SUMMARY
All known protein kinases, except CASK, require Mg2+ to stimulate phosphotransfer from ATP to a protein substrate. The CaM-kinase domain of CASK exhibits activity in the absence of Mg2+, moreover it is inhibited by divalent ions including Mg2+. Here, we converted the Mg2+-inhibited wild type CASK kinase (CASKWT) into a Mg2+-stimulated kinase (CASK4M) by substituting four residues within the ATP-binding pocket. Crystal structures of CASK4M with and without bound nucleotide and Mn2+, together with kinetic analyses demonstrate that Mg2+ accelerates catalysis of CASK4M by stabilizing the transition state, enhancing the leaving group properties of ADP and by productive positioning the γ-phosphate of ATP. Phylogenetic analysis revealed that the four residues conferring Mg2+-mediated stimulation were lost from CASK during early animal evolution, converting a primordial, Mg2+-coordinating CASK into a Mg2+-inhibited kinase. This emergence of Mg2+-sensitivity conferred divalent ion-driven regulation to CASK, in parallel with the evolution of animal nervous systems.
INTRODUCTION
Protein kinases comprise approximately 1.7 % of the protein-coding genes in the human genome (1) and are valuable targets for therapeutics (2). Structural and functional similarities among diverse eukaryotic protein kinases suggest that they evolved from a common ancestor. Thus, protein kinases exhibit an N-terminal lobe, comprised of five-stranded, antiparallel β-sheet and a regulatory helix, αC, and a largely α-helical C-terminal lobe (3). The enzymes employ a number of highly conserved functional motifs to exert substrate peptide binding, nucleotide binding and catalysis (3). These motifs include an Asp-Phe-Gly (DFG) sequence at the beginning of the activation segment and a conserved asparagine in the catalytic loop of the C-terminal lobe, which are involved in Mg2+-binding and were believed to be indispensable for the catalysis of phosphotransfer by kinases (4,3,5). During evolution, some kinases acquired mutations in some of the conserved functional motifs. Non-canonical motifs may satisfy unique functional requirements in particular kinases, such as an unusual substrate specificity, or confer particular catalytic properties (6,7). Some changes may also be detrimental to catalysis and about 10 % of the human protein kinases bearing such changes are presently classified as “pseudokinases” (8). However, despite their presumed catalytic inactivity, most pseudokinases, for example Her-3, Jak-2, CCK4 and IRAK2, are essential and are thought to regulate the function of other active kinases.
Many protein kinases bear additional domains or regions, which can regulate a kinase via autoinhibition, oligomerization or recruitment of substrates (9). Thus, while a fundamental level of regulation is implemented via the conserved functional motifs within the kinase domain, additional domains provide another layer of regulation from outside of the kinase core.
CASK is an essential protein that contains an N-terminal protein kinase domain, followed by elements characteristic of membrane-associated guanylate kinases (MAGUKs), including a PDZ-, an SH3- and an inactive guanylate kinase domain. CASK is highly enriched in brain where it binds to cell-adhesion molecules, including neurexins (10), syndecans (11,12,13) and SynCAM (14). In mice, genetic disruption of CASK causes a cleft palate, synaptic dysfunction and lethality (15). In humans, mutations in the CASK gene also produce a cleft palate syndrome with optic atrophy and mental retardation (16,17,18,19,20).
The N-terminal kinase domain of CASK most closely resembles members of the sub-family of Ca2+/calmodulin (CaM)-dependent protein kinases. However, human CASK exhibits a Gly162-Phe163-Gly164 (GFG) instead of the Mg2+-binding DFG motif and a cysteine (Cys146) instead of the conserved, Mg2+-binding asparagine in the catalytic loop. Consistent with these substitutions, Mg2+-binding in CASK is disrupted (21). Since Mg2+ was considered an indispensable cofactor for catalytic phosphotransfer, CASK was categorized as a pseudokinase (Boudeau et al., 2006). Recently, however, we found that CASK binds ATP and catalyzes phosphotransfer to the synaptic adhesion molecule neurexin-1, even in the absence of Mg2+ (21). Indeed, CASK is fully active only in the absence of divalent cations such as Mg2+ and Ca2+ (21). The inhibition of CASK by divalent metal ions would provide an effective mechanism to allow CASK activity in inactive neurons (low divalent ion concentration) and to shut down the enzyme in active neurons (high divalent ion concentration) (21).
To gain insight into how the unique Mg2+-sensitive catalytic activity of CASK evolved, we have carried out systematic mutagenesis, mechanistic and structural studies. We found that substitutions of four residues, generating CASK4M, turn CASK into a Mg2+-stimulated kinase. However unlike conventional kinases, CASK4M retained significant Mg2+-independent phosphotransfer activity. Structural analysis revealed that in CASK4M, Mg2+ accelerates catalysis by stabilizing the transition state, by enhancing the leaving group properties of ADP and by indirectly positioning the γ-phosphate of ATP, suggesting a similar role for Mg2+ in conventional protein kinases. Although strongly stimulated by Mg2+, CASK4M exhibited the comparable activity towards neurexin-1 as CASKWT in vivo, suggesting that the divalent ion-sensitive kinase activity of CASK is an adaptive innovation that serves to convert CASK from a general kinase into a kinase specific for substrates that are recruited via its MAGUK domains. Strikingly, evolutionary comparison of CASK sequences demonstrates that CASK initially emerged as a Mg2+-utilizing kinase, and later became Mg2+-sensitive. The amino acid substitutions that rendered CASK sensitive to Mg2+ occurred during early animal evolution, in parallel with the appearance of the nervous system. Thus, CASK represents a case, in which apparently detrimental changes in conserved functional motifs do not lead to the loss of catalytic activity but instead may have implemented a novel regulatory mechanism.
RESULTS
Designing a Mg2+-coordinating version of CASK
The CASK CaM-kinase domain is highly homologous to the corresponding domains of canonical CaM-kinases, such as CaMKI ( ≈ 37% identity) and CaMKII (≈ 44% identity) (1). CaMKI and CaMKII need Mg2+ and Ca2+ for optimal activity. Mg2+ activates phosphotransfer from ATP, while Ca2+ is required for activation of calmodulin, which counteracts autoinhibition of the enzymes. In contrast, the CASK CaM-kinase domain is inhibited by Mg2+ or Ca2+ and no metal ion was found in crystal structures of the CASK CaM-kinase domain in complex with adenine nucleotides (21).
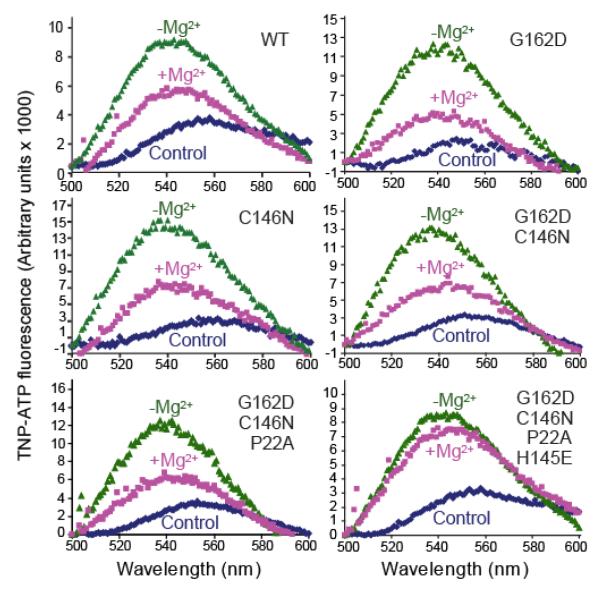
Sequence alignments of vertebrate CASKs with human CaMKI and CaMKII reveal substitutions in CASK of residues that are highly conserved in canonical CaM-kinases; i.e. the Mg2+-coordinating aspartate of the DFG motif is replaced by a glycine (Gly162), and the Mg2+-binding asparagine of the catalytic-loop is replaced by a cysteine (Cys146). In addition to coordinating Mg2+, this Asn is known to position an essential aspartate of the catalytic loop (22,23). We hypothesized that amino acid changes at these two positions are responsible for the loss of Mg2+-binding by CASK, following the initial merger of a Mg2+-coordinating CaM-kinase domain with the MAGUK domains during evolution. To test this hypothesis, we converted Gly162 and Cys146 of CASK to the canonical Asp and Asn residues, respectively, and tested binding of the mutants to an ATP analog (TNP-ATP) in the absence and presence of Mg2+. TNP-ATP becomes fluorescent when inserted into the hydrophobic ATP-binding pocket of protein kinases (24). However, neither the single Gly162Asp or Cys146Asn mutants nor the double mutant conferred Mg2+-TNP-ATP binding onto CASK (Figure 1).
