Abstract
Four flavonoids including luteolin, apigenin, 3′-hydroxygenkwanin and genkwanin were isolated and purified from Daphne genkwa Sieb. et Zucc. by high-speed countercurrent chromatography (HSCCC). Preparative HSCCC with a two-phase solvent system composed of n-hexane-ethyl acetate-methanol-water (5:7:5:5, v/v) was successfully performed by increasing the flow rate of the mobile phase from 1.2 to 2.0 mL/min after 260 min. In a one-step operation, 150 mg of the extracts of D. genkwa was separated to yield 8 mg of luteolin, 25.8 mg of apigenin, 23.6 mg of 3′-hydroxygenkwanin and 35.3 mg genkwanin with the purities of 91.2, 97.4, 94.3 and 95.8%, respectively, analyzed by HPLC using area normalization method. The chemical structures of the four compounds were identified by HPLC, ESI-MS, and 1H NMR.
Keywords: Daphne genkwa Sieb. et Zucc., Flavonoid, High-speed countercurrent chromatography
INTRODUCTION
Daphne genkwa Sieb. et Zucc. (Thymelaeaceae) is a medicinal plant widely distributed in China and has long been known as the medicinal agents for anti-inflammation, anti-leukemia, anti-tumor, analgesia, and tranquilization[1-3]. The plant has also been used as the effective remedy for treating edema, asthma, and cancer in Korea and China[4]. Chemical investigations have disclosed that the flower buds of D. genkwa contain several types of compounds, including flavonoids, daphne diterpene esters, volatile oil, biscoumarin[3,5-9] ,in which apigenin, luteolin, genkwanin, 3-hydroxygenkwanin, are the four major flavonoids and show anti-inflammatory, anti-leukemia, and antitussive activities[5,6,10,11].In view of their pharmacological activities and broad medical applications, a large quantity of pure compounds is needed for further pharmacological study. Therefore, an effective preparative method for isolation and purification of flavonoids from D. genkwa is indispensable. The separation and purification of flavonoids from D. genkwa by classical methods requires multiple chromatographic steps using silica gel, diaion HP-20, polyamide column, sephadex LH-20, etc.[3,7,9,12-15]. These methods are tedious and time consuming with a potential risk of loss of target compounds due to the highly-adsorptive effect of the solid matrix. High-speed countercurrent chromatography (HSCCC) is a unique liquid–liquid partition chromatographic technique without using solid matrix, thereby eliminating irreversible adsorption. Therefore, HSCCC separation can yield a highly efficient separation of a large amount of samples in several hours. The method also permits introduction of crude samples directly into the column without extensive preparation, and has been successfully applied for isolation and purification of a large number of natural products[16-24]. In the past, three flavonoids have been purified from D. genkwa by combined use of macroporuous resin column chromatography and HSCCC[25]. In this paper, four flavonoids were purified by HSCCC by a sinle run, thus eliminating the sample loss by irreversible adsorption onto the solid support.
EXPERIMENTAL
Apparatus
The preparative HSCCC apparatus (GS10A, Beijing UE Biotech., Beijing, China) was equipped with a polytetrafluroethylene (PTFE) multilayer coil (110 m × 1.6 mm I.D.) with a total column capacity of 240 mL. The β value of the preparative column varied from 0.5 at the internal terminal to 0.7 at the external terminal (β = r/R, where r is the rotation radius or the distance from the coil to the holder shaft, and R is the revolution radius or the distances between the holder axis and central axis of the centrifuge). A 10-mL-loop manual sample injection valve was used to introduce the sample into the coil. The rotation speed of the apparatus can be regulated from 0 to 1000 rpm. The system was also equipped with an NS-1007 constant-flow pump, a Model 8823A-UV detector (Beijing Institute of New Technology Application, Beijing, China) monitoring at 280 nm, and a Yakogawa 3057 recorder.
The analytical HPLC system consisted of a Shimadzu LC-10ATvp Multisolvent Delivery System, a Shimadzu SPD-M10Avp UV detector, an injection valve (Model 7726) with a 20-μL loop, a system controller (SCL-10AVP) and a Shimadzu LC Solution Workstation (Shimadzu, Japan). The nuclear magnetic resonance (NMR) spectrometer was a Mercury Plus 400 NMR system (Varian Inc., USA). The electro-spray mass spectrometer was a LCQ Deca XP Max system (Finnigan, USA).
Reagents and Materials
All organic solvents used for preparation of crude samples and HSCCC separation were of analytical grade (Guangzhou Chemical Reagent Co. Ltd., Guangzhou, China). Methanol used for HPLC was of chromatographic grade (Dikma Co., Ltd., Beijing, China), and water was distilled. D. genkwa was purchased from Bozhou Chigusa Pharmaceutical Factory in Pieces. (Anhui, China) and indentified by Doctor Qingzhen Ran (Guangzhou University of Chinese Medicine, Guangzhou, China).
Preparation of Crude Extract
One thousand grams of dry D. genkwa was shattered to powder ( 20-40 mesh) and thrice extracted with 3500 mL of 80% ethanol at 80°C for 3, 2, and 1 h, consecutively. After filtration, the extracts were combined and evaporated to dryness under reduced pressure at 60°C, and the residue was redissolved in 20% ethanol (total volume 500 mL). This enriched extract was then thrice extracted with ethyl acetate (600 mL each time). The ethyl acetate solutions were combined and evaporated to dryness under reduced pressure at 60°C, yielding 30 g of the crude sample, which was stored in a refrigerator at 4°C for subsequent HSCCC separation.
Selection of Two-Phase Solvent System
n-Hexane-ethyl acetate-methanol-water was used as the two-phase solvent system for HSCCC. The suitable volume ratio of the two-phase solvent system was determined according to the partition coefficient (K) of each target component. The K value of each component was determined by HPLC as follows: a suitable amount of crude extract was dissolved in the lower phase of the two-phase solvent system. The solution was then analyzed by HPLC. The peak area was recorded as A1. An equal volume of the upper phase was then added to the solution which was mixed thoroughly. After partition equilibration was reached, the lower phase solution was analyzed by HPLC again, and the peak area was recorded as A2. The K value was calculated using the following equation:
| (1) |
Preparation of Two-Phase Solvent System
N-hexane–ethyl acetate–methanol–water (5:7:5:5, v/v) was used as the two-phase solvent system for the HSCCC separation. It was prepared by adding the solvent to a separatory funnel in the prescribed volume ratios and thoroughly equilibrated by repeated shaking and degassing. The upper phase and the lower phase were separated and degassed by sonication for 30 min shortly before use. The sample solution was prepared by dissolving 150 mg of the crude extract in 10 mL of two-phase solvent system (1:1, v/v) for isolation and purification by HSCCC.
