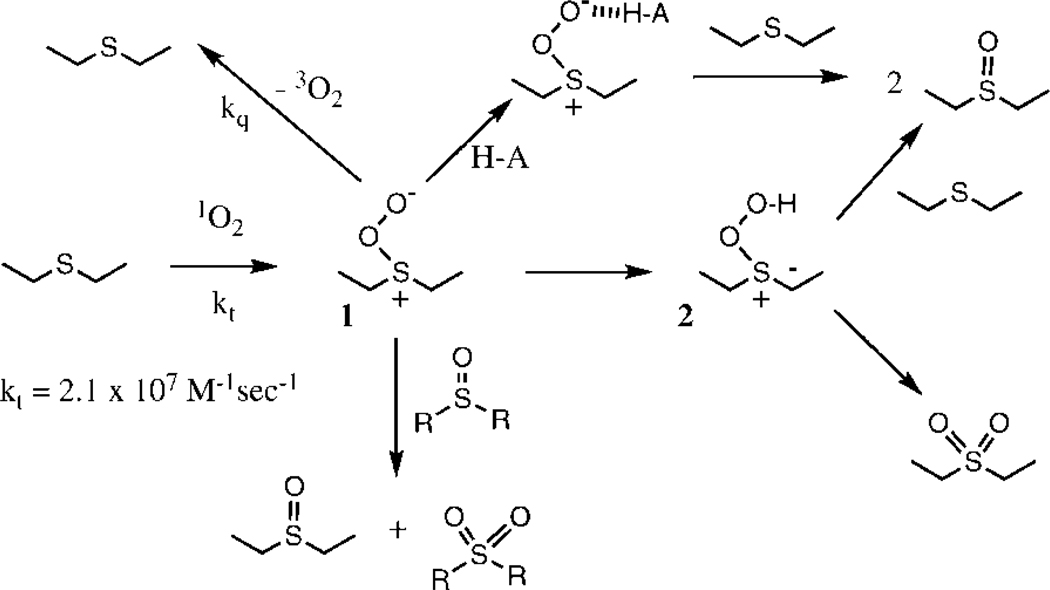
The primary adduct between singlet oxygen and organic sulfides such as diethylsulfide is almost certainly a zwitterionic persulfoxide (1) that exhibits nucleophilic reactivity. In a seminal paper (1), Liang et al. established that this primary intermediate is converted to a secondary intermediate that exhibits electrophilic reactivity. More recent studies by Jensen et al. strongly suggest that this second intermediate may be a hydroperoxy sulfonium ylide (2) (2); A cyclic thiadioxirane analogous to the recently observed (6) phosphadioxirane has been ruled out since the barrier of conversion from the persulfoxide to a thiadioxirane would be at least 20 kcal/mol which is incompatible with the experimental observation of the fleeting nature of the persulfoxide (7). The hydroperoxy sulfonium ylide subsequently reacts with starting material to give two equivalents of sulfoxide (usually, this is the major pathway) or intramolecularly collapses to sulfone. Since formation of the hydroperoxy sulfonium ylide requires abstraction of an α proton by the persulfoxide, this reactive channel is not available to arylsulfides. Indeed, the rate of product formation for arylsulfides is much lower than for alkylsulfides.
Another interesting feature of the photooxidation of organic sulfides is the low rate of conversion to product under aprotic conditions. The rate of conversion of the persulfoxide intermediate to final product can be less than 1% in dry aprotic solvents, but may approach 100% under protic conditions. Under aprotic conditions, most of the persulfoxide simply falls apart releasing ground state (triplet) dioxygen and the starting sulfide; this represents physical quenching (kq) of singlet oxygen. The overall mechanistic scheme for these various processes is depicted in Scheme 1, using diethyl sulfide as an example. Bonesi et al. have shown that for para-substituted methyl arylsulfides, the rate of singlet oxygen removal (kt), i.e. the process leading to the formation of persulfoxide, correlates linearly with the σ values of the substituents on the aryl ring (ρ = −1.97) (8). This result is reasonable, given that singlet oxygen is a strong electrophile. There does not appear to be any variation of the kt values with solvent. On the other hand, Albini and Bonesi et al. have shown (9, 10) that addition of acid dramatically increases the rate of product formation under aprotic conditions. This has been rationalized by protonation of the nucleophilic oxygen atom of the persulfoxide, thereby converting the protonated persulfoxide into a strong electrophile (ρ = −1.78 for the protonated persulfoxide of para-substituted thioanisoles) (8–10).
Scheme 1.
Mechanistic pathways during the photooxidation of diethyl sulfide.
