Abstract
The histone chaperone Asf1 and the checkpoint kinase Rad53 are found in a complex in budding yeast cells in the absence of genotoxic stress. Our data suggest that this complex involves at least three interaction sites. One site involves the H3-binding surface of Asf11 with an as yet undefined surface of Rad53. A second site is formed by the Rad53-FHA1 domain binding to Asf1-T270 phosphorylated by casein kinase II. The third site involves the C-terminal 21 amino acids of Rad53 bound to the conserved Asf1 N-terminal domain. The structure of this site showed that the Rad53 C-terminus binds Asf1 in a remarkably similar manner to peptides derived from the histone cochaperones HirA and CAF-I. We call this binding motif, (R/K)R(I/A/V) × (L/P), the AIP box for Asf1-Interacting Protein box. Furthermore, C-terminal Rad53-F820 binds the same pocket of Asf1 as does histone H4-F100. Thus Rad53 competes with histones H3-H4 and cochaperones HirA/CAF-I for binding to Asf1. Rad53 is phosphorylated and activated upon genotoxic stress. The Asf1-Rad53 complex dissociated when cells were treated with hydroxyurea but not methyl-methane-sulfonate, suggesting a regulation of the complex as a function of the stress. We identified a rad53 mutation that destabilized the Asf1-Rad53 complex and increased the viability of rad9 and rad24 mutants in conditions of genotoxic stress, suggesting that complex stability impacts the DNA damage response.
Keywords: checkpoint, chromatin, DNA damage, NMR, X-ray structure
Asf1 is a highly conserved chaperone of histones H3 and H4 that has been implicated in histone modification and nucleosome assembly/disassembly during DNA transcription, replication, recombination, and repair (1). In addition to H3-H4, Asf1 interacts with several other chromatin-associated proteins in a conserved manner, including the HirA and CAF-I histone cochaperones (2) and the Bdf transcription factors (3). In budding yeast, Asf1 also forms a complex with the DNA damage checkpoint kinase Rad53 (4, 5). Curiously, in mammalian cells, Asf1 does not appear to interact with Chk2 (6), the mammalian ortholog of Rad53, but rather with Tousled-like kinases that are also implicated in DNA damage responses but that are not conserved in yeast (7). The Asf1-Rad53 complex was reportedly dissociated when yeast cells were subjected to genotoxic stress in a Mec1-dependent manner (4, 5). Mec1 activates Rad53 by phosphorylation in response to genotoxic stress. It was suggested that Rad53 could target Asf1 to sites of DNA damage where Rad53 activating phosphorylation would then liberate Asf1 to facilitate DNA repair (4, 5). This model is compelling, but no experimental verification has yet appeared. Rad53 binding to Asf1 was also suggested to inhibit the ability of Asf1 to promote transcriptional silencing on the basis of genetic data (8). To test the functional importance of the Asf1-Rad53 complex, we mapped the interaction surfaces and solved the X-ray structure between the conserved Asf1 N-terminal domain (Asf1N) and the C-terminal peptide of Rad53. These structural data allowed us to identify a mutation destabilizing the complex. Interestingly, this mutant increased the resistance to genotoxic stress of rad9 and rad24 mutants that are partially defective in Rad53 activation.
Results
Rad53 and Asf1 Interact Through At Least Two Binding Sites.
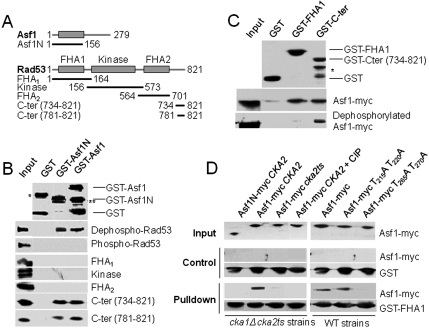
We used GST pull-down experiments to characterize the Asf1-Rad53 interaction in vitro. Different constructs of Asf1 (full-length and N-terminal domain) and Rad53 (full-length, and fragments containing the FHA1 domain, the kinase domain, the FHA2 domain, or C-terminal peptides) were expressed and purified from Escherichia coli with a GST or a 6His tag, respectively (Fig. 1A). Full-length Rad53 undergoes extensive autophosphorylation when it is expressed in E. coli (9) and this form of the protein was unable to bind Asf1 (Fig. 1B). In vitro, dephosphorylation of Rad53 allowed its binding to both GST-Asf1 and GST-Asf1N, but not to GST alone (Fig. 1B and Fig. S1A). Thus, Rad53 autophosphorylation blocks its binding to Asf1. We further analyzed binding of Rad53 domains to the GST-Asf1 constructs. Interestingly, both GST-Asf1 and GST-Asf1N specifically bound C-terminal peptides of Rad53, but not the FHA1, kinase, or FHA2 domains (Fig. 1B). Schwartz et al. previously showed that the Rad53 FHA1 domain could pull down Asf1 in yeast extracts, but treatment of such extracts with protein phosphatase prevented binding (10). We confirmed this observation, and we further verified that GST-Rad53-Cter peptides (aa 734–821 and 781–821) could pull down Asf1 from yeast extracts treated with protein phosphatase (Fig. 1C and Fig. S1B). These results suggest that Rad53 and Asf1 interact through at least two binding sites: Rad53-FHA1 binds a phosphorylated site of Asf1, and the C-terminal peptide of Rad53 binds Asf1N.
Fig. 1.