HSCCC Separation Procedure
HSCCC separation was performed as follows: The multilayer coiled column was first entirely filled with the upper phase as the stationary phase. The lower aqueous mobile phase was then pumped into the head end of the column inlet at a flow rate of l.2 mL/min, while the apparatus was run at a revolution speed of 700 rpm. After hydrodynamic equilibrium was reached, 10 mL of sample solution containing 150 mg of the crude extract was injected through the injection value. The effluent from the tail end of the column was continuously monitored with a UV detector at 280 nm and the chromatogram recorded. Each peak fraction was collected according to the elution profile and analyzed by HPLC. After the separation was completed, retention of the stationary phase was measured by collecting the column contents into a graduated cylinder by forcing them out of the column with pressurized nitrogen gas.
HPLC Analysis and Identification of Crude Sample and HSCCC Peak Fractions
The ethyl acetate extract from D. genkwa and the peak fraction obtained by HSCCC were analyzed by analytical HPLC. The analysis was performed with a Shimadzu ODS C18 column (250 mm × 4.6 mm I.D., 5 μm). The mobile phase, composed of methanol-0.1% aqueous acetic acid was eluted with gradient elution (0-10 min, 30-50%, 10-23 min 50%, 23-43 min 50-100%, 43-50 min 100% methanol), at a flow rate of 1.0 mL/min, with a column temperature of 30°C. The effluent was monitored at 330 nm by a Shimadzu photodiode array detector .
Identification of the HSCCC peak fractions was carried out by HPLC, ESI-MS, 1H NMR.
RESULTS AND DISCUSSION
Optimization the HPLC Conditions
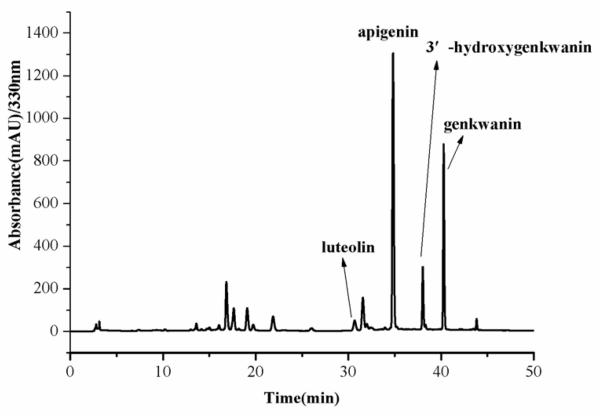
The crude extract, the partition coefficient (K) of each component in crude extract and the fractions obtained by HSCCC were analyzed by HPLC, thus an effective HPLC method was sought, beginning with the crude extract. Some papers have been reported for detection of the chemical from D. genkwa by HPLC[25–27], in which only three compounds (apigenin, 3′-hydroxygenkwanin, genkwanin) have been determined. Various combinations of mobile phase (methanol–water, methanol–aqueous acetic acid with different concentrations of acetic acid), flow rate, and detection wavelength were tested in both the isocratic- and gradient-elutionmodes. The optimum separation conditions were achieved using methanol–0.1% aqueous acetic acid as a pair of solvents in the gradient mode(methanol: 0-10 min, 30-50%;10-23 min, 50%; 23-43 min, 50-100%, 43-50 min, 100%) at a flow rate of 1.0 mL/min, with a column temperature of 30°C and an effluent monitoring wavelength at 330 nm. The ethyl acetate extract and the fractions separated by HSCCC were analyzed by HPLC under the optimum analytical conditions. The HPLC chromatogram of the crude extract is given in Fig. 2.
Fig.2.
HPLC chromatograms of ethyl acetate extract from D. genkwa Conditions: column, reversed-phase Shimadzu ODS C18 column (250 mm × 4.6 mm I.D., 5μm). HPLC conditions are in the text.
Optimization of Two-Phase Solvent System
Successful separation of the target compounds using HSCCC requires a suitable two-phase solvent system. In this work, a simple method was used to select a suitable two-phase solvent system. As suggested by Ito[28], if the sample is moderately polar, the search may start at n-hexane-ethyl acetate–methanol–water (1:1:1:1,v/v) but if the sample is of strong polarity start at n-butanol–ethyl acetate–water (4:1:4,v/v). In our research, the sample was an ethyl acetate extract which would contain moderately polar compounds. Then, n-hexane–ethyl acetate–methanol–water was selected as the two-phase solvent system, since this two-phase solvent system can be used to separate the compounds with a broad range of their hydrophobicity by modifying the volume ratio of the four solvents. Table 1 shows K values of four target compounds presented in the crude extract in the two-phase solvent system composed of n-hexane–ethyl acetate–methanol–water at various volume ratios at 4:5:4:5, 1:2:1:2, 7:3:5:5, 6:4:5:5, 5:7:5:5, and 1:1:1:1(v/v).
Table 1.
K-values of the target compounds in several two-phase solvent systems.
| Solvent system (n-hexane–ethyl acetate–methanol –water) (v/v) |
K-values |
|||
|---|---|---|---|---|
| luteolin | apigenin | 3′-hydroxygenkwanin | genkwanin | |
| 4:5:4:5 | 0.35 | 0.69 | 0.55 | 0.40 |
|
| ||||
| 1:2:1:2 | 0.41 | 0.39 | 0.38 | 0.19 |
|
| ||||
| 7:3:5:5 | 0.05 | 0.31 | 0.31 | 0.51 |
|
| ||||
| 6:4:5:5 | 0.24 | 0.25 | 0.51 | 1.13 |
| 5:7:5:5 | 0.70 | 0.86 | 1.66 | 2.60 |
| 1:1:1:1 | 0.27 | 0.47 | 1.11 | 1.48 |
The K-values were calculated according to the equation (1).
