Abstract
Arthropod-borne viruses (arboviruses) are of paramount concern as a group of pathogens at the forefront of emerging and re-emerging diseases. Although some arboviral infections are asymptomatic or present with a mild influenza-like illness, many are important human and veterinary pathogens causing serious illness ranging from rash and arthritis to encephalitis and hemorrhagic fever. Here, we discuss arboviruses from diverse families (Flaviviruses, Alphaviruses, and the Bunyaviridae) that are causative agents of encephalitis in humans. An understanding of the natural history of these infections as well as shared mechanisms of neuroinvasion and neurovirulence is critical to control the spread of these viruses and for the development of effective vaccines and treatment modalities.
Keywords: arbovirus, Flavivirus, Bunyaviridae, Alphavirus, encephalitis
The arthropod-borne viruses (arboviruses): transmission, emergence, and re-emergence
Arthropod-borne viruses (arboviruses) are propagated through biological transmission between vertebrate hosts by hematophagous (blood feeding) arthropod vectors such as mosquitoes, biting midges, phlebotomine flies, and ticks. Arboviruses are found in diverse viral families, including Togaviridae (genus Alphavirus); Bunyaviridae (genera Nairovirus, Orthobunyavirus, Phlebovirus, and Tospovirus); Flaviviridae (genus Flavivirus); Rhabdoviridae (genus Vesiculovirus); Orthomyxoviridae (genus Thogotovirus); Reoviridae (genus Orbivirus); and Asfarviridae (genus Asfarvirus) (Weaver and Reisen 2010). Many arboviral infections in humans are asymptomatic or present with a mild influenza-like illness. However, several arboviruses have become increasingly important human and veterinary pathogens, causing respiratory illness, arthritis, febrile illness, encephalitis, hemorrhagic syndrome, shock, and death. In this review, we describe the natural history of arboviral infections and focus on arboviruses that cause neurologic disease in humans, specifically members of the genera Flavivirus and Alphavirus and the family Bunyaviridae.
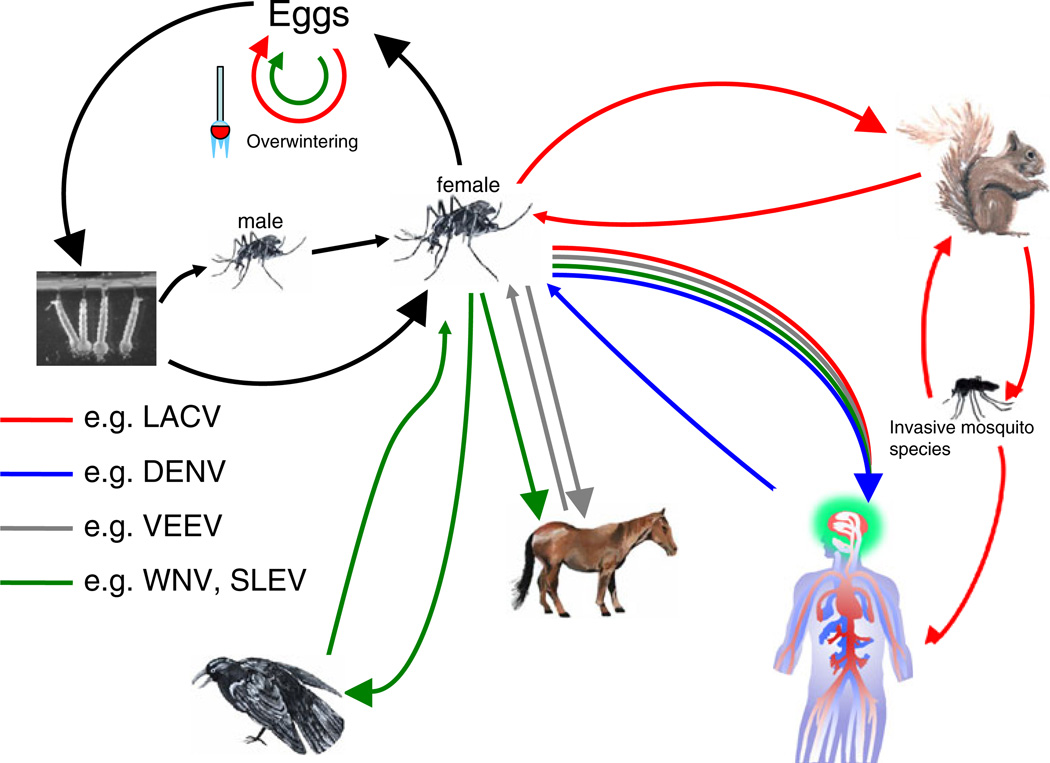
Most arboviruses are spread through sylvatic transmission cycles between the invertebrate vector(s) and an enzootic vertebrate reservoir—typically rodents, birds, and non-human primates (Fig. 1). In the amplifying vertebrate host, arboviruses must achieve a high viremia that is sufficient for a permissive arthropod to become infected during a blood meal. In this enzootic cycle, transmission to dead-end hosts (humans or domestic animals) may occur. Viremia is low or transient in the dead-end host, and there is little chance of passing enough virus to establish an infection in an arthropod vector. However, some arboviruses (e.g., Venezuelan equine encephalitis virus [VEEV] and Japanese encephalitis virus [JEV]) have expanded their range by establishing epizootic amplification cycles in domestic animals such as horses and pigs (Brault et al. 2002, Weaver et al. 1999, van den Hurk et al. 2009). In most cases, humans serve as dead-end hosts and do not play a major role in maintaining the arbovirus lifecycle (Fig. 1). However, an urban epidemic cycle in which humans have become the primary amplifying host has been described for dengue virus (DENV), yellow fever virus, and chikungunya virus (CHIKV; Weaver and Barrett 2004; Barrett and Higgs 2007; Nimmannitya et al. 1969; Padbidri and Gnaneswar 1979).
Fig. 1.
Representative life cycles of arboviruses. Arboviruses are maintained in nature through an invertebrate reservoir cycle and a vertebrate amplification cycle. Mosquitoes are the representative vectors in this figure. However, other hematophagous invertebrates can serve as arboviral vectors. For many arboviruses, vertical transmission from an infected female mosquito to her progeny occurs via transovarial infection of eggs (e.g., LACV [red] and WNV [green]; Patrican and DeFoliart 1987; Watts et al. 1974; Young et al. 2008). Infected male mosquitoes can infect naïve female mosquitoes by venereal transmission. Upon taking a blood meal, infected mosquitoes may transmit virus to amplifying vertebrate hosts. These amplifying hosts develop brief, but high viremias that may lead to subsequent infection of other hematophagous mosquitoes from either the same or different mosquito species. In the enzootic cycle, arboviruses use sylvatic (red and green) transmission (e.g., LACV, small rodents and WNV and St. Louis encephalitis virus (SLEV), birds). In most cases, humans serve as dead-end hosts and do not play a major role in maintaining the arbovirus lifecycle. However, some arbovirus (e.g., VEEV, horses and JEV, pigs) have established epizootic amplification cycles in domestic animals (gray). Of increasing concern, some arboviruses (e.g., DENV) can rely on human amplification (blue) in an urban setting
In addition to vector/host dynamics, climate, geography, and immune status of host populations play a significant role in the arbovirus lifecycle. Two climate-dependent transmission patterns for arboviruses have been described. In tropical areas, virus circulates throughout most of the year, often with a broad seasonal peak. In more temperate climates, however, virus is transmitted between the vector and vertebrate host species only during the warmer months and arboviral disease is absent in the colder months. For arboviruses in these cooler climates, the virus often persists by overwintering in mosquito eggs (Fig. 1; Gubler 2002).
Arboviruses are distributed worldwide, representing nearly 30% of all emerging infectious diseases in the last decade (Jones et al. 2008). While the variables contributing to the epidemiology of each virus are unique, some common socio-economic, environmental, and ecological factors contribute to the emergence of arboviral diseases (Morens et al. 2004). Human behavior has played a significant role in the emergence and re-emergence of arboviral diseases. Modern travel and trade has greatly facilitated the spread of arboviruses and highly efficient, anthropophilic mosquitoes, including Aedes aegypti and Aedes albopictus, and mosquitoes in the Culex pipiens complex (Benedict et al. 2007, Fonseca et al. 2004). In addition, environmental factors, including agricultural development, urban expansion, and population growth have increased human contact with arboviruses and their vectors. Unplanned urbanization accompanied by crowded, substandard living conditions with inadequate water and waste management provides ideal breeding sites for mosquitoes. Finally, climate change is thought to play a significant role in the emergence and re-emergence of arboviral diseases (Elliott 2009). Longer durations of warm weather have created conditions that are conducive to increased vector populations and provided opportunities for changes in vector range, vertebrate host, and vector composition and dynamics (Weaver and Reisen 2010). Notably, cycles of increased precipitation, including those experienced during the warm phase of the El Niño/Southern Oscillation (ENSO) phenomenon, have been associated with arbovirus outbreaks and an increased abundance and prolonged hostseeking activity of mosquito vectors (Linthicum et al. 1999; Heft and Walton 2008).
Flaviviruses
The genus Flavivirus contains some of the most well-studied agents of arboviral encephalitides. The history and international prominence of this group of viruses have made it a paradigm for the emergence potential of arboviruses. The type species of the genus Flavivirus, yellow fever virus (flavus means yellow in Latin), spread into the Americas from West Africa through the slave trade beginning in the sixteenth century and serves as one of the earliest documented examples of an arboviral disease’s spread to a new continent by human travel (Gould et al. 2003). More recently, the re-emergence of DENV and emergence of neurotropic flaviviruses, such as West Nile virus (WNV) and JEV, have underscored the potential for this group of viruses to cause widespread human disease (Morens et al. 2004).
The flaviviruses are spherical, enveloped viruses with a positive-strand, nonsegmented RNA genome that produces ten mature viral proteins via proteolytic processing of a single polyprotein. These mature viral proteins include three structural proteins (capsid [C], envelope [E], and premembrane/ membrane [prM/M]) and seven nonstructural proteins (NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5; Stadler et al. 1997; Yu et al. 2008). During the maturation process, prM is transported through the cellular secretory pathway where cellular furin cleaves prM resulting in the release of the pr peptide from the M protein and, consequently, the formation of mature virions. Flaviviruses enter the cell via receptor-mediated endocytosis, and fusion of the viral membrane with the endosomal vesicle membrane occurs after exposure to low pH in the endosome following internalization through a class II fusion mechanism shared with both the Alphaviruses and the Bunyaviridae (Kielian 2006; Plassmeyer et al. 2007).
A majority of the 75+ identified viruses contained in the genus Flavivirus are arboviruses; approximately 40 are mosquito-borne, while 16 are tick-borne, and 18 have no known vector (Heinz et al. 2000). Of interest, genomic sequence analysis and base substitution rates have demonstrated that the mosquito-borne, tick-borne, and the majority of the flaviviruses without a known vector can be separated in to three distinct evolutionary lineages that diverged early in the evolution of the genus (Billoir et al. 2000). Additionally, all flaviviruses can be grouped serologically. Here, we will focus mainly on the Japanese encephalitis (JE) serogroup whose members are major etiologic agents of arboviral encephalitides nearly worldwide (Porterfield 1975).
Japanese encephalitis virus
JEV is the leading cause of viral encephalitis in Asia with an estimated 35,000 to 50,000 cases and 15,000 deaths reported annually (Tsai 2000) (Misra and Kalita 2010). Case-fatality rates range from 0.3% to 60%, depending on age and the population. JE is primarily a disease of children in endemic areas, but it affects both adults and children in newly affected areas because of the lack of protective immunity (Misra and Kalita 2010). JEV is a member of the JE serogroup and is antigenically related to WNV, St. Louis encephalitis virus, Kunjin virus, Usutu virus, and Murray Valley encephalitis virus (Broom et al. 2002; Day 2001; Kramer et al. 2007). Minor members of the JE serogroup include the Koutango and Yaounde viruses in Africa and Cacipacore virus in South America. Most of the JE serogroup viruses have an avian amplifying host and are transmitted by Culex mosquitoes. The prototype Nakayama strain of JEV was first isolated from a fatal case of encephalitis in Tokyo, Japan in 1935. However, encephalitis epidemics in humans and horses, ostensibly resulting from JEV infection, have been reported in Japan since the 1870s (Solomon et al. 2000). In the twentieth century, the virus spread across Asia and can now be found in most of China, Southeast Asia, the Asian subcontinent, and the Pacific Rim, reaching northern Australia in 1998 (van den Hurk et al. 2009; Gao et al. 2010).