Figure 1. Designing a Mg2+-coordinating version of CASK CaM-kinase domain.
Fluorescence emission spectra of TNP-ATP in the presence of WT and mutant CASK. The protein analyzed (WT or mutant) is indicated in the upper right corner. Dark blue trace: Control spectrum of TNP-ATP (1 μM) in Tris-HCl buffer (pH 7.0) with EDTA (4 mM). Green trace: Spectra of samples containing 1 μM of the indicated recombinant CASK CaM-kinase domain, TNP-ATP (1 μM) and EDTA (4 mM) in Tris-HCl buffer (pH 7.0). Magenta trace: Spectra of samples containing 1 μM of the indicated recombinant CASK CaM-kinase domain, TNP-ATP (1 μM) and 100 μM MgCl2 in Tris-HCl buffer (pH 7.0). Samples were excited at 410 nm, and spectra were recorded between 500 and 600 nm. The spectra are representatives of experiments repeated three times with essentially identical results.
Upon further sequence analysis, we observed two additional deviations from canonical CaM-kinases in residues that line the nucleotide-binding pocket of CASK, and could affect Mg2+-ATP binding. First, Pro22 (which corresponds to a conserved alanine in standard CaM-kinases) could stiffen the Gly-rich loop of CASK, which as a consequence may lack sufficient flexibility to accommodate the Mg2+-ATP complex. Moreover, Pro22 is unable to form a hydrogen bond with ATP phosphates, as it lacks a backbone NH group. Second, His145 in CASK replaces a negatively charged glutamate in the catalytic loop of standard CaM-kinases immediately preceding the Mg2+-coordinating Asn.
To examine whether these additional changes in conserved residues contribute to the loss of Mg2+-coordination in CASK, we reverted Pro22 and His145 to the canonical Ala and Glu residues, respectively, in addition to the initial Gly162Asp and Cys146Asn mutations. A CASK mutant containing Gly162Asp, Cys146Asn, and Pro22Ala was still incapable of coordinating TNP-ATP in the presence of Mg2+ (Figure 1). Only the quadruple mutant containing Gly162Asp, Cys146Asn, Pro22Ala and His145Glu, which we termed CASK4M, displayed TNP-ATP binding in the presence of Mg2+ (Figure 1). The increase in fluorescence upon interaction of TNP-ATP with the CASK4M CaM-kinase domain was completely inhibited in the presence of excess ATP, indicating that TNP-ATP specifically mimics ATP (Supplemental Figure S1A).
Comparison of the catalytic properties of CASKWT and CASK4M
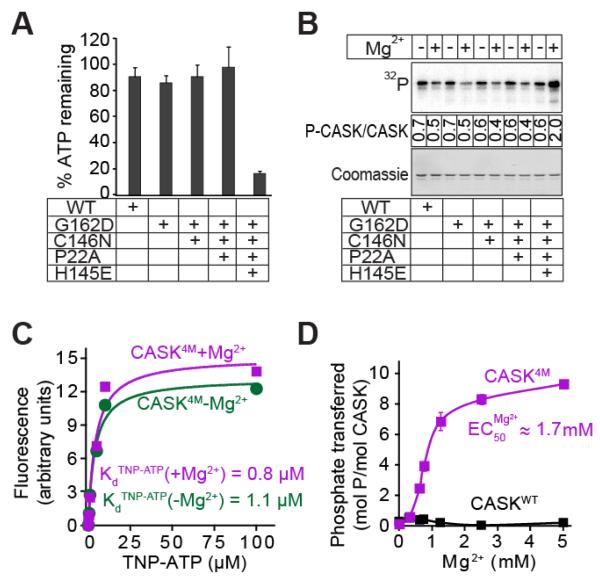
ATP binding of CASK4M in the presence of Mg2+ suggested that it may behave catalytically like a conventional, Mg2+-dependent CaM-kinase. To test this notion, we probed CASK mutants for kinase activity in the presence of Mg2+-ATP and excess autocamtide-2 (a synthetic peptide substrate for CaMKII). Consistent with the results from Mg2+-ATP binding, only the quadruple CASK4M mutant exhibited ATP-consumption in the presence of Mg2+ and substrate (Figure 2A) and an increase in autophosphorylation in presence of Mg2+ (about threefold increase under saturating conditions; Figure 2B; about 30-fold increase under limiting conditions compared to the reactions without Mg2+; Supplemental Figure S2A). Interestingly, all CASK mutants, including CASK4M, still bound to ATP (Figure 1) and autophosphorylated (Figure 2B) in the absence of Mg2+, retaining a unique activity of CASK not present in canonical CaM-kinases. Thus, CASK4M appears to bridge the Mg2+-stimulated activity of classical CaM-kinases and the Mg2+-independent activity of CASKWT. It, therefore, represents a valuable tool to decipher the catalytic roles of the divalent metal ions in kinase reactions.
Figure 2. Effect of divalent ions on nucleotide-binding and hydrolysis.
A. ATP consumption by WT or mutant CASK CaM-kinase domain. In Tris-HCl buffer (pH 7.0) supplemented with Ca2+ (1 mM), CaM (4 μM), and Mg2+ (2 mM), indicated variant of CASK CaM-kinase domain (1 μM), Autocamtide-2 (100 μM) and ATP (50 μM) were incubated for 60 min. The amount of ATP remaining was detected with KinaseGlotm. Data represents means ± standard deviation of three independent experiments.
B. Autophosphorylation of WT and mutant CASK CaM-kinase domains. The indicated variants of CASK CaM-kinase domain (1 μM) were incubated in Tris-HCl buffer (pH 7.0) with Na+-γ32P-ATP (50 cpm/pmol; -Mg2+) or 10 mM Mg2+-ATP (+Mg2+) at 30°C with shaking for 2 h. The proteins were separated by SDS-PAGE, transferred onto a nitrocellulose membrane, and visualized by phosphorimager scanning (upper panel). Ponceau staining was used for loading control (lower panel). Mean stoichiometry of phosphate incorporation (phosphates/CASK molecule) from three independent experiments are shown between the panels.
C. TNP-ATP binding. Increasing amounts of TNP-ATP were added to cuvettes containing 10 mM Tris-HCl pH 7.0, 1 μM CASK4M, and either 4 mM EDTA (magenta symbols) or 200 μM Mg2+ (green symbols). The TNP-ATP fluorescence of the samples (excitation: 410 nm; emission: 541 nm) is plotted after subtracting background TNP-ATP fluorescence obtained with parallel samples, which contained the same TNP-ATP, Tris-HCl, EDTA, or Mg2+ concentrations but lysozyme instead of CASK. The plot is a representative of three independent experiments.
D. Effect of Mg2+ on kinase activity of CASK4M. Indicated variants of CASK CaM-kinase domain (2 μM), autocamtide-2 (100 μM) and γ32P-ATP (250 μM; 250 cpm/pmol) in Tris-HCl buffer (pH 7.0) were incubated for 10 min with increasing amounts of Mg2+. Amount of phospho-autocamtide-2 generated was estimated by scintillation counting of dot-blots on nitrocellulose membrane. Data shown are means ± standard errors of the means (SEMs; n=3).
Mg2+ enhances the catalytic efficiency of CASK4M
Various functions have been suggested for Mg2+ in kinase catalysis, including nucleotide binding, association and/or dissociation of the substrate peptide, and stabilization of the phosphotransfer transition state (22,25,26,27). To clarify the contribution of Mg2+ to phosphotransfer catalysis, we first tested whether the Mg2+ coordinating ability of CASK4M alters ATP binding (represented by interaction with the analog TNP-ATP).