The results indicated that the solvent systems composed of n-hexane–ethyl acetate–methanol–water at the volume ratios of 4:5:4:5, 1:2:1:2, 7:3:5:5 (v/v) give too small K values and were not suitable for separation of the four target compounds. In Table 1, the K values of genkwanin in the solvent systems composed of n-hexane–ethyl acetate–methanol–water at the volume ratios of 4: 5: 4: 5 and 1: 2: 1: 2 (v/v) were lower than other compounds, because genkwanin is the lowest polarity compound. When n-hexane–ethyl acetate–methanol–water at the ratio of 6:4:5:5 and 1:1:1:1 (v/v) were used as the two-phase solvent system, the K values were much improved, but luteolin was eluted near solvent front with polar impurities. When n-hexane–ethyl acetate–methanol–water at a volume ratio of 5:7:5:5 (v/v)were used as the two-phase solvent system, the K values became suitable and all target compounds could be well separated from each other. Therefore, the two-phase solvent system composed of n-hexane–ethyl acetate–methanol–water 5:7:5:5 (v/v) was selected in the present study. A preliminary HSCCC experiment was carried out at a constant flow rate of 1.5 mL/min. As shown in Fig. 3A, peaks I, II and III were not well resolved, while genkwanin (peak IV) was retained in the column over 400 min. In the subsequent studies, the flow rate of the mobile phase was increased stepwise in such a way that the elution was started at 1.2 mL/min up to 260 min which was then increased to 2.0 mL/min to quickly elute peak IV. Consequently, four fractions were obtained from 150 mg crude extract in 400 min as shown in Fig. 3B. The retention of stationary phase in this separation was 67%.
Fig. 3.
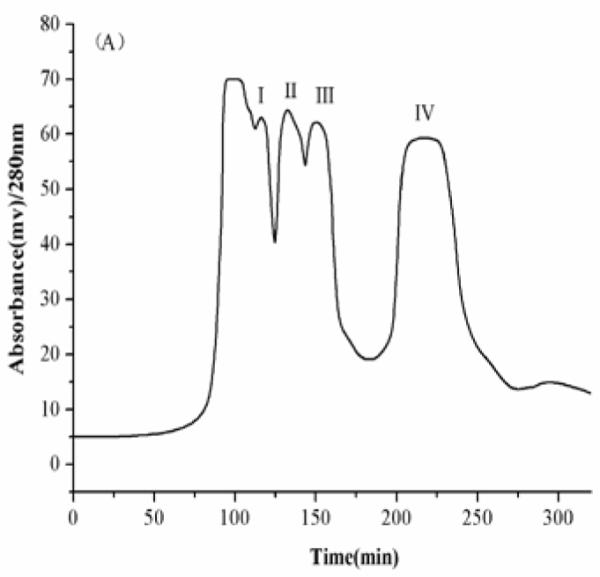
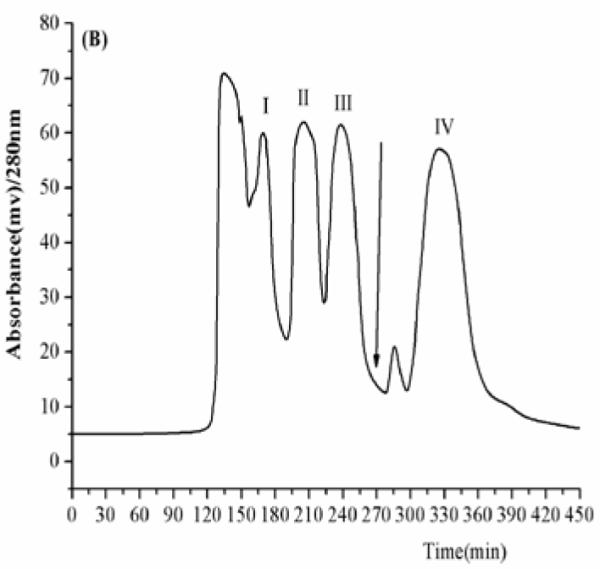
Preparative HSCCC chromatogram of the ethyl acetate extract from D. genkwa; solvent system: n-hexane-ethyl acetate–methanol–water (5:7:5:5, v/v); stationary phase: upper phase; mobile phase: lower phase; revolution speed: 700 rpm; detection wavelength: 280 nm; sample size: 150 mg; injection volume: 10 mL; (A) flow rate: 1.5 mL/min; retention of the stationary phase: 60%.; (B) flow rate: 0-260 min at 1.2 mL/min and 260-450 min at 2.0 mL/min; retention of the stationary phase: 67%. The arrow indicates that the flow rate of the mobile phase was increased stepwise from 1.2 to 2.0 mL/min after 260 min.
As shown in Fig. 4, the HPLC analysis of each HSCCC fraction revealed that from 150 mg of the crude sample four flavonoids were obtained as follows fraction I (luteolin, 8mg), fraction II (apigenin, 25.8 mg), fraction III (3′-hydroxygenkwanin, 23.6 mg), fraction IV (genkwanin, 35.3 mg) at purities of 91.2, 97.4, 94.3 and 95.8%, respectively.
Fig. 4.