JEV is maintained in an enzoonotic cycle between ardeid wading birds and Culex mosquitoes (Table 1; Sucharit et al. 1989). Water birds are believed to both serve as maintenance hosts and contribute to the spread of JEV to new geographical regions (Solomon et al. 2000). Culex tritaeniorrhynchus, the primary mosquito vector for JEV, breeds in rice paddy fields or pools of stagnant water (Innis 1995) and coincidental infection of humans living or traveling in close proximity to the enzootic cycle of JEV, usually in rural areas, may occur (Solomon et al. 2000; Cao et al. 2010). However, pigs have become an important host for the maintenance, amplification, and spread of JEV because of the high frequency of a long-lasting viremia in infected pigs, the preference that C. tritaeniorrhynchus mosquitoes have shown for feeding on pigs, and the frequent replenishing of susceptible pigs in endemic areas each year owing to the commercial pork industry (Misra and Kalita 2010). JEV antibodies have also been reported in horses, cattle, sheep dogs, and monkeys. These animals are generally asymptomatic; however, fatal encephalitis can occur in infected horses and (Yamanaka et al. 2006) and fatal encephalitis, abortion and stillbirth have been observed in pregnant sows infected with JEV (Li et al. 2010).
Table 1.
Major etiological agents of arboviral encephalitides
| Virus Family | Vertebrate Reservoir |
Primary Vector | Distribution | References |
|---|---|---|---|---|
| Flaviviridae | ||||
| Japanese encephalitis virus | Birds, Pigs | Culex tritaeniorrhynchus | SE Asia, Pacific Rim | (van den Hurk et al. 2009) |
| Murray Valley encephalitis virus | Birds | Culex annulirostris | Australia | (Broom et al. 2002) |
| St. Louis encephalitis virus | Birds | Culex species | Americas | (Day 2001) |
| West Nile Virus | Birds | Culex species, Aedes albopictus | North America, Africa, Europe, Asia, Australia | (Kramer et al. 2007) |
| Bunyaviridae | ||||
| California encephalitis virus | Rodents, rabbits | Aedes melanimon | North America | (Griot et al. 1993) |
| La Crosse virus | Chipmunks, squirrels | Ochleratus triseriatus and A. albopictus | North America | (Borucki et al. 2002) |
| Rift Valley fever virus | Sheep, cattle | Aedes and Culex species | Africa | (Bird et al. 2009) |
| Toscana virus | Bats, humans | Phlebotomine flies | Europe | (Dionisio et al. 2003) |
| Alphaviridae | ||||
| Chikungunya virus | Primates, humans | Aedes aegypti | Africa, India, SE Asia | (Cavrini et al. 2009) |
| Eastern equine encephalitis virus | Birds | C. melanura, Psorophora, Ochlerotatus, and Aedes species | Americas | (Zacks and Paessler 2010) |
| Venezuelan equine encephalitis virus | Rodents, horses | A. aegypti | Americas | (Zacks and Paessler 2010) |
| Western equine encephalitis virus | Birds, mammals | Culex tarsalis | North America | (Zacks and Paessler 2010) |
In humans, the incubation period of JEV is 5–15 days, and the vast majority of infections are asymptomatic. Clinical disease from JEV infections varies from non-specific febrile illness to meningoencephalitis, aseptic meningitis, acute flaccid paralysis, and severe encephalitis (Solomon et al. 1998; Solomon and Vaughn 2002). Symptoms of non-specific febrile illness caused by JEV may include headache, cough, nausea, vomiting, or diarrhea. In the case of the more severe JE, patients develop a Parkinsonian syndrome with wide, unblinking eyes, tremor, and cogwheel rigidity (Solomon and Vaughn 2002). Seizures are common in affected individuals, especially children. Upper motor neuron signs, cerebellar signs, and cranial nerve palsies are common in JE patients and cognitive and language impairments occur in approximately 30% of those affected (Mackenzie et al. 2004).
The mechanisms used by JEV to penetrate the blood brain barrier (BBB) are not fully understood. After an individual acquires JEV from an infected mosquito, peripheral amplification of the virus occurs primarily in the epidermis and lymph nodes. Primary infection typically leads to a rapid and strong IgM response in serum and cerebrospinal fluid within days of infection (Burke et al. 1985). Both the humoral and cellular immune responses are believed to protect the host by restricting viral replication during the viremic phase, before the virus crosses the BBB (Hammon and Sather 1973). However, upon achieving a sufficient viremia, JEV is thought to enter the central nervous system (CNS) via the olfactory bulb or the choroid plexus (Liou and Hsu 1998). Of interest, the JEV E protein, which plays a major role in virus fusion and entry, has been demonstrated to have a major role in determining neuroinvasiveness and neurovirulence of the virus in the murine model (Ni and Barrett 1996). Once in the brain, JEV infection appears to be widespread. Necropsies have shown that the thalamus, basal ganglia, and midbrain are heavily affected, providing anatomical correlates to tremor and other Parkisonian-like syndromes (Miyake 1964; Johnson et al. 1985). JEV is believed to cause neuronal injury through a proinflammatory cytokine and chemokine cascade that occurs upon activation of astrocytes and microglia. It has been demonstrated that various proinflammatory molecules including IL-1β, MCP-1 (CCL-2), IFN-α, TNF-α, RANTES (CCL-5), IL-6, and IL-8 are elevated in JE patients (Babu et al. 2006; Winter et al. 2004).
There are no specific antivirals for JE and treatment focuses on supportive care. However, a number of drugs including minocycline and curcumin are under investigation as potential therapeutics for JEV and other arboviral infections for both their antiviral and neuroprotective effects (Dutta et al. 2009; Mishra and Basu 2008; Richardson-Burns and Tyler 2005). Fortunately, JEV is one of the few arboviruses for which a vaccine is available. Most vaccines for JEV are derived from infected mouse brain, and multiple doses are required to achieve efficacies of at least 80% (Rojanasuphot et al. 1989). The widespread use of vaccination has greatly improved control of JEV in Japan, Korea, Taiwan, and Singapore. In March 2009, the Food and Drug Administration approved a new, inactivated cell-culture-derived JEV vaccine (IXIARO) for use in adult travelers over the age of 17 (Kurane and Takasaki 2000; Fischer et al. 2010; Duggan and Plosker 2009).
West Nile virus
First isolated in 1937 in the West Nile district of Uganda, WNV was not detected in the Western hemisphere until 1999 when an outbreak in New York City and surrounding areas resulted in 62 cases of WNV encephalitis and seven deaths (Briese et al. 1999; Jia et al. 1999; Gubler 2007). The introduction of WNV to North America was accompanied by avian mortalities, particularly among American crows and other corvids. Upon isolation and analysis of the virus responsible for the outbreak, a strain of WNV with a close genetic relationship to WNV isolates from Israel suggested that the virus was imported from the Middle East (Jia et al. 1999; Linthicum et al. 1999), possibly by travelers from Israel or migratory or exotic birds transported in an airplane (Davis et al. 2006). Subsequent to the introduction of WNV to New York City, the virus has expanded its range to include the 48 contiguous states and spread throughout Canada, Mexico, the Caribbean, and Colombia (Davis et al. 2006; Murray et al. 2010). WNV is now the leading cause of arboviral encephalitis in the United States of America (Davis et al. 2006; Brinton 2002).
Birds are the amplifying hosts for WNV and, although the virus infects over 300 species of birds, the American robin and American crow are the most common carriers of the virus in the Western hemisphere. Infection in wild birds is often asymptomatic, and vulnerability to lethal infection varies between species. Infected birds can develop a prolonged viremia, sometimes lasting more than 100 days, which allows for repeated cycles of mosquito infection (Komar 2003; Malkinson and Banet 2002; Reisen and Brault 2007). WNV has been identified in over 50 mosquito species, but as with JEV, the main vectors for transmission of WNV are Culex mosquitoes (Table 1). Although the virus is promiscuous in its vector usage, the main species involved in WNV transmission are C. pipiens, Culex restuans, Culex quinnquefasciatus, and Culex tarsalis (Davis et al. 2006; Smithburn et al. 1940). Currently, it is unclear which mosquito species primarily transmit WNV to humans. Alternative routes of transmission to humans have been described including transfusion of blood products, organ transplantation from an infected donor, and mother-to-child transmission either across the placenta or through breast-feeding (Harrington et al. 2003; Iwamoto et al. 2003; Davis et al. 2006). Serological data show that numerous mammalian species can become infected with WNV. However, with the exception of horses who are also susceptible to WNV meningoencephalitis, these non-avian, dead-end hosts do not significantly contribute to the maintenance of the virus (Marfin and Gubler 2001; McLean et al. 2002; Trevejo and Eidson 2008).
Although most human WNV infections are asymptomatic, West Nile fever occurs in approximately 20–30% of infections. West Nile fever consists of a flu-like illness that develops after incubation for 3–14 days and is characterized by fever, headache, back pain, fatigue, anthralgia, and myalgia persisting for 3 days to several weeks (Hayes et al. 2005). Retro-orbital pain, anorexia, nausea, vomiting, diarrhea, and pharyngitis can also occur (Petersen and Marfin 2002). Additionally, a maculopapular rash occurs in about half of West Nile fever cases, especially in children. Severe infections have even led to myocarditis, pancreatitis, and hepatitis (Solomon and Vaughn 2002). Neuroinvasion of WNV results in meningitis or encephalitis in approximately 0.7% of infections and may result in tremor or parkinsonian features (Petersen and Marfin 2002) (Sejvar et al. 2003). Approximately 60% of patients with neuroinvasive WNV disease have encephalitis and 40% have meningitis (Gyure 2009). The mortality rate is about 10% among patients with neuroinvasive disease, with increased age being the most important risk factor for the development of neuroinvasion and death. Muscle weakness occurs in approximately half of patients with WNV encephalitis, a marked increase in comparison to other arboviral encephalitides. Additionally, WNV infection may cause an acute flaccid paralysis syndrome, seizures, or cerebellar ataxia (Petersen and Marfin 2002; Solomon and Vaughn 2002). Survivors of WNV encephalitis often suffer long-term cognitive and neurological impairments including muscle weakness, insomnia, depression, confusion, headache, and myalgia (Petersen and Marfin 2002). In general, better outcomes are observed in younger patients.
Again, the mechanisms leading to neuroinvasion of WNV are not completely understood. After virus inoculation by mosquitoes, the virus replicates in Langerhans and dendritic cells. Infected Langerhans cells migrate to draining lymph nodes, allowing the virus to enter the bloodstream (primary viremia). Dissemination of virus to the reticuloendothelial system further augments viremia (secondary viremia; Gyure 2009). It is believed that invasion of the nervous system may occur after direct infection of endothelial cells in the cerebral microvasculature or after viremic dissemination to the olfactory bulb and direct axonal retrograde transport from infected peripheral neurons (Samuel and Diamond 2006). Innate (complement and interferon), humoral, and cell-mediated immunity limit WNV dissemination and facilitate viral clearance (Klein and Diamond 2008). However, it has been suggested that, in addition to their protective role, CD8+ T cells contribute to the neuropathogenesis of WNV infection by secreting proinflammatory cytokines and lysing virus-infected neurons (Sitati and Diamond 2006). Host genetics play a role in disease susceptibility and individuals homozygous for a defective form of the chemokine receptor CCR5 (CCR5Δ32) are at an increased risk of neuroinvasive WNV disease (Glass et al. 2006). Moreover, several virus encoded proteins are directly involved in WNV-mediated CNS disease including the NS3, which induces apoptosis through the extrinsic, death receptor-linked caspase-8 pathway, and the NS1, NS2A, and NS4B proteins which function as interferon antagonists (Diamond 2009). Notably, as we will discuss below, pro-apoptotic, host-protein synthesis inhibitory, and type I interferon blocking properties have been attributed to several non-structural proteins of arboviruses from different families suggesting that the ability of these viruses to induce death and subvert the innate immune system is critical for their maintenance in nature and may hold the key to uncovering shared mechanisms of neuropathogenesis (Blakqori et al. 2007; Billecocq et al. 2004; Yin et al. 2009). Moreover, the characterization of common mechanisms that these viruses use to induce apoptosis and counter the innate immune system may lead to the development of broadly applicable treatment modalities for arboviral encephalitides.