Photooxidation of metal thiolato complexes
In contrast with the photooxidation of organic sulfides, the chemistry of singlet oxygen with metal-bound thiolates has only received attention during the past decade. Reactions of triplet dioxygen with metal-bound thiolate appear to be quite common, and in the few cases for which data for both triplet and singlet dioxygen is known, attack of triplet dioxygen on metal-bound thiolate leads to similar products as the corresponding reaction with singlet oxygen (11, references therein). This chemistry is of interest to bioinorganic chemists, since the M–S–cys motif is ubiquitous in nature. Indeed, there are numerous reports of oxidative damage and irreversible deactivation of sulfur-rich metalloenzymes. In many cases, there is a strong evidence that reaction of molecular oxygen with the thiolate ligand (i.e. S-bound cysteine) is responsible for the damage. For example, Xu and Wilcox have reported that exposure of Zinc fingers to oxygen leads to an increase in the mass by 32 mass units (12), suggesting formation of zinc-bound sulfinate. Similarly, the activities of CO-dehydrogenase and [NiFe] hydrogenase are inhibited by exposure to oxygen, apparently via oxidation of the thiolate ligand (13). Cornman et al. have reported that a V(V)-thiolate complex undergoes irreversible oxidation to a disulfenate complex upon reaction with H2O2 (14, 15). The oxidized complex shows an unusual η2-coordination of the sulfenate ligands. The mechanism of this oxidation is unknown, as is the exact role of the metal in this oxidation. Similar to the case for the reaction of singlet oxygen with organic sulfides, peroxidic intermediates have not been observed in any of these examples. It is quite possible that such peroxidic intermediates may actually be responsible for oxidative damage in biological systems, as these species are more reactive than the initial oxidant (hence the inability to observe these species). Understanding the reactivity of such intermediates is therefore essential to predict how S-bound cysteinate may be modified by dioxygen. On the other hand, in some cases, oxidized cysteine ligands may be essential for the function of an enzyme. For example, nitrile hydratase posses a mono-oxidized (i.e. sulfenic) and double-oxidized (i.e. sulfinic) cysteine ligand at the active site (16 and references therein). Kovacs et al. have published some elegant studies on how modified (i.e. oxidized) Co-bound cysteine affects the function of Nitrile hydratase (17–19). Unanswered questions in all of the work on oxidized Co-bound cysteine in Nitrile Hydratase are – How does nature control the oxidation of the cysteine such that one cysteine ligand is oxidized to a sulfenate while another one is oxidized to a sulfinate? What are the intermediates in this process, and how is their reactivity controlled? As clear from the review of previous work on the photooxidation of metal-bound thiolate and our own data presented in this paper, we are far from being able to formulate a general mechanism for this process. Superficially, the photooxidation of metal-bound thiolates appears to be similar to that of organic sulfides, but it is already known that there are important kinetic differences, as well as differences concerning the reactivity of the peroxidic intermediate(s).
Reaction of metal thiolato complexes with singlet oxygen
Most of the researches with respect to the chemistry of singlet oxygen with metal-bound thiolates have focused on metal complexes to which two thiolate ligands are bound. A priori, reaction of such a complex with singlet oxygen can lead to several products, viz:
Formation of sulfenate at one of the sulfur sites only. Formation of this product necessarily implies intermolecular oxygen atom transfer from a peroxidic intermediate.
Formation of a bis-sulfenate. This could occur via intramolecular oxygen atom transfer from the peroxidic intermediates or by sequential formation of sulfenate at one sulfur site via intermolecular oxygen atom transfer.
Formation of mono or bis sulfinate. This could occur via collapse of a peroxidic intermediate without oxygen atom transfer or by a secondary photooxidation of a sulfenate, or by attack of a persulfooxide intermediate on a sulfenate ligand.
Formation of a mixed sulfenato/sulfinato complex.
Photooxidation of Ni(II)-and Pd(II)-dithiolato complexes
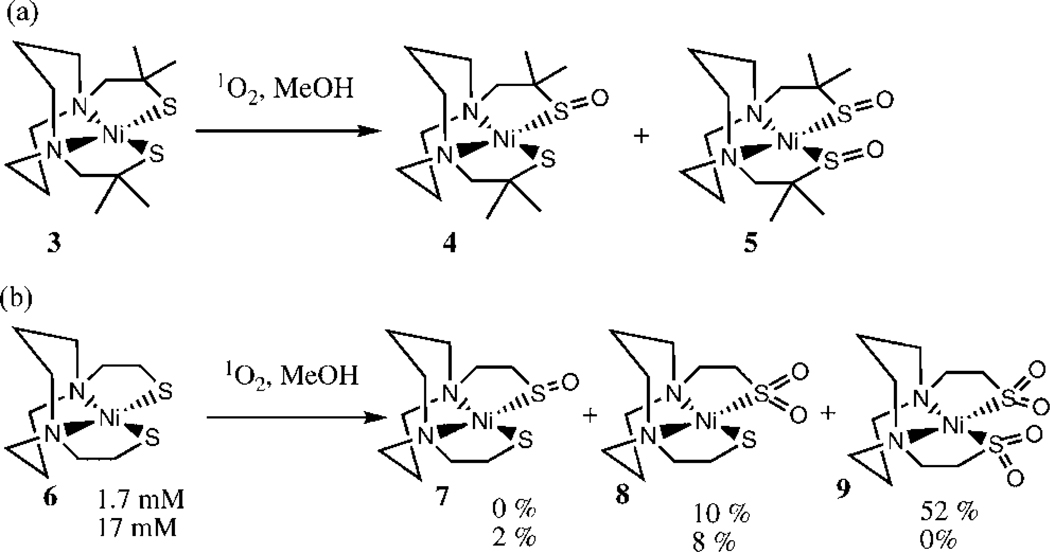
One of the most detailed studies of the photooxidation of dithiolate complexes was published by Grapperhaus et al. (20, 21). Remarkably, depending on conditions, all of the different possible products noted above were observed: reaction of the sterically hindered Ni(II)-dithiolate complex 3 in methanol with singlet oxygen led to formation of the monosulfento and the bissulfenato complexes 4 and 5, respectively (Scheme 2a). In contrast, the Ni(II) complex 6 forms the mono sulfenate 7, the mono sulfinato 8, and the bis sulfinato complex 9 upon photooxidation in methanol. At first glance, this result may seem rather puzzling. Formation of the mono sulfenato complexes 4 and 7 unambiguously establishes that a peroxidic intermediate is indeed capable of intermolecular oxygen atom transfer. It therefore seems rather surprising that no bis-sulfenate (which would result from intermolecular oxygen atom transfer) is observed for complex 6. Grapperhaus et al. suggested that such a bis sulfenato complex could indeed be formed, but that it would rapidly isomerize to the monosulfinato adduct 8 (21). For complex 3, this isomerization would be much more difficult due to the steric bulk of the four methyl groups, thus making it possible to observe complex 5.1
Scheme 2.
Photooxidation of Ni(II)-dithiolato complexes in methanol (20): (a) formation of bis-sulfenate with hindered dithiolate ligand; (b) no formation of bis-sulfenate with less hindered ligand.
