Abstract
Homeobox 9 (HOXB9), a nontransforming transcription factor overexpressed in breast cancer, alters tumor cell fate and promotes tumor progression and metastasis. Here we show that HOXB9 confers resistance to ionizing radiation by promoting DNA damage response. In nonirradiated cells, HOXB9 induces spontaneous DNA damage, phosphorylated histone 2AX and p53 binding protein 1 foci, and increases baseline ataxia telangiectasia mutated (ATM) phosphorylation. Upon ionizing radiation, ATM is hyperactivated in HOXB9-expressing cells during the early stages of the double-stranded DNA break (DSB) response, accelerating accumulation of phosphorylated histone 2AX, mediator of DNA-damage checkpoint 1, and p53 binding protein 1, at DSBs and enhances DSB repair. The effect of HOXB9 on the response to ionizing radiation requires the baseline ATM activity before irradiation and epithelial-to-mesenchymal transition induced by TGF-β, a HOXB9 transcriptional target. Our results reveal the impact of a HOXB9–TGF-β–ATM axis on checkpoint activation and DNA repair, suggesting that TGF-β may be a key factor that links tumor microenvironment, tumor cell fate, DNA damage response, and radioresistance in a subset of HOXB9-overexpressing breast tumors.
Homeobox B9 (HOXB9), a member of the class I homeobox (HOX) genes, is overexpressed in breast cancer, and elevated levels correlate with high tumor grade. HOXB9 transactivates several angiogenic factors and erythroblastic leukemia viral oncogene homolog (ErbB) and TGF-β ligands, leading to epithelial-to-mesenchymal transition (EMT), increased angiogenesis, and distal metastasis. Thus, although not a transforming oncogene, HOXB9 promotes breast cancer progression. HOXB9-induced ErbB ligands and TGF-β activate human epidermal growth factor receptor 2/3 (HER2/HER3) phosphorylation and TGF-β receptor pathways, respectively; although activation of the HER/AKT and TGF-β axes promotes cell motility, induction of the TGF-β pathway promotes EMT of these cells (1).
EMT, an embryonic process leading to loss of cell–cell contact and invasion, is associated with resistance to chemotherapeutic drugs and radiation (2, 3). Several embryonic transcription factors, including HOX, that are deregulated in breast cancer induce EMT (4). However, their mechanistic role in DNA damage response (DDR) and radiation resistance is unclear. Exposure of tumor cells to ionizing radiation causes double-stranded DNA breaks (DSBs), triggering DDR through activation of the ataxia telangiectasia mutated (ATM) kinase, which induces cell-cycle arrest and also promotes DNA repair to maintain chromosome stability. When activated, ATM autophosphorylates itself at Ser1989 and phosphorylates the histone variant H2AX at Ser139 around DSBs, which recruits DNA repair proteins such as mediator of DNA-damage checkpoint 1 (MDC1), p53 binding protein 1 (53BP1), and breast cancer type 1 susceptibility protein to sites of DNA damage. The phosphorylated histone H2AX (known as γ-H2AX) and the recruited DNA repair proteins form discrete nuclear foci at DSBs, providing surrogate markers to characterize the dynamic process of DNA repair (5–8). In addition to DSBs, the oncogenic stress induced by various oncogenes activates ATM (9). How exactly ATM is activated by oncogenic stress and whether such ATM activation affects the response of cancer cells to extrinsic DNA damage still is unclear.
Tumoral secretion of growth factors and cytokines alters the tumor microenvironment and modulates tumor cell fate, resulting in increased tumor aggressiveness and resistance to chemotherapy and radiation (10, 11). However, the consequence of this process on DDR is not clearly defined. Activation or aberrant overexpression of transcription factors such as HOXB9 in tumors may induce tumoral expression of growth factors, resulting in their local increase in the tumor microenvironment (1). TGF-β, a pleiotropic cytokine and a potent inducer of EMT, promotes tumor progression through autocrine and paracrine mechanisms (3, 12). Ionizing radiation has been shown to induce EMT and invasion of cells through chronic activation of the TGF-β pathway (13, 14), suggesting that TGF-β may influence radiation responses by modulating tumor cell fate.