TNP-ATP binding to CASK4M was similar with or without Mg2+ (Figure 2C) and similar to that of CASKWT without Mg2+ (Supplemental Figure S3A). However, ATP competed with TNP-ATP for binding to CASK4M better in the presence of Mg2+, indicating that Mg2+ alters the affinity of ATP to CASK4M (Supplemental Figure S3B). We surmise that the effect of Mg2+ on ATP affinity is comparatively small; as it is masked in direct TNP-ATP affinity measurements, perhaps by additional contacts of the ATP analog. We also examined the enzymatic parameters of CASK4M-mediated phosphotransfer in the absence and presence of Mg2+. Efficient autophosphorylation was achieved in the presence of Mg2+ (Supplemental Figure S4). Moreover, catalysis, as measured by phosphate transferred onto autocamtide-2 by CASK4M was robustly enhanced by Mg2+ (Figure 2D). It is unlikely that the strong enhancement of catalytic efficiency of CASK4M in the presence of Mg2+ is due to the mild effect of Mg2+ on the nucleotide affinity we observed (see above). Therefore, our results suggest additional roles for Mg2+ downstream of ATP binding.
Overall structural comparison of CASKWT, CASK4M and CaMKII
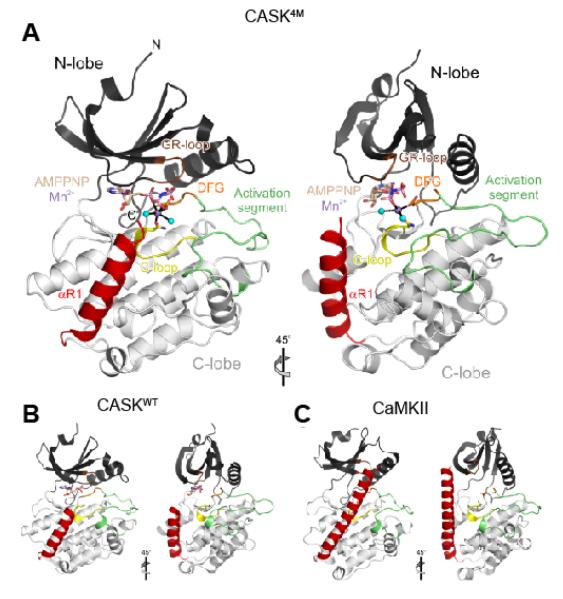
In order to trace the sources of the mechanistic differences in CASKWT, CASK4M and CaMKII, we conducted crystal structure analyses. We crystallized the CaM-kinase domain of CASK4M under similar conditions as the CaM-kinase domain of CASKWT, and solved the structure by molecular replacement at 2.0 Å resolution (Table 1). The CASK4M CaM-kinase domain exhibits a typical protein kinase fold, with an N-terminal lobe dominated by a five-stranded β-sheet and a primarily α-helical C-terminal lobe (Figure 3A). The C-terminal lobe is followed by a loop (residues 286-288) and an α-helix, αR1 (residues 289-303; Figure 3A, B), which are not part of the canonical kinase core. The overall structure of CASK4M CaM-kinase domain is significantly closer to that of CASKWT (PDB IDs 3C0G and 3C0I; RMSD 0.43-0.61 Å for 302 matching Cα atoms; (21)) than to that of CaMKII crystallized in an autoinhibited conformation (PDB ID 2BDW; RMSD 1.47-1.95 Å for 260-276 matching Cα atoms; (28)) (Figure 3A-C). Therefore, the four amino acid substitutions that confer Mg2+-stimulated kinase activity onto CASK4M do not alter the overall structure of the CaM-kinase domain.
Table 1.
Crystallographic Data and Refinement
| Data Collection | Native | AMPPNP | Mn2+ | AMPPNP-Mn2+ |
|---|---|---|---|---|
| Wavelength (Å) | 0.91841 | 0.91841 | 1.87856 | 1.87856 |
| Temperature (K) | 100 | 100 | 100 | 100 |
| Space Group | P212121 | P212121 | P212121 | P212121 |
| Unit Cell (Å) a, b, c | 58.7, 61.8, 100.2 | 59.0, 62.2, 97.9 | 58.9, 61.8, 101.5 | 58.9, 62.0, 100.6 |
| Resolution (Å) | 30.0-1.95 | 50.0-2.05 | 50.0-2.20 | 20.0-2.30 |
| (1.98-1.95)a | (2.09-2.05) | (2.24-2.20) | (2.36-2.30) | |
| Reflections | ||||
| Unique | 26982 (1269) | 22576 (1094) | 35224 (1513)b | 31058 (1968)b |
| Completeness (%) | 98.4 (94.7) | 97.7 (97.4) | 96.9 (84.4) | 98.2 (83.8) |
| Redundancy | 6.7 (6.1) | 5.2 (4.9) | 4.7 (2.7) | 4.5 (2.7) |
| I/σ(I) | 29.0 (1.9) | 15.9 (2.3) | 11.3 (1.3) | 14.4 (2.2) |
| Rsym(I) c | 3.6 (68.4) | 5.7 (66.4) | 9.0 (56.8) | 6.3 (49.7) |
|
| ||||
| Refinement | ||||
|
| ||||
| Resolution (Å) | 30.0-2.00 | 30.0-2.10 | 30.0-2.20 | 20.0-2.30 |
| (2.05-2.00) | (2.15-2.10) | (2.26-2.20) | (2.36-2.30) | |
| Reflections | ||||
| Number | 24959 (1727) | 21153 (1516) | 18858 (1180) | 16714 (1046) |
| Completeness (%) | 98.6 (94.3) | 97.7 (96.6) | 97.0 (84.2) | 100.0 (100.0) |
| Test Set (%) | 5.1 | 5.1 | 5.1 | 5.0 |
| Rwork d | 19.6 (24.8) | 21.9 (29.7) | 21.0 (28.5) | 17.9 (20.9) |
| Rfree d | 24.1 (31.5) | 26.8 (29.6) | 27.0 (39.9) | 23.4 (30.9) |
| ESU (Å)e | 0.14 | 0.20 | 0.19 | 0.17 |
| Refined Atoms | ||||
| Protein | 2461 | 2446 | 2422 | 2430 |
| Water | 235 | 199 | 141 | 196 |
| Ligands | 19 | 31 | - | 32 |
| Mean B-Factors (Å2) | ||||
| Wilson | 30.4 | 33.7 | 35.1 | 51.1 |
| Protein | 38.0 | 46.1 | 35.6 | 43.1 |
| Water | 46.2 | 46.3 | 38.5 | 46.9 |
| Ligand | 65.5 | 63.5 | - | 54.2 |
| Ramachandran Plot (%) f | ||||
| Favored | 96.0 | 94.4 | 97.0 | 95.7 |
| Outliers | 1.0 | 1.0 | 0.7 | 1.0 |
| RMSDa Target Geometry | ||||
| Bond Lengths (Å) | 0.011 | 0.011 | 0.010 | 0.010 |
| Bond Angles (°) | 1.322 | 1.209 | 1.248 | 1.324 |
| RMSD B-Factors (Å2) | ||||
| Main Chain Bonds | 0.661 | 0.437 | 0.549 | 0.589 |
| Main Chain Angles | 1.064 | 0.741 | 0.942 | 1.033 |
| Side Chain Bonds | 1.554 | 1.099 | 1.316 | 1.523 |
| Side Chain Angles | 2.277 | 1.719 | 2.110 | 2.493 |
| PDB ID | XXXX | XXXX | XXXX | XXXX |
Data for the highest resolution shell in parentheses
Mn2+ and AMPPNP-Mn2+ data sets were processed without merging Friedel pairs
Rsym(I) = ∑hkl∑i∣Ii(hkl) - <I(hkl)>∣ / ∑hkl∑i∣Ii(hkl)∣; for n independent reflections and i observations of a given reflection; <I(hkl)> - average intensity of the i observations
R = ∑hkl∥Fobs∣ - ∣Fcalc∥ / ∑hkl∣Fobs∣; Rwork - hkl ∉ T; Rfree - hkl ∊ T; T test set
ESU - estimated overall coordinate error based on maximum likelihood
Calculated with MolProbity (http://molprobity.biochem.duke.edu/)
RMSD - root-mean-square deviation
Figure 3. Overview of the CASK4M-Mn2+-AMPPNP crystal structure.