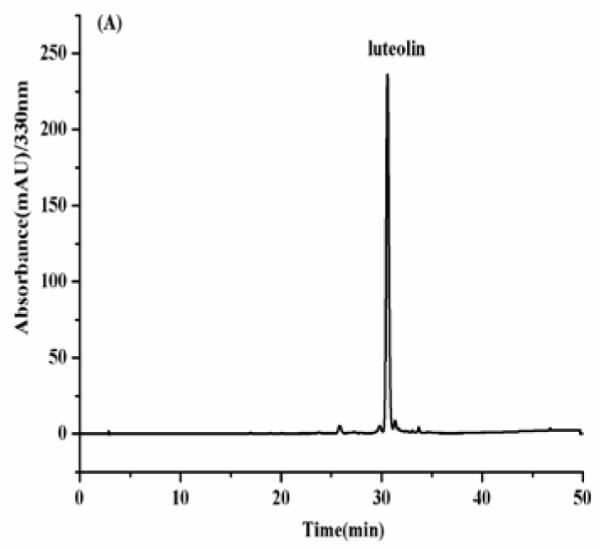
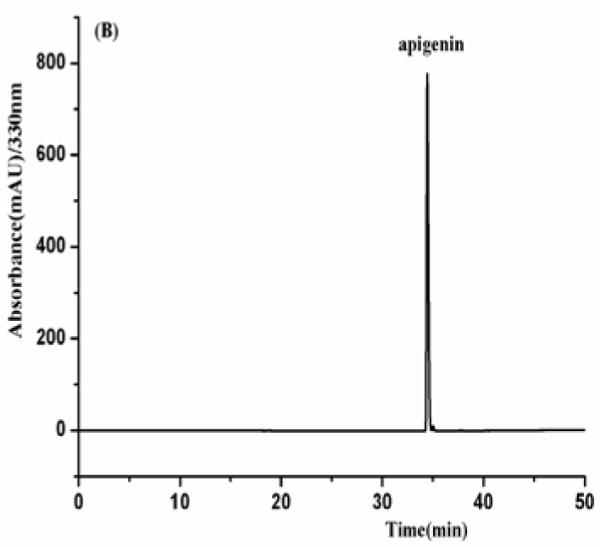
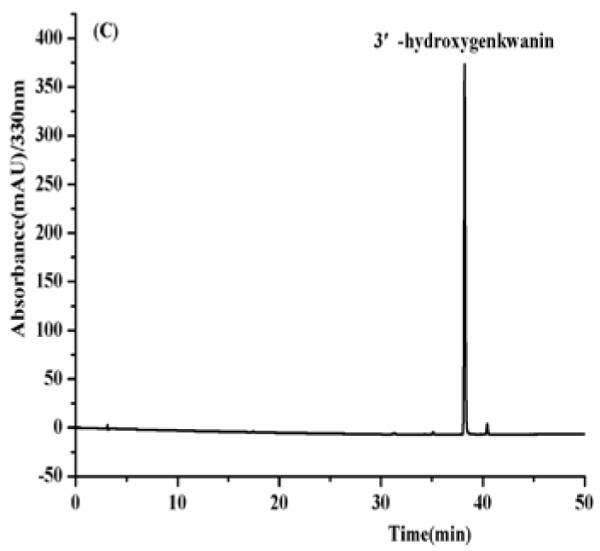
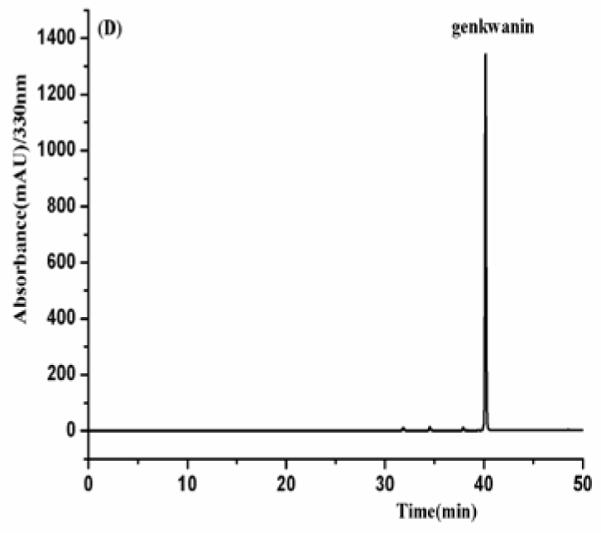
(A-D) HPLC chromatogram of the purified compounds by HSCCC. HPLC conditions: reversed-phase Shimadzu ODS C18 column (250 mm × 4.6 mm I.D., 5μm). HPLC conditions are in the text.
The chemical structures of components present in each peak fraction purified by HSCCC were identified from ESI-MSn and 1H-NMR data. Initially, the ESI-MS experiments were performed in the negative mode for determining molecular weights. Then the ESI-MSn were obtained in the negative mode for further structural elucidation and to facilitate comparison with a previous study[6,9,29]. Data collected from the ESI-MSn experiment provided much more detailed structural information about these four compounds than the MS methods reported in the literature[9], shown in Figs. 5-8. The subsequent structural identification of the peak fractions collected from the HSCCC was performed by comparison with previous 1H-NMR data[6,9,29,30]. The detailed data is given below:
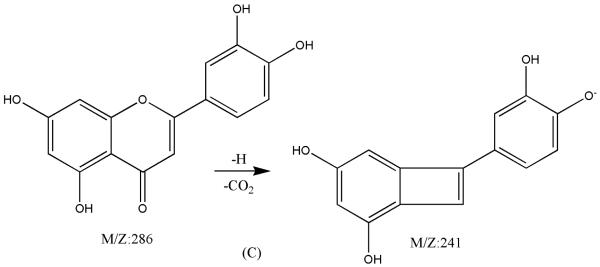
Figure 5.