Rad53 and Asf1 interact through at least two binding sites. (A) Schematic representation of the different constructs of Asf1 and Rad53 produced in E. coli for pull-down assays. (B) GST pull-down assays with GST, GST-Asf1, GST-Asf1N, and different 6His-tagged fragments of Rad53. The asterisk indicated a dimeric form of GST and the double asterisk indicates a GST-Asf1 degradation product. (C) GST-pull-down assays with GST, GST-FHA1, GST-Rad53-C-ter, and yeast extracts expressing Asf1-myc. The asterisk corresponds to a degradation product of GST-C-ter. (D) GST pull-down assay with GST (control) and GST-FHA1 (Pull-down) of yeast extracts expressing Asf1-myc full-length, Asf1N-myc, or Asf1-myc mutated on threonine residues of the C-terminal tail as indicated. The pull-down was performed with extracts from the wild-type or cka1Δ cka2-ts (thermosensitive mutant of CKA2) mutants expressing or not WT CKA2 from a plasmid, and with the addition of calf intestinal phosphatase to cell extracts where indicated (CKA2 + CIP).
The Rad53 FHA1 Domain Binds Asf1 Phosphorylated on T270.
The Rad53 FHA1 domain was previously shown to bind pTxxD phospho-threonine peptides (11, 12). We sought Asf1 phospho-threonines recognized by FHA1. Rad53-FHA1 could pull down full-length Asf1, but not Asf1N from yeast extracts, indicating that the phospho-peptide bound by FHA1 is within the acidic C-terminal tail of Asf1 that contains two pairs of threonines nearby acidic residues (Fig. 1D and Fig. S2A). We created two mutants in which threonines were changed to alanine in a pairwise fashion (T215A + T220A and T265A + T270A) and expressed them in yeast. The Rad53-FHA1 domain could pull down the T215A + T220A mutant, but not the T265A + T270A mutant (Fig. 1D). Interestingly, of these four threonine residues, only T270 is conserved in the Asf1 sequence from different yeast species (Fig. S2A), and was the only site predicted to bind FHA1 by the STRIP program that we developed for the prediction of phospho-binding sites (13). Importantly, phospho-T270 was found on Asf1 that copurified with Rad53 from yeast extracts (14). Finally, a synthetic Asf1 peptide phosphorylated solely on T270 interacted with the FHA1 domain as determined by NMR chemical shift perturbation experiments (Fig. S2 B and C). The FHA1 binding mode was similar to that of other FHA1 partners (see Supporting Information) (11, 12, 15). Isothermal titration calorimetry (ITC) indicated that FHA1 bound the phospho-T270 peptide with a dissociation constant of 5.3 μM (Fig. S2D). The Rad53-FHA1 domain was previously shown to interact with peptides that had been phosphorylated by casein kinase II (15) whose catalytic subunits are encoded by the yeast CKA1 and CKA2 genes (16). We thus examined if this was also the case for Asf1. We found that CK2 was able to phosphorylate in vitro the C-terminal tail of Asf1 (Fig. S2E). We also found that FHA1 could not pull down Asf1 from a thermosensitive yeast cka1Δ cka2-ts mutant (16) (Fig. 1D). Furthermore, yeast Cka1 and Cka2 were found to copurify with Asf1 in a high-throughput screen (17). Altogether, these results suggest that CKA2 phosphorylates at least T270 in Asf1 and that this creates a binding site for the Rad53-FHA1 domain.
The Rad53 C-terminal Peptide (aa 800–821) Binds to Asf1N on the HirA/CAF-I and Histone H4 Binding Surfaces.
We used ITC to define more precisely the minimal Rad53 C-terminal fragment sufficient for Asf1N binding. Peptides from 8–40 residues were synthesized and tested for their binding affinity to Asf1N (Table 1). The fragment containing the last 21 aa of Rad53 (800–821) was the minimum fragment retaining the maximum binding affinity to Asf1N. The dissociation constant was 0.08 (±0.03) μM, which is 2 orders of magnitude lower than for the Rad53 FHA1-phospho-Asf1 interaction (Fig. S3A). We then solved the structure of the complex between Asf1N (1–156) and the Rad53 C-terminal peptide (800–821) by X-ray crystallography at 2.9 Å resolution (see Table S1 for statistics). The peptide contacts Asf1 in two distinct regions defining two binding epitopes (Fig. 2A). The first binding epitope corresponds to Rad53 K804-T811 that lies in an extended conformation on the hydrophobic surface of Asf1 between the fourth and the fifth beta strands (residues D58 to F72). Three Rad53 residues (K804 CO, A806 NH, and D809 NH, CO) establish backbone pairing hydrogen bonds with Asf1 L61 NH, CO and K71 NH, CO, respectively. The side chains of two Rad53 residues, A806 and L808, point toward the Asf1 conserved hydrophobic surface composed of I60, L61, V62, and F72 side chains. In addition, the side chains of two basic residues, K804 and R805 form salt bridges with Asf1-D58 and D37, respectively (Fig. 2B). Interestingly, despite a low sequence identity, this binding epitope overlaps that of the B domain-like peptides of HirA/p60-CAF-I (2, 18) with a similar binding mode (Fig. 2C), suggesting that Rad53 binding to Asf1 is competitive with HirA and CAF-I. Pull-down experiments provided biochemical evidence for this competitive interaction (Fig. S3B). Based on the alignment of HirA, CAF-I, and Rad53 C-terminal peptides, we defined an Asf1 binding motif that is less stringent than previously proposed (2, 18). We call this motif, (R/K)R(I/A/V)x(L/P), the AIP box for Asf1-Interacting Protein box. The motif is centered on one strictly conserved arginine residue, corresponding to R805 in the case of Rad53, preceded by a basic residue and followed by a hydrophobic residue (I/A/V) in position i + 1, and (L/P) in position i + 3 with respect to the conserved arginine (Fig. S3C). Interestingly, the AIP box is present in a subset of proteins that interact with Asf1 in an unknown manner, and may thus potentially bind to the same surface of Asf1 as HirA, p60-CAF-I, and Rad53 (Table S2). These proteins include yeast Kap123, Spt15 and human codanin-1.