In this study we demonstrate that HOXB9, a transcription factor overexpressed in breast cancer (1), induces the formation of γ-H2AX and 53BP1 nuclear foci in nonirradiated cells and confers radioresistance by promoting ATM activation and DNA repair via a TGF-β–dependent mechanism. Thus, we identify a mechanism by which overexpression of a transcription factor through transactivation of TGF-β alters tumor cell fate and contributes to DDR in a subset of breast tumors.
Results
HOXB9 Induces Radiation Resistance and Spontaneous Nuclear γ-H2AX and 53BP1 Foci.
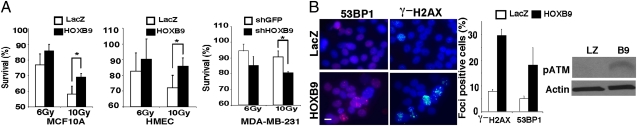
We previously demonstrated that HOXB9 expression in mammary epithelial cells leads to mesenchymal transition (1). To determine whether HOXB9-induced EMT is associated with radioresistance, we analyzed the survival of HOXB9-expressing MCF10A cells and human mammary epithelial cells (HMEC) exposed to ionizing radiation. Compared with cells expressing β-galactosidase (LacZ), cells expressing HOXB9 displayed a significant increase in survival after ionizing radiation in both survival and colony-formation assays (Fig. 1A and Fig. S1A). Knockdown of HOXB9 expression in a breast cancer cell line, MDA-MB-231, which exhibits mesenchymal features and expresses high levels of HOXB9 (1), increased the sensitivity of these cells to ionizing radiation (Fig. 1A). These results suggest that HOXB9 expression is associated with radiation resistance.
Fig. 1.
HOXB9 expression promotes radiation resistance. (A) MCF10A (Left) and HMECs (Center) expressing either HOXB9 or LacZ were irradiated with 6 Gy or 10 Gy of ionizing radiation, and cell survival was measured after 24 h. (Right) MDA-MB-231 cells in which HOXB9 expression was knocked down with shHOXB9 were irradiated with 6 Gy or 10 Gy of ionizing radiation, and cell survival was measured after 24 h. Cells infected with shGFP were used as control. *P < 0.001. (B) HOXB9 expression promotes the formation of γ-H2AX and 53BP1 foci. (Left) The expression of γ-H2AX and 53BP1 was evaluated in MCF10A cells expressing either LacZ or HOXB9. (Scale bar, 10 μM.) (Middle) Bar graph demonstrates the percentage of nuclei positive for γ-H2AX or 53BP1 foci. The mean was derived from the percentage of cells that scored positive for γ-H2AX or 53BP1 foci across nine fields. (Right) Western blot analysis demonstrates increased phospho-ATM expression in HOXB9-expressing cells. B9, HOXB9; LZ, LacZ.
To investigate whether HOXB9 alters DDR, we monitored the focus formation of γ-H2AX and 53BP1, two markers of DNA damage sites, in HOXB9-expressing cells. Even before ionizing radiation treatment, HOXB9-expressing MCF10A cells (Fig. 1B) and HMECs (Fig. S1B) displayed a significant increase in γ-H2AX and 53BP1 foci compared with the respective LacZ-expressing control cells. Furthermore, the levels of phosphorylated ATM (pATM) were increased by HOXB9 expression (Fig. 1B). Consistent with these observations, HOXB9 knockdown in MDA-MB-231 cells led to a reduction in baseline γ-H2AX and 53BP1 foci (Fig. S1C). These results suggest that HOXB9 increases the level of intrinsic genomic stress or the response to such stress. Consistent with the increased γ-H2AX and 53BP1 foci, we detected increased amounts of single- and double-stranded DNA breaks in HOXB9-expressing cells using the comet assay (Fig. S1D). We noted that the number of DNA breaks detected in HOXB9-expressing cells was much lower than that induced by 20 Gy of ionizing radiation (Fig. S1D), suggesting that HOXB9 induces only a low level of genomic instability.