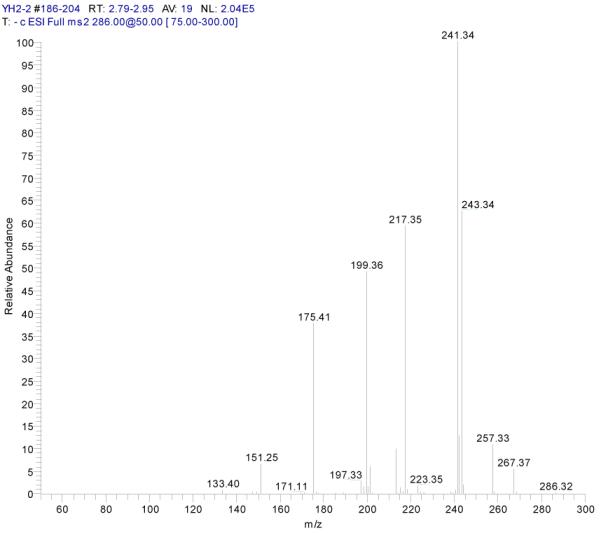
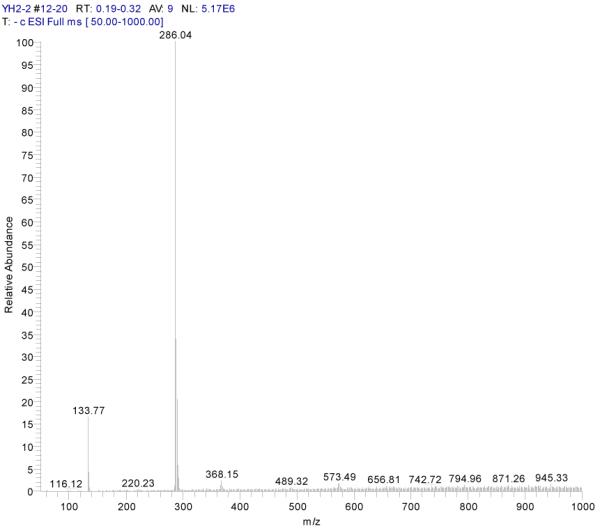
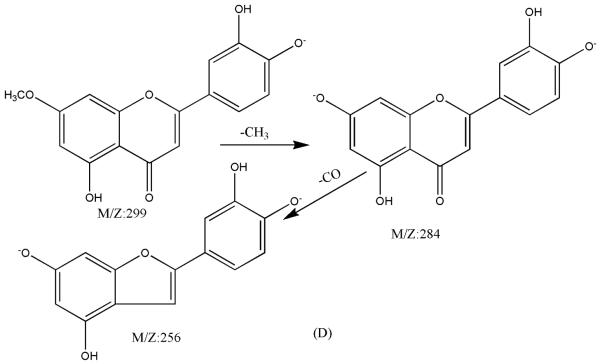
ESI-MSn mass spectra of fraction I (HSCCC fraction I in Fig. 3): (A) ESI-MS spectrum of the [M-H]− ion of fraction I; (B) MS2 on product ion m/z 241; (C) Scheme proposed fragmentation mechanisms of [M-H]− ion of fraction I.
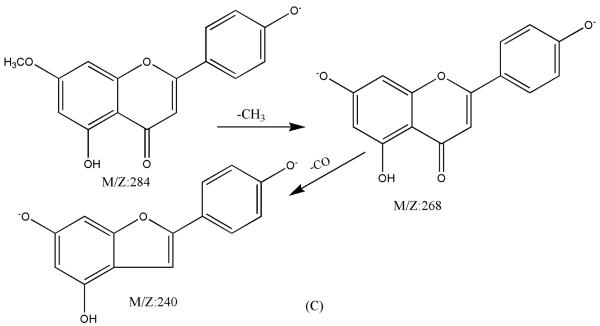
Figure 8.
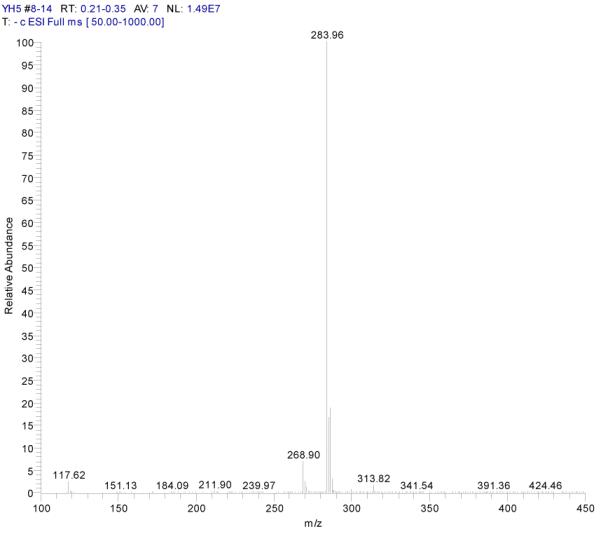
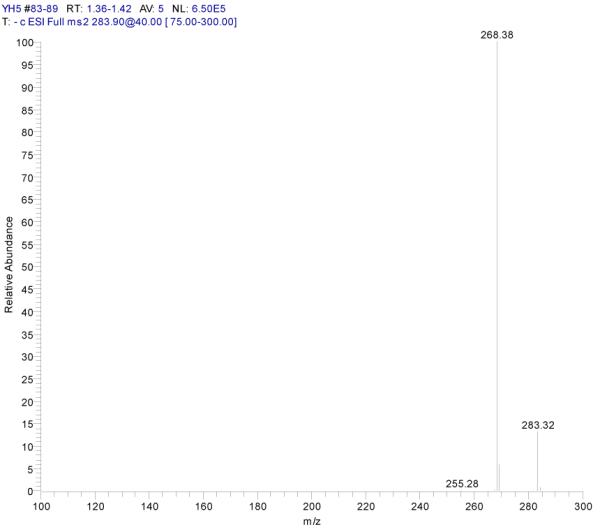
ESI-MSn mass spectra of fraction IV (HSCCC fraction III in Fig. 3): (A) ESI-MS spectrum of the [M-H]− ion of fraction IV(B) MS2 on product ion m/z 268; (C) MS3 on product ion m/z 240; (D) Scheme proposed fragmentation mechanisms of [M-H]− ion of fraction IV.
Fraction I yellow powder, ESI-MS1 m/z 286, ESI-MS2 m/z 241 [M - H]−, 1H-NMR (DMSO-d6), 6.16 (1H, s, H-6), 6.42 (1H, s, H-8), 6.65 (1H, s, H-3), 6.87(1H, d, H-5′), 7.41(2H, d, H-2′, 6′).
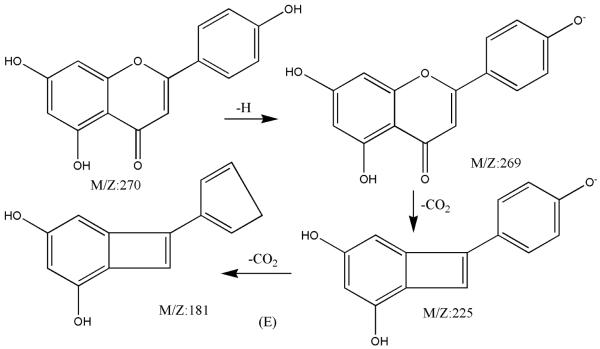
Fraction II yellow powder, ESI-MS1 m/z 270, ESI-MS2 m/z 225, ESI-MS3 m/z 181 [M - H]−, 1H-NMR (DMSO-d6), 6.42 (1H, s, H-2), 6.13(1H, s, H-6), 6.72 (1H, s, H-8), 6.89 (2H, d, H-3′,5′), 7.87(2H, d, H-2′, 6′).