Table 1.
Summary of ITC data for Asf1N binding to C-terminal fragments of Rad53
| Peptide | Kd (μM) * | Kd ratio † | ΔH (kCal.M-1) |
| Rad53 800–821 | 0.08(±0.03) | 1 | −10.8(±0.1) |
| Rad53 781–821 | 0.28(±0.05) | 3 | −9.1(±1.2) |
| Rad53 800–811 | 0.42 (±0.06) | 5 | −3.5(±0.1) |
| Rad53 811–821 | undetectable | ND § | ND § |
| Rad53 800–821 A806R-L808R‡ | 7.1 (±0.5) | 89 | −3.1(±0.2) |
| Rad53 800–821 R805A | 11.6 (±1) | 145 | −3.8(±0.9) |
| Rad53 800–821 R805D | undetectable | ND § | ND § |
| Rad53 800–821 F820A | 0.58 (±0.4) | 7 | −2.9(±0.9) |
*binding stoichiometry was found N ≈ 1 (± 0.1) for all peptides
†ratio of the Kd with that of the reference peptide Rad53 800–821
‡ND = not determined.
§also referred in the text as AIP box mutant or rad53-ALRR
Fig. 2.
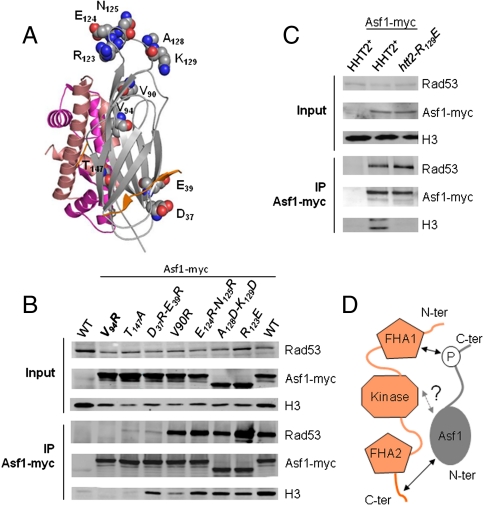
Crystal structure of Asf1N in complex with the C-terminus of Rad53(800-821). (A) Cartoon representation of Asf1 (in gray) bound to Rad53(800-821) in orange. Dashed lines are used to materialize the peptide chain between the two binding epitopes. Important Rad53 residues are labeled. (B) Detail of the interface delineated in (A). In the crystal structure, the peptide bridges epitopes on two different Asf1 molecules, but NMR solution analyses indicate that the peptide normally binds a single Asf1 molecule (see Supporting Information and Fig. S4). Hydrophobic side chains are shown as spheres, polar and charged residues as sticks. Polar contacts are shown as yellow dashed lines. Residue labels are indicated. (C) Cartoon representation of Asf1 (in gray) bound to the HirA B domain (in green) and to the H3 (in rose)-H4 (in magenta) complex. Residues overlapping with Rad53 are indicated.
The second Asf1-binding epitope of the Rad53 C-terminal peptide involves its last three residues (819–821), and in particular F820 that burrows in the hydrophobic cavity formed by the first and last Asf1 beta strands (residues L6, I9, and P144) (Fig. 2B). The position of this aromatic residue overlaps that of histone H4-F100 (Fig. 2C), suggesting that this interaction competes with binding of histone H4 to Asf1.
In the crystal structure, the Rad53(800–821) peptide swaps between two Asf1 molecules (Supporting Information and Fig. S4). Analysis of the dynamic behavior of the complex using NMR spectroscopy showed that bridging of Asf1 dimers by the Rad53 peptide is an artefact of the crystal packing (Supporting Information and Fig. S5). Furthermore, the second epitope is highly dynamic, consistent with its ability to swap with a second Asf1 molecule in the crystal.
We analyzed by ITC the relative importance of residues found in contact with Asf1 in the structure (Table 1). Consistent with the dynamic analysis, deletion of the second binding epitope or mutation of the aromatic F820 residue had a minor impact on the binding affinity, while deletion or mutation of hydrophobic or charged residues inside the first epitope (the AIP box) prevented binding or dramatically decreased the affinity (Table 1). Thus, the AIP box predominates in Asf1-Rad53 binding.
Mutations in the H3, H4, and HirA/CAF-I Binding Surfaces of Asf1N Affect the Stability of the Asf1-Rad53 Complex.