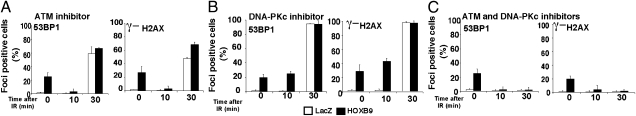
To characterize further the baseline DDR in nonirradiated HOXB9-expressing cells, we asked whether the γ-H2AX and 53BP1 foci in these cells are dependent upon ATM, ataxia telangiectasia and Rad3 related (ATR), or DNA-dependent protein kinase catalytic subunit (DNA-PKcs). Inhibition of ATM and DNA-PKcs using kinase-specific inhibitors, either individually or in combination, did not eliminate the γ-H2AX and 53BP1 foci, suggesting that these kinases are not essential for the maintenance of the foci (see 0-min time points in Fig. 2 A–C). Because ATR is essential for cell survival, the effect of ATR on γ-H2AX and 53BP1 foci cannot be assessed directly. To investigate whether the γ-H2AX and 53BP1 foci are associated with DNA replication problems, we analyzed the cell-cycle distribution of HOXB9-expressing cells. Consistent with mesenchymal differentiation, HOXB9 expression increased the G1 fraction in MCF10A cells and HMECs compared with cells expressing LacZ but caused no significant change in the fraction of S-phase cells (Fig. S2). Conversely, HOXB9 knockdown in MDA-MB-231 cells led to a reduction of G1 cells but not S-phase cells (Fig. S2). These results suggest that DNA replication is not perturbed significantly by HOXB9 expression. Thus, the DNA damage induced by HOXB9 leads to ATM activation and increases baseline DDR in nonirradiated cells. The exact cause of the genomic instability in nonirradiated HOXB9-expressing cells remains to be elucidated (Discussion).
Fig. 2.
ATM is required for the rapid DDR observed in HOXB9-expressing cells. MCF10A cells expressing LacZ or HOXB9 were treated with the ATM inhibitor KU55933 (10 μM) (A), the DNA PKcs inhibitor Nu7062 (10 μM) (B), or a combination of the ATM and DNA PKcs inhibitors for 1 h before irradiation (10 Gy) (C). 53BP1 and γ-H2AX expression was visualized 0, 10, and 30 min after γ-irradiation (IR). Bar graphs demonstrate the percentage of nuclei positive for γ-H2AX or 53BP1. The mean was derived from the percentage of cells that scored positive for γ-H2AX or 53BP1 across nine fields.
HOXB9 Expression Accelerates DDR by Priming ATM Activation.
In response to treatment with 10 Gy of ionizing radiation, the percentage of nuclei exhibiting γ-H2AX and 53BP1 foci increased to ∼80% within 10 min in cells expressing HOXB9, whereas focus formation in LacZ-expressing MCF10A (Fig. 3) and HMECs (Fig. S3A) did not reach the same levels until 30 min after ionizing radiation. These results suggest that the formation of γ-H2AX and 53BP1 foci induced by DNA damage is accelerated in cells expressing HOXB9. The levels of histone H4, lysine 20 dimethylation, a histone mark that associates directly with 53BP1 (15), were not altered substantially after ionizing radiation treatment in cells expressing HOXB9 (Fig. S3B). These results raise the possibility that HOXB9 expression specifically promotes an early step of DSB response and accelerates H2AX phosphorylation on chromatin, thereby indirectly enhancing the recruitment of 53BP1.
Fig. 3.
HOXB9 expression leads to rapid radiation-induced DDR. MCF10A cells expressing LacZ or HOXB9 were irradiated (10 Gy), and γ-H2AX or 53BP1 expression was visualized 0, 10, and 30 min after ionizing radiation (IR). Bar graphs demonstrate the percentage of nuclei positive for γ-H2AX or 53BP1 foci. The mean was derived from the percentage of cells that scored positive for γ-H2AX or 53BP1 foci across nine fields. (Scale bar, 10 μM.)