Fraction III: yellow powder, ESI-MS1 m/z 300, ESI-MS2 m/z 284, ESI-MS3 m/z 256[M - H]−. 1H-NMR (DMSO-d6) 3.86 (3H, s, 7-OCH3), 6.35 (1H, s, H-6), 6.70 (2H, s, H-3, 8), 6.86 (1H, d, H-5′), 7.42 (2H, d, H-2′, 6′).
Fraction IV: yellow powder. ESI-MS1 m/z 284, ESI-MS2 m/z 268, ESI-MS3 m/z 240 [M - H]−. 1H-NMR (DMSO-d6 3.80 (3H, s, 7-OCH3), 6.29 (1H, d, 6-H),. 6.67 (1H, d, H-8), 6.76 (1H, s, H-3), 6.90 (2H, d, H-3′, 5′), 7.88 (2H, d, H-2′, 6′).
Thereby, the purified peak fractions were identified as follows: fraction I, luteolin; fraction II, apigenin; fraction III, 3′-hydroxygenkwanin; and fraction IV, genkwanin, compared with the data given in ref.[6,9,30]
In conclusion, four flavonoids, the main bioactive constituents in D. genkwa, a well known Chinese herbal medicine, were isolated and purified successfully by HSCCC.
Fig.1.
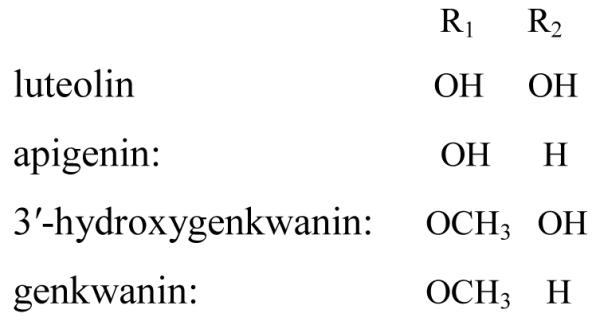
The chemical structures of four flavonoids from D. genkwa.
Figure 6.
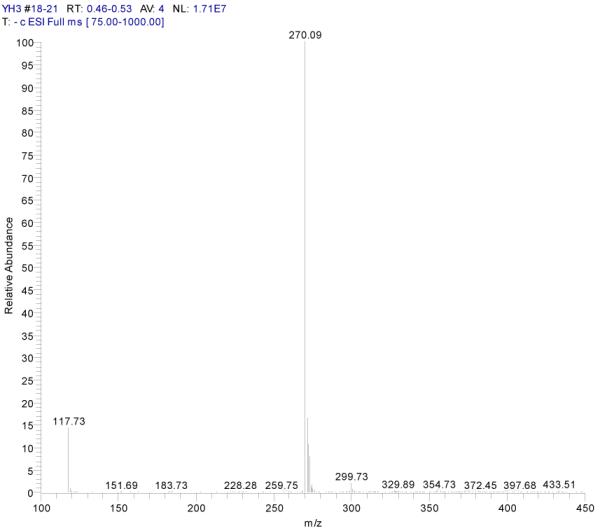
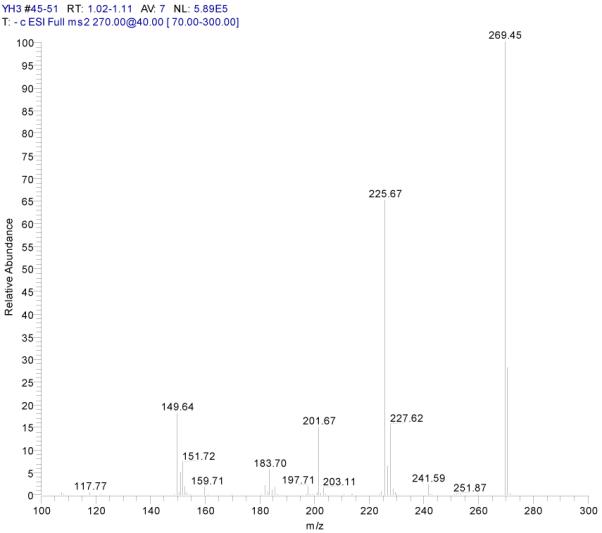
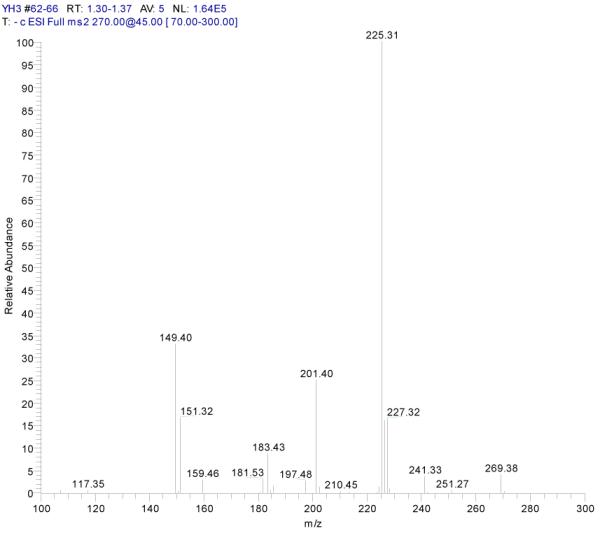
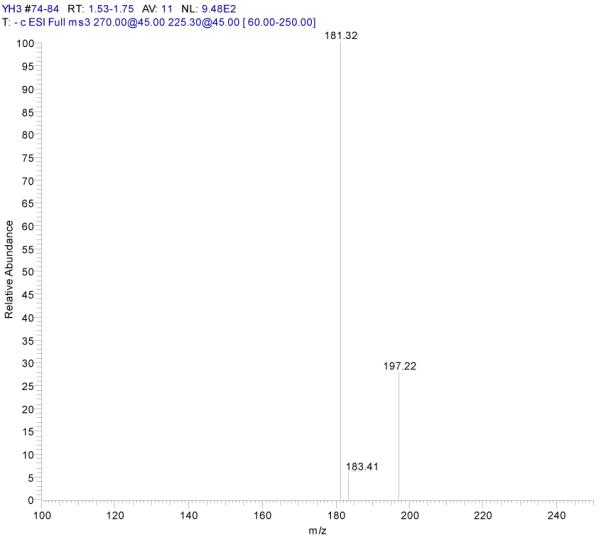
ESI-MSn mass spectra of fraction II (HSCCC fraction II in Fig. 3): (A) ESI-MS spectrum of the [M-H]− ion of fraction II (B) MS2 on product ion m/z 269; (C) MS2 on product ion m/z 225; (D) MS3 on product ion m/z 181; (E) Scheme proposed fragmentation mechanisms of [M-H]− ion of fraction II.