The concentration dependence for the photooxidation of 6 (Scheme 2b) is consistent with several competing pathways. At high concentration, a small amount (2%) of mono sulfenate 7 is observed indicating that intermolecular oxygen transfer is one of the reactive channels during the reaction. However, the relatively large (8%) amount of sulfinate 8 indicates that collapse of the intermediate and/or rapid isomerization of a bis sulfenate are two major pathways. The absence of the sulfenate 7 at low concentration is consistent with a competition between an intramolecular and an intermolecular pathway from the same intermediate.
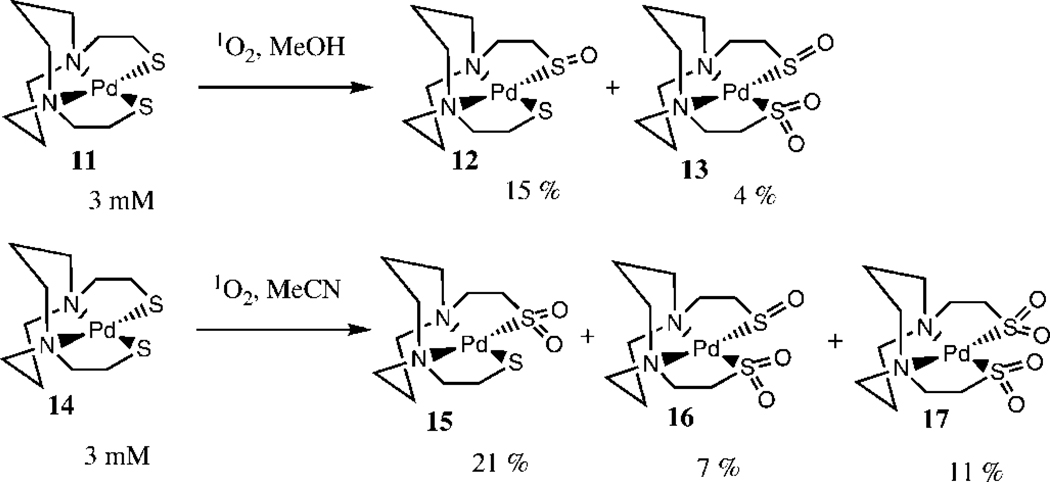
As noted previously, during the photooxidation of organic sulfides, protic solvents are believed to stabilize the primary intermediate, i.e. the persulfoxide. The photooxidation of complex 6 was also carried out in dry acetonitrile, and no mono sulfenate 7 was observed. It therefore seems possible that protic solvents may also prolong the lifetime of the peroxidic intermediate, so that it can undergo intermolecular oxygen atom transfer. Further support for this hypothesis can be derived from Darensbourg’s study of the photooxidation of related Pd-dithiolato complexes 11 and 14 (Scheme 3) (21). In methanol, formation of the mono-sulfenate 12 (which necessarily implies intermolecular oxygen atom transfer) was observed, while in acetonitrile this species could not be detected, and instead an appreciable amount of the mono-sulfinate 15 was formed.
Scheme 3.
Photooxidation of Pd-dithiolato complexes (21).
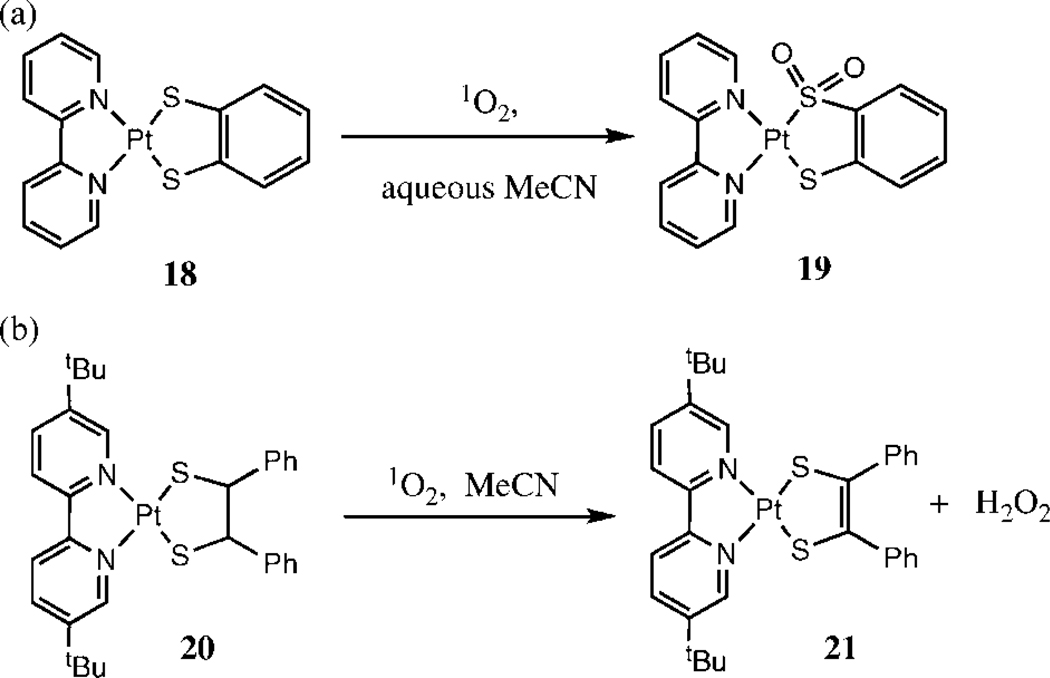
Several interesting Platinum (II)-dithiolato complexes have been studied by the groups of Connick and Gray (22) and Zhang et al. (23). These systems contain a bipyridine (bpy) ligand and a dithiolate. They are self-sensitized (24) and therefore no external photosensitizers were employed during these studies. Photooxidation of Pt(II)(bpy)(bdt) (18) (bdt = 1,2-benzenedithiolate) in wet acetonitrile leads to formation of the corresponding mono- and bis-sulfinato complexes; no sulfenate has been observed (Scheme 4a). On the other hand, photooxidation of Pt(II)(4,4’-ditert-butyl-2,2’-bipyridine)(meso-1,2-diphenyl-1,2-ethanedithiolate) (20, Pt(II)dbbpy)(dpdt) leads to the formation of an elimination product (21) (Scheme 4b). It has been suggested that the elimination product is formed by α-hydrogen abstraction by a persulfoxide intermediate, followed by the loss of and base-catalyzed eliminiation (23). Complex 18 does not have any α-hydrogen atoms to either sulfur atom, which would explain why S-oxygenated product 19 instead of the elimination product was formed (22).