There are currently no human vaccines or antiviral therapeutics with proven efficacy for WNV, although equine WNV vaccines are currently in use (Reisen and Brault 2007). Several potential treatment modalities including ribavirin (used in Hepatitis C, another member of the Flaviviridae, in combination with pegylated interferon drugs), interferon-〈2b, immunoglobulin with high titer against West Nile virus (Omr-IgG-aM), and humanized monoclonal antibodies (e.g., Mab E16) are candidate therapeutic agents based on animal models and limited clinical experience (Thompson et al. 2009; Gyure 2009; Levi et al. 2010).
Bunyaviridae
The Bunyaviridae are the largest viral family with over 350 viral species encompassed by five genera (Orthobunyavirus, Hantavirus, Nairovirus, Phlebovirus, and Tospovirus; Calisher 1986; Nichol et al. 2005). The terminal 10–15 nucleotides of each RNA genome segment, which are highly conserved and partially complementary, play a major role in delineating these genera along with antigenic, serological, molecular, and structural differences (Calisher 1986). The bunyavirus genome is composed of three negative-sense, single-stranded RNA segments. The genome segments, designated by size as large (L), medium (M), and small (S), are encapsidated by nucleocapsid (N) protein to form helical ribonucleoprotein complexes. The L segment encodes the viral polymerase that is responsible for both the transcription and replication of the viral genome. A polyprotein is encoded in a single open reading frame (ORF) by the M segment and is cotranslationally processed into the viral glycoproteins, Gc (G1) and Gn (G2), and in the case of the orthobunyaviruses and some phleboviruses, a non-structural protein (NSm) of unknown function. The S segment encodes the N protein. For the orthobunyaviruses, phleboviruses, and tospoviruses, the S segment also encodes another non-structural protein (NSs) through an ORF (orthobunyaviruses) or an ambisense coding mechanism (phleboviruses and tospoviruses; Schmaljohn and Hooper 2001).
Bunyaviruses are disseminated worldwide and infect a broad range of invertebrate and vertebrate hosts. With the exception of the hantaviruses (Plyusnin and Morzunov 2001), all of the viruses in the family Bunyaviridae are vector-borne and utilize hematophagous arthropod vectors including mosquitoes, phlebotomine flies, and ticks. Many bunyaviruses are significant pathogens in humans and animals with the exception of the tospoviruses, which are plant pathogens. Human illnesses resulting from bunyavirus infection range from mild, asymptomatic infection to more severe disease including pulmonary disease, hemorrhagic fever, and fatal encephalitis (Soldan and Gonzalez-Scarano 2005; Dionisio et al. 2003). For example, Crimean-Congo hemorrhagic fever virus (CCHFV; Nairovirus) is the second most widespread of all medically important arboviruses after DENV and is endemic in much of Africa, Asia, and Europe (Ergonul 2006; Hoogstraal 1979). Notably, mortality rates of up to 30% in humans have been reported for CCHFV (Ergonul 2006). In addition, La Crosse virus (LACV; Orthobunyavirus) and Rift Valley fever virus (RVFV; Phlebovirus) are important causes of arboviral encephalitides and will be discussed at length in this review.
Currently, there are no effective therapeutics or vaccines for bunyaviruses with the exception of vaccines for veterinary use developed to protect against RVFV infection. Unfortunately, the RVFV vaccines have been relatively unsuccessful due to vaccine shortages, incomplete protection, and because of deleterious effects of vaccination (Caplen et al. 1985; Morrill et al. 1997b; Morrill et al. 1997a; Morrill and Peters 2003; Randall et al. 1964). In this era of rapidly changing local and global environments (Nichol et al. 2000), several factors including the promiscuous use of arthropod vectors and vertebrate hosts have made the Bunyaviridae prominent among emerging and re-emerging infectious agents and have exacerbated the need for effective therapeutics and vaccines.
La Crosse virus
LACV was first isolated from the brain of a fatal case of encephalitis in a young girl who died in La Crosse, Wisconsin, after becoming infected in Minnesota (Thompson et al. 1965). LACV is recognized as a major cause of pediatric arboviral encephalitis in the Midwestern United States of America, where its primary mosquito vector, Ochleratus (formerly Aedes) triseriatus is native. Recently, LACV has spread south into Tennessee, North Carolina, and West Virginia due to invasive mosquito species including the aggressive, day-feeding Asian tiger mosquito, A. albopictus (Gerhardt et al. 2001; Elliott 2009). The expanding distribution of LACV into North Carolina and Tennessee, along with the ability of LACV to use A. albopictus as a secondary vector, could result in an increase in both the incidence and distribution of LACV throughout the southeastern United States of America (Gerhardt et al. 2001) (Haddow et al. 2009).
The amplification and maintenance of LACV predominantly involves O. triseriatus (Table 1) as the principal arthropod vector with chipmunks and squirrels serving as the primary vertebrate hosts. In endemic areas of LACV, a large proportion of squirrels (30%) and chipmunks (60%) are LACV seropositive (Gauld et al. 1974; Soldan and Gonzalez-Scarano 2005). Infection of chipmunks and squirrels results from an infected mosquito taking a blood meal and entry of LACV into the vertebrate host from the mosquito’s saliva. Subsequent to infection, the rodent develops a high viremia capable of infecting naïve blood-feeding mosquitoes. However, the rodent hosts do not exhibit overt illness (Borucki et al. 2002). Furthermore, LACV can be transovarially transmitted from an infected female mosquito to her progeny, and recent studies have suggested that female mosquitoes infected with LACV mate more efficiently than uninfected mosquitoes (Reese et al. 2009). Virus can be amplified substantially by this mechanism of transmission since each gonadotropic cycle leads to a multitude of infected progeny (Borucki et al. 2002, Watts et al. 1973). Importantly, LACV is able to overwinter through this transovarial transmission (Watts et al. 1974; Thompson and Beaty 1977).
Most human LACV infections occur from July through September in children under the age of 15 (McJunkin et al. 2001). The incidence of LACV encephalitis in endemic areas is approximately 20 to 30 cases per 100,000 children younger than 15 years of age (McJunkin et al. 2001). Reports of LACV encephalitis in the United States of America have historically ranged from 42 to 174 cases per year, which is likely an underreporting due to clinical similarities with herpes simplex virus encephalitis (Borucki et al. 2002; McJunkin et al. 2001). A recent review of LACV infections in the eastern United States of America (Arkansas, Delaware, Florida, Maryland, Missouri, New Jersey, and Pennsylvania) from 2003–2007 reported that there were a total of 282 confirmed LACV infections during the study period (Haddow and Odoi 2009). Clinically, these infections presented as uncomplicated fever (5.0%), meningitis (17.2%), meningioencephalitis (56.3%), or encephalitis (20.7%). Further, this study confirmed that the incidence risk and case fatality rates of LACV infection may be higher than previously reported with a county level incidence risk ranging from 0.2 to 228.7 per 100,000 (Haddow and Odoi 2009; Haddow et al. 2009). Human illness resulting from LACV infection is typically a nonspecific febrile illness; however, in a small proportion of children, acute encephalitis follows. Symptoms associated with LACV encephalitis include headache, fever, vomiting, stiff neck, and, rarely, coma (McJunkin et al. 2001; Gonzalez-Scarano et al. 1996; Kalfayan 1983). Approximately half of the cases of LACV encephalitis have seizures during the acute illness with about 10% developing epilepsy. About 2% of LACV encephalitis cases develop persistent paresis, learning disabilities, or cognitive defects, as well as, neurobehavioral sequelae such as attention deficits and hyperactivity (McJunkin et al. 2001, Gunderson and Brown 1983, Rust et al. 1999). However, LACV encephalitis rarely results in death with a case-fatality rate of about 0.3% (McJunkin et al. 2001, Rust et al. 1999).
Many features of the human disease of LACV encephalitis are recapitulated in an established mouse model of LACV encephalitis. As with humans, the susceptibility of mice to LACV is age-dependent where younger hosts are significantly more susceptible to CNS infection (Janssen et al. 1984; Janssen et al. 1986; Gonzalez-Scarano et al. 1985). This model enables the study of the neuropathogenesis and progression of LACV from the periphery into the CNS following subcutaneous viral inoculation. In this model, newborn mice are sensitive to subcutaneous inoculation of as little as one plaque-forming unit (PFU) of LACV, whereas adult mice are resistant to much higher doses administered by the same route. However, intracranial inoculation of LACV is fatal to mice regardless of age. This demonstrates that LACV is neurovirulent even when not neuroinvasive. Following peripheral inoculation of LACV into suckling mice, the virus replicates predominantly in the striated muscle. This replication leads to a high viremia, which allows the virus to cross the BBB and is a critical determinant in the neuroinvasion of LACV. Studies using the murine model identified that the neuroinvasiveness of LACV is determined by its ability to generate a high viremia, and this viremia correlates with virus replication in striated muscle (Janssen et al. 1984). Even after intracranial inoculation, there are age-related differences in viral antigen in the brain. Here, suckling mouse brains demonstrate disseminated viral antigen, while in adult mice viral antigen is more restricted to specific neuroanatomical regions, specifically the pyramidal cell layer of the hippocampus, rostral midbrain, and hypothalamus (Janssen et al. 1984).
The murine model for LACV is particularly informative because it allows for the distinction between neuroinvasion and neurovirulence of LACV by peripheral or intracranial virus inoculation, respectively. Further studies in the murine model of LACV encephalitis using a monoclonal antibody LACV escape variant (V22), which had decreased fusion and a lower pH threshold to mediate fusion, demonstrated that LACV V22 had decreased replication in peripheral tissues (e.g., striated muscle) critical for the generation of a high viremia following peripheral inoculation (Gonzalez-Scarano et al. 1985). However, LACV V22 still replicated almost as robustly as wild-type LACV and remained neurovirulent in mouse brains after intracranial inoculation. LACV V22 showed similar antigen distribution in the brain as well as similar, though less severe, neuropathological changes (Gonzalez-Scarano et al. 1985; Griot et al. 1993). Most recently, recombinant LACV with mutations in the fusion peptide domain demonstrated decreased replication in differentiated muscle cells (murine cell line G8) and primary rat neuronal cultures as well as decreased fusion phenotypes in vitro. These viruses, however, retained their ability to cause neuronal loss in primary rat neuronal cultures (Soldan et al. 2010). These studies suggest that attenuation of LACV neuroinvasiveness does not necessarily correlate with decreased neurovirulence. Similarly, a mutation in the fusion loop (L107F), but not the receptor-binding domain III (A316V) or a stem-helix (K440R), of the WNV fusion peptide attenuated neuroinvasiveness of WNV when inoculated peripherally. Nevertheless, none of the mutant viruses had a significant attenuation in neurovirulence following intracerebral inoculation (Zhang et al. 2006). While the role of virus fusion in neuroinvasion and neurovirulence has not been fully elucidated, these studies provide evidence for fusion as a critical determinant of a neuroinvasive phenotype from members of two different viral families (Flaviviridae and Bunyaviridae). The structural similarities between the fusion peptide regions of disparate arboviruses and the sensitivity of these highly conserved regions to single amino acid mutations may suggest that the ability of arboviruses to invade the CNS is subject to high selective pressure exerted on viral fusion peptides. This possible connection between virus fusion and neuroinvasion could potentially be exploited to identify novel targets to prevent CNS invasion for a broad range of arboviral encephalitides.
The specific pathways used by LACV to mediate neurotoxicity are not well described. However, it has been demonstrated that LACV infection induces neuronal apoptosis in the brains of infected mice (Pekosz et al. 1996). Further, the presence of apoptotic cells in LACV-infected mouse brains correlated with neuronal dropout, suggesting that LACV-induced apoptosis may play an important role in the pathogenesis of LACV encephalitis. In a more recent study, the Orthobunyavirus nonstructural protein, NSs, was demonstrated to induce apoptosis as a general inhibitor of translation that induces cytochrome c release from mitochondria and results in the activation of caspases (Colon-Ramos et al. 2003). Moreover, neuronal apoptosis is a hallmark of viral encephalitides caused by HIV, Herpes Simplex Virus-1, WNV, Toscana virus, and reoviruses (Shrestha et al. 2003; Richardson-Burns et al. 2002). Therefore, determining the pathways and mechanisms of virus-induced neuronal apoptosis will be of critical importance in understanding viral pathogenesis of LACV infection and developing effective therapeutics for LACV encephalitis. Currently, there is no standard therapy for LACV encephalitis. However, ribavirin has been shown to inhibit LACV infection, probably by targeting the RNA-dependent RNA polymerase, and it has been suggested that oral ribavirin may be useful in limiting the severity and improving the prognosis of children infected with LACV (Cassidy and Patterson 1989; McJunkin et al. 1997). To date, ribavirin has only been used off-label, and a pilot trial using the intravenous form of ribavirin is underway (McJunkin et al. 1997; Haddow 2009).