We next asked how DDR is accelerated in HOXB9-expressing cells. In marked contrast to the spontaneous γ-H2AX and 53BP1 foci, γ-H2AX and 53BP1 foci induced by ionizing radiation in these cells were abolished completely by ATM inhibition up to 10 min after ionizing radiation and were partially suppressed by DNA-PKcs inhibition (Fig. 2 A and B). These results suggest that ATM, but not DNA-PKcs, is the primary kinase that promotes the formation of γ-H2AX and 53BP1 foci in the early stage of response to ionizing radiation in HOXB9-expressing cells. Inhibition of both ATM and DNA-PKcs abolished all DDR in LacZ- and HOXB9-expressing cells 30 min after ionizing radiation (Fig. 2C), suggesting that ATM and DNA-PKcs play functionally redundant roles in the late stage of response to ionizing radiation. Surprisingly, although ATM is not essential for the γ-H2AX and 53BP1 foci in nonirradiated HOXB9-expressing cells, these foci disbursed rapidly after ionizing radiation when ATM was inhibited (Fig. 2 A and C). The reason for this change is still unclear. Nonetheless, these results suggest that accelerated DSB response in HOXB9-expressing cells is mediated primarily by ATM, whereas both ATM and DNA-PKcs contribute to the late-stage responses.
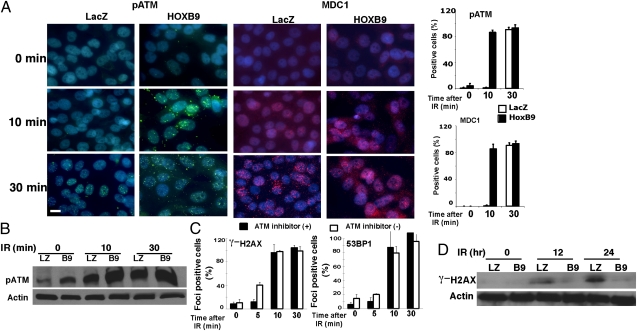
Because the early DDR in HOXB9-expressing cells depends on ATM, we analyzed the kinetics of ATM activation in these cells. Nuclear foci of pATM were observed in HOXB9-MCF10A cells within 10 min of ionizing radiation. MDC1, a protein that directly binds γ-H2AX and promotes pATM and 53BP1 recruitment, also is recruited rapidly to sites of DNA damage in these cells (Fig. 4A). The enhanced ionizing radiation-induced phosphorylation of ATM also was confirmed by Western blot (Fig. 4B). Thus, HOXB9 promotes an early event during DSB response that facilitates pATM recruitment, H2AX phosphorylation, and MDC1 recruitment.
Fig. 4.
HOXB9-expressing cells exhibit rapid ATM activation and early termination of DDR. (A) MCF10A cells expressing LacZ or HOXB9 were irradiated (10 Gy), and pATM and MDC1 expression were visualized 0, 10, and 30 min after γ-irradiation (IR). Bar graphs show the percentage of nuclei positive for pATM or MDC1. The mean was derived from the percentage of cells that scored positive for pATM or MDC1 across nine fields. (Scale bar, 10 μM.) (B) pATM is elevated in HOXB9-expressing cells. Western blot analysis of pATM expression in MCF10A cells expressing LacZ (LZ) or HOXB9 (B9) after 0, 10, and 30 min of irradiation. (C) MCF10A cells expressing HOXB9 were treated with KU55933 (10 μM) for 2 h before irradiation. The inhibitor was washed out, and γ-H2AX expression (Left) and 53BP1 expression (Right) was visualized 0, 5, 10, and 30 min after γ-irradiation. (D) Western blot analysis of γ-H2AX expression in MCF10A cells expressing LacZ or HOXB9 after 0, 12, and 24 h of γ-irradiation.
The activation and function of ATM at DSBs are enhanced by a pATM- and MDC1-mediated feed-forward loop (16, 17). The initial activation of ATM at DSBs leads to ATM autophosphorylation and limited H2AX phosphorylation. The phosphorylated H2AX on chromatin recruits MDC1, which in turn recruits pATM and enables it to phosphorylate more H2AX and propagate its functions. HOXB9 not only accelerates ionizing radiation-induced ATM phosphorylation but also increases the baseline of pATM in nonirradiated cells (Figs. 1B and 4B). The preexistence of low levels of pATM in HOXB9-expressing cells may allow the pATM- and MDC1-mediated feed-forward loop to be established more rapidly in response to DSBs. To test this possibility, we pretreated HOXB9-expressing cells with a highly reversible ATM inhibitor (KU55933) (18) for 2 h and then washed out the inhibitor and irradiated the cells (Fig. 4C). Inhibition of the preexisting ATM activity in HOXB9-expressing cells before ionizing radiation delayed the ATM-mediated early response to ionizing radiation at 5 min but not 10 min, suggesting that these events depend partially on preexistent pATM and that other unknown mechanisms could contribute to accelerated DDR in HOXB9-expressing cells. These results are consistent with the hypothesis that HOXB9 expression accelerates DSB response at least in part by priming a fraction of ATM molecules in the phosphorylated active state.