Scheme 4.
Photooxidation of Pt-dithiolato complexes: (a) formation of a sulfinato complex (no α-hydrogen adjacent to sulfur atom) (22). (b) formation of elimination product (23).
Nature of the peroxidic intermediates in the photooxidation of dithiolate complexes
Very little is known about the nature of the intermediates in the photooxidations discussed above. For example, it is not clear whether the primary intermediate is always sufficiently nucleophilic to attack sulfenate, or whether or not it is in fact capable of α-hydrogen abstraction. Trapping experiments with diphenyl sulfoxide failed to trap any intermediate for the Ni(II) complexes (11). Several published mechanisms have invoked the formation of a metal-bound thiadioxirane as a secondary intermediate, in contrast with the photooxidation of organic sulfides. However, no compelling evidence has been presented for or against this formulation. One fascinating observation by Connick and Gray (22) during the photooxidation of complex 18 was a long-lived transient (τ > 1μs). Albini’s work on quenching of the persulfoxide by protic agents has established that the lifetime of the persulfoxide intermediate for organic sulfides must be at least 10−7 s(10). Assuming that the lifetime of a metal-bound persulfoxide is not significantly shorter than that of its organic counterpart, it seems possible that the transient observed by Gray et al. is indeed a metal-bound persulfoxide.
Photooxidation of Co(III)-thiolato complexes
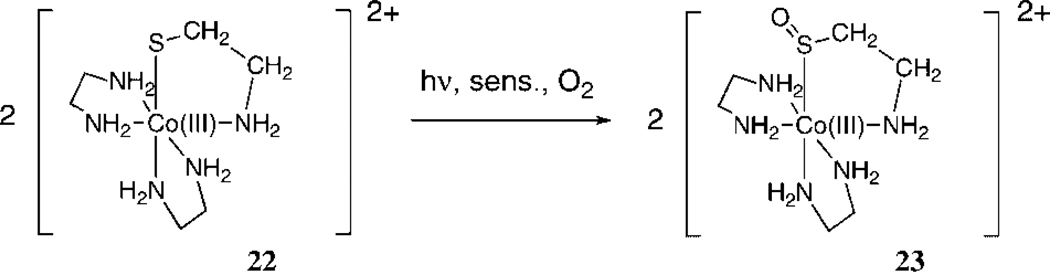
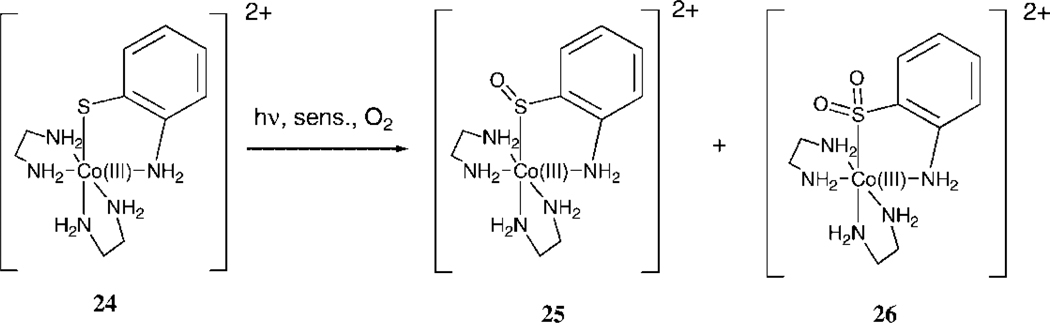
We have been studying the reaction of singlet oxygen with octahedral Co(III) complexes bearing one thiolate ligand only, i.e. (2-mercaptoethylamine-N,S)bis(ethylenediamine) Co(III)tetrafluoroborate (22) [Co(en)2(N,S-Aet)]2+ and (2-mercapto aniline-N,S)bis (ethylenediamine)Co(III) tetrafluoroborate (24) [Co(en)2(N,S-Mer)]2+ , and (Cysteinato-N, S)bis(ethylenediamine)cobalt(III) (25, 26).