Rift valley fever virus
RVFV was first described in Kenya by Daubney and Hudson (1931) during an outbreak of enzootic hepatitis in a herd of ewes (Daubney and Hudson 1931). This Phlebovirus is a significant emerging pathogen responsible for recurrent epizootics and epidemics in Africa that have resulted in substantial economic losses owing to human illness and loss of livestock (Bird et al. 2009, Balkhy and Memish 2003). RVFV was restricted to sub-Saharan Africa until 1977, where it was responsible for a massive epidemic/epizootic in the Nile River and in the delta region of Egypt (Meegan 1979a, b; Meegan et al. 1979). This RVFV outbreak was the largest on record with an estimated 200,000 human infections and at least 594 deaths (Meegan et al. 1979). Since this outbreak, RVFV infection has occurred sporadically in Egypt (Abd el-Rahim et al. 1999, Arthur et al. 1993).
In the year 2000, RVFV was reported outside of Africa for the first time. In Yemen and Saudi Arabia, a RVFV outbreak resulted in approximately 2,000 human infections and almost 250 deaths along with substantial loss of livestock (CDC 2000). Outbreaks of RVFV have continued to occur in Africa. As recently as 2010, there was a RVFV outbreak in South Africa with at least 63 human cases reported including two deaths (WHO 2010b). RVFV is transmitted by numerous mosquito species (Aedes, Anopheles, Culex, Eretmapodites, Mansonia, and Coquillettidia) and can be transmitted by other vectors including sandflies (Fontenille et al. 1998; Moutailler et al. 2008). Human infections typically result from either bites from infected mosquitoes or close contact with sick animals (Wilson et al. 1994; Morrill and McClain 1996).
RVFV has two distinct but overlapping transmission cycles: a low-level enzootic cycle and an epizootic/epidemic cycle (Bird et al. 2009). The transition from a low-level enzootic cycle to the epizootic/epidemic cycle is dependent on large-scale weather events such as the warm ENSO that can result in heavy rainfall over Eastern and Southern Africa (Bird et al. 2009; Linthicum et al. 1987). The low-level enzootic cycle predominates during non-excessive rainfall where RVFV is likely maintained within the mosquito vector population by transovarial transmission and occasional amplification in wildlife (Bird et al. 2009). The transition to the epizootic/epidemic cycle occurs during periods of extended rainfall that results in increased mosquito activity (Bird et al. 2009).
RVFV can cause severe disease in livestock and outbreaks of RVFV can be identified by the sudden development of abortion “storms” in livestock following heavy rainfall (Swanepoel and JAW 2004). Susceptible livestock include sheep, cattle, Asian water buffalo, camels, and goats. After an incubation period of 2–4 days, disease in livestock due to RVFV is characterized by acute onset of inappetence, nasal discharge, and diarrhea as well as fever, hepatitis, and frequent abortion (Bird et al. 2009, Balkhy and Memish 2003). These animals are highly viremic (1 × 106 to 1 × 108 PFU/mL; Bird et al. 2009). Sheep are the most susceptible of livestock. In lambs, the mortality rates can reach above 90%, while adult sheep mortality rates are approximately 10% to 30% (Bird et al. 2009; Daubney and Hudson 1931).
RVFV infection of humans occurs most often in individuals in contact with livestock, and human transmission is thought to occur by mosquito vectors, aerosols of blood, or other direct contact with infected animals (Abu-Elyazeed et al. 1996). Human infection with RVFV is associated with a broad range of diseases, from asymptomatic infection to more severe disease including retinitis, hepatitis, renal failure, necrotic encephalitis, severe hemorrhagic fever, and death (Bird et al. 2009; Madani et al. 2003). Symptoms associated with RVFV encephalitis include disorientation, drowsiness, severe headache, stiff neck, paraparesis or hemiparesis, convulsions, and coma (Madani et al. 2003; Soldan and Gonzalez-Scarano 2005). Interestingly, the mortality rate for humans was reported to be about 1%, but it has increased in recent outbreaks (WHO 2010a). For example, the RVFV outbreak in 2006 and 2007 in Eastern Africa had a mortality rate of 30% (WHO 2007). Furthermore, a study of a RVFV epidemic in Saudi Arabia reported a 14% mortality rate and a high incidence of neurological manifestations (17.1%) in infected individuals (Madani et al. 2003).
RVFV encephalitis occurs in <1% of infected humans and the pathogenesis of RVFV encephalitis is poorly understood. Examination of the brain material from RVFV infected calves shows diffuse perivascular infiltrates of lymphocytes and macrophages, multifocal meningitis, and focal areas of neuronal necrosis. Neurons and glial cells in these brains are positive for viral antigen throughout the CNS (Rippy et al. 1992). Furthermore, post-mortem examination of fatally infected rhesus monkeys showed mild, multifocal, perivascular encephalitis in the cerebral cortex. Moreover, a significant correlation was found between a delayed interferon response and mortality providing evidence that suggest the appearance of interferon is important in limiting the severity of disease and CNS infection (Morrill et al. 1990). Of interest, the interferon response is inhibited by RVFV. It has been demonstrated that the RVFV NSs protein blocks interferon production by targeting the RNA polymerase II complex and downregulating host transcription (Billecocq et al. 2004; Thomas et al. 2004). Recently, the LACV NSs was also shown to suppress interferon induction suggesting that there are commonalities in mechanisms of pathogenesis for the Bunyaviridae (Blakqori et al. 2007).
RVFV outbreaks outside endemic countries would lead to serious public health and agricultural problems. RVFV spread could potentially occur by movement of infected human, animals, or mosquitoes. The ability of RVFV to be transmitted by numerous mosquito species could aid in producing reservoirs in non-endemic areas where it is introduced (Elliott 2009). Even though the spread of RVFV can be somewhat controlled by effective vaccination of animals, there are no approved RVFV vaccines for humans. As suggested by Ikegami and Makino, an ideal RVFV vaccine for both humans and animals would be able to elicit rapid humoral immune responses that neutralize RVFV, induce long-term protective immunity, and be well tolerated (Ikegami and Makino 2009).
Alphaviruses
Currently, there are 29 known alphaviruses (each virus often consisting of several variants or strains) distributed around the world and grouped by geographic distribution into Old World and New World viruses (Peters and Dalrymple 1990). Alphaviruses have a broad host range and cellular tropism and cause a wide range of animal and human diseases. In general, most Old World alphaviruses are associated with arthritis and rash illness, while New World arboviruses cause encephalitis. Exceptions to this statement include Sindbis virus (the prototype alphavirus), which causes encephalomyelitis in mice, and Ross River Virus and CHIKV, which are also neuroinvasive and cause neurological disease in humans (Zacks and Paessler 2010).
The alphaviruses are small, enveloped viruses with a positive sense RNA genome containing two ORFs. The 5’ORF encodes a replication protein precursor that is processed by proteases to generate four different replicase polypeptide proteins for transcription and replication of viral RNA and the 3’ ORF encodes the four major structural proteins including the capsid (C) and the three envelope glycoproteins (E1, E2, and E3). Of interest, amino acid substitutions in the E2 glycoprotein that forms the tips of spikes on the surface of VEEV have been found to enhance equine amplification (Brault et al. 2002).
The alphaviruses are of interest not only as important pathogens of humans and horses, but also for their potential use in gene therapy and as conceivable agents of bioterrorism (Lundstrom 2005; Sidwell and Smee 2003). Here, we will discuss alphaviruses that cause encephalitis in humans and pose a serious threat to human health in many areas: including, Western equine encephalitis virus (WEEV), Eastern equine encephalitis virus (EEEV), VEEV, and CHIKV.
Western-, eastern-, and Venezuelan equine encephalitis viruses
WEEV, VEEV, and EEEV are New World viruses that, as the name suggests, cause encephalitis in horses and humans. The primary amplifying vector for these viruses include birds (EEEV and WEEV), rodents (WEEV and VEEV), and horses (VEEV; Table 1). These viruses are transmitted primarily by mosquitoes in the genera Culex, Culiseta, and Aedes (Table 1). While all three viruses (EEEV, WEEV, and VEEV) cause encephalitis in horses and humans, their virulence and incidence vary greatly within these hosts. EEEV is the most virulent of the equine encephalitis trio with human case-fatality rates estimated in the range of 50% to 70% and horse case-fatality rates estimated at 70% to 90% (Zacks and Paessler 2010). However, EEEV has the lowest incidence of human cases of the three with only 257 confirmed human cases occurring in the United States from 1964 to 2008 (CDC 2010).
In both murine and hamster models of EEEV, neuroinvasion and subsequent encephalitis develop rapidly (Paessler et al. 2004). EEEV appears to invade the CNS of infected animals via the vascular route by passive transfer across the BBB or possibly by infected leukocytes (Vogel et al. 2005). The first antigen positive neuronal cells in the hamster model of EEEV are in the basal ganglia and brain stem and the virus spreads infecting periventricular and perivascular neurons as well as the hippocampus (Paessler et al. 2004). In contrast, VEEV is the least virulent in humans with case-fatality rates of 1% or less, but is significantly more transmissible than WEEV and EEEV in human populations and is capable of producing epidemics (Zacks and Paessler 2010). Humans are extremely susceptible to VEEV infection and nearly all infected humans develop clinical signs, but most infections are mild. Case-fatality rates for WEEV fall between those of EEEV and VEEV. In horses, case-fatality rates range from 3% to 50%; in humans, they range from 3% to 7% with 639 confirmed human cases of WEEV from 1964 to 2005 (CDC 2005).
Charles et al. have proposed two mechanisms of neuroinvasion for VEEV in a mouse model (Charles et al. 1995). In the first mechanism, VEEV has direct access to the unmyelinated olfactory sensory neurons from the blood by the capillary bed that underlies the olfactory neuroepithelium. The other proposed route of neuroinvasion is through the trigeminal nerve. VEEV replicates in the tooth, including the nervous tissue of the pulp, subsequently spreading down the trigeminal nerve. Interestingly, CNS invasion was first observed via the trigeminal nerve in animals whose olfactory system had been ablated. This finding demonstrates that neuroinvasion in alphaviruses is strongly influenced by the availability of intact peripheral nerve tracts (Charles et al. 1995).
Currently, there are no vaccines against WEEV, EEEV, or VEEV that are effective in humans; however, several are under development. Perhaps the most promising are live-attenuated vaccines that use alphavirus vectors expressing VEEV and EEEV proteins. These vaccines, which are highly immunogenic, have been shown to be safe and effective in mice and hamsters and have potential for use in humans (Zacks and Paessler 2010; Ni et al. 2007; Paessler et al. 2003; Paessler et al. 2006; Wang et al. 2007). There are equine vaccines against WEEV, EEEV, and VEEV in some countries. While these vaccines provide protection against these viruses in horses, they do little to prevent spread since horses are not their primary vertebrate reservoirs.
Chikungunya virus
CHIKV is an Old World Alphavirus that has been implicated in a number of outbreaks of painful polyarthralgia and myalgia in East and Southern Africa and Southeast Asia over the last 50 years (Schuffenecker et al. 2006) (Pialoux et al. 2007). In the Makonde language, chikungunya means “that which bends up” reflecting the painful, contorted stance assumed by those who are infected with this arbovirus. In Africa, CHIKV relies on wild primates and squirrels in its amplification cycle. CHIKV is transmitted by Aedes species; A. aegypti and A. albopictus are the main vectors in Asia where the virus is maintained in an urban amplification cycle (Kumar et al. 2008). Recently, A. albopictus was implicated as the primary vector in an outbreak on La Réunion Island. Molecular studies suggest that a mutation (A226V) in the viral envelope (E1) glycoprotein increased the infectivity of CHIKV for A. albopictus (Schuffenecker et al. 2006). A. albopictus is widely distributed in Europe and the United States of America, increasing the likelihood of CHIKV outbreaks throughout Europe and North America.