HOXB9 Expression Promotes DNA Repair and Cell-Cycle Reentry.
When DSBs are repaired successfully in cells, γ-H2AX is dephosphorylated gradually. Western blot analysis of γ-H2AX in HOXB9-MCF10A cells demonstrated that DNA repair was largely completed 12 h after radiation, whereas it persisted in LacZ-MCF10A cells (Fig. 4D). Consistent with these observations, cell-cycle analysis demonstrated that at 3 d after irradiation HOXB9-expressing cells had recovered from the G2/M arrest and reentered S phase, whereas a major fraction of irradiated LacZ-MCF10A cells remained arrested at G2/M and did not enter S phase (Fig. S4A). BrdU-incorporation studies also show that irradiated HOXB9-MCF10A cells reenter S phase faster than irradiated LacZ-MCF10A cells (Fig. S4A). Together these results suggest that DSBs are repaired more efficiently in HOXB9-expressing cells.
To evaluate further the effects of HOXB9 on DSB repair, we introduced the reporter constructs DR-GFP [a reporter for homologous recombination (HR)] and pEJ [a reporter for nonhomologous end joining (NHEJ)] into HOXB9- or LacZ-MCF10A cells (19, 20). Upon expression of the I-SceI endonuclease, the efficiencies of HR and NHEJ were determined by the GFP+ cell populations. HOXB9 increased the efficiency of HR and, to a lesser extent, the efficiency of NHEJ (Fig. 4 B and C). These results suggest that, although HOXB9 induces spontaneous DNA damage, cells expressing HOXB9 can repair DSBs more efficiently.
DDR in HOXB9-Expressing Cells Is Dependent on TGF-β.
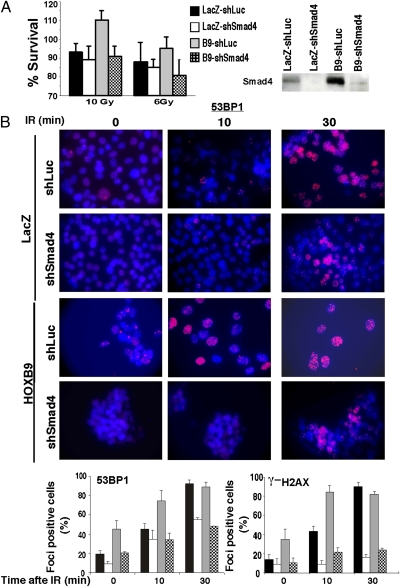
Because HOXB9-mediated EMT is driven by activation of TGF-β, a transcriptional target of HOXB9 (1), we tested whether inhibition of TGF-β activation in HOXB9-expressing cells would reverse radiation resistance exhibited by these cells. TGF-β activation in cells was abrogated by shRNA knockdown of mothers against decapentaplegic homolog 4 (Smad4), a key effector of TGF-β signaling. Suppression of TGF-β activation, which reversed HOXB9-induced mesenchymal phenotype (1), restored radiation sensitivity in HOXB9-MCF10A cells (Fig. 5A). Moreover, inhibition of the TGF-β pathway suppressed the DDR observed in nonirradiated cells and the rapid response seen 10 min after irradiation (Fig. 5B and Fig. S5A). The formation of 53BP1 and γ-H2AX foci observed in cells 30 min after irradiation was mitigated significantly but not abolished completely in shSmad4 cells (Fig. 5B and Fig. S5A), suggesting that TGF-β may influence the functions of both ATM and DNA-PKcs in focus formation. Together, the results strongly suggest that the effects of HOXB9 on DDR, like those on EMT, involve the TGF-β pathway.