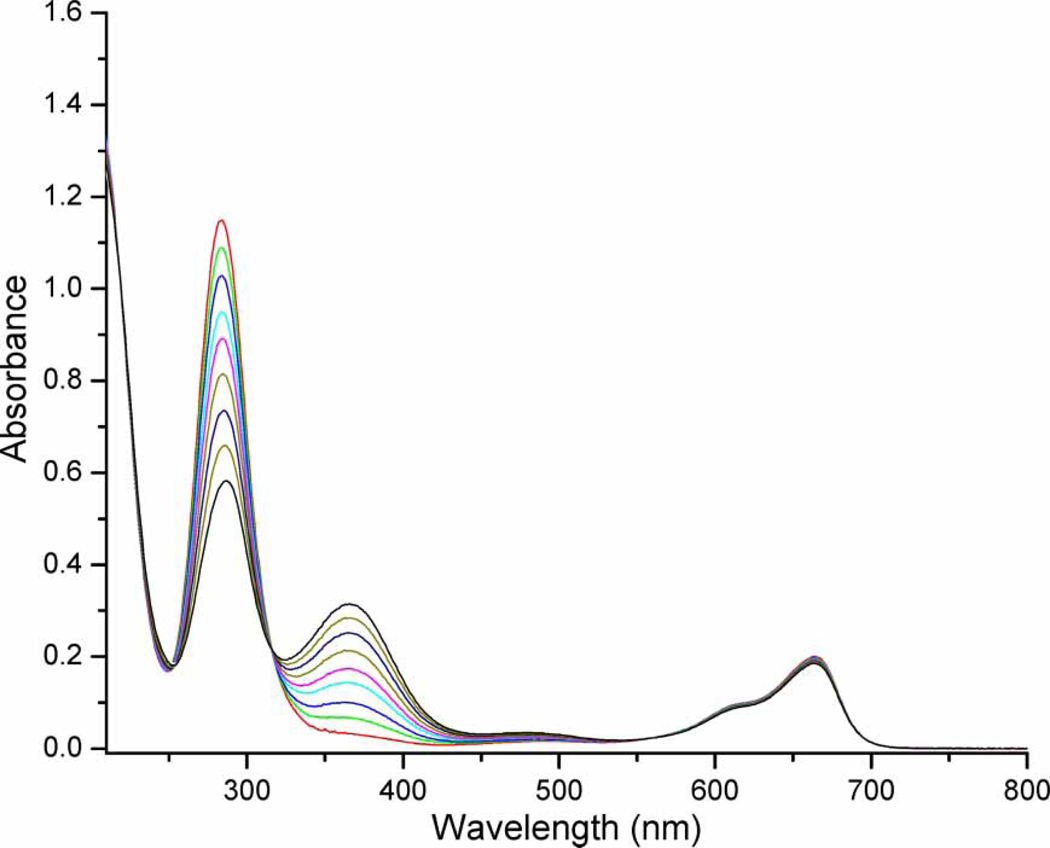
As previously published by our group, complex 22 reacts with singlet dioxygen to form the corresponding sulfenato complexe [Co(en)2(N,S(O)-Aet)]2+ (23) (23). Methylene Blue or Rose Bengal were used as sensitizers. The reaction proceeds in the same way for either sensitizer, except at high concentrations of 22 with Rose Bengal. In that case, aggregation was observed, and the reaction slowed dramatically. The photooxidation of 22 can be easily followed by UV/VIS spectroscopy. Figure 1 shows the electronic spectra of the photooxidation of complex 22.
Figure 1.
Photooxidation of [Co(en)2(N, S-Aet)]2+ (22) in water, total irradiation time = 3 min, sensitizer = methylene blue.
The band near 280 nm is typical for a Co(III)-SulfurLMCTband, and this peak decreases during the photooxidation; the peak at 365 nm corresponds to the sulfenate charge-transfer band (27) which increases during the photooxidation. Thus, intermolecular oxygen atom transfer appears to be the main pathway of reactivity for the peroxidic intermediate. Control experiments demonstrate that unlike for the Pt complexes 18 and 20, this reaction is not a self-sensitized photooxygenation. No reaction takes place upon irradiation under an oxygen atmosphere without the presence of the sensitizer. During the initial stages of the photooxidation of 22 (up to 80% conversion), there is no formation of the corresponding sulfinato complexes, regardless of solvent (see 1H NMR spectra in supplemental material). Small amounts of the sulfinate are observed at the later stages of the reaction, possibly via photooxidation of the sulfenate 23. Photooxidation of [Co(en)2(N,S-Mer)]2+ (24) also results in formation of the corresponding sulfenate 25, accompanied by formation of an appreciable amount of sulfinate [Co(en)2(N,S(O2)-Mer)]2+ (26). Other minor unidentified byproducts are also noted in the 1H NMR spectrum after the photooxidation of complex 24. Unlike for complex 22, some sulfinate 26 is formed during the early stages of the photooxidation of 24, regardless of solvent or sensitizer (i.e. Methylene Blue or Rose Bengal). Singlet oxygen reacts much faster with both Co(thiolato) complexes 22 and 24 than with their corresponding sulfenato adducts, and therefore the sulfinato complex 26 could not have been formed by a secondary reaction of the sulfenato complex 25 with singlet oxygen. It seems likely that two entirely different mechanistic pathways exist for the formation of these two products.
As previously reported by our group (26), we have measured the rates of singlet dioxygen disappearance (kt) by complexes 22 and 24 in several protic and aprotic solvents. The value of the rate constant kt thus obtained is the sum of all processes by which singlet dioxygen is removed. Thus the value of kt corresponds to the susceptibility of the cobalt-thiolato complex towards attack by 1O2. For complexes 22 and 24, the value of kt is highly solvent dependant: the rate of attack by singlet oxygen in DMF is about twice as high as in methanol and four times higher than in water (Table 1). This is in dramatic contrast with the photooxidation of organic sulfides, where there is a very little variation of kt for different solvents. If all of the peroxidic intermediate formed during the initial reaction of the thiolate with singlet oxygen were converted to the sulfenato product, then the rate of product formation kr would equal twice the total rate of singlet oxygen removal by the starting complex, i.e. kr = 2kt. Results for the values of the rates of singlet oxygen disappearance (kt) and chemical reaction rate constants (kr) are summarized in Table 1.
Table 1.