A. Orthogonal ribbon plot of CASK4M CaM-kinase domain with landmark functional elements colored. Gly-rich loop (GR-loop) - brown, catalytic loop (C-loop) - yellow; DFG motif of the Mg2+-binding loop - orange; activation segment - green; C-terminal Ca2+/CaM-binding element - red. AMPPNP and residues Asn146 and Asp162, which coordinate the Mn2+ ion, are shown as sticks and colored by atom type. Carbon - beige; oxygen - red; nitrogen - blue; phosphorus - pink. B. Structure of CASK CaM-kinase domain in complex with AMPPNP (sticks) lacking a divalent metal ion (β and γ phosphates disordered; pdb ID 3C0H; (21)) in the same orientation as the CASK4M CaM-kinase domain in A. Cys146 is shown as sticks. Functional elements are colored as in A. C. Structure of CaMKII (pdb ID 2BDW; (28)) in the same orientation as the CASK4M CaM-kinase domain in A. Asn140 and Asp156, whose equivalents in CASK4M coordinate the Mn2+ ion, are shown as sticks. Functional elements are colored as in A.
Divalent metal ions alter the positioning of the ATP phosphate moieties
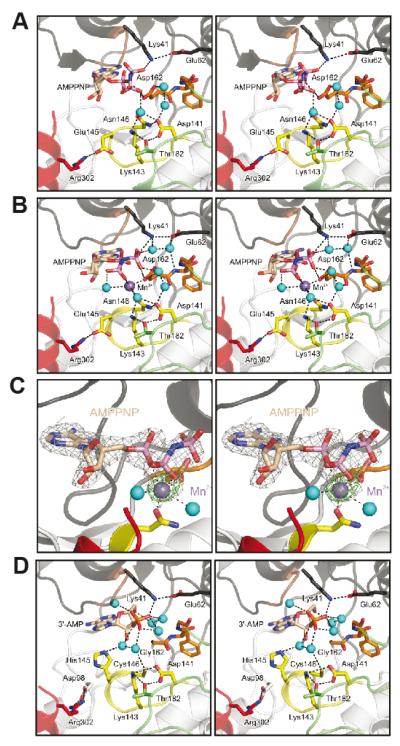
To investigate the presumed additional role of Mg2+ beyond nucleotide binding, we determined the crystal structures of CASK4M in complex with either Na+-AMPPNP or Mn2+-AMPPNP (Table 1). AMPPNP is a non-hydrolyzable analog of ATP. Mn2+ is considered a stereochemical equivalent of Mg2+ (22,23). Consistent with the TNP-ATP binding data (Figures 1and 2C), CASK4M coordinated AMPPNP even in the absence of divalent metal ions (Figure 4A). Conversely, after soaking CASK4M crystals with Mn2+ solution, we failed to discern a bound divalent ion in the absence of AMPPNP (Supplemental Figure S5A), suggesting that Mg2+ is coordinated only when complexed with the nucleotide.
Figure 4. Nucleotide-binding pocket of the CASK4M CaM-kinase domain.
A. CASK4M CaM-kinase in complex with AMPPNP without a divalent metal ion.
B. CASK4M CaM-kinase in complex with AMPPNP-Mn2+. Residues of the Mg2+-binding loop are shown in orange, residues of the catalytic loop are in yellow and residues of the C-terminal Ca2+/CaM-binding element are in red (as in Figure 3). Selected residues and the nucleotides are shown as sticks and colored by atom type; carbon - as the respective fragment; oxygen - red; nitrogen - blue; phosphorus - pink. Water molecules (cyan) and the Mn2+ ion (purple) are shown as spheres. The orientations are the same as in Figure 3A left panel. The orientations of the AMPPNP β and γ-phosphates differ in the complexes without and with Mn2+ (compare panels A and B).
C. Stereo plot showing the final 2Fo-Fc electron density around the AMPPNP-Mn2+ complex contoured at the 1 σ level (gray mesh) and the anomalous difference Fourier map contoured at the 5 σ level (green mesh), indicating the position of the Mn2+ ion. AMPPNP is shown as sticks and colored by atom type as before. The Mn2+ ion (purple) and two coordinating water molecules (cyan) are shown as spheres. The orientation is the same as in Figure 3A left panel.
D. CASKWT CaM-kinase domain in complex with co-purified 3′-AMP (21). Color-coding as above. The orientations are the same as in Figure 3B left panel.
The overall orientation of the adenosine moiety of AMPPNP was similar in the presence or absence of Mn2+ (Figure 4A, B) and AMPPNP could be modeled in a similar orientation into the nucleotide-binding pocket of CASKWT without steric clashes, suggesting that the global positioning of ATP and the orientation of the base moiety is not affected by the divalent cation. However, the orientation of β- and γ-phosphates of AMPPNP was specifically altered by Mn2+ in CASK4M (Figure 4A, B). We therefore conclude that Mg2+ positions the phosphates of ATP in the nucleotide-binding pocket of CASK4M independent of the positioning of the adenosine moiety, which is consistent with previous studies on PKA (29,30).
Role of Mg2+ in kinase catalysis
Kinases vary in the manner by which Mg2+ ions bind the ATP phosphates in their active sites (22,31). In order to unequivocally locate divalent metal ion(s) in the Mn2+-AMPPNP complex of CASK4M CaM-kinase domain, we measured diffraction at an X-ray wavelength of 1.88 Å, where Mn2+ exhibits a measurable anomalous signal, and calculated anomalous difference Fourier maps. This procedure unequivocally revealed a single coordinated Mn2+ ion in the Mn2+-AMPPNP co-crystal structure of CASK4M CaM-kinase domain (Figure 4C).
Similar to some conventional kinases, such as DAPKI and MEK1, (32,33) the Mn2+ ion in the CASK4M CaM-kinase domain, coordinates the β-phosphate and indirectly orients the γ-phosphate in a puppet-master fashion (Figure 4C). Therefore, we surmise that the role of Mg2+ in enhancing kinase catalysis is to position the γ-phosphate in proximity of the conserved Lys41, as well as to stabilize the transition state. Mg2+ may also enhance the leaving group properties of ADP by compensating for additional negative charge that evolves at the β-phosphate during the reaction. Taken together, our observations are consistent with the notion that Mg2+ coordinates the ATP phosphates to favor a catalytically productive kinase-ATP complex (22,25,23,34,35).
His145 may act as a Mg2+ sensor in CASKWT
The conformation of residues lining the ATP-binding pockets of the CASK4M (this work) and CASKWT (21) kinase domains are very similar. Differences are strictly limited to the four amino acid exchanges. Ala22 and Asn146 in CASK4M adopt very similar conformations as the corresponding Pro22 and Cys146 in CASKWT. Apart from the introduction of the Asp162 side chain (instead of Gly162 in CASKWT), the most pronounced difference is a change in side chain orientation of Glu145 in CASK4M compared to the corresponding His145 in CASKWT (compare Figure 4A with D). In CASKWT, His145 protrudes into the nucleotide-binding pocket (Figure 4D) and spatially overlaps with a water molecule in the coordination sphere of the Mn2+ ion bound in the CASK4M-Mn2+-AMPPNP co-crystal structure (Figure 4B, C). In CASK4M, Glu145 is sequestered by Arg302 from the C-terminal extension and is thereby turned away from the ATP pocket (Figure 4A, B and Supplemental Figure S5A, B). A similar situation is seen in CaMKII (28). Since the triple mutant Gly162Asp/Cys146Asn/Pro22Ala of CASK is still inhibited by Mg2+ (Figure 2B), these observations suggest that His145 is not tolerated in the coordination sphere of the divalent metal ion. TNP-ATP binding to CASKWT is partially inhibited by Mg2+ at pH 8.8, where most His side chains are expected to be neutral (Supplemental Figure S1B). However, the local pH is difficult to estimate and His145 may still be protonated within the CASKWT ATP-binding pocket. Thus, our data suggest that His145 does not allow metal coordination in CASKWT by invading the coordination sphere of the in-coming metal ion.