Fig. 5.
Effects of HOXB9 on DDR are mediated through the TGF-β pathway. (A) MCF10A cells expressing LacZ-shLuciferase (Luc), LacZ-shSmad4, HOXB9-shLuc, or HOXB9-shSmad4 were irradiated with 6 Gy or 10 Gy of ionizing radiation, and cell survival was measured after 24 h. (Right) Western blot demonstrates the knockdown of Smad4 protein in cells. (B) (Upper) MCF10A cells expressing LacZ-shLuc, LacZ-shSmad4, HOXB9-shLuc, or HOXB9-shSmad4 were irradiated (10 Gy), and γ-H2AX or 53BP1 expression was visualized 0, 10, and 30 min after ionizing radiation. Representative images of cells stained with 53BP1 are shown. (Scale bar, 10 μM.) (Lower) Bar graphs demonstrate the percentage of nuclei positive for γ-H2AX or 53BP1 foci. The mean was derived from the percentage of cells that scored positive for γ-H2AX or 53BP1 foci across nine fields. The key for the bar graphs is shown in A. IR, ionizing radiation.
To assess directly whether activation of the TGF-β pathway is sufficient to recapitulate the effects of HOXB9 expression, cells were treated with TGF-β, and radiation resistance was monitored. Cells treated with TGF-β were more resistant to radiation than untreated cells (Fig. S5B). Moreover, TGF-β also increased nuclear pATM expression in nonirradiated cells (Fig. S5C); consistent with this observation, cells treated with TGF-β exhibited increased γ-H2AX and 53BP1 foci before irradiation and responded to ionizing radiation more efficiently 10 min after irradiation (Fig. S5C). These results demonstrate that HOXB9 potentiates DDR by activating the TGF-β pathway.
Discussion
Several human tumors, including those of the breast, exhibit activated DDR and display ATM activation and γ-H2AX and 53BP1 foci attributable to oncogenic stress (21). In this study, we demonstrate that HOXB9, a nontransforming transcription factor that promotes tumor progression and metastasis (1), induces DDR through induction of TGF-β, its transcriptional target (1). This observation reveals a mechanism that contributes to DDR in a subset of breast tumors that overexpress this transcription factor, which through transactivation of a cytokine elevates baseline ATM phosphorylation and accelerates DDR and radiation resistance.
Comet assays demonstrate that before irradiation HOXB9 induces detectable spontaneous DNA damage in cells leading to enhanced ATM phosphorylation. Although DNA replication stress and DNA damage are induced in cells by oncogene expression (21), HOXB9 is not a transforming oncogene. Despite being overexpressed in 40% of breast cancers, HOXB9 by itself neither transforms mammary epithelial cells nor renders them tumorigenic in vivo. However, it enhances the invasive properties of cells in vitro and promotes tumor progression and metastasis in vivo when coexpressed with transforming oncogenes such as activated rat sarcoma viral oncogene (Ras) (1). Our observations demonstrate that a nontransforming gene-transcription factor (HOXB9) involved in tumor progression and metastasis can increase spontaneous DNA damage through transactivation of its target, TGF-β, and identify an important mechanism of genomic instability in a subset of breast tumors.
The formation of γ-H2AX and 53BP1 foci can be mediated by ATM, ATR, or DNA-PKcs in different contexts (22, 23). Although the levels of pATM are elevated in nonirradiated HOXB9-expressing cells, inhibition of ATM or DNA-PKcs does not interfere with the formation of γ-H2AX and 53BP1 foci in these cells. Although this result might be caused by foci not being disassembled quickly enough after addition of the inhibitor, it also could suggest the involvement of ATR in mediating the HOXB9 effects. Loss of TGF-β expression in epithelial cells has been associated with decreased ATM autophosphorylation and kinase activity and attenuated DDR (24). In addition, TGF-β also induces DNA damage signaling by the “bystander effect”; irradiated glioma cells induce γ-H2AX foci in neighboring nontargeted bystander glioma cells through a TGF-β–mediated process that is independent of ATM and DNA-PKcs but is dependent on ATR (22). The mechanism by which TGF-β induces DDR is unclear. However, TGF-β is known to induce reactive oxygen species that can promote EMT, oxidative damage to DNA, and genomic instability in mouse mammary epithelial cells (25, 26). Our data demonstrate that in HOXB9-expressing cells the induction of γ-H2AX and 53BP1 foci before irradiation and accelerated DDR after ionizing radiation are mediated in part through the HOXB9–TGF-β–ATM axis.