Figure 7.
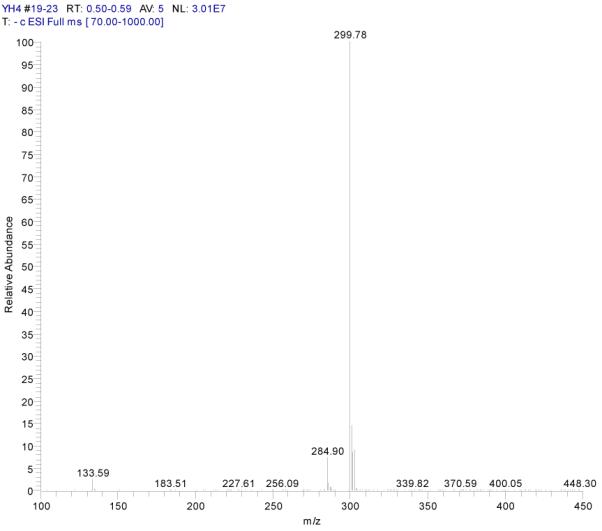
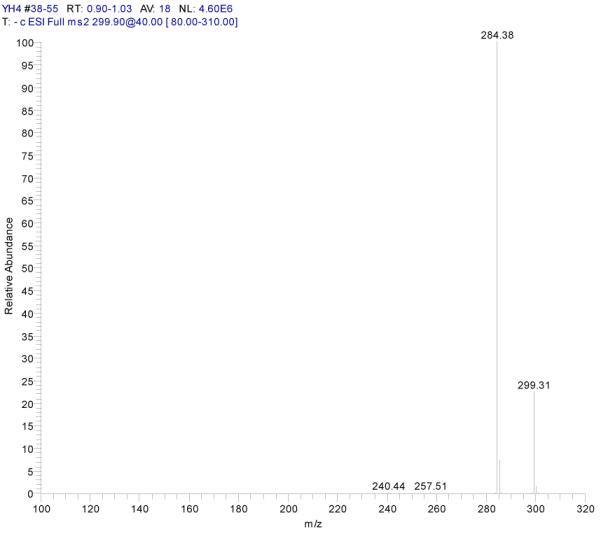
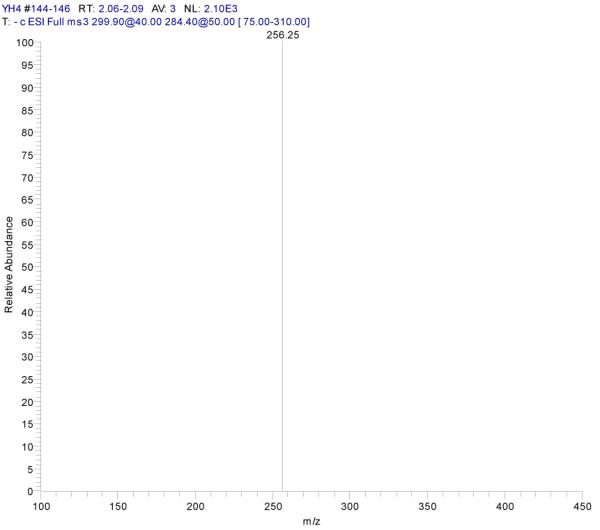
ESI-MSn mass spectra of fraction III (HSCCC fraction III in Fig. 3): (A) ESI-MS spectrum of the [M-H]− ion of fraction III (B) MS2 on product ion m/z 284; (C) MS3 on product ion m/z 256; (D) Scheme proposed fragmentation mechanisms of [M-H]− ion of fraction III.