Additional features supporting the Mg2+-independent activities of CASKWT and CASK4M
Although the phosphorylation activity of the CASK4M CaM-kinase domain is strongly stimulated by Mg2+, the enzyme still exhibits catalytic activity in the absence of Mg2+ (Figure 2B). Similarly, CASKWT binds ATP and performs phosphotransfer in the absence of Mg2+ (21). Therefore, CASK must have undergone evolutionary adaptions, which are retained in CASK4M and which allow catalysis without Mg2+. As one possibility, CASK may have acquired features that promote the kinase-ATP-substrate ternary complex formation and elevate substrate phosphorylation under chemically sub-optimal conditions (i.e. in the absence of Mg2+).
Both CASKWT (21) and CASK4M CaM-kinase domains retained bound nucleotide during purification and crystallization in the absence of added nucleotides (Figure 4D and Supplemental Figure S5B), unlike other constitutively active kinases such as casein kinase II and PIM1, which are purified in their apo-forms (36,37). We modeled this nucleotide as an adenosine-3′-phosphate molecule (3′-AMP), likely a product of bacterial RNA degradation during cell rupture. The binding mode of 3′-AMP clearly differs from the binding of AMPPNP in either enzyme (compare Figure 4A, B with Figure 4D and Supplemental Figure S5B), and 3′-AMP is easily detached from the pocket since no residual electron density was discerned in the nucleotide-binding pocket of CASK4M after washing of the crystals in Mn2+-containing buffer (see above; Supplemental Figure S5A). Nevertheless, co-purification of the nucleotide attests the general accessibility of the nucleotide-binding pockets of CASKWT and CASK4M. These observations indirectly support the idea that CASKWT and CASK4M adopt a nucleotide-receptive fold that ensures an unregulated occupancy of their nucleotide-binding pockets by ATP even in the absence of Mg2+. A constitutive supply of ATP may partly compensate for the inability of CASKWT to utilize the co-factor Mg2+.
Features supporting the Ca2+-independent activities of CASKWT and CASK4M
In their active states, protein kinases adopt a conserved conformation of the two lobes, in which their functional elements are poised for substrate binding and catalysis (3,38). By default, archetypical CaM-kinases adopt autoinhibited conformations (39,28). The activation of CaM-kinases requires binding of Ca2+/CaM to the autoregulatory domain leading to their detachment from the kinase domain and restoration of the active conformation. Therefore, Ca2+ is required for optimal catalysis by CaM-kinases (39,28).
Immediately following the kinase domain, CASK contains a sequence homologous to the autoregulatory domain of CaMKII (residues 281-310) which binds to Ca2+/CaM (10). Similar to CaMKI and CaMKII, residues 289-302 of CASK form a helical extension (αR1) which packs against the C-terminal lobe (Figure 3 and Supplemental Figure S6). However, neither in CASKWT (21) nor in CASK4M, helix αR1 or its C-terminal extension engages in direct contacts with residues of the ATP-binding cleft (Figure 3A)(39). Also, CASK displays an arginine to leucine substitution in the RXXT/S motif of its autoregulatory domain. A similar mutation in the CaMKII autoinhibitory segment can reduce the affinity of the autoinhibitory segment to the substrate-binding site by up to 200-fold (40,41). As a consequence, the CASKWT and CASK4M CaM-kinase domains, including the putative autoinhibitory regions, remain compatible with constitutive substrate binding. This allows increased formation of the ternary kinase-ATP-substrate complexes, which partly compensates for the failure of CASKWT to utilize Mg2+.
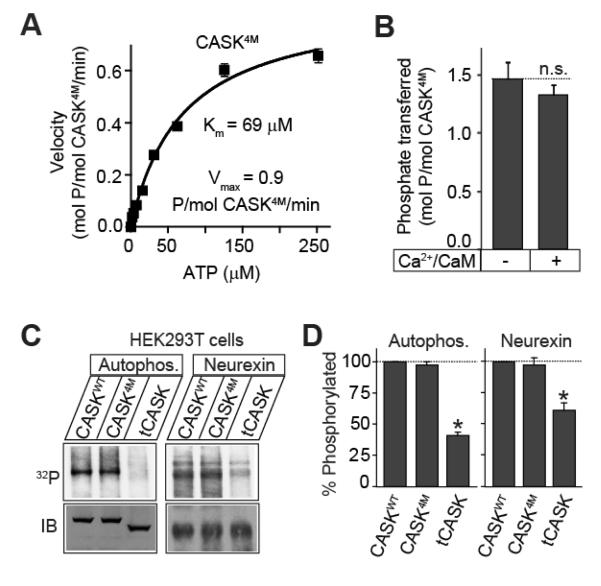
Since CASK4M is not inhibited by divalent ions, we were able to directly test the effect of Ca2+/CaM on the rate of catalysis. We first measured the Mg2+-stimulated catalytic kinetics of CASK4M towards autocamtide-2 (a CaMKII specific substrate) in absence of Ca2+/CaM. Under these conditions, CASK4M has a maximum velocity of ≈ 1 μmol/μmol/min and Michaelis constant for ATP is ≈ 70 μM (Figure 5A). These parameters are comparable with those of Ca2+/Calmodulin-activated CaMKIV for Synapsin I (also a CAMKII substrate) (42). Importantly, addition of Ca2+/CaM had no stimulating effect on the kinase activity of CASK4M (Figure 5B). Together, the structural and the enzymological data suggest that CASK retains a non-functional autoinhibitory domain, possibly as an evolutionary vestige of its CaM-kinase ancestors. Correspondingly, both CASKWT and CASK4M exhibited autophosphorylation activity in complete absence of divalent ions, unlike CaMKII (Supplemental Figure S2C). Whether Ca2+/CaM binding to CASK has another physiological role remains to be tested.
Figure 5. Compensation for slow kinetics in CASK kinase activity.
A. Catalytic kinetics of CASK4M. Purified CASK4M CaM-kinase domain (2 μM) was incubated with increasing amount of γ32P-ATP (400 cpm/pmol) in Tris-HCl buffer pH 7.0, containing Mg2+ (10 mM) and autocamtide (100 μM) as the substrate peptide for 10 min at 30°C. Amount of phospho-Autocamtide-2 generated was estimated by scintillation counting dot-blots on nitrocellulose membrane. Data shown are means ± SEMs (n=3). Michaelis constant (K ATP m ) and Vmax were calculated using Graph-Pad Prism software. Data shown are means ± SEMs (n=3).
B. Effect of Ca2+/CaM on the catalytic rate. CASK4M CaM-kinase domain (2μM) was incubated with γ32P-ATP (200 μM, 800 cpm/pmol) in Tris-HCl buffer (pH 7.0) supplemented with Mg2+ (2 mM) and autocamtide-2 (100 μM), for 2 min in the presence or absence of Ca2+ (1 mM) and CaM (10 μM). Amount of phospho-autocamtide-2 generated was estimated with scintillation counting of dot blots on a nitrocellulose membrane. Data are represented as means ± SEMs, n = 3. n.s. (not significant)
C, D. Neurexin phosphorylation. HEK293T cells were transfected with Flag epitope-tagged neurexin and either EGFP-CASK, EGFP-CASK4M or truncated EGFP-tCASK. 48 h later, transfected cells were labeled with 32Pi, followed by anti-Flag immunoprecipitation of neurexin. Immunoprecipitates were separated by SDS-PAGE and visualized by phosphorimager scanning. Autophosphorylation of the co-precipitated CASK (Autophos.) and phosphorylated neurexin (Neurexin) are shown. Immunoblotting (IB) for neurexin and CASK was performed to show expression. Bar-graph depicts the comparison of autophosphorylation or neurexin phosphorylation levels in cells co-expressing the indicated CASK variants. Data are represented as means ± SEMs, n = 3; asterisks indicate P < 0.05.