In the 2005–2006 La Réunion outbreak, 40% of the 785,000 inhabitants were infected with CHIKV. The epidemic subsequently spread to the Indian subcontinent, where outbreaks involving nearly 1.5 million people occurred in India, before expanding to Sri Lanka and Indonesia. It was during this outbreak that the ability of CHIKV to cause encephalitis, particularly among newborns and the elderly, was first appreciated. A recent review of 23 cases with CNS manifestations from La Réunion demonstrated that 95% of patients had altered mental status; 30%, headaches; 26%, seizures; 9%, sensory abnormalities; and 4%, motor dysfunction, with an overall mortality of 10% (Tournebize et al. 2009). Notably, specific abnormalities were not found by neuroimaging studies (Tournebize et al. 2009). Both encephalitis and febrile seizures were common in CHIKV infected children with CNS complications. Frequent CNS complications were again observed in a 2007 outbreak in Ravenna, Italy where there were 337 suspected cases (Angelini et al. 2008).
The incubation period for CHIKV is 2 to 6 days, and 95% of adults are symptomatic after CHIKV infection. The acute phase of infection includes a maculopapular rash, fever, and intense muscular and joint pain (Queyriaux et al. 2008). This acute phase is, in some patients, marked by polyarthralgias that can last for weeks to years beyond the initial infection. The very old and the young are at greatest risk for severe CHIKV illness, and the disease is fatal in 33% of those infected over the age of 65 (Queyriaux et al. 2008). Mechanisms for neuroinvasion and neurovirulence for CHIKV are not well described. A recently developed mouse model recapitulates the age-dependent susceptibility to CHIKV-induced CNS disease observed in humans. Of interest, increased susceptibility was also observed in interferon deficient mice (Couderc et al. 2008). In the mouse, CHIKV enters the CNS through the choroid plexus after achieving sufficient viremia. The virus does not appear to infect neurons or glial cells, but does infect macrophages (Couderc et al. 2008; Labadie et al. 2010). There are currently no specific antiviral treatments for CHIKV. However, several vaccines are under development including a novel, envelope-based DNA vaccine (Thiboutot et al. 2010).
Conclusion
Global factors including increasing volume of international trade and transportation, migration, climate change, changes to ecosystems (deforestation, loss of biodiversity) intensified livestock production, and cultivation of infected animals all favor the emergence of arbovirus infections. Members of the genera Flavivirus, Alphavirus, and the Bunyaviridae are important causes of human disease as emerging agents of arboviral encephalitides. Increased awareness of arboviruses in endemic areas, education regarding modes of disease transmission and necessary precautions, and implementation of vector control are critical in the prevention of future outbreaks. Moreover, the development of effective vaccines and therapeutics is of great importance.
Acknowledgments
This work was supported by PHS grants 1R01AI074626-01 and T32-NS07180.
Footnotes
statement regarding conflicts of interest
The authors declare that there are no conflicts of interest.
Contributor Information
Bradley S. Hollidge, Department of Neurology, University of Pennsylvania, Philadelphia, PA 19104-4283, USA Neuroscience Graduate Group, University of Pennsylvania, Philadelphia, PA 19104-4283, USA.
Francisco González-Scarano, Department of Neurology, University of Pennsylvania, Philadelphia, PA 19104-4283, USA; Department of Microbiology, University of Pennsylvania, Philadelphia, PA 19104-4283, USA.
Samantha S. Soldan, Email: sssoldan@mail.med.upenn.edu, Department of Neurology, University of Pennsylvania, Philadelphia, PA 19104-4283, USA.
References
- Outbreak of Rift Valley fever—Saudi Arabia, August–October 2000. MMWR Morb Mortal Wkly Rep. 49:905–908. [PubMed] [Google Scholar]
- Abd el-Rahim IH, Abd el-Hakim U, Hussein M. An epizootic of Rift Valley fever in Egypt in 1997. Rev Sci Tech. 1999;18:741–748. doi: 10.20506/rst.18.3.1195. [DOI] [PubMed] [Google Scholar]
- Abu-Elyazeed R, El-Sharkawy S, Olson J, Botros B, Soliman A, Salib A, Cummings C, Arthur R. Prevalence of anti-Rift-Valley-fever IgM anitbody in abattoir workers in the Nile delta during the 1993 outbreak in Egypt. Bull World Health Organ. 1996;74:155–158. [PMC free article] [PubMed] [Google Scholar]
- Angelini P, Macini P, Finarelli AC, Pol C, Venturelli C, Bellini R, Dottori M. Chikungunya epidemic outbreak in Emilia-Romagna (Italy) during summer 2007. Parassitologia. 2008;50:97–98. [PubMed] [Google Scholar]
- Arthur RR, El-Sharkawy MS, Cope SE, Botros BA, Oun S, Morrill JC, Shope RE, Hibbs RG, Darwish MA, Imam IZ. Recurrence of Rift Valley fever in Egypt. Lancet. 1993;342:1149–1150. doi: 10.1016/0140-6736(93)92128-g. [DOI] [PubMed] [Google Scholar]
- Babu GN, Kalita J, Misra UK. Inflammatory markers in the patients of Japanese encephalitis. Neurol Res. 2006;28:190–192. doi: 10.1179/016164106X98062. [DOI] [PubMed] [Google Scholar]
- Balkhy HH, Memish ZA. Rift Valley fever: an uninvited zoonosis in the Arabian peninsula. Int J Antimicrob Agents. 2003;21:153–157. doi: 10.1016/s0924-8579(02)00295-9. [DOI] [PubMed] [Google Scholar]
- Barrett AD, Higgs S. Yellow fever: a disease that has yet to be conquered. Annu Rev Entomol. 2007;52:209–229. doi: 10.1146/annurev.ento.52.110405.091454. [DOI] [PubMed] [Google Scholar]
- Benedict MQ, Levine RS, Hawley WA, Lounibos LP. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 2007;7:76–85. doi: 10.1089/vbz.2006.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billecocq A, Spiegel M, Vialat P, Kohl A, Weber F, Bouloy M, Haller O. NSs protein of Rift Valley fever virus blocks interferon production by inhibiting host gene transcription. J Virol. 2004;78:9798–9806. doi: 10.1128/JVI.78.18.9798-9806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billoir F, De Chesse R, Tolou H, De Micco P, Gould EA, De Lamballerie X. Phylogeny of the genus flavivirus using complete coding sequences of arthropod-borne viruses and viruses with no known vector. J Gen Virol. 2000;81:781–790. doi: 10.1099/0022-1317-81-3-781. [DOI] [PubMed] [Google Scholar]
- Bird BH, Ksiazek TG, Nichol ST, Maclachlan NJ. Rift Valley fever virus. J Am Vet Med Assoc. 2009;234:883–893. doi: 10.2460/javma.234.7.883. [DOI] [PubMed] [Google Scholar]
- Blakqori G, Delhaye S, Habjan M, Blair CD, Sanchez-Vargas I, Olson KE, Attarzadeh-Yazdi G, Fragkoudis R, Kohl A, Kalinke U, Weiss S, Michiels T, Staeheli P, Weber F. La Crosse bunyavirus nonstructural protein NSs serves to suppress the type I interferon system of mammalian hosts. J Virol. 2007;81:4991–4999. doi: 10.1128/JVI.01933-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borucki MK, Kempf BJ, Blitvich BJ, Blair CD, Beaty BJ. La Crosse virus: replication in vertebrate and invertebrate hosts. Microbes Infect. 2002;4:341–350. doi: 10.1016/s1286-4579(02)01547-2. [DOI] [PubMed] [Google Scholar]
- Brault AC, Powers AM, Holmes EC, Woelk CH, Weaver SC. Positively charged amino acid substitutions in the e2 envelope glycoprotein are associated with the emergence of venezuelan equine encephalitis virus. J Virol. 2002;76:1718–1730. doi: 10.1128/JVI.76.4.1718-1730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briese T, Jia XY, Huang C, Grady LJ, Lipkin WI. Identification of a Kunjin/West Nile-like flavivirus in brains of patients with New York encephalitis. Lancet. 1999;354:1261–1262. doi: 10.1016/s0140-6736(99)04576-6. [DOI] [PubMed] [Google Scholar]
- Brinton MA. The molecular biology of West Nile Virus: a new invader of the western hemisphere. Annu Rev Microbiol. 2002;56:371–402. doi: 10.1146/annurev.micro.56.012302.160654. [DOI] [PubMed] [Google Scholar]
- Broom AK, Lindsay MD, Plant AJ, Wright AE, Condon RJ, Mackenzie JS. Epizootic activity of Murray Valley encephalitis virus in an aboriginal community in the southeast Kimberley region of Western Australia: results of cross-sectional and longitudinal serologic studies. Am J Trop Med Hyg. 2002;67:319–323. doi: 10.4269/ajtmh.2002.67.319. [DOI] [PubMed] [Google Scholar]
- Burke DS, Nisalak A, Ussery MA, Laorakpongse T, Chantavibul S. Kinetics of IgM and IgG responses to Japanese encephalitis virus in human serum and cerebrospinal fluid. J Infect Dis. 1985;151:1093–1099. doi: 10.1093/infdis/151.6.1093. [DOI] [PubMed] [Google Scholar]
- Calisher CH. History, Classification, and Taxonomy of Viruses in the Family Bunyaviridae. In: Elliot R, editor. The Bunyaviridae. New York: Plenum Press; 1986. [Google Scholar]
- Cao M, Feng Z, Zhang J, Ma J, Li X. Contextual risk factors for regional distribution of Japanese encephalitis in the People’s Republic of China. Trop Med Int Health. 2010 doi: 10.1111/j.1365-3156.2010.02563.x. (in press) [DOI] [PubMed] [Google Scholar]
- Caplen H, Peters CJ, Bishop DH. Mutagen-directed attenuation of Rift Valley fever virus as a method for vaccine development. J Gen Virol. 1985;66(Pt 10):2271–2277. doi: 10.1099/0022-1317-66-10-2271. [DOI] [PubMed] [Google Scholar]
- Cassidy LF, Patterson JL. Mechanism of La Crosse virus inhibition by ribavirin. Antimicrob Agents Chemother. 1989;33:2009–2011. doi: 10.1128/aac.33.11.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavrini F, Gaibani P, Pierro AM, Rossini G, Landini MP, Sambri V. Chikungunya: an emerging and spreading arthropod-borne viral disease. J Infect Dev Ctries. 2009;3:744–752. doi: 10.3855/jidc.169. [DOI] [PubMed] [Google Scholar]
- CDC. Fact Sheet: Western Equine Encephalitis. 2005 [Google Scholar]
- CDC. Eastern Equine Encephalitis. 2010 [Google Scholar]
- Charles PC, Walters E, Margolis F, Johnston RE. Mechanism of neuroinvasion of Venezuelan equine encephalitis virus in the mouse. Virology. 1995;208:662–671. doi: 10.1006/viro.1995.1197. [DOI] [PubMed] [Google Scholar]
- Colon-Ramos DA, Irusta PM, Gan EC, Olson MR, Song J, Morimoto RI, Elliott RM, Lombard M, Hollingsworth R, Hardwick JM, Smith GK, Kornbluth S. Inhibition of translation and induction of apoptosis by Bunyaviral nonstructural proteins bearing sequence similarity to reaper. Mol Biol Cell. 2003;14:4162–4172. doi: 10.1091/mbc.E03-03-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couderc T, Chretien F, Schilte C, Disson O, Brigitte M, Guivel-Benhassine F, Touret Y, Barau G, Cayet N, Schuffenecker I, Despres P, Arenzana-Seisdedos F, Michault A, Albert ML, Lecuit M. A mouse model for Chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog. 2008;4:e29. doi: 10.1371/journal.ppat.0040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubney R, Hudson JR. Enzootic hepatitis or Rift Valley fever: an undescribed virus disease of sheep cattle and man from east Africa. J Path Bacteriol. 1931;34:545–579. [Google Scholar]