Increased ATM phosphorylation in nonirradiated cells may enable this protein to localize to DSBs more efficiently after irradiation through binding to MDC1, thereby facilitating the recruitment of additional DNA repair factors, and to be responsible for the rapid DDR and radiation resistance observed in HOXB9-expressing cells. Consistent with this concept, preexisting pATM in nonirradiated HOXB9-expressing cells is important for the accelerated DSB response after ionizing radiation. As a result of the enhanced ATM activation and accelerated recruitment of repair factors in HOXB9-expressing cells, DNA repair is carried out more efficiently in these cells. This increased efficiency was confirmed using reporter constructs to characterize DNA damage repair (19), demonstrating that HR is enhanced in HOXB9 cells compared with LacZ-expressing control cells. Furthermore, NHEJ also may be enhanced modestly in HOXB9-expressing cells.
In conclusion, through the induction of TGF-β, expression of HOXB9 in breast cancers alters the tumor microenvironment and tumor cell fate, leading to enhanced DDR and resistance to γ-irradiation. Further studies are needed to determine whether HOXB9 expression can serve as a biomarker to determine radiation responses in breast cancer.
Methods
Cell Culture.
MCF10A cells or HMECs were infected with HOXB9-expressing lentiviral construct, and MDA-MB-231 cells were infected with shHOXB9 lentiviral construct (1). Smad4 expression in cells was knocked down similarly with shSmad4.
Western Blot Analysis and Immunofluorescence.
Antibodies against phospho-ATM (#2152–1; Epitomics) and γ- H2AX (ab18311; Abcam) were used for Western blot analysis. The immunofluorescence protocol is described in SI Methods.
Cell-Cycle Analysis.
Cell cycle analysis was done as described in SI Methods.
BrdU-Incorporation Assay.
Cells were fixed and permeabilized with reagents from the BrdU Flow Kit (BD Pharmingen) according to the manufacturer's instructions. BrdU was detected using an APC-BrdU Flow Kit according to the manufacturer's instructions.
Supplementary Material
Acknowledgments
We thank Drs. Isselbacher and Ellisen for critical reading of the manuscript. This work was supported by National Institutes of Health/National Cancer Institute Grant CA89138, National Cancer Institute Federal Share Program and Income (L.Z. and S.M.), and by Susan G. Komen for the Cure Grants PDF0600282 and KG090412 (to S.M.) and GM076388 (to L.Z.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. K.P. is a guest editor invited by the EditorialBoard.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018867108/-/DCSupplemental.
References
- 1.Hayashida T, et al. HOXB9, a gene overexpressed in breast cancer, promotes tumorigenicity and lung metastasis. Proc Natl Acad Sci USA. 2010;107:1100–1105. doi: 10.1073/pnas.0912710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Creighton CJ, Chang JC, Rosen JM. Epithelial-mesenchymal transition (EMT) in tumor-initiating cells and its clinical implications in breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:253–260. doi: 10.1007/s10911-010-9173-1. [DOI] [PubMed] [Google Scholar]
- 3.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briegel KJ. Embryonic transcription factors in human breast cancer. IUBMB Life. 2006;58:123–132. doi: 10.1080/15216540600686870. [DOI] [PubMed] [Google Scholar]
- 5.Abraham RT, Tibbetts RS. Cell biology. Guiding ATM to broken DNA. Science. 2005;308:510–511. doi: 10.1126/science.1112069. [DOI] [PubMed] [Google Scholar]
- 6.Bakkenist CJ, Kastan MB. Initiating cellular stress responses. Cell. 2004;118:9–17. doi: 10.1016/j.cell.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 7.Harper JW, Elledge SJ. The DNA damage response: Ten years after. Mol Cell. 2007;28:739–745. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Li L, Zou L. Sensing, signaling, and responding to DNA damage: Organization of the checkpoint pathways in mammalian cells. J Cell Biochem. 2005;94:298–306. doi: 10.1002/jcb.20355. [DOI] [PubMed] [Google Scholar]