We mutated Asf1 at a series of residues dispersed over the surface of the N-terminal domain (Fig. 3A). Immunoprecipitation of wild-type and mutant Asf1 from yeast cell extracts showed that specific residues located in the histone H3, histone H4, and HirA/CAF-I binding surfaces of Asf1 were important for its binding to Rad53, whereas other sites had no effect (Fig. 3B). The D37R + E39R and the T147A mutants are located in the HirA/CAF-I (2, 18) and histone H4 (19, 20) binding surfaces of Asf1, respectively, and both mutants showed reduced affinity for Rad53 as predicted by the structure of the Rad53 C-terminal peptide bound to Asf1. Interestingly, the V94R mutant that blocks binding to histone H3 (21) is also defective in binding to Rad53. This residue is too far from the Rad53 C-terminal peptide to be able to affect its binding directly. Furthermore, NMR experiments showed that the V94R mutation has little effect on the overall Asf1N tertiary structure (21), so it is unlikely that this mutation affects Rad53 binding through indirect conformational changes. We considered two explanations for these results. One possibility was that histone H3 bound to Asf1 contributes to the binding of Rad53. To test this possibility, we immunoprecipitated Asf1 from yeast cell extracts containing the histone H3-R129E mutant in place of the wild type. We previously showed that this mutant is unable to bind Asf1 (22, 23). We found that Rad53 was still immunoprecipitated with Asf1 in these extracts in which H3-R129E was not associated with Asf1 (Fig. 3C). This result suggests that Rad53 binding to Asf1 does not require bridging by histone H3. The second possibility is that Asf1 may directly interact with Rad53 through a third binding site overlapping the histone H3 binding surface of Asf1N. Schwartz et al. showed that a Rad53-K227A + D339A kinase-dead mutant coimmunoprecipitated weakly with Asf1 in yeast extracts compared to wild-type Rad53 (10). We confirmed this result and showed that the Rad53-K227A single mutant was also affected in its interaction with Asf1 (Fig. S6A). These observations suggest that the Rad53 kinase domain might be able to interact with Asf1, although we did not observe an obvious interaction of the Rad53 kinase domain with GST-Asf1 or GST-Asf1N when all proteins were purified from E. coli (Fig. 1B). Alternatively, Rad53 kinase activity may be indirectly required for this putative third interaction site. Our current working model is that Asf1 and Rad53 interact at three distinct surfaces to form a complex that precludes binding of histones and histone cochaperones to Asf1 (Fig. 3D).
Fig. 3.
Putative third interaction surface involving the H3-binding surface of Asf1 and the kinase domain of Rad53. (A) Cartoon representation of Asf1 (in gray) bound to the Rad53-K804-S812 peptide (in orange) and to the H3 (in rose)-H4 (in magenta) complex. Residues that were mutated to test their effect on Rad53 binding are shown with spherical atoms for N (blue), O (red), and C (gray). (B) Coimmunoprecipitation of Rad53 and histone H3 with the indicated mutants of Asf1-myc. Input indicates proteins in total cell extracts. IP indicates proteins coprecipitating with Asf1-myc on anti-myc beads. Asf1 is tagged with a 13-myc epitope, except for the A128D-K129D and R123E mutants that have fewer repetitions of the myc epitope. (C) Rad53 coprecipitates with Asf1 in extracts from a histone H3-R129E (hht2-R129E) mutant that cannot bind Asf1. (D) Schema of tripartite model for the Asf1-Rad53 interaction. The question mark indicates that the putative interface between Asf1N and the kinase domain of Rad53 awaits further experimental verification.
Phosphorylation of C-terminal Rad53 Serine and Threonine Residues Cannot Explain Dissociation of the Rad53-Asf1 Complex Upon Treatment of Yeast Cells with Hydroxyurea.
Some previous work suggested that Rad53 and Asf1 are found in a complex in yeast cells in the absence of genotoxic stress, but the complex was dissociated upon treatment of cells with hydroxyurea (HU) or methyl-methane-sulfonate (MMS) (4, 5). We confirmed that Rad53 was no longer immunoprecipitated with Asf1 when cells were treated with HU (Fig. 4A, Left). However, we found that phosphorylated forms of Rad53 still coimmunoprecipitated with Asf1 when cells were treated with MMS (Fig. 4A, Middle), although the most highly phosphorylated species were not coimmunoprecipitated. These results are consistent with a mass spectroscopy study showing that Asf1 remained associated with Rad53 after treating cells with MMS (14). It is possible that earlier work showing a dissociation of the complex after MMS treatment of cells used immunoprecipitation conditions that were overly stringent. Our results suggest that the complex is differentially regulated in response to HU or MMS treatment, presumably to allow a tailored cellular response to these distinct genotoxic stresses.
Fig. 4.
Dissociation of the Asf1-Rad53 complex when cells are treated with HU but not MMS, and destabilization of the complex by the rad53-ALRR mutation and its phenotypic consequences. (A) Asf1-myc was immunoprecipitated from extracts of control cells and cells treated with 200 mM HU for 2 h at 30 °C (Left) or with increasing MMS (0.05%, 0.1%, 0.15%, 0.2% final concentration) for 1 h at 30 °C (Middle). Right: Wild-type Rad53 and the Rad53-TSSDEE (T811D + S812E + S821E) mutant coprecipitate with the Asf1 - T265A + T270A-myc nonphosphorylatable mutant that is defective in binding Rad53-FHA1. (B) Rad53-ALRR (A806R + L806R) does not coimmunoprecipitate with Asf1-myc in conditions allowing efficient coimmunoprecipitation of wild-type Rad53. (C) rad53-ALRR increases the growth of rad9 and rad24 mutants in the presence of MMS or HU. (D) Only minor differences in Rad53 phosphorylation in W303 wild-type, rad9 rad24, rad53-ALRR, and rad53-ALRR rad9 rad24 yeast strains during normal growth or after treatment with 200 mM HU for 2 h at 30 °C or with 0.05% MMS for 1 h at 30 °C.