CASKWT and CASK4M show similar intracellular kinase activities
The CASK4M activity was stimulated by Mg2+ and was considerably higher than the activity of CASKWT in the presence or absence of Mg2+ in vitro (Figure 2B, D and Supplemental Figures S2A, B). In cells, the majority of ATP is bound to Mg2+. We therefore asked whether in a cytosolic milieu, full-length CASK4M would also yield higher levels of phosphorylated target compared to full-length CASKWT. Apart from CASK itself, neurexin-1 is presently the only characterized in vivo substrate of CASK (21). Neurexin-1 was co-transfected with full-length CASKWT and CASK4M into HEK 293T cells, and the steady-state phosphorylation status of neurexin-1 was quantified (Figure 5C, D). As a control, we used tCASKWT, in which amino acids 1-161 have been deleted, thus severely truncating the kinase domain but leaving the MAGUK domains intact. Surprisingly, neither autophosphorylation nor neurexin-1 phoshorylation levels at steady state level were significantly augmented in CASK4M compared to CASKWT (Figure 5C, D). Thus in the cytosol, CASK activity seems sufficient for maximum neurexin-1 phosphorylation. Most likely, the PDZ domain of CASK, which binds neurexin-1 (10), ensures a constant supply of substrate (21).
Based on the above observations, we suggest that the MAGUK scaffolding domains of CASK spatially constrain the CASK kinase activity to the vicinity of the membrane-bound cell adhesion protein complexes, to which CASK binds. This scaffolding interaction not only raises the local substrate concentration and specificity, but also sustains stoichiometry between CASK and its substrate.
Evolution of CASK from a Mg2+-coordinating kinase
The inability of Mg2+-coordinating CASK4M to increase neurexin-1 phosphorylation in a cellular environment raises the possibility that the original CaM-kinase domain, which merged with a MAGUK to give rise to an ancestral CASK kinase, could have been a canonical Mg2+-coordinating enzyme. Thus, we searched for CASK-like sequences in evolutionarily ancient metazoan and animal species (Supplemental Figure S7).
We detected the most ancient CASK proteins in metazoans that characterize the emergence of animals in evolution. A CASK ortholog was detected in the placozoan Trichoplax adherens, (Figure 6B) which lacks tissue differentiation but contains multiple neuronal proteins (43). Placozoan CASK exhibits canonical residues at three of the four positions, we investigated herein as a source of Mg2+-sensitivity in vertebrate CASK; at the 145-equivalent position in the catalytic loop, where vertebrate CASK carries a histidine instead of a glutamate, placozoan CASK features a glutamine. A similar Glu-to-Gln exchange is found in some active human CaM-kinases, e.g. DRAK-1 or -2, and is therefore compatible with a canonical Mg2+-dependent kinase mechanism. Thus, placozoan CASK resembles CASK4M and may represent a Mg2+-stimulated evolutionary CASK relic.
Figure 6. Evolution of CASK.
A. Evolutionary changes in the nucleotide-binding pocket of CASK CaM-kinase domain. CASK CaM-kinase domain sequences from various animal species were aligned, and the residues corresponding to those mutated in CASK4M were identified and are shown. Corresponding human CaMKIIα residues are shown on the left for comparison.
B. Sequence conservation (identities) of CASK domains between human and placozoan CASK (from T. adhaerens). See Supplemental Figure S7 for a full sequence alignment.
C. Model comparing CASK and CaMKII catalytic cycles. Typically, CaM-kinases are held in an autoinhibited conformation by the autoregulatory domain (yellow) with an open, inactive nucleotide binding cleft. Upon binding of Ca2+ (purple)/CaM (green), this autoinhibition is released and the enzyme attains an active closed conformation amenable to Mg2+ (yellow)/ATP (blue) binding and substrate binding (lower panel). CASK CaM-kinase, on other hand, constitutively binds ATP, and is regulated by the recruitment of its substrates via the MAGUK scaffolding domains, especially the PDZ-domain.
Cnidarians like Nematostella vectensis, the sea anemone, contain differentiated tissues, including neuronal tissue. The CASK ortholog of the sea anemone exhibits a single Glu to His substitution in the catalytic loop (Figure 6A). Our CASK triple mutant (Gly162Asp/Cys146Asn/Pro22Ala), which is not stimulated by Mg2+ (Figure 1) is most similar to the Cnidarian CASK.
All four of the amino acid changes we have investigated herein first appear together in the subkingdom bilateria, as seen in platyhelminthes (Schistosoma japonicum), and are conserved thereafter. Curiously, ecdysozoans (moulting animals) like nematodes (Caenorhabditis elegans) and arthropods (Drosophila melanogaster) exhibit sporadic variations in these substitutions (Figure 6A), whose functional implications remain to be investigated.
Taken together, our phylogenetic analysis indicates that CASK arose from the fusion of a Mg2+-coordinating CaM-kinase domain with a membrane palmitoylated protein (MPP)-like scaffolding MAGUK. This fusion happened concurrently with the development of the basal metazoans. The substrate-scaffolding function provided by the MAGUK domains then could have allowed CASK to gradually shed its dependence on Mg2+. These changes rendered CASK completely dependent on substrate recruitment via its MAGUK domains, such as on the PDZ-domain-dependent binding of neurexins.
DISCUSSION
Mammals contain at least 22 MAGUK proteins that vary in size and domain structure (44). MAGUKs are absent from bacterial, plant, fungal and protozoan genomes, suggesting that their evolution coincides with the emergence of animals. CASK, the only MAGUK bearing a CaM-kinase domain at its N-terminus, first appeared evolutionarily either simultaneous to, or shortly after, the emergence of MAGUKs in basal metazoans. Mutations in CASK are associated with numerous human developmental anomalies (16,17,18,19,20,45) and the CASK gene is essential in mice (15). Although CASK was considered a pseudokinase due to its lack of Mg2+-binding (8,1), we recently found that CASK is an active kinase, and represents the first known kinase inhibited by Mg2+, a co-factor of conventional kinases (21). In the present study, we examined the structural mechanism that confers onto CASK its unique Mg2+-sensitivity. We identified four evolutionarily conserved residues that determine this property, and show that mutation of these residues converts Mg2+-inhibition of CASK into Mg2+-stimulation.
Pseudokinases constitute 10 % of the human kinome. Many pseudokinases perform critical cellular functions, although prior to the identification of kinase activity in CASK, no pseudokinase was shown to be catalytically active. Moreover, prior to the current study, back mutation attempts to convert pseudokinases into standard kinases had failed, possibly due to incomplete understanding of the precise evolutionary changes involved (46,47). Indeed, there are other pseudokinases (such as the Trb-family of Ser/Thr kinases, and CCK4 tyrosine kinase) with substitutions in the Mg2+-binding motifs analogous to those observed in CASK (8), and it is possible that these other pseudokinases may also be catalytically active under defined conditions. The mutations described here for CASK4M may also convert these other enzymes into Mg2+-dependent kinases. Thus, similar to CASK, these atypical kinase domains may have accumulated changes, which specialize them for a particular physiological niche.
Mg2+ has been postulated to promote kinase catalysis in multiple ways, including nucleotide binding, γ-phosphate positioning and stabilization of the transition state. Our ability to generate a Mg2+-coordinating mutant of CASK, CASK4M, in which Mg2+ strongly accelerated phosphotransfer, allowed us to directly address the mechanisms of Mg2+-dependent catalytic enhancement. Analysis of crystal structures together with enzymatic studies strongly suggested that Mg2+ acts primarily downstream of ATP binding, and positions the γ-phosphate of bound ATP optimally for catalysis by binding to its ß-phosphate. In addition, direct binding to the ß-phosphate suggests that stabilization of the transition state and compensation of the additional negative charge evolving at the ß-phosphate during phosphate transfer (thus enhancing the property of ADP as a leaving group) are also possible roles of Mg2+ during catalysis.
Phylogenetically, the CASK CaM-kinase domain falls near the CaMKII cluster (1), whose members are autoinhibited by a Ca2+/CaM dependent regulatory domain (28). Yet, unlike CaMKII, a Arg-to-Leu substitution that is seen even in the most primitive placozoan CASK leaves its autoregulatory segment suboptimal for competing with substrate binding. Moreover, unlike CaMKII (28), CASK exhibits no dimerization of the autoregulatory domain and constitutively adopts an active, closed conformation. This conformation permits uninterrupted ATP-binding to the nucleotide-binding pocket of CASK possibly in part compensating for the suboptimal, Mg2+-independent phosphotransfer chemistry that the enzyme employs.