- Davis LE, Debiasi R, Goade DE, Haaland KY, Harrington JA, Harnar JB, Pergam SA, King MK, Demasters BK, Tyler KL. West Nile virus neuroinvasive disease. Ann Neurol. 2006;60:286–300. doi: 10.1002/ana.20959. [DOI] [PubMed] [Google Scholar]
- Day JF. Predicting St. Louis encephalitis virus epidemics: lessons from recent, and not so recent, outbreaks. Annu Rev Entomol. 2001;46:111–138. doi: 10.1146/annurev.ento.46.1.111. [DOI] [PubMed] [Google Scholar]
- Diamond MS. Virus and host determinants of West Nile virus pathogenesis. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000452. e1000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionisio D, Esperti F, Vivarelli A, Valassina M. Epidemiological, clinical and laboratory aspects of sandfly fever. Curr Opin Infect Dis. 2003;16:383–388. doi: 10.1097/00001432-200310000-00003. [DOI] [PubMed] [Google Scholar]
- Duggan ST, Plosker GL. Japanese encephalitis vaccine (inactivated, adsorbed) [IXIARO] Drugs. 2009;69:115–122. doi: 10.2165/00003495-200969010-00008. [DOI] [PubMed] [Google Scholar]
- Dutta K, Ghosh D, Basu A. Curcumin protects neuronal cells from Japanese encephalitis virus-mediated cell death and also inhibits infective viral particle formation by dysregulation of ubiquitin-proteasome system. J Neuroimmune Pharmacol. 2009;4:328–337. doi: 10.1007/s11481-009-9158-2. [DOI] [PubMed] [Google Scholar]
- Elliott RM. Bunyaviruses and climate change. Clin Microbiol Infect. 2009;15:510–517. doi: 10.1111/j.1469-0691.2009.02849.x. [DOI] [PubMed] [Google Scholar]
- Ergonul O. Crimean-Congo haemorrhagic fever. Lancet Infect Dis. 2006;6:203–214. doi: 10.1016/S1473-3099(06)70435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Lindsey N, Staples JE, Hills S. Japanese encephalitis vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2010;59:1–27. [PubMed] [Google Scholar]
- Fonseca DM, Keyghobadi N, Malcolm CA, Mehmet C, Schaffner F, Mogi M, Fleischer RC, Wilkerson RC. Emerging vectors in the Culex pipiens complex. Science. 2004;303:1535–1538. doi: 10.1126/science.1094247. [DOI] [PubMed] [Google Scholar]
- Fontenille D, Traore-Lamizana M, Diallo M, Thonnon J, Digoutte JP, Zeller HG. New vectors of Rift Valley fever in West Africa. Emerg Infect Dis. 1998;4:289–293. doi: 10.3201/eid0402.980218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Nasci R, Liang G. The neglected arboviral infections in mainland China. PLoS Negl Trop Dis. 2010;4:e624. doi: 10.1371/journal.pntd.0000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauld LW, Hanson RP, Thompson WH, Sinha SK. Observations on a natural cycle of La Crosse virus (California group) in Southwestern Wisconsin. Am J Trop Med Hyg. 1974;23:983–992. doi: 10.4269/ajtmh.1974.23.983. [DOI] [PubMed] [Google Scholar]
- Gerhardt RR, Gottfried KL, Apperson CS, Davis BS, Erwin PC, Smith AB, Panella NA, Powell EE, Nasci RS. First isolation of La Crosse virus from naturally infected Aedes albopictus. Emerg Infect Dis. 2001;7:807–811. doi: 10.3201/eid0705.017506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass WG, Mcdermott DH, Lim JK, Lekhong S, Yu SF, Frank WA, Pape J, Cheshier RC, Murphy PM. CCR5 deficiency increases risk of symptomatic West Nile virus infection. J Exp Med. 2006;203:35–40. doi: 10.1084/jem.20051970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Janssen RS, Najjar JA, Pobjecky N, Nathanson N. An avirulent G1 glycoprotein variant of La Crosse bunyavirus with defective fusion function. J Virol. 1985;54:757–763. doi: 10.1128/jvi.54.3.757-763.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Bupp K, Nathanson N. Pathogenesis of diseases caused by viruses of the bunyavirus genus. In: Elliot R, editor. The Bunyaviridae. New York: Plenum Press; 1996. [Google Scholar]
- Gould EA, De Lamballerie X, Zanotto PM, Holmes EC. Origins, evolution, and vector/host coadaptations within the genus Flavivirus. Adv Virus Res. 2003;59:277–314. doi: 10.1016/s0065-3527(03)59008-x. [DOI] [PubMed] [Google Scholar]
- Griot C, Gonzalez-Scarano F, Nathanson N. Molecular determinants of the virulence and infectivity of California serogroup bunyaviruses. Annu Rev Microbiol. 1993;47:117–138. doi: 10.1146/annurev.mi.47.100193.001001. [DOI] [PubMed] [Google Scholar]
- Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002;33:330–342. doi: 10.1016/s0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- Gubler DJ. The continuing spread of West Nile virus in the western hemisphere. Clin Infect Dis. 2007;45:1039–1046. doi: 10.1086/521911. [DOI] [PubMed] [Google Scholar]
- Gunderson CB, Brown KL. Clinical aspects of La Crosse encephalitis: preliminary report. In: Calisher CH, T WH, editors. California Serogroup Viruses. New York: A.R. Liss, Inc.; 1983. [PubMed] [Google Scholar]
- Gyure KA. West Nile virus infections. J Neuropathol Exp Neurol. 2009;68:1053–1060. doi: 10.1097/NEN.0b013e3181b88114. [DOI] [PubMed] [Google Scholar]
- Haddow AD. The use of oral ribavirin in the management of La Crosse viral infections. Med Hypotheses. 2009;72:190–192. doi: 10.1016/j.mehy.2008.05.043. [DOI] [PubMed] [Google Scholar]
- Haddow AD, Odoi A. The incidence risk, clustering, and clinical presentation of La Crosse virus infections in the eastern United States 2003–2007. PLoS ONE. 2009;4:e6145. doi: 10.1371/journal.pone.0006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow AD, Jones CJ, Odoi A. Assessing risk in focal arboviral infections: are we missing the big or little picture? PLoS ONE. 2009;4:e6954. doi: 10.1371/journal.pone.0006954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammon WM, Sather GE. Passive immunity for arbovirus infection. I. Artificially induced prophylaxis in man and mouse for Japanese (B) encephalitis. Am J Trop Med Hyg. 1973;22:524–534. doi: 10.4269/ajtmh.1973.22.524. [DOI] [PubMed] [Google Scholar]
- Harrington T, Kuehnert MJ, Kamel H, Lanciotti RS, Hand S, Currier M, Chamberland ME, Petersen LR, Marfin AA. West Nile virus infection transmitted by blood transfusion. Transfusion. 2003;43:1018–1022. doi: 10.1046/j.1537-2995.2003.00481.x. [DOI] [PubMed] [Google Scholar]
- Hayes EB, Komar N, Nasci RS, Montgomery SP, O'leary DR, Campbell GL. Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis. 2005;11:1167–1173. doi: 10.3201/eid1108.050289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heft DE, Walton WE. Effects of the El Nino–Southern Oscillation (ENSO) cycle on mosquito populations in southern California. J Vector Ecol. 2008;33:17–29. doi: 10.3376/1081-1710(2008)33[17:eotens]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Heinz FX, Collett MS, Purcell RH, Gould EA, Howard CR, Houghton M, Moormann RJM, Rice CM, Thiel HJ. Family Flaviviridae. In: van Regenmortel MH, Fauquet CM, Bishop DHL, Carstens E, Estes MK, Lemon S, Maniloff J, Mayo MA, McGeoch D, Pringle CR, Wickner RB, editors. Virus taxonomy 7th report of the International Committee for the Taxonomy of Viruses. San Diego, CA: Academic Press; 2000. [Google Scholar]
- Hoogstraal H. The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J Med Entomol. 1979;15:307–417. doi: 10.1093/jmedent/15.4.307. [DOI] [PubMed] [Google Scholar]
- Ikegami T, Makino S. Rift valley fever vaccines. Vaccine. 2009;27 Suppl 4:D69–D72. doi: 10.1016/j.vaccine.2009.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis BL. Japanese Encephalitis. In: Porterfield JS, editor. Exotic viral infections. London: Chapman and Hall; 1995. [Google Scholar]
- Iwamoto M, Jernigan DB, Guasch A, Trepka MJ, Blackmore CG, Hellinger WC, Pham SM, Zaki S, Lanciotti RS, Lance-Parker SE, Diazgranados CA, Winquist AG, Perlino CA, Wiersma S, Hillyer KL, Goodman JL, Marfin AA, Chamberland ME, Petersen LR. Transmission of West Nile virus from an organ donor to four transplant recipients. N Engl J Med. 2003;348:2196–2203. doi: 10.1056/NEJMoa022987. [DOI] [PubMed] [Google Scholar]
- Janssen R, Gonzalez-Scarano F, Nathanson N. Mechanisms of bunyavirus virulence. Comparative pathogenesis of a virulent strain of La Crosse and an avirulent strain of Tahyna virus. Lab Invest. 1984;50:447–455. [PubMed] [Google Scholar]
- Janssen RS, Nathanson N, Endres MJ, Gonzalez-Scarano F. Virulence of La Crosse virus is under polygenic control. J Virol. 1986;59:1–7. doi: 10.1128/jvi.59.1.1-7.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia XY, Briese T, Jordan I, Rambaut A, Chi HC, Mackenzie JS, Hall RA, Scherret J, Lipkin WI. Genetic analysis of West Nile New York 1999 encephalitis virus. Lancet. 1999;354:1971–1972. doi: 10.1016/s0140-6736(99)05384-2. [DOI] [PubMed] [Google Scholar]
- Johnson RT, Burke DS, Elwell M, Leake CJ, Nisalak A, Hoke CH, Lorsomrudee W. Japanese encephalitis: immunocyto-chemical studies of viral antigen and inflammatory cells in fatal cases. Ann Neurol. 1985;18:567–573. doi: 10.1002/ana.410180510. [DOI] [PubMed] [Google Scholar]
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalfayan B. Pathology of La Crosse virus infection in humans. In: Calisher CH, Thompson WH, editors. California serogroup viruses. New York: Liss; 1983. [PubMed] [Google Scholar]
- Kielian M. Class II virus membrane fusion proteins. Virology. 2006;344:38–47. doi: 10.1016/j.virol.2005.09.036. [DOI] [PubMed] [Google Scholar]
- Klein RS, Diamond MS. Immunological headgear: antiviral immune responses protect against neuroinvasive West Nile virus. Trends Mol Med. 2008;14:286–294. doi: 10.1016/j.molmed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Komar N. West Nile virus: epidemiology and ecology in North America. Adv Virus Res. 2003;61:185–234. doi: 10.1016/s0065-3527(03)61005-5. [DOI] [PubMed] [Google Scholar]
- Kramer LD, Li J, Shi PY. West Nile virus. Lancet Neurol. 2007;6:171–181. doi: 10.1016/S1474-4422(07)70030-3. [DOI] [PubMed] [Google Scholar]
- Kumar NP, Joseph R, Kamaraj T, Jambulingam P. A226V mutation in virus during the 2007 chikungunya outbreak in Kerala, India. J Gen Virol. 2008;89:1945–1948. doi: 10.1099/vir.0.83628-0. [DOI] [PubMed] [Google Scholar]
- Kurane I, Takasaki T. Immunogenicity and protective efficacy of the current inactivated Japanese encephalitis vaccine against different Japanese encephalitis virus strains. Vaccine. 2000;18 Suppl 2:33–35. doi: 10.1016/s0264-410x(00)00041-4. [DOI] [PubMed] [Google Scholar]