Rate constants of singlet oxygen disappearance (kt) and chemical reaction rate constants (kr) for metal thiolato complexes under various conditions.
| Compound | Solvemt | kt (× 108 M−1 s−1) | kr (×108 M−1 s−1) | kr/2kt × 100 % |
|---|---|---|---|---|
| 22a | DMF-d7 | 6.4 ± 0.8 | 0.01 ± 0.007 | 0.08% |
| 22a | CD3OD | 3.3 ± 0.9 | 1.2 ± 0.5 | 18% |
| 22a | D2O | 1.4 ± 0.2 | 0.84 ± 0.16 | 30% |
| 24a | DMF-d7 | 6.7 ± 0.4 | 0.01 ± 0.002 | 0.07% |
| 24a | CD3OD | 2.5 ± 0.3 | 0.12 ± 0.03 | 2.6% |
| 24a | D2O | 1.7 ± 0.8 | 0.36 ± 0.15 | 11% |
| 22b | CD3OD pH = 3 | 2.5 ± 0.2 | 0.6 ± 0.2 | 12% |
| 24b | CD3OD pH = 3 | 0.8 ± 0.1 | 0.3 ± 0.1 | 19 % |
| 18c | CH3CN | 1.0 | ||
| 20d | CH3CN | 3.0 |
As previously reported (25), values of the rates of total singlet oxygen removal (kt) and chemical reaction rates (kr) (Table 1) indicate that protic solvents have two opposite effects on the photooxidation of the thiolato complexes: protons reduce the value of kt, that is the rate of initial attack by the singlet oxygen molecule. Conversely, protic solvents dramatically increase the fraction of product formation vs. singlet oxygen removal, i.e. the ratio of kr/2kt. The latter effect is very similar to the effect on the reaction of organic sulfides with singlet oxygen (1–4) discussed above. For organic solvents, the effect has been attributed to the stabilization of the persulfoxide intermediate by hydrogen bonding (or formation of a sulfurane), and, based on our data, it is likely that the same type of effect occurs during the photooxidation of the cobalt complexes. On the other hand, the decrease in the value of kt under protic conditions for complexes 22 and 24 is different from what is observed for organic sulfides. The decrease in the rate of initial attack by singlet oxygen (kt) in protic solvents could be due to a decrease in the nucleophilicity of the thiolate ligand due to hydrogen bonding2.
Effect of acidity
We now report additional observations on how protic solvents and acidity affect the reaction of singlet oxygen with the sulfur atom of metal thiolate. We have found that in deuterated methanol at pH smaller than 7, the total rate of singlet oxygen removal can be somewhat reduced. For example, at pH = 3, the rate constant for singlet oxygen removal for complex 24 is only 0.8 ± 0.1 × 108 M−1s−1, or slightly less than one-third of the value in deuterated methanol at neutral pH. On the other hand, for complex 22, the decrease of kt does not appear to be statistically significant (Table 1). No decomposition of the unreacted complex is noted in either case, nor are there any changes in the UV/VIS spectrum. This observation is in agreement with the suggestion that the magnitude of kt is simply an expression of the nucleophilicity of the metal-bound sulfur atom. We have also investigated the effect of protons on the rate of product formation. For complex 22, there was no change in the kr value within the limits of error for kr under neutral and acidic conditions. The rate of product formation (kr) in methanol does increase by a factor of about three at pH = 3 for complex 24 under acidic conditions. Likewise, addition of protons in DMF appeared to increase the rate of product formation, although this effect is much more modest (not more than 2- to 3-fold) compared to the addition of acids to organic sulfides in aprotic solvents noted by Bonesi and Albini (9).
Trapping experiments
We have also attempted to establish whether or not a nucleophilic peroxidic intermediate is formed. We had previously noted that a very large amount of DMSO (20% by vol) is need to alter the balance of sulfenate vs. sulfinate formation for the complex (Cysteinato-N, S)bis(ethylenediamine)cobalt(III) (26). Nahm and Foote (28) have used trimethyl phosphate as a highly efficient trap for nucleophilic peroxides. In particular, they noted that the phosphite is a much more efficient trap for the nucleophilic persulfoxide than DMSO. However, our attempts to trap any peroxidic intermediate formed during the photooxygenation of 24 with P(OMe)3 have uniformly failed, even when a 10-fold excess of the phosphite was used. This result is similar to Darensbourg’s failure to trap intermediates in the Ni dithiolate systems with diphenyl sulfoxide.
Discussion
One of the most important mechanistic issues in the photooxidation of metal-bound thioate ligands is the formation of sulfenate vs. sulfinate. Formation of sulfenate unambiguously implies that peroxidic intermediates are capable of transferring an oxygen atom to external substrates. Clearly, a large number of variables determine which process predominates. Furthermore, in the presence of a second thiolate ligand, isomerization of an initially formed bis-sulfenate to a sulfinate and thiolate appears to be a possible complication, i.e. the observed products may in fact be secondary products. Nevertheless, Darensbourg’s results seem to indicate that high dilution favors sulfinate formation (cf. Scheme 2), as would be expected if both products can be formed from the same intermediate. The effect of protons on the course of the reaction seems to be even more complex than in the reaction with organic sulfides. It is fairly clear that the susceptibility of an unoxidized thiolate ligand towards photooxidation depends on hydrogen bonding, resulting in a large solvent effect on the value kt. The decrease of kt under acidic conditions noted in this study is in agreement with this hypothesis, as are the recent observation by Smith et al. who noted that intramolecular hydrogen bonding decreases the nucleophilic attack of Zn-thiolates on methyl iodide (29, 30). On the other hand, protic solvents do increase the conversion of the peroxidic intermediate to form oxygenated products, as does the addition of acid. However, the latter effect is much less pronounced than for organic sulfides. In some cases, protic solvents appear to favor formation of sulfenate over sulfinate (cf. Scheme 3), consistent with their apparent role in stabilizing the persulfoxide intermediate. However, it is not known if this effect on the product distribution is a general one. The role of α-hydrogens adjacent to the sulfur atom is even more difficult to ascertain. The results of the monthiolato Co(III) complexes presented in this study seem to indicate that product formation is more efficient in the presence of such hydrogen atoms (cf. the kr/2kt ratios for complex 22 vs. 24 in Table 1). This may indicate that a hydroperoxy sulfonium ylide type intermediate may be a viable second intermediate formed from the metal-bound persulfoxide. On the other hand, the presence of such hydrogens clearly is not required (and therefore formation of such an intermediate is not the only pathway), as the formation of the Pt complex 19 and our results for complex 24 attest. The observation that an appreciable amount of the sulfinato complex 26 is formed from 24 indicates that the lack of an α-hydrogen may make an intramolecular reaction of the peroxidic intermediate competitive with intermolecular oxidation pathways. Finally, the peroxidic intermediate for the metal-bound thiolate photooxidations appears to be somewhat less nucleophilic, as indicated by the failure of several trapping experiments.