Moreover, the merger of an ancestral CASK kinase domain with a MAGUK linked the enzyme activity to the MAGUK scaffolding domains, which could recruit its substrate, thereby a) facilitating phosphotransfer by increasing the local substrate-concentration, b) increasing substrate-specificity (9) and c) eliminating the need for fast catalytic turnover (Figure 6C). Based on the above features, CASK enjoys continuous access to both ATP and its protein substrate, rendering the enzyme independent of both Mg2+ and Ca2+.
The presence of Mg2+-coordinating residues in placozoan CASK suggests that the lack of stimulation of CASK by Mg2+ was acquired following, and possibly as a result of, its independence from divalent ions. The primary change in this direction seems to be the acquisition of a His145-equivalent in the nucleotide-binding pocket, which appears to be critical to counteract Mg2+ coordination. This change may have caused a lack of evolutionary pressure to maintain a flexible glycine-rich (GR) loop or Mg2+-coordinating residues elsewhere in the domain, thus inviting secondary changes in the nucleotide-binding pocket. Once optimized over the evolutionary time scale, this domain architecture was maintained in all Chordates.
Why was CASK transformed from a putative Mg2+-stimulated to a Mg2+-inhibited kinase early in evolution? One possibility is that Mg2+ was simply unnecessary in the context of the substrate recruiting mechanism implemented by the MAGUK domains of CASK (21). Consistent with this idea, our experiments in cells (Figure 5C) demonstrate that Mg2+-stimulation does not confer increased steady state phosphorylation in a cellular context when the substrate is recruited by the PDZ-domain. An additional possibility is that divalent ion-sensitivity evolved with emergence of excitable cells like those in the nervous tissue, as a mechanism of negative regulation. Localized influx of divalent ions might significantly reduce the free ATP concentration at a synapse, thereby negatively regulating the catalytic rate of CASK (Figure 6C). Taken together, it appears that the multi-domain structure of CASK allowed the CaM-kinase domain to shed its Mg2+ dependence, which led to evolution of CASK into a hybrid kinase, exhibiting a substrate recruitment module (PDZ domain) fused to a slow, regulated catalytic module (CaM-kinase domain).
Supplementary Material
ACKNOWLEDGEMENTS
We thank members of the Südhof and Jahn laboratories for discussions and Reinhard Lührmann for providing generous access to crystallography equipment. We are grateful to the support by the staff of beamline BL14.2 (BESSY, Berlin, Germany) for help during diffraction data collection. This work was supported by a grant from the NIMH (R37 MH52804-08 to TCS) and the Max-Planck-Society. MS is a Human Frontiers Long Term Fellow.
Footnotes
EXPERIMENTAL PROCEDURES See supplemental material for Methods.
REFERENCES
- 1.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–34. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 2.Noble ME, Endicott JA, Johnson LN. Protein kinase inhibitors: insights into drug design from structure. Science. 2004;303:1800–5. doi: 10.1126/science.1095920. [DOI] [PubMed] [Google Scholar]
- 3.Huse M, Kuriyan J. The conformational plasticity of protein kinases. Cell. 2002;109:275–82. doi: 10.1016/s0092-8674(02)00741-9. [DOI] [PubMed] [Google Scholar]
- 4.Hanks SK, Quinn AM, Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988;241:42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- 5.Taylor SS, Radzio-Andzelm E. Three protein kinase structures define a common motif. Structure. 1994;2:345–55. doi: 10.1016/s0969-2126(00)00036-8. [DOI] [PubMed] [Google Scholar]
- 6.Higgins JM. Haspin-like proteins: a new family of evolutionarily conserved putative eukaryotic protein kinases. Protein Sci. 2001;10:1677–84. doi: 10.1110/ps.49901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayans O, van der Ven PF, Wilm M, Mues A, Young P, Furst DO, Wilmanns M, Gautel M. Structural basis for activation of the titin kinase domain during myofibrillogenesis. Nature. 1998;395:863–9. doi: 10.1038/27603. [DOI] [PubMed] [Google Scholar]
- 8.Boudeau J, Miranda-Saavedra D, Barton GJ, Alessi DR. Emerging roles of pseudokinases. Trends Cell Biol. 2006 doi: 10.1016/j.tcb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Ubersax JA, Ferrell JE., Jr. Mechanisms of specificity in protein phosphorylation. Nat Rev Mol Cell Biol. 2007;8:530–41. doi: 10.1038/nrm2203. [DOI] [PubMed] [Google Scholar]
- 10.Hata Y, Butz S, Sudhof TC. CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J Neurosci. 1996;16:2488–94. doi: 10.1523/JNEUROSCI.16-08-02488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen AR, Woods DF, Marfatia SM, Walther Z, Chishti AH, Anderson JM. Human CASK/LIN-2 binds syndecan-2 and protein 4.1 and localizes to the basolateral membrane of epithelial cells. J Cell Biol. 1998;142:129–38. doi: 10.1083/jcb.142.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsueh YP, Wang TF, Yang FC, Sheng M. Nuclear translocation and transcription regulation by the membrane-associated guanylate kinase CASK/LIN-2. Nature. 2000;404:298–302. doi: 10.1038/35005118. [DOI] [PubMed] [Google Scholar]
- 13.Hsueh YP, Yang FC, Kharazia V, Naisbitt S, Cohen AR, Weinberg RJ, Sheng M. Direct interaction of CASK/LIN-2 and syndecan heparan sulfate proteoglycan and their overlapping distribution in neuronal synapses. J Cell Biol. 1998;142:139–51. doi: 10.1083/jcb.142.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Sudhof TC. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–31. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- 15.Atasoy D, Schoch S, Ho A, Nadasy KA, Liu X, Zhang W, Mukherjee K, Nosyreva ED, Fernandez-Chacon R, Missler M, Kavalali ET, Sudhof TC. Deletion of CASK in mice is lethal and impairs synaptic function. Proc Natl Acad Sci U S A. 2007;104:2525–30. doi: 10.1073/pnas.0611003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimitratos SD, Stathakis DG, Nelson CA, Woods DF, Bryant PJ. The location of human CASK at Xp11.4 identifies this gene as a candidate for X-linked optic atrophy. Genomics. 1998;51:308–9. doi: 10.1006/geno.1998.5404. [DOI] [PubMed] [Google Scholar]
- 17.Froyen G, Van Esch H, Bauters M, Hollanders K, Frints SG, Vermeesch JR, Devriendt K, Fryns JP, Marynen P. Detection of genomic copy number changes in patients with idiopathic mental retardation by high-resolution X-array-CGH: important role for increased gene dosage of XLMR genes. Hum Mutat. 2007;28:1034–42. doi: 10.1002/humu.20564. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi S, Mizuno S, Migita O, Okuyama T, Makita Y, Hata A, Imoto I, Inazawa J. The CASK gene harbored in a deletion detected by array-CGH as a potential candidate for a gene causative of X-linked dominant mental retardation. Am J Med Genet A. 2008;146A:2145–51. doi: 10.1002/ajmg.a.32433. [DOI] [PubMed] [Google Scholar]
- 19.Najm J, Horn D, Wimplinger I, Golden JA, Chizhikov VV, Sudi J, Christian SL, Ullmann R, Kuechler A, Haas CA, Flubacher A, Charnas LR, Uyanik G, Frank U, Klopocki E, Dobyns WB, Kutsche K. Mutations of CASK cause an X-linked brain malformation phenotype with microcephaly and hypoplasia of the brainstem and cerebellum. Nat Genet. 2008 doi: 10.1038/ng.194. [DOI] [PubMed] [Google Scholar]