Rad53 is phosphorylated at more than 20 serine or threonine residues, some of which are phosphorylated differentially depending on the type of genotoxic stress (9, 14). Dissociation of the Rad53-Asf1 complex may be induced by phosphorylation of Rad53 on specific sites after treatment of cells with HU. We noticed that there are three serine/threonine residues in the Rad53 C-terminal peptide (T811, S812, and S821) that binds Asf1N. Relative to the Asf1N binding epitopes in the Rad53 C-terminal sequence (Fig. 2A), T811 and S812 are located at the very end of the first binding epitope, and S821 is in the second binding epitope. We mutated each of these serines/threonine to acidic residues to produce a phospho-mimetic mutant (Rad53-T811D + S812D + S821E, abbreviated as Rad53-TSSDEE). If phosphorylation of one or more of these residues is sufficient to induce dissociation of the complex in response to HU, we would expect that the phospho-mimetic Rad53-TSSDEE mutant would not be associated with Asf1 even in the absence of genotoxic stress. However, we found that Rad53-TSSDEE coprecipitated with Asf1 in the absence of genotoxic stress, even in the context of the nonphosphorylatable Asf1 - T265A + 270A mutant that is defective in binding Rad53-FHA1 (Fig. 4A, Right and Fig. S6B). Thus, phosphorylation of C-terminal Rad53 T811, S812, or S821 residues may potentially contribute to destabilizing the complex, but is not sufficient to explain dissociation of the complex in response to HU treatment.
The AIP Box Mutant rad53-ALRR Destabilizes the Asf1-Rad53 Complex and Increases the Resistance of rad9 and rad24 Mutants to Genotoxic Stress.
Rad53 A806 and L808 contribute to a hydrophobic surface that is important in the binding affinity of the Rad53-C-terminal peptide with Asf1N (Fig. 2B). Rad53 A806 and L808 also constitute the two hydrophobic residues of the AIP box. Mutation of these residues to arginine greatly decreased the affinity of the Rad53 C-terminal peptide for Asf1N (Table 1). Consistently, the yeast Rad53-A808R + L808R (abbreviated Rad53-ALRR) mutant did not coimmunoprecipitate with Asf1 from yeast extracts (Fig. 4B). These observations are consistent with a destabilization of the Asf1-Rad53 complex in the rad53-ALRR mutant. In contrast, Asf1-T265A + T270A was still bound to Rad53 despite its defective interaction with Rad53-FHA1 (Fig. 4A, Right). Thus, the interaction of the Rad53-C-terminal peptide with Asf1 makes a more important contribution to the stability of the complex than does the FHA1 interaction, consistent with the ITC data. We tested the rad53-ALRR and asf1-T265A + T270A mutants as well as the combined triple mutant for sensitivity to HU and MMS but found no obvious differences with the wild type (Fig. 4C and Fig. S6C). The rad53-K227A kinase-dead mutant is sensitive to histone gene overexpression (8, 24). Interestingly, we found that the rad53-ALRR mutant is slightly sensitive to HHT1 (histone H3) overexpression, although not to the same extent as rad53-K227A (Fig. S6D). Rad53 is activated in response to genotoxic stress by two parallel pathways. One pathway is mediated by Mrc1 in response to blocked replication forks (25), and the other depends on Rad9, the Rad24 clamp loader complex, and a PCNA-like clamp complex (26). Strikingly, we found that Rad53-ALRR increased the resistance of rad9, rad24, and rad9 rad24 double mutants to MMS (Fig. 4C). This effect was not observed for the asf1-T265A + T270A mutant (Fig. S6C). Rad53-ALRR also increased the resistance of the rad9 rad24 double mutant to HU. In contrast, it did not modify the resistance of the mrc1 mutant to HU or to MMS.
We compared activating phosphorylation of Rad53 in response to HU and MMS in the wild type, the rad53-ALRR mutant, the rad9 rad24 double mutant, and the rad53-ALRR rad9 rad24 triple mutant. We found no dramatic differences in Rad53 levels or phosphorylation in any of these contexts, but a modest decrease in Rad53 phosphorylation was observed in the rad9 rad24 double mutant, the rad53-ALRR mutant, and the rad53-ALRR rad9 rad24 triple mutant compared to the wild type in response to HU and MMS (Fig. 4D). This slight difference in phosphorylation was not correlated with sensitivity of these strains to HU or MMS exposure. Since defects in Rad53 dephosphorylation have also been implicated in sensitivity to genotoxic stress, we compared Rad53 dephosphorylation in wild type and rad53-ALRR mutant cells after a transient exposure to MMS, but found no obvious difference between the two strains (Fig. S6E). Thus, the ability of the Rad53-ALRR mutant to increase the resistance of rad9 and rad24 mutants to genotoxic stress does not appear to be due to obvious differences in Rad53 activation or inactivation.