In summary, while the mechanisms of the photooxidation of metal thiolates and organic sulfide appear to be similar, there are in fact significant differences. Protic solvents decrease the susceptibility of the metal-bound thiolate toward attack by singlet oxygen, and the peroxidic intermediate appears to be less nucleophilic. Further experiments are needed to establish the factors that determine the reaction pathways from the primary peroxidic intermediate formed upon attack of singlet dioxygen on metal-bound thiolate.
Experimental section
Instrumentation
1H and 31P NMR spectra were obtained on a Bruker DRX400 instrument. Low-temperature spectra were obtained by standard VT NMR techniques. Absorption spectra were recorded on a Carey 300UV–VIS spectrophotometer. The apparatus for time-resolved singlet oxygen luminescence quenching experiments has been described elsewhere (31). Methylene blue was used as a sensitizer during the luminescence quenching experiments, and substrate concentrations ranged from 1 × 10−5 M to 1 × 10−3 M.
Materials
All solvents and materials were commercially available and used as received. Cobalt(II) tetrafluoroborate hexahydrate, ethylenediamine, cystamine dihydrochloride, 2,2’-dithiodianiline, sodium tetrafluoroborate, and tetrafluoroboric acid solution (48% in H2O), were purchased from Sigma-Aldrich. NMR solvents were obtained from Cambridge Isotope Laboratories.
General procedures for photooxidation of complexes 22 and 24
Singlet oxygen was produced via continuous irradiation with a Oriel Tungsten–Halogen 300 W lamp, using Methylene blue or Rose Bengal as sensitizers. Rose Bengal was only used at low substrate concentration (1 mmol or less), to avoid aggregation. The starting Cobalt complexes 22 and 24 were prepared by literature procedures (32, 33); their concentrations during the photooxidation experiments ranged from 0.1 to 50 mmol. A cut-off filter at 492 nm was employed to prevent excitation of the Co complexes. The reactions can be carried out in simple test tubes or directly in NMR tubes. Products were monitored by UV/VIS and 1H NMR spectroscopy, as well as comparison with authentic samples that were obtained from 22 and 24 via oxidation with 30% aqueous hydrogen peroxide. For 1H NMR experiments of complex 22, the methylene protons of the aet ligand are two multiplets at 2.14 and 2.23 ppm, respectively. These peaks disappear during the photooxidation of 22, while two new peaks at 3.17 and 3.24 ppm appear, corresponding to the protons of the sulfeante 23 (see supplemental information). For complex 24, the aromatic region of the spectrum is most useful to monitor the course of the reaction (see supplemental information). The protons of the ethylene diamine ligands of both cobalt complexes and their photooxidation products overlap each other and consequently these signals provide no useful information during the course of the reactions.
Competition experiments
In order to evaluate the rate of product formation (kr), competition experiments were carried out between the thiolate complexes and two anthracene derivatives, namely 9,10-dimethylanthracene in DMF and methanol and 3-(10-(2-carboxy-ethyl)-anthracen-9-yl)-propionate in water; their kr values have been previously remeasured (23). Methylene Blue was used as a sensitizer. These two anthracene derivatives react with singlet dioxygen only via chemical reaction to give the corresponding 9,10-endoperoxides. Products were analyzed by monitoring the 1H NMR signals of the alkyl groups for the anthracence derivatives and by UV/VIS spectrsocopy for the Co complexes. For the competetion experiments under acidic conditions, a few µl of aqueous HCl was added to the methanol solution until the pH was adjusted to 3. The competition experiments were carried out under continuous irradition such that the two substrates compete for one reactive intermediate (i.e. singlet oxygen) that is produced under steady-state conditions (i.e. by continuos irradiation). All results were fitted into the equation by Higgins et al. (34). The rate ratio thus obtained is independant of the singlet oxygen concentration in solution. A control experiment demonstrated that no oxidation of complexes 22 or 24 by the 9,10-dimethylanthracene endoperoxide product occurs.
Trapping experiments with trimethyl phosphite
Trimethyl phosphite is nearly unreactive toward 1O2, but very efficient in trapping the nucleophilic intermediate in the photooxidation of organic sulfides (28). To trap the intermediate in the reaction of 1O2 with Cobalt complex 24 in CD3OD, up to 10 equivalents of trimethyl phosphite were photooxidized in an NMR tube (sensitizer: Methylene blue). The reactions were monitored by 31P NMR. There was no difference in the ratio of trimethyl phosphate (31P NMR: 3 ppm) and trimethyl phosphite (31P NMR: 140 ppm) as compared to a sample in which only trimethyl phosphite was photooxidized under the same conditions.
Scheme 5.
Photooxidation of thiolato complex 22.
Scheme 6.
Photooxidation of thiolato complex 24.
Acknowledgements
This research was supported by a Henry Dreyfus Teacher-Scholar Award. Support by the NIH-NIGMS MBRS program (Award number GM08101) is also gratefully acknowledged.