- Labadie K, Larcher T, Joubert C, Mannioui A, Delache B, Brochard P, Guigand L, Dubreil L, Lebon P, Verrier B, De Lamballerie X, Suhrbier A, Cherel Y, Le Grand R, Roques P. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J Clin Invest. 2010;120:894–906. doi: 10.1172/JCI40104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi ME, Quan D, Ho JT, Kleinschmidt-Demasters BK, Tyler KL, Grazia TJ. Impact of rituximab-associated B-cell defects on West Nile virus meningoencephalitis in solid organ transplant recipients. Clin Transplant. 2010;24:223–228. doi: 10.1111/j.1399-0012.2009.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hou L, Ye J, Liu X, Dan H, Jin M, Chen H, Cao S. Development of a convenient immunochromatographic strip for the diagnosis of infection with Japanese encephalitis virus in swine. J Virol Methods. 2010 doi: 10.1016/j.jviromet.2010.04.015. (in press) [DOI] [PubMed] [Google Scholar]
- Linthicum KJ, Bailey CL, Davies FG, Tucker CJ. Detection of Rift Valley fever viral activity in Kenya by satellite remote sensing imagery. Science. 1987;235:1656–1659. doi: 10.1126/science.3823909. [DOI] [PubMed] [Google Scholar]
- Linthicum KJ, Anyamba A, Tucker CJ, Kelley PW, Myers MF, Peters CJ. Climate and satellite indicators to forecast Rift Valley fever epidemics in Kenya. Science. 1999;285:397–400. doi: 10.1126/science.285.5426.397. [DOI] [PubMed] [Google Scholar]
- Liou ML, Hsu CY. Japanese encephalitis virus is transported across the cerebral blood vessels by endocytosis in mouse brain. Cell Tissue Res. 1998;293:389–394. doi: 10.1007/s004410051130. [DOI] [PubMed] [Google Scholar]
- Lundstrom K. Biology and application of alphaviruses in gene therapy. Gene Ther. 2005;12 Suppl 1:S92–S97. doi: 10.1038/sj.gt.3302620. [DOI] [PubMed] [Google Scholar]
- Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10:S98–S109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- Madani TA, Al-Mazrou YY, Al-Jeffri MH, Mishkhas AA, Al-Rabeah AM, Turkistani AM, Al-Sayed MO, Abodahish AA, Khan AS, Ksiazek TG, Shobokshi O. Rift Valley fever epidemic in Saudi Arabia: epidemiological, clinical, and laboratory characteristics. Clin Infect Dis. 2003;37:1084–1092. doi: 10.1086/378747. [DOI] [PubMed] [Google Scholar]
- Malkinson M, Banet C. The role of birds in the ecology of West Nile virus in Europe and Africa. Curr Top Microbiol Immunol. 2002;267:309–322. doi: 10.1007/978-3-642-59403-8_15. [DOI] [PubMed] [Google Scholar]
- Marfin AA, Gubler DJ. West Nile encephalitis: an emerging disease in the United States. Clin Infect Dis. 2001;33:1713–1719. doi: 10.1086/322700. [DOI] [PubMed] [Google Scholar]
- Mcjunkin JE, Khan R, De Los Reyes EC, Parsons DL, Minnich LL, Ashley RG, Tsai TF. Treatment of severe La Crosse encephalitis with intravenous ribavirin following diagnosis by brain biopsy. Pediatrics. 1997;99:261–267. doi: 10.1542/peds.99.2.261. [DOI] [PubMed] [Google Scholar]
- Mcjunkin JE, De Los Reyes EC, Irazuzta JE, Caceres MJ, Khan RR, Minnich LL, Fu KD, Lovett GD, Tsai T, Thompson A. La Crosse encephalitis in children. N Engl J Med. 2001;344:801–807. doi: 10.1056/NEJM200103153441103. [DOI] [PubMed] [Google Scholar]
- Mclean RG, Ubico SR, Bourne D, Komar N. West Nile virus in livestock and wildlife. Curr Top Microbiol Immunol. 2002;267:271–308. doi: 10.1007/978-3-642-59403-8_14. [DOI] [PubMed] [Google Scholar]
- Meegan JM. Rift Valley fever in Egypt: an overview of the epizootics in 1977 and 1978. Contrib Epidemiol Biostat. 1979a:100–113. [Google Scholar]
- Meegan JM. The Rift Valley fever epizootic in Egypt 1977–78. 1 Description of the epizzotic and virological studies. Trans R Soc Trop Med Hyg. 1979b;73:618–623. doi: 10.1016/0035-9203(79)90004-x. [DOI] [PubMed] [Google Scholar]
- Meegan JM, Watten RH, Laughlin LW. Clinical experience with Rift Valley fever in humans during the 1977 Egyptian epizootic. Contrib Epidemiol Biostat. 1979;3:114–123. [Google Scholar]
- Mishra MK, Basu A. Minocycline neuroprotects, reduces microglial activation, inhibits caspase 3 induction, and viral replication following Japanese encephalitis. J Neurochem. 2008;105:1582–1595. doi: 10.1111/j.1471-4159.2008.05238.x. [DOI] [PubMed] [Google Scholar]
- Misra UK, Kalita J. Overview: Japanese encephalitis. Prog Neurobiol. 2010;91:108–120. doi: 10.1016/j.pneurobio.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Miyake M. The Pathology of Japanese Encephalitis. A Review. Bull World Health Organ. 1964;30:153–160. [PMC free article] [PubMed] [Google Scholar]
- Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430:242–249. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrill JC, Mcclain DJ. Epidemiology and pathogenesis of Rift Valley fever and other phleboviruses. In: Elliot R, editor. The Bunyaviridae. New York: Plenum Press; 1996. [Google Scholar]
- Morrill JC, Peters CJ. Pathogenicity and neurovirulence of a mutagen-attenuated Rift Valley fever vaccine in rhesus monkeys. Vaccine. 2003;21:2994–3002. doi: 10.1016/s0264-410x(03)00131-2. [DOI] [PubMed] [Google Scholar]
- Morrill JC, Jennings GB, Johnson AJ, Cosgriff TM, Gibbs PH, Peters CJ. Pathogenesis of Rift Valley fever in rhesus monkeys: role of interferon response. Arch Virol. 1990;110:195–212. doi: 10.1007/BF01311288. [DOI] [PubMed] [Google Scholar]
- Morrill JC, Mebus CA, Peters CJ. Safety and efficacy of a mutagen-attenuated Rift Valley fever virus vaccine in cattle. Am J Vet Res. 1997a;58:1104–1109. [PubMed] [Google Scholar]
- Morrill JC, Mebus CA, Peters CJ. Safety of a mutagen-attenuated Rift Valley fever virus vaccine in fetal and neonatal bovids. Am J Vet Res. 1997b;58:1110–1114. [PubMed] [Google Scholar]
- Moutailler S, Krida G, Schaffner F, Vazeille M, Failloux AB. Potential vectors of Rift Valley fever virus in the Mediterranean region. Vector Borne Zoonotic Dis. 2008;8:749–753. doi: 10.1089/vbz.2008.0009. [DOI] [PubMed] [Google Scholar]
- Murray KO, Mertens E, Despres P. West Nile virus and its emergence in the United States of America. Vet Res. 2010;41:67. doi: 10.1051/vetres/2010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni H, Barrett AD. Molecular differences between wild-type Japanese encephalitis virus strains of high and low mouse neuroinvasiveness. J Gen Virol. 1996;77(Pt 7):1449–1455. doi: 10.1099/0022-1317-77-7-1449. [DOI] [PubMed] [Google Scholar]
- Ni H, Yun NE, Zacks MA, Weaver SC, Tesh RB, Da Rosa AP, Powers AM, Frolov I, Paessler S. Recombinant alphaviruses are safe and useful serological diagnostic tools. Am J Trop Med Hyg. 2007;76:774–781. [PubMed] [Google Scholar]
- Nichol ST, Arikawa J, Kawaoka Y. Emerging viral diseases. PNAS. 2000;97:12411–12412. doi: 10.1073/pnas.210382297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol ST, Beaty BJ, Elliot RM, Al E. Bunyaviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus taxonomy eight report of the International Committee on Taxonomy of Viruses. Amsterdam: Academic; 2005. [Google Scholar]
- Nimmannitya S, Halstead SB, Cohen SN, Margiotta MR. Dengue and chikungunya virus infection in man in Thailand, 1962–1964. I. Observations on hospitalized patients with hemorrhagic fever. Am J Trop Med Hyg. 1969;18:954–971. doi: 10.4269/ajtmh.1969.18.954. [DOI] [PubMed] [Google Scholar]
- Padbidri VS, Gnaneswar TT. Epidemiological investigations of chikungunya epidemic at Barsi, Maharashtra state, India. J Hyg Epidemiol Microbiol Immunol. 1979;23:445–451. [PubMed] [Google Scholar]
- Paessler S, Fayzulin RZ, Anishchenko M, Greene IP, Weaver SC, Frolov I. Recombinant sindbis/Venezuelan equine encephalitis virus is highly attenuated and immunogenic. J Virol. 2003;77:9278–9286. doi: 10.1128/JVI.77.17.9278-9286.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paessler S, Aguilar P, Anishchenko M, Wang HQ, Aronson J, Campbell G, Cararra AS, Weaver SC. The hamster as an animal model for eastern equine encephalitis–and its use in studies of virus entrance into the brain. J Infect Dis. 2004;189:2072–2076. doi: 10.1086/383246. [DOI] [PubMed] [Google Scholar]
- Paessler S, Ni H, Petrakova O, Fayzulin RZ, Yun N, Anishchenko M, Weaver SC, Frolov I. Replication and clearance of Venezuelan equine encephalitis virus from the brains of animals vaccinated with chimeric SIN/VEE viruses. J Virol. 2006;80:2784–2796. doi: 10.1128/JVI.80.6.2784-2796.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrican LA, Defoliart GR. Aedes triseriatus and La Crosse virus: similar venereal infection rates in females given the first bloodmeal immediately before mating or several days after mating. Am J Trop Med Hyg. 1987;36:648–652. doi: 10.4269/ajtmh.1987.36.648. [DOI] [PubMed] [Google Scholar]
- Pekosz A, Phillips J, Pleasure D, Merry D, Gonzalez-Scarano F. Induction of apoptosis by La Crosse virus infection and role of neuronal differentiation and human bcl-2 expression in its prevention. J Virol. 1996;70:5329–5335. doi: 10.1128/jvi.70.8.5329-5335.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters CJ, Dalrymple JM. Alphaviruses. In: Fields BN, Knipe DM, Chanok RM, editors. Virology. 2nd edn. New York, NY: Raven Press; 1990. [Google Scholar]
- Petersen LR, Marfin AA. West Nile virus: a primer for the clinician. Ann Intern Med. 2002;137:173–179. doi: 10.7326/0003-4819-137-3-200208060-00009. [DOI] [PubMed] [Google Scholar]
- Pialoux G, Gauzere BA, Jaureguiberry S, Strobel M. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis. 2007;7:319–327. doi: 10.1016/S1473-3099(07)70107-X. [DOI] [PubMed] [Google Scholar]
- Plassmeyer ML, Soldan SS, Stachelek KM, Roth SM, Martin-Garcia J, Gonzalez-Scarano F. Mutagenesis of the La Crosse Virus glycoprotein supports a role for Gc (1066–1087) as the fusion peptide. Virology. 2007;358:273–282. doi: 10.1016/j.virol.2006.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plyusnin A, Morzunov SP. Virus evolution and genetic diversity of hantaviruses and their rodent hosts. Curr Top Microbiol Immunol. 2001;256:47–75. doi: 10.1007/978-3-642-56753-7_4. [DOI] [PubMed] [Google Scholar]
- Porterfield JS. The basis of arbovirus classification. Med Biol. 1975;53:400–405. [PubMed] [Google Scholar]
- Queyriaux B, Simon F, Grandadam M, Michel R, Tolou H, Boutin JP. Clinical burden of chikungunya virus infection. Lancet Infect Dis. 2008;8:2–3. doi: 10.1016/S1473-3099(07)70294-3. [DOI] [PubMed] [Google Scholar]
- Randall R, Binn LN, Harrison VR. Immunization against Rift Valley Fever Virus Studies on the Immunogenicity of Lyophilized Formalin-Inactivated Vaccine. J Immunol. 1964;93:293–299. [PubMed] [Google Scholar]
- Reese SM, Beaty MK, Gabitzsch ES, Blair CD, Beaty BJ. Aedes triseriatus females transovarially infected with La Crosse virus mate more efficiently than uninfected mosquitoes. J Med Entomol. 2009;46:1152–1158. doi: 10.1603/033.046.0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen W, Brault AC. West Nile virus in North America: perspectives on epidemiology and intervention. Pest Manag Sci. 2007;63:641–646. doi: 10.1002/ps.1325. [DOI] [PubMed] [Google Scholar]