Discussion
We discovered a remarkable complexity to the Asf1-Rad53 interaction that appears to involve three distinct interaction surfaces. First, we show that the Rad53-FHA1 domain binds Asf1 phosphorylated at T270 in its C-terminal acidic tail domain in a casein kinase II-dependent manner. Rad53-FHA1 binds multiple phospho-proteins (13), so there is likely to be dynamic competitive interactions that contribute to the functions of Rad53 in DNA replication and repair. The affinity of the pT270 Asf1 phospho-peptide is modest (5 μM) compared to other known FHA1 partners like Ptc2 and Cdc45 (approximately 0.5 μM) (13). Mutation of Asf1-T265 and T270 to alanine prevented binding of Asf1 to Rad53-FHA1, but this mutant had no apparent phenotype and still coimmunoprecipitated with Rad53. We defined a second interaction surface comprised of the C-terminal 21 aa of Rad53 that binds the same surfaces of the conserved Asf1 N-terminal domain as do the histone cochaperones HirA/CAF-I and histone H4. Indeed, the Rad53 C-terminal peptide has a strikingly similar binding mode to the HirA and p60-CAF-I B-domain peptides (2, 18), and it is clear that the three proteins must compete for binding to the same surface of Asf1N. Considering the relative affinity of the corresponding peptides for Asf1, the Saccharomyces cerevisiae Rad53 C-terminal peptide presents the highest affinity (approximately 100 nM, Table 1) compared to the FHA1 binding site and also compared to that of human and Schizosaccharomyces pombe HirA/p60-CAF-I B-domain peptides for Asf1 (approximately 2 μM) (2, 18). Based on the structure of Rad53, HirA, and p60-CAF-I B-domain peptides in complex with Asf1, we derived a new minimal sequence motif (R/K)R(I/A/V)x(L/P) that we call the AIP box (Asf1-Interacting Protein box), for peptides potentially able to bind this same surface of Asf1 (Fig. S3C). In the three founding members of the AIP box family, namely Rad53, HirA, and p60-CAF-I, mutation of the central arginine residue or the two hydrophobic residues in positions i + 1 and i + 3 abolishes the binding of the peptide to Asf1 (Table 1) (2, 18). This small degenerate binding motif could be compared to the well-characterized PCNA PIP box that is found in many proteins associated with the replication fork with binding affinities to PNCA in the same range as the AIP box to Asf1 (100 nM to 50 μM) (27). The AIP box is too degenerate to identify novel Asf1 binding partners by searching in the large database of nonredundant protein sequences. However, we found this motif in a subset of proteins that bind Asf1 in an unknown manner (Table S2). It is now possible to mutate these potential AIP boxes to assess their functional importance. Interestingly, the Rad53 AIP box is conserved only in yeast species, consistent with the fact that the human Chk2 ortholog was not found in a complex with Asf1 (6).
Phe-820 of Rad53 also competes with Phe-100 of histone H4 for binding to a distinct surface of Asf1N. It is unlikely that the Rad53 C-terminal peptide is able to compete with binding of the heterodimeric H3-H4 to Asf1N that occupies a much greater surface than that of the F820 binding pocket. However, the Asf1-V94R mutant is defective in both histone H3 and Rad53 binding. Since Asf1-V94 is distal to the surface bound by the Rad53 C-terminus, an additional region of Rad53 likely interacts with the H3 binding epitope on Asf1N. Similarly, it was suggested that in addition to its AIP box, other regions of HirA must contribute to its interaction with Asf1 and impart specificity for binding to the mammalian Asf1a isoform (2).
Rad53 and Asf1 are found in a complex in yeast cells in the absence of genotoxic stress. Our data indicate that Rad53 competes with histones H3-H4 and the cochaperones HirA and CAF-I for binding to Asf1. We found that the Asf1-Rad53 complex was dissociated when cells were treated with hydroxyurea, a ribonucleotide reductase inhibitor, but not when cells were treated with the methylating agent MMS. Rad53 is activated by phosphorylation at multiple sites in response to genotoxic stress. Some sites, such as T-loop phosphorylation, are probably necessary for Rad53 activation in all situations. However, other phosphorylations are specific to cells treated with the UV-mimetic 4-nitro-quinoline 1-oxide or with MMS (9, 14). Some phosphorylation sites specific to HU treatment may explain the dissociation of the Asf1-Rad53 complex in this condition relative to MMS. A phospho-mimetic mutant at putative phosphorylation sites in the C-terminus of Rad53 did not lead to dissociation of Asf1. Thus, we suggest that phosphorylation within the putative third interaction surface in the Asf1-Rad53 complex is required for dissociation of the complex in presence of HU. Dissociation of the Asf1-Rad53 complex would increase the pool of Asf1 competent for binding histones and other partners, and could also modify the ability of Rad53 to phosphorylate specific substrates. The identification of Rad53 phosphorylation sites that are specific to HU-treated cells would allow further testing of this model.
Our structure of Asf1N in association with the Rad53 C-terminal peptide allowed us to identify residues important for the stability of the complex. The rad53-ALRR AIP box mutations disrupt an important hydrophobic contact and destabilize the Asf1-Rad53 complex in yeast cells. The rad53-ALRR mutant did not have an obvious phenotype with regard to genotoxic stress, however, it did show some sensitivity to overexpression of histone H3 for reasons yet to be determined. Rad53-ALRR also increased the resistance to genotoxic stress of rad9 and rad24 mutants. The Asf-1-T265A+270A mutant does not detectably destabilize the Asf1-Rad53 complex and did not increase the resistance of rad9 and rad24 to genotoxic stress, so we suggest that decreased stability of the Asf1-Rad53 complex is required for this effect. Rad9 and Rad24 are implicated in activation of Rad53 in response to DNA double-strand breaks (26). In asynchronously growing cells, we observed only modest decreases in Rad53 phosphorylation in the rad9 rad24 mutant treated with HU or MMS compared to wild-type cells, presumably because Rad53 is still efficiently activated by Mrc1 at stalled replication forks in these cells (25). The rad53-ALRR mutation did not significantly modify the profile of Rad53 phosphorylation, so the increased viability of rad53-ALRR rad9 rad24 mutants exposed to MMS or HU may not be through effects on Rad53 activity. We favor the hypothesis that the decreased stability of the Rad53-Asf1 complex in the rad53-ALRR mutant increases cell viability through increased tolerance or repair of lesions (including reconstitution of chromatin structure) provoked by HU or MMS in the rad9 rad24 mutants. This could presumably occur by the increased availability of Rad53 or Asf1 to interact with its multiple alternative partners at the levels of DNA metabolism and chromatin.