Footnotes
Supporting information available
This material is available free of charge via the internet at www.informaworld.com
References
- 1.Liang JJ, Gu CL, Kacher ML, Foote CS. J. Am. Chem. Soc. 1983;105:4717–4721. [Google Scholar]
- 2.Jensen F, Greer A, Clennan EL. J. Am. Chem. Soc. 1998;120:4439–4449. [Google Scholar]
- 3.Clennan EL. Acc. Chem. Res. 2001;34:875–884. doi: 10.1021/ar0100879. [DOI] [PubMed] [Google Scholar]
- 4.Clennan EL, Pace A. Tetrahedron. 2005;61:6665–6691. [Google Scholar]
- 5.Sawwan N, Greer A. Chem. Rev. 2007;107:3247–3285. doi: 10.1021/cr0400717. [DOI] [PubMed] [Google Scholar]
- 6.Ho DG, Gao R, Celaje J, Chung H-Y, Selke M. Science. 2003;302:259–262. doi: 10.1126/science.1089145. [DOI] [PubMed] [Google Scholar]
- 7.Jensen F. J. Org. Chem. 1992;57:6478–6487. [Google Scholar]
- 8.Bonesi SM, Fagnoni M, Albini A. J. Org. Chem. 2004;69:928–935. doi: 10.1021/jo035679e. [DOI] [PubMed] [Google Scholar]
- 9.Bonesi SM, Albini A. J. Org. Chem. 2000;65:4532–4536. doi: 10.1021/jo000069p. [DOI] [PubMed] [Google Scholar]
- 10.Albini A, Bonesi SM. J. Photosci. 2003;10:1–7. [Google Scholar]
- 11.Grapperhaus CA, Darensbourg MY. Acc. Chem. Res. 1998;31:451–459. [Google Scholar]
- 12.Xu Y, Wilcox DE. J. Am. Chem. Soc. 1998;120:7375–7376. [Google Scholar]
- 13.Henderson RK, Bouwman E, Spek AL, Reedijk J. Inorg. Chem. 1997;36:4616–4617. doi: 10.1021/ic9707218. [DOI] [PubMed] [Google Scholar]
- 14.Huyer G, Liu S, Kelly J, Moffat J, Payette P, Kennedy B, Tsaprailis G, Gresser MJ, Ramachandran C. J. Biol. Chem. 1997;272:843–851. doi: 10.1074/jbc.272.2.843. [DOI] [PubMed] [Google Scholar]
- 15.Cornman RC, Stauffer TC, Boyle PD. J. Am. Chem. Soc. 1997;119:5986–5987. [Google Scholar]
- 16.Harrop TC, Mascharak PK. Acc. Chem. Res. 2004;37:253–260. doi: 10.1021/ar0301532. [DOI] [PubMed] [Google Scholar]
- 17.Kitagawa T, Dey A, Lugo-Mas P, Solomon EI, Kovacs JA. J. Am. Chem. Soc. 2006;128:14448–14449. doi: 10.1021/ja064870d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lugo-Mas P, Dey A, Xu L, Davin SD, Benedict SJ, Kaminsky N, Hodgson KO, Hedman B, Solomon EI, Kovac JA. J. Am. Chem. Soc. 2006;128:11211–11221. doi: 10.1021/ja062706k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theisen RM, Shearer J, Kaminsky W, Kovacs JA. Inorg. Chem. 2004;43:7682–7690. doi: 10.1021/ic0491884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grapperhaus CA, Darensbourg MY, Sumner LW, Russell DH. J. Am. Chem. Soc. 1996;118:1791–1792. [Google Scholar]
- 21.Grapperhaus CA, Maguire MJ, Tuntulani T, Darensbourg M. Inorg. Chem. 1997;36:1860–1866. doi: 10.1021/ic970050d. [DOI] [PubMed] [Google Scholar]
- 22.Connick WB, Gray HB. J. Am. Chem. Soc. 1997;119:11620–11627. [Google Scholar]
- 23.Zhang Y, Ley KD, Schanze KS. Inorg. Chem. 1996;35:7102–7110. doi: 10.1021/ic960685x. [DOI] [PubMed] [Google Scholar]
- 24.Shukla S, Kamath SS, Srivastava TS. J. Photochem. Photobiol. A. Chem. 1989;50:199–207. [Google Scholar]
- 25.Hernandez B, Wang Y, Zhang D, Selke M. Chem. Commun. 2006;9:997–999. doi: 10.1039/b514601a. [DOI] [PubMed] [Google Scholar]
- 26.Galvez C, Ho DG, Azod A, Selke M. J. Am. Chem. Soc. 2001;123:3381–3382. doi: 10.1021/ja003993+. [DOI] [PubMed] [Google Scholar]
- 27.Heeg MJ, Elder RC, Deutsch E. Inorg. Chem. 1979;18:2036–2039. [Google Scholar]
- 28.Nahm K, Foote CS. J. Am. Chem. Soc. 1989;111:1909–1911. [Google Scholar]
- 29.Smith JN, Shirin Z, Carrano CJ. J. Am. Chem. Soc. 2003;125:868–869. doi: 10.1021/ja029418i. [DOI] [PubMed] [Google Scholar]
- 30.Smith JN, Hoffman JT, Shirin Z, Carrano CJ. Inorg. Chem. 2005;44:2012–2017. doi: 10.1021/ic048630f. [DOI] [PubMed] [Google Scholar]
- 31.Zhang D, Ye B, Ho DG, Gao R, Selke M. Tetrahedron. 2006;62:10729–10733. [Google Scholar]
- 32.Lane RH, Sedor FA, Gilroy MJ, Eisenhard PF, Bennett RX, Ewall RX, Bennett LE. Inorg. Chem. 1977;16:93–101. [Google Scholar]
- 33.Dickman K, Doedens RJ, Deutsch E. Inorg. Chem. 1980;19:945–950. [Google Scholar]
- 34.Higgins R, Foote CS, Cheng H. Adv. Chem. Ser. 1968;77:102–117. [Google Scholar]