- Richardson-Burns SM, Tyler KL. Minocycline delays disease onset and mortality in reovirus encephalitis. Exp Neurol. 2005;192:331–339. doi: 10.1016/j.expneurol.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Richardson-Burns SM, Kominsky DJ, Tyler KL. Reovirus-induced neuronal apoptosis is mediated by caspase 3 and is associated with the activation of death receptors. J Neurovirol. 2002;8:365–380. doi: 10.1080/13550280260422677. [DOI] [PubMed] [Google Scholar]
- Rippy MK, Topper MJ, Mebus CA, Morrill JC. Rift Valley fever virus-induced encephalomyelitis and hepatitis in calves. Vet Pathol. 1992;29:495–502. doi: 10.1177/030098589202900602. [DOI] [PubMed] [Google Scholar]
- Rojanasuphot S, Charoensuk O, Kitprayura D, Likityingvara C, Limpisthien S, Boonyindee S, Jivariyavej V, Ugchusak K. A field trial of Japanese encephalitis vaccine produced in Thailand. Southeast Asian J Trop Med Public Health. 1989;20:653–654. [PubMed] [Google Scholar]
- Rust RS, Thompson WH, Matthews CG, Beaty BJ, Chun RW. La Crosse and other forms of California encephalitis. J Child Neurol. 1999;14:1–14. doi: 10.1177/088307389901400101. [DOI] [PubMed] [Google Scholar]
- Samuel MA, Diamond MS. Pathogenesis of West Nile Virus infection: a balance between virulence, innate and adaptive immunity, and viral evasion. J Virol. 2006;80:9349–9360. doi: 10.1128/JVI.01122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaljohn CS, Hooper JW. Bunyaviridae: the viruses and their replication. In: Howley DM, editor. Fields virology. 4th edn. Philadelphia: Lippincott Williams and Wilkins; 2001. [Google Scholar]
- Schuffenecker I, Iteman I, Michault A, Murri S, Frangeul L, Vaney MC, Lavenir R, Pardigon N, Reynes JM, Pettinelli F, Biscornet L, Diancourt L, Michel S, Duquerroy S, Guigon G, Frenkiel MP, Brehin AC, Cubito N, Despres P, Kunst F, Rey FA, Zeller H, Brisse S. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006;3:e263. doi: 10.1371/journal.pmed.0030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejvar JJ, Haddad MB, Tierney BC, Campbell GL, Marfin AA, Van Gerpen JA, Fleischauer A, Leis AA, Stokic DS, Petersen LR. Neurologic manifestations and outcome of West Nile virus infection. JAMA. 2003;290:511–515. doi: 10.1001/jama.290.4.511. [DOI] [PubMed] [Google Scholar]
- Shrestha B, Gottlieb D, Diamond MS. Infection and injury of neurons by West Nile encephalitis virus. J Virol. 2003;77:13203–13213. doi: 10.1128/JVI.77.24.13203-13213.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidwell RW, Smee DF. Viruses of the Bunya- and Togaviridae families: potential as bioterrorism agents and means of control. Antiviral Res. 2003;57:101–111. doi: 10.1016/s0166-3542(02)00203-6. [DOI] [PubMed] [Google Scholar]
- Sitati EM, Diamond MS. CD4+ T-cell responses are required for clearance of West Nile virus from the central nervous system. J Virol. 2006;80:12060–12069. doi: 10.1128/JVI.01650-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithburn KC, Hughes TP, Burke AW, Paul JH. A neurotropic virus isolated from the blood of a native of Uganda. Am J Trop Med. 1940;20:471–492. [Google Scholar]
- Soldan SS, Gonzalez-Scarano F. Emerging infectious diseases: the Bunyaviridae. J Neurovirol. 2005;11:412–423. doi: 10.1080/13550280591002496. [DOI] [PubMed] [Google Scholar]
- Soldan SS, Hollidge BS, Wagner V, Weber F, Gonzalez-Scarano F. La Crosse virus (LACV) Gc fusion peptide mutants have impaired growth and fusion phenotypes, but remain neurotoxic. Virology. 2010;404:139–147. doi: 10.1016/j.virol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon T, Vaughn DW. Pathogenesis and clinical features of Japanese encephalitis and West Nile virus infections. Curr Top Microbiol Immunol. 2002;267:171–194. doi: 10.1007/978-3-642-59403-8_9. [DOI] [PubMed] [Google Scholar]
- Solomon T, Kneen R, Dung NM, Khanh VC, Thuy TT, Ha DQ, Day NP, Nisalak A, Vaughn DW, White NJ. Poliomyelitis-like illness due to Japanese encephalitis virus. Lancet. 1998;351:1094–1097. doi: 10.1016/S0140-6736(97)07509-0. [DOI] [PubMed] [Google Scholar]
- Solomon T, Dung NM, Kneen R, Gainsborough M, Vaughn DW, Khanh VT. Japanese encephalitis. J Neurol Neurosurg Psychiatry. 2000;68:405–415. doi: 10.1136/jnnp.68.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler K, Allison SL, Schalich J, Heinz FX. Proteolytic activation of tick-borne encephalitis virus by furin. J Virol. 1997;71:8475–8481. doi: 10.1128/jvi.71.11.8475-8481.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucharit S, Surathin K, Shrestha SR. Vectors of Japanese encephalitis virus (JEV): species complexes of the vectors. Southeast Asian J Trop Med Public Health. 1989;20:611–621. [PubMed] [Google Scholar]
- Swanepoel R, Jaw C. Rift Valley fever. In: Jaw C, Thompson GR, Tustin RD, Al E, editors. Infectious diseases of livestock with special reference to southern Africa. 2nd edn. Cape Town, South Africa: Oxford University Press; 2004. [Google Scholar]
- Thiboutot MM, Kannan S, Kawalekar OU, Shedlock DJ, Khan AS, Sarangan G, Srikanth P, Weiner DB, Muthumani K. Chikungunya: a potentially emerging epidemic? PLoS Negl Trop Dis. 2010;4:623. doi: 10.1371/journal.pntd.0000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D, Blakqori G, Wagner V, Banholzer M, Kessler N, Elliott RM, Haller O, Weber F. Inhibition of RNA polymerase II phosphorylation by a viral interferon antagonist. J Biol Chem. 2004;279:31471–31477. doi: 10.1074/jbc.M400938200. [DOI] [PubMed] [Google Scholar]
- Thompson W, Beaty BJ. Venereal transmission of La Crosse (California encephalitis) arbovirus in Aedes triseriatus mosquitoes. Science. 1977;196:530–531. doi: 10.1126/science.850794. [DOI] [PubMed] [Google Scholar]
- Thompson WH, Kalfayan B, Anslow RO. Isolation of California Encephalitis Group Virus from a Fatal Human Illness. Am J Epidemiol. 1965;81:245–253. doi: 10.1093/oxfordjournals.aje.a120512. [DOI] [PubMed] [Google Scholar]
- Thompson BS, Moesker B, Smit JM, Wilschut J, Diamond MS, Fremont DH. A therapeutic antibody against west nile virus neutralizes infection by blocking fusion within endosomes. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000453. e1000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournebize P, Charlin C, Lagrange M. Neurological manifestations in Chikungunya: about 23 cases collected in Reunion Island. Rev Neurol (Paris) 2009;165:48–51. doi: 10.1016/j.neurol.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Trevejo RT, Eidson M. Zoonosis update: West Nile virus. J Am Vet Med Assoc. 2008;232:1302–1309. doi: 10.2460/javma.232.9.1302. [DOI] [PubMed] [Google Scholar]
- Tsai TF. New initiatives for the control of Japanese encephalitis by vaccination: minutes of a WHO/CVI meeting, Bangkok, Thailand, 13–15 October 1998. Vaccine. 2000;18 Suppl 2:1–25. doi: 10.1016/s0264-410x(00)00037-2. [DOI] [PubMed] [Google Scholar]
- van den Hurk AF, Ritchie SA, Mackenzie JS. Ecology and geographical expansion of Japanese encephalitis virus. Annu Rev Entomol. 2009;54:17–35. doi: 10.1146/annurev.ento.54.110807.090510. [DOI] [PubMed] [Google Scholar]
- Vogel P, Kell WM, Fritz DL, Parker MD, Schoepp RJ. Early events in the pathogenesis of eastern equine encephalitis virus in mice. Am J Pathol. 2005;166:159–171. doi: 10.1016/S0002-9440(10)62241-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Petrakova O, Adams AP, Aguilar PV, Kang W, Paessler S, Volk SM, Frolov I, Weaver SC. Chimeric Sindbis/eastern equine encephalitis vaccine candidates are highly attenuated and immunogenic in mice. Vaccine. 2007;25:7573–7581. doi: 10.1016/j.vaccine.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts D, Pantuwatana S, Defoliart GR, Yuill TM, Thompson WH. Transovarial transmission of La Crosse virus (California encephalitis group) in the mosquito, Aedes triseriatus. Science. 1973:1140–1141. doi: 10.1126/science.182.4117.1140. [DOI] [PubMed] [Google Scholar]
- Watts DM, Thompson WH, Yuill TM, Defoliart GR, Hanson RP. Overwintering of La Crosse virus in Aedes triseriatus. Am J Trop Med Hyg. 1974:694–700. doi: 10.4269/ajtmh.1974.23.694. [DOI] [PubMed] [Google Scholar]
- Weaver SC, Barrett AD. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat Rev Microbiol. 2004;2:789–801. doi: 10.1038/nrmicro1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral Res. 2010;85:328–345. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver SC, Powers AM, Brault AC, Barrett AD. Molecular epidemiological studies of veterinary arboviral encephalitides. Vet J. 1999;157:123–138. doi: 10.1053/tvjl.1998.0289. [DOI] [PubMed] [Google Scholar]
- WHO. Outbreaks of Rift Valley fever in Kenya, Somalia, and United Republic of Tanzania, December 2006–April 2007. Wkly Epidemiol Rec. 2007;82:169–178. [PubMed] [Google Scholar]
- WHO. Rift Valley fever. 2010a
- WHO. Rift Valley fever in South Africa. 2010b
- Wilson ML, Chapman LE, Hall DB, Dykstra EA, Ba K, Zeller HG, Traore-Lamizana M, Hervy JP, Linthicum KJ, Peters CJ. Rift Valley fever in rural northern Senegal: human risk factors and potential vectors. Am J Trop Med Hyg. 1994;50:663–675. doi: 10.4269/ajtmh.1994.50.663. [DOI] [PubMed] [Google Scholar]
- Winter PM, Dung NM, Loan HT, Kneen R, Wills B, Thu Le T, House D, White NJ, Farrar JJ, Hart CA, Solomon T. Proinflammatory cytokines and chemokines in humans with Japanese encephalitis. J Infect Dis. 2004;190:1618–1626. doi: 10.1086/423328. [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Tsujimura K, Kondo T, Yasuda W, Okada A, Noda K, Okumura T, Matsumura T. Isolation and genetic analysis of Japanese encephalitis virus from a diseased horse in Japan. J Vet Med Sci. 2006;68:293–295. doi: 10.1292/jvms.68.293. [DOI] [PubMed] [Google Scholar]
- Yin J, Gardner CL, Burke CW, Ryman KD, Klimstra WB. Similarities and differences in antagonism of neuron alpha/beta interferon responses by Venezuelan equine encephalitis and Sindbis alphaviruses. J Virol. 2009;83:10036–10047. doi: 10.1128/JVI.01209-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young DS, Kramer LD, Maffei JG, Dusek RJ, Backenson PB, Mores CN, Bernard KA, Ebel GD. Molecular epidemiology of eastern equine encephalitis virus, New York. Emerg Infect Dis. 2008;14:454–460. doi: 10.3201/eid1403.070816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu IM, Zhang W, Holdaway HA, Li L, Kostyuchenko VA, Chipman PR, Kuhn RJ, Rossmann MG, Chen J. Structure of the immature dengue virus at low pH primes proteolytic maturation. Science. 2008;319:1834–1837. doi: 10.1126/science.1153264. [DOI] [PubMed] [Google Scholar]
- Zacks MA, Paessler S. Encephalitic alphaviruses. Vet Microbiol. 2010;140:281–286. doi: 10.1016/j.vetmic.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li L, Woodson SE, Huang CY, Kinney RM, Barrett AD, Beasley DW. A mutation in the envelope protein fusion loop attenuates mouse neuroinvasiveness of the NY99 strain of West Nile virus. Virology. 2006;353:35–40. doi: 10.1016/j.virol.2006.05.025. [DOI] [PubMed] [Google Scholar]