REFERENCES
- 1.CC Z, MQ S, ZHL W. China’s Naturopathy. 1996 [Google Scholar]
- 2.CC Z, SHL Q, WM Z. Chin. J. Ethnomed Ethnopharm. 2000 [Google Scholar]
- 3.Park B-Y, Min B-S, Ahn K-S, Kwon O-K, Joung H, Bae K-H, Lee H-K, Oh S-R. Daphnane diterpene esters isolated from flower buds of Daphne genkwa induce apoptosis in human myelocytic HL-60 cells and suppress tumor growth in Lewis lung carcinoma (LLC)-inoculated mouse model [J] Journal of Ethnopharmacology. 2007;111:496. doi: 10.1016/j.jep.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 4.College JNM. The Encyclopedia of Traditional Chinese Medicine. 2nd ed Shanghai Science & Technology Press; Shanghai: 1985. [Google Scholar]
- 5.Li L, Song S, Gao P. Research progress in the chemical constituents and pharmacological activities of Daphne genkwa Sieb. et Zucc. Journal of Shenyang Pharmaceutical University. 2007;24:587. [Google Scholar]
- 6.Wang C, Li R, Huang L, Zhong L, Yuan S. Studies on Chemical Constituents of Daphne genkwa. Journal of Chinese Medicinal Materials. 2009;32:508. [PubMed] [Google Scholar]
- 7.Park BY, Min BS, Oh SR, Kim JH, Bae KH, Lee HK. Isolation of flavonoids, a biscoumarin and an amide from the flower buds Daphne genkwa and the evaluation of their anti-complement activity. Phytotherapy Research. 2006;20:610. doi: 10.1002/ptr.1915. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W, Shi Q, Shen Y, Chen H. A new flavanol from Daphne genkwa. Fitoterapia. 2007;78:596. doi: 10.1016/j.fitote.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Song L. analytical chemistry. Jilin University; Jilin: 2008. Studies on Flavonoids from Daphne Genkwa Sieb. et Zucc. p. 1. [Google Scholar]
- 10.Haidi W, Ailin LI, Guanhua H. New progress in the pharmacology of apigenin. Chinese Journal of New Drugs. 2008;17:1561. [Google Scholar]
- 11.Carlo G.. Di, Mascolo N, Izzo AA, Capasso F. Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sciences. 1999;65:337. doi: 10.1016/s0024-3205(99)00120-4. [DOI] [PubMed] [Google Scholar]
- 12.Baba K, Taniguchi M, Kozawa M. A third spirobiflavonoid genkwanol C from Daphne genkwa. Phytochemistry. 1993;33:913. [Google Scholar]
- 13.Nikaido T, Ohmoto T, Sankawa U. Inhibitiors of Adenosine 3′,5′-Cyclic Monophosphate Phosphodiesterase in Daphne genkwa Sieb. et Zucc. Chemical and Pharmaceutical Bulletin. 1987;35:675. doi: 10.1248/cpb.35.675. [DOI] [PubMed] [Google Scholar]
- 14.Zheng W, Gao X, Chen C, Tan R. Total flavonoids of Daphne genkwa root significantly inhibit the growth and metastasis of Lewis lung carcinoma in C57BL6 mice [J] International Immunopharmacology. 2007;7:117. doi: 10.1016/j.intimp.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Okunishi T, Umezawa T, Shimada M. Isolation and enzymatic formation of lignans of Daphne genkwa and Daphne odora. Journal of Wood Science. 2001;47:383. [Google Scholar]
- 16.Xiao W, Han L, Shi B. Isolation and purification of flavonoid glucosides from Radix Astragali by high-speed counter-current chromatography] Journal of Chromatography B. 2009;877:697. doi: 10.1016/j.jchromb.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 17.Xin X, Yang Y, Zhong J, Aisa HA, Wang H. Preparative isolation and purification of isobenzofuranone derivatives and saponins from seeds of Nigella glandulifera Freyn by high-speed counter-current chromatography combined with gel filtration. Journal of Chromatogr. A. 2009;1216:4258. doi: 10.1016/j.chroma.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 18.Han Q-B, Zhou Y, Feng C, Xu C, Huang S-X, Li S-L, Qiao C-F, Song J-Z, Chang DC, Luo KQ, Xu H-X. Bioassay guided discovery of apoptosis inducers from gamboge by high-speed counter-current chromatography and high-pressure liquid chromatography/electrospray ionization quadrupole time-of-flight mass spectrometry. Journal of Chromatogr. B. 2009;877:401. doi: 10.1016/j.jchromb.2008.12.046. [DOI] [PubMed] [Google Scholar]
- 19.Wei Y, Xie Q, Dong W, Ito Y. Separation of epigallocatechin and flavonoids from Hypericum perforatum L. by high-speed counter-current chromatography and preparative high-performance liquid chromatography. Journal of Chromatogr. A. 2009;1216:4313. doi: 10.1016/j.chroma.2008.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang K, Liu Z, Huang J-A, Dong X, Song L, Pan Y, Liu F. Preparative isolation and purification of theaflavins and catechins by high-speed countercurrent chromatography. Journal of Chromatogr. B. 2008;867:282. doi: 10.1016/j.jchromb.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Han X, Ma X, Zhang T-Y, Zhang Y, Liu Q, Ito Y. Isolation of high-purity casticin from Artemisia annua L. by high-speed counter-current chromatography. Journal of Chromatogr. A. 2007;1151:180. doi: 10.1016/j.chroma.2007.02.105. [DOI] [PubMed] [Google Scholar]
- 22.Peng J, Fan G, Chai Y, Wu Y. Efficient new method for extraction and isolation of three flavonoids from Patrinia villosa Juss. by supercritical fluid extraction and high-speed counter-current chromatography. Journal of Chromatogr. A. 2006;1102:44. doi: 10.1016/j.chroma.2005.10.045. [DOI] [PubMed] [Google Scholar]
- 23.Zhou X, Peng J, Fan G, Wu Y. Isolation and purification of flavonoid glycosides from Trollius ledebouri using high-speed counter-current chromatography by stepwise increasing the flow-rate of the mobile phase. Journal of Chromatogr. A. 2005;1092:216. doi: 10.1016/j.chroma.2005.07.064. [DOI] [PubMed] [Google Scholar]
- 24.Liu R, Feng L, Sun A, Kong L. Preparative isolation and purification of coumarins from Peucedanum praeruptorum Dunn by high-speed counter-current chromatography. Journal of Chromatogr. A. 2004;1057:89. doi: 10.1016/j.chroma.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Yin L, Xu L, Peng J. A simple and efficient protocol for largescale preparation of three flavonoids from the flower of Daphne genkwa by combination of macroporous resin and counter-current chromatography. Journal of Sep. Sci. 2010;33:2168. doi: 10.1002/jssc.201000054. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S, Zhang F, LI X, Dong W, Wen L, Wang S. Evaluation of Daphne genkwa Diterpenes: Fingerprint and Quantitative Analysis by High Performance Liquid Chromatography Phytochemical Analysis. Phytochem. Anal. 2007;18:91. doi: 10.1002/pca.953. [DOI] [PubMed] [Google Scholar]
- 27.Pang N, Bi K, Yan B, Chen X. Fingerprint analysis of Flos Genkwa by HPLC. Chinese Traditional and Herbal Drugs. 2010;41(5):818. [Google Scholar]
- 28.Ito Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. Journal of Chromatogr. A. 2005;1065:145. doi: 10.1016/j.chroma.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 29.Xu X, Yang N, Qian S, Xie N, Yu M, Duan J-A. Study on Flavonoids in Ligustrum lucidum. Journal of Chinese Medicinal Materials. 2007;30:538. [PubMed] [Google Scholar]
- 30.Zeng Y, Xiao J, Li X, Wang J. Isolation and identification of flavanoids from buds of Daphne genkwa Sieb. et Zucc. processed by rice vinegar. Journal of Shenyang Pharmaceutical University. 2009;26:353. [Google Scholar]