- 20.Samuels BA, Hsueh YP, Shu T, Liang H, Tseng HC, Hong CJ, Su SC, Volker J, Neve RL, Yue DT, Tsai LH. Cdk5 promotes synaptogenesis by regulating the subcellular distribution of the MAGUK family member CASK. Neuron. 2007;56:823–37. doi: 10.1016/j.neuron.2007.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukherjee K, Sharma M, Urlaub H, Bourenkov GP, Jahn R, Sudhof TC, Wahl MC. CASK Functions as a Mg2+-independent neurexin kinase. Cell. 2008;133:328–39. doi: 10.1016/j.cell.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams JA. Kinetic and catalytic mechanisms of protein kinases. Chem Rev. 2001;101:2271–90. doi: 10.1021/cr000230w. [DOI] [PubMed] [Google Scholar]
- 23.Hanks SK, Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. Faseb J. 1995;9:576–96. [PubMed] [Google Scholar]
- 24.Stewart RC, VanBruggen R, Ellefson DD, Wolfe AJ. TNP-ATP and TNP-ADP as probes of the nucleotide binding site of CheA, the histidine protein kinase in the chemotaxis signal transduction pathway of Escherichia coli. Biochemistry. 1998;37:12269–79. doi: 10.1021/bi980970n. [DOI] [PubMed] [Google Scholar]
- 25.Adams JA, Taylor SS. Divalent metal ions influence catalysis and active-site accessibility in the cAMP-dependent protein kinase. Protein Sci. 1993;2:2177–86. doi: 10.1002/pro.5560021217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng Y, Zhang Y, McCammon JA. How does the cAMP-dependent protein kinase catalyze the phosphorylation reaction: an ab initio QM/MM study. J Am Chem Soc. 2005;127:1553–62. doi: 10.1021/ja0464084. [DOI] [PubMed] [Google Scholar]
- 27.Saylor P, Wang C, Hirai TJ, Adams JA. A second magnesium ion is critical for ATP binding in the kinase domain of the oncoprotein v-Fps. Biochemistry. 1998;37:12624–30. doi: 10.1021/bi9812672. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg OS, Deindl S, Sung RJ, Nairn AC, Kuriyan J. Structure of the autoinhibited kinase domain of CaMKII and SAXS analysis of the holoenzyme. Cell. 2005;123:849–60. doi: 10.1016/j.cell.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 29.Herberg FW, Doyle ML, Cox S, Taylor SS. Dissection of the nucleotide and metal-phosphate binding sites in cAMP-dependent protein kinase. Biochemistry. 1999;38:6352–60. doi: 10.1021/bi982672w. [DOI] [PubMed] [Google Scholar]
- 30.Narayana N, Cox S, Nguyen-huu X, Ten Eyck LF, Taylor SS. A binary complex of the catalytic subunit of cAMP-dependent protein kinase and adenosine further defines conformational flexibility. Structure. 1997;5:921–35. doi: 10.1016/s0969-2126(97)00246-3. [DOI] [PubMed] [Google Scholar]
- 31.Waas WF, Rainey MA, Szafranska AE, Cox K, Dalby KN. A kinetic approach towards understanding substrate interactions and the catalytic mechanism of the serine/threonine protein kinase ERK2: identifying a potential regulatory role for divalent magnesium. Biochim Biophys Acta. 2004;1697:81–7. doi: 10.1016/j.bbapap.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Ohren JF, Chen H, Pavlovsky A, Whitehead C, Zhang E, Kuffa P, Yan C, McConnell P, Spessard C, Banotai C, Mueller WT, Delaney A, Omer C, Sebolt-Leopold J, Dudley DT, Leung IK, Flamme C, Warmus J, Kaufman M, Barrett S, Tecle H, Hasemann CA. Structures of human MAP kinase kinase 1 (MEK1) and MEK2 describe novel noncompetitive kinase inhibition. Nat Struct Mol Biol. 2004;11:1192–7. doi: 10.1038/nsmb859. [DOI] [PubMed] [Google Scholar]
- 33.Tereshko V, Teplova M, Brunzelle J, Watterson DM, Egli M. Crystal structures of the catalytic domain of human protein kinase associated with apoptosis and tumor suppression. Nat Struct Biol. 2001;8:899–907. doi: 10.1038/nsb1001-899. [DOI] [PubMed] [Google Scholar]
- 34.Niefind K, Guerra B, Ermakowa I, Issinger OG. Crystal structure of human protein kinase CK2: insights into basic properties of the CK2 holoenzyme. Embo J. 2001;20:5320–31. doi: 10.1093/emboj/20.19.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valiev M, Yang J, Adams JA, Taylor SS, Weare JH. Phosphorylation reaction in cAPK protein kinase-free energy quantum mechanical/molecular mechanics simulations. J Phys Chem B. 2007;111:13455–64. doi: 10.1021/jp074853q. [DOI] [PubMed] [Google Scholar]
- 36.Battistutta R, De Moliner E, Sarno S, Zanotti G, Pinna LA. Structural features underlying selective inhibition of protein kinase CK2 by ATP site-directed tetrabromo-2- benzotriazole. Protein Sci. 2001;10:2200–6. doi: 10.1110/ps.19601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qian KC, Wang L, Hickey ER, Studts J, Barringer K, Peng C, Kronkaitis A, Li J, White A, Mische S, Farmer B. Structural basis of constitutive activity and a unique nucleotide binding mode of human Pim-1 kinase. J Biol Chem. 2005;280:6130–7. doi: 10.1074/jbc.M409123200. [DOI] [PubMed] [Google Scholar]
- 38.Nolen B, Taylor S, Ghosh G. Regulation of protein kinases; controlling activity through activation segment conformation. Mol Cell. 2004;15:661–75. doi: 10.1016/j.molcel.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 39.Goldberg J, Nairn AC, Kuriyan J. Structural basis for the autoinhibition of calcium/calmodulin-dependent protein kinase I. Cell. 1996;84:875–87. doi: 10.1016/s0092-8674(00)81066-1. [DOI] [PubMed] [Google Scholar]
- 40.Fong YL, Soderling TR. Studies on the regulatory domain of Ca2+/calmodulin-dependent protein kinase II. Functional analyses of arginine 283 using synthetic inhibitory peptides and site-directed mutagenesis of the alpha subunit. J Biol Chem. 1990;265:11091–7. [PubMed] [Google Scholar]
- 41.Smith MK, Colbran RJ, Brickey DA, Soderling TR. Functional determinants in the autoinhibitory domain of calcium/calmodulin-dependent protein kinase II. Role of His 282 and multiple basic residues. J Biol Chem. 1992;267:1761–8. [PubMed] [Google Scholar]
- 42.Miyano O, Kameshita I, Fujisawa H. Purification and characterization of a brain-specific multifunctional calmodulin-dependent protein kinase from rat cerebellum. J Biol Chem. 1992;267:1198–203. [PubMed] [Google Scholar]
- 43.Srivastava M, Begovic E, Chapman J, Putnam NH, Hellsten U, Kawashima T, Kuo A, Mitros T, Salamov A, Carpenter ML, Signorovitch AY, Moreno MA, Kamm K, Grimwood J, Schmutz J, Shapiro H, Grigoriev IV, Buss LW, Schierwater B, Dellaporta SL, Rokhsar DS. The Trichoplax genome and the nature of placozoans. Nature. 2008;454:955–60. doi: 10.1038/nature07191. [DOI] [PubMed] [Google Scholar]
- 44.te Velthuis AJ, Admiraal JF, Bagowski CP. Molecular evolution of the MAGUK family in metazoan genomes. BMC Evol Biol. 2007;7:129. doi: 10.1186/1471-2148-7-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Q, Lu J, Yang C, Wang X, Cheng L, Hu G, Sun Y, Zhang X, Wu M, Liu Z. CASK and its target gene Reelin were co-upregulated in human esophageal carcinoma. Cancer Lett. 2002;179:71–7. doi: 10.1016/s0304-3835(01)00846-1. [DOI] [PubMed] [Google Scholar]
- 46.Boudeau J, Scott JW, Resta N, Deak M, Kieloch A, Komander D, Hardie DG, Prescott AR, van Aalten DM, Alessi DR. Analysis of the LKB1-STRAD-MO25 complex. J Cell Sci. 2004;117:6365–75. doi: 10.1242/jcs.01571. [DOI] [PubMed] [Google Scholar]
- 47.Prigent SA, Gullick WJ. Identification of c-erbB-3 binding sites for phosphatidylinositol 3′-kinase and SHC using an EGF receptor/c-erbB-3 chimera. Embo J. 1994;13:2831–41. doi: 10.1002/j.1460-2075.1994.tb06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.