Materials and Methods
Production and Purification of Recombinant Proteins for Biophysical and Structural Studies.
Production and purification of recombinant proteins were performed as described in (21). Briefly, recombinant soluble (His)6-tagged GST fusion proteins were purified on GSH agarose (Sigma) and cleaved using a (His)6-tagged TEV protease. A Ni-NTA agarose column (Qiagen) was used to trap the (His)6-tagged TEV protease and the (His)6-tagged GST.
Crystallization, Data Collection, Structure Determination, and Refinement.
Crystals of the complex were grown by sitting drop vapour diffusion at 20 °C against reservoir solution containing 35% PEG 4000, 0.1M Na Acetate-pH 4.6, 0.2 M NH4 sulphate. Diffraction data were collected on the Proxima1 beamline at the SOLEIL synchrotron (Gif-sur-Yvette, France). All data were processed and integrated using XDS (Table S1). Structure resolution was carried out using molecular replacement using the structure of yeast Asf1 as search model (PDB ID code 1ROC). The structure was refined and the peptide model was built using the software Buster and visualized with the software Coot.
NMR Experiments
NMR experiments were carried out on Bruker DRX-600 MHz and 700 MHz spectrometers equipped with cryoprobes. All NMR data were processed using Xwinnmr (Bruker) and analyzed using Sparky (T. D. Goddard and D. G. Kneller, University of California, San Francisco). NMR samples of the complex between Asf1 (1–156) and Rad53 (800–821) were prepared in the following buffer: Tris D11 10 mM, pH 7.4, NaN3 0.1%, EDTA 1 mM, DSS 0.1 mM. D2O 10% or 100%. The buffer phosphate 10 mM, pH 6.5, DTT 1 mM, NaN3 0.1%, EDTA 1 mM, DSS 0.1 mM. D2O 10% was used for the complex between Rad53 FHA1 (1–164) and Asf1 (266–277).
Phenotypic Analysis.
For spotting analyses, cells were resuspended at 107/mL, subjected to 10-fold serial dilutions, and 3 μL of each dilution was spotted on plates of YPD, YPD + 100 mM HU, and YPD + 0.0025% MMS. Growth was assayed at 72 h.
More detailed information on Materials and Methods including isothermal titration calorimetry (ITC) experiments, GST pull-down assays, phenotypic analysis, yeast strains, construction of mutants and analysis of Rad53 phosphorylation and dephosphorylation is provided in the Supporting Information.
Supplementary Material
ACKNOWLEDGMENTS.
We thank J.D. Diffley for the generous gift of anti-Rad53 serum, and O.M. Aparicio, P.D. Kaufman, R.A. Sclafani, K. Sugimoto for plasmids, and S.J. Elledge, C.V. Glover, M.P. Longhese, and N.F. Lowndes for yeast strains. We thank the Imagif platform, B. Murciano, M.H. LeDu, J.B. Charbonnier, and P. Legrand for their help in crystallogenesis and crystallography. We thank the SOLEIL synchrotron (PROXIMA1 beamline) and European Synchrotron Radiation Facility (beamlines ID29 and ID23) for beam time. We thank the Très Grandes Infrastructures de Recherche for providing NMR time at 800 MHz and we thank C. van Heijenoort for her help in setting up NMR filtered experiments. We thank O. Rechiche and S. Miron for their help in calorimetry. We are grateful to J-Y. Thuret and A. Lengronne for comments on this manuscript. Y.J. was supported by the Commissariat à l’Energie Atomique (CEA) Irtelis doctoral program. K. S. was supported by the CEA. This work was supported by grants from the French National Research Agency ANR-07-BLAN-0098-01 to C.M and ANR-07-JCJC-0126 to F.O. and the French Association for Research on Cancer ARC 4916 to C.M. and ARC 3828, 8006 to F.O.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1106023109/-/DCSupplemental.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank in Europe (PDBe), www.ebi.ac.uk/pdbe (PDB ID code 2YGV).