- 9.Bartkova J, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- 10.Barcellos-Hoff MH, Park C, Wright EG. Radiation and the microenvironment—tumorigenesis and therapy. Nat Rev Cancer. 2005;5:867–875. doi: 10.1038/nrc1735. [DOI] [PubMed] [Google Scholar]
- 11.Shinohara ET, Maity A. Increasing sensitivity to radiotherapy and chemotherapy by using novel biological agents that alter the tumor microenvironment. Curr Mol Med. 2009;9:1034–1045. doi: 10.2174/156652409789839107. [DOI] [PubMed] [Google Scholar]
- 12.Taylor MA, Parvani JG, Schiemann WP. The pathophysiology of epithelial-mesenchymal transition induced by transforming growth factor-beta in normal and malignant mammary epithelial cells. J Mammary Gland Biol Neoplasia. 2010;15:169–190. doi: 10.1007/s10911-010-9181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andarawewa KL, et al. Ionizing radiation predisposes nonmalignant human mammary epithelial cells to undergo transforming growth factor beta induced epithelial to mesenchymal transition. Cancer Res. 2007;67:8662–8670. doi: 10.1158/0008-5472.CAN-07-1294. [DOI] [PubMed] [Google Scholar]
- 14.Jung JW, et al. Ionising radiation induces changes associated with epithelial-mesenchymal transdifferentiation and increased cell motility of A549 lung epithelial cells. Eur J Cancer. 2007;43:1214–1224. doi: 10.1016/j.ejca.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 15.Sakaguchi A, Steward R. Aberrant monomethylation of histone H4 lysine 20 activates the DNA damage checkpoint in Drosophila melanogaster. J Cell Biol. 2007;176:155–162. doi: 10.1083/jcb.200607178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lou Z, et al. MDC1 maintains genomic stability by participating in the amplification of ATM-dependent DNA damage signals. Mol Cell. 2006;21:187–200. doi: 10.1016/j.molcel.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 17.So S, Davis AJ, Chen DJ. Autophosphorylation at serine 1981 stabilizes ATM at DNA damage sites. J Cell Biol. 2009;187:977–990. doi: 10.1083/jcb.200906064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White JS, Choi S, Bakkenist CJ. Irreversible chromosome damage accumulates rapidly in the absence of ATM kinase activity. Cell Cycle. 2008;7:1277–1284. doi: 10.4161/cc.7.9.5961. [DOI] [PubMed] [Google Scholar]
- 19.Mansour WY, et al. Hierarchy of nonhomologous end-joining, single-strand annealing and gene conversion at site-directed DNA double-strand breaks. Nucleic Acids Res. 2008;36:4088–4098. doi: 10.1093/nar/gkn347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierce AJ, Johnson RD, Thompson LH, Jasin M. XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev. 1999;13:2633–2638. doi: 10.1101/gad.13.20.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartkova J, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 22.Burdak-Rothkamm S, Short SC, Folkard M, Rothkamm K, Prise KM. ATR-dependent radiation-induced gamma H2AX foci in bystander primary human astrocytes and glioma cells. Oncogene. 2007;26:993–1002. doi: 10.1038/sj.onc.1209863. [DOI] [PubMed] [Google Scholar]
- 23.DiTullio RA, Jr, et al. 53BP1 functions in an ATM-dependent checkpoint pathway that is constitutively activated in human cancer. Nat Cell Biol. 2002;4:998–1002. doi: 10.1038/ncb892. [DOI] [PubMed] [Google Scholar]
- 24.Kirshner J, et al. Inhibition of transforming growth factor-beta1 signaling attenuates ataxia telangiectasia mutated activity in response to genotoxic stress. Cancer Res. 2006;66:10861–10869. doi: 10.1158/0008-5472.CAN-06-2565. [DOI] [PubMed] [Google Scholar]
- 25.Radisky DC, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhyu DY, et al. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol. 2005;16:667–675. doi: 10.1681/ASN.2004050425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.