References
- 1.Mousson F, Ochsenbein F, Mann C. The histone chaperone Asf1 at the crossroads of chromatin and DNA checkpoint pathways. Chromosoma. 2007;116:79–93. doi: 10.1007/s00412-006-0087-z. [DOI] [PubMed] [Google Scholar]
- 2.Tang Y, et al. Structure of a human ASF1a-HIRA complex and insights into specificity of histone chaperone complex assembly. Nat Struct Mol Biol. 2006;13:921–929. doi: 10.1038/nsmb1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akai Y, et al. Structure of the histone chaperone CIA/ASF1-double bromodomain complex linking histone modifications and site-specific histone eviction. Proc Natl Acad Sci USA. 2010;107:8153–8158. doi: 10.1073/pnas.0912509107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emili A, Schieltz DM, Yates JR, 3rd, Hartwell LH. Dynamic interaction of DNA damage checkpoint protein Rad53 with chromatin assembly factor Asf1. Mol Cell. 2001;7:13–20. doi: 10.1016/s1097-2765(01)00150-2. [DOI] [PubMed] [Google Scholar]
- 5.Hu F, Alcasabas AA, Elledge SJ. Asf1 links Rad53 to control of chromatin assembly. Genes Dev. 2001;15:1061–1066. doi: 10.1101/gad.873201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groth A, et al. Human Asf1 regulates the flow of S phase histones during replicational stress. Mol Cell. 2005;17:301–311. doi: 10.1016/j.molcel.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 7.Sillje HH, Nigg EA. Identification of human Asf1 chromatin assembly factors as substrates of Tousled-like kinases. Curr Biol. 2001;11:1068–1073. doi: 10.1016/s0960-9822(01)00298-6. [DOI] [PubMed] [Google Scholar]
- 8.Sharp JA, Rizki G, Kaufman PD. Regulation of histone deposition proteins Asf1/Hir1 by multiple DNA damage checkpoint kinases in S. cerevisiae. Genetics. 2005;171:885–899. doi: 10.1534/genetics.105.044719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sweeney FD, et al. Saccharomyces cerevisiae Rad9 acts as a Mec1 adaptor to allow Rad53 activation. Curr Biol. 2005;15:1364–1375. doi: 10.1016/j.cub.2005.06.063. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz MF, Lee SJ, Duong JK, Eminaga S, Stern DF. FHA domain-mediated DNA checkpoint regulation of Rad53. Cell Cycle. 2003;2:384–396. [PubMed] [Google Scholar]
- 11.Liao H, et al. Structure of the FHA1 domain of yeast Rad53 and identification of binding sites for both FHA1 and its target protein Rad9. J Mol Biol. 2000;304:941–951. doi: 10.1006/jmbi.2000.4291. [DOI] [PubMed] [Google Scholar]
- 12.Durocher D, et al. The molecular basis of FHA domain:phosphopeptide binding specificity and implications for phospho-dependent signaling mechanisms. Mol Cell. 2000;6:1169–1182. doi: 10.1016/s1097-2765(00)00114-3. [DOI] [PubMed] [Google Scholar]
- 13.Aucher W, et al. A strategy for interaction site prediction between phospho-binding modules and their partners identified from proteomic data. Mol Cell Proteomics. 2010;9:2745–2759. doi: 10.1074/mcp.M110.003319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smolka MB, et al. Dynamic changes in protein-protein interaction and protein phosphorylation probed with amine-reactive isotope tag. Mol Cell Proteomics. 2005;4:1358–1369. doi: 10.1074/mcp.M500115-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guillemain G, et al. Mechanisms of checkpoint kinase Rad53 inactivation after a double-strand break in Saccharomyces cerevisiae. Mol Cell Biol. 2007;27:3378–3389. doi: 10.1128/MCB.00863-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang R, Chen W, Adams PD. Molecular dissection of formation of senescence-associated heterochromatin foci. Mol Cell Biol. 2007;27:2343–2358. doi: 10.1128/MCB.02019-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krogan NJ, et al. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature. 2006;440:637–643. doi: 10.1038/nature04670. [DOI] [PubMed] [Google Scholar]
- 18.Malay AD, Umehara T, Matsubara-Malay K, Padmanabhan B, Yokoyama S. Crystal structures of fission yeast histone chaperone Asf1 complexed with the Hip1 B-domain or the Cac2 C terminus. J Biol Chem. 2008;283:14022–14031. doi: 10.1074/jbc.M800594200. [DOI] [PubMed] [Google Scholar]
- 19.Natsume R, et al. Structure and function of the histone chaperone CIA/ASF1 complexed with histones H3 and H4. Nature. 2007;446:338–341. doi: 10.1038/nature05613. [DOI] [PubMed] [Google Scholar]
- 20.English CM, Adkins MW, Carson JJ, Churchill ME, Tyler JK. Structural basis for the histone chaperone activity of Asf1. Cell. 2006;127:495–508. doi: 10.1016/j.cell.2006.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mousson F, et al. Structural basis for the interaction of Asf1 with histone H3 and its functional implications. Proc Natl Acad Sci USA. 2005;102:5975–5980. doi: 10.1073/pnas.0500149102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agez M, et al. Structure of the histone chaperone ASF1 bound to the histone H3 C-terminal helix and functional insights. Structure. 2007;15:191–199. doi: 10.1016/j.str.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Galvani A, et al. In vivo study of the nucleosome assembly functions of ASF1 histone chaperones in human cells. Mol Cell Biol. 2008;28:3672–3685. doi: 10.1128/MCB.00510-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunjan A, Verreault A. A Rad53 kinase-dependent surveillance mechanism that regulates histone protein levels in S. cerevisiae. Cell. 2003;115:537–549. doi: 10.1016/s0092-8674(03)00896-1. [DOI] [PubMed] [Google Scholar]
- 25.Alcasabas AA, et al. Mrc1 transduces signals of DNA replication stress to activate Rad53. Nat Cell Biol. 2001;3:958–965. doi: 10.1038/ncb1101-958. [DOI] [PubMed] [Google Scholar]
- 26.Navadgi-Patil VM, Burgers PM. A tale of two tails: Activation of DNA damage checkpoint kinase Mec1/ATR by the 9-1-1 clamp and by Dpb11/TopBP1. DNA Repair (Amst) 2009;8:996–1003. doi: 10.1016/j.dnarep.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruning JB, Shamoo Y. Structural and thermodynamic analysis of human PCNA with peptides derived from DNA polymerase-delta p66 subunit and flap endonuclease-1. Structure. 2004;12:2209–2219. doi: 10.1016/j.str.2004.09.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.