Abstract
Multiple factors including long-term treatment with tamoxifen are involved in the development of selective estrogen receptor (ER) modulator resistance in ERα-positive breast cancer. Many underlying molecular events that confer resistance are known but a unifying theme is yet to be revealed. In this report, we provide evidence that HOXB7 overexpression renders MCF-7 cells resistant to tamoxifen via cross-talk between receptor tyrosine kinases and ERα signaling. HOXB7 is an ERα-responsive gene. Extended treatment of MCF-7 cells with tamoxifen resulted in progressively increasing levels of HOXB7 expression, along with EGFR and EGFR ligands. Up-regulation of EGFR occurs through direct binding of HOXB7 to the EGFR promoter, enhancing transcriptional activity. Finally, higher expression levels of HOXB7 in the tumor significantly correlated with poorer disease-free survival in ERα-positive patients with breast cancer on adjuvant tamoxifen monotherapy. These studies suggest that HOXB7 acts as a key regulator, orchestrating a major group of target molecules in the oncogenic hierarchy. Functional antagonism of HOXB7 could circumvent tamoxifen resistance.
In postmenopausal women with early-stage estrogen receptor-α (ERα)-positive breast cancer, the selective estrogen-receptor modulator (SERM), tamoxifen (TAM) represents a major adjuvant treatment in clinical practice. Many of the breast tumors that initially respond to the tamoxifen therapy eventually develop resistance and recur (1). Among the patients with breast cancer with acquired resistance, only 20% of patients who progress on tamoxifen respond to the selective ER down-regulator, fulvestrant, or to aromatase inhibitors even if ERα expression is maintained and regulates tumor proliferation (2–4). Thus, far, studies of tamoxifen resistance have revealed that increased levels of ErbB/HER family members can directly alter the cellular response to tamoxifen.
Homeobox genes are regulatory genes encoding nuclear proteins that act as transcription factors during normal development and differentiation (5). One of these, HOXB7, is involved in a variety of developmental processes, including hematopoietic differentiation and lymphoid and mammary gland development. The role of HOX genes in breast cancer development is largely unexplored. Recently, we identified HOXB7 through microarray analysis as one of the genes whose expression was significantly elevated in both the primary cancer and distant metastasis (6). HOXB7 overexpression promoted cell proliferation in SKBR3 breast cancer cells that acquired the ability to grow as robustly vascularized xenografts in immunodeficient mice (7). In culture, HOXB7 transformed mammary epithelial cells, MCF10A, and promoted epithelial/mesenchymal transition and invasion in a variety of cell lines through activation of the RHO/RAC pathway (6).
Here, we present evidence that HOXB7 overexpression in ER+ breast cancer cells confers TAM resistance through increased expression and signaling of EGFR. By studying ER+, TAM-resistant cells, and MCF-7 cells over time as they acquired TAM resistance, we show that elevation of HOXB7 expression might be one of the key steps in the acquisition and maintenance of SERM resistance in breast cancer. Therefore, HOXB7 could serve as a critical regulator in the transition of breast cancer cells to estrogen independence, TAM resistance, and acquisition of an aggressive phenotype. Further, HOXB7 might be an attractive target for breast cancer therapeutics.
Results
HOXB7 Expression Promotes Breast Tumorigenesis.
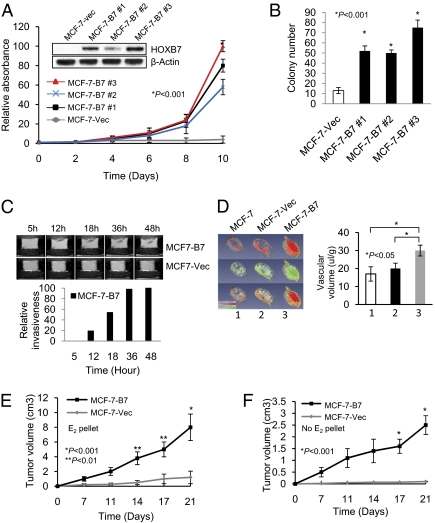
Breast cancer cells, MCF-7, are estrogen dependent for growth in vitro and in vivo and are susceptible to the cytostatic/cytotoxic effects of TAM. Stable expression of a HOXB7 expression vector in MCF-7 cells (three clones: MCF-7-B7 1, 2, and 3) enabled the cells to proliferate much faster than the vector control cells (MCF-7-Vec) in monolayer cultures and significantly enhanced colony formation (Fig. 1 A and B). Magnetic resonance imaging (MRI) analysis of invasion of cells through the extracellular matrix revealed that MCF-7-B7 cells, but not MCF-7 cells were highly invasive in vitro (8) and were significantly hypervascular in vivo (Fig. 1 C and D) (9) without any significant change in permeability. Consistent with these observations, transplanted to the athymic nude mice s.c. in the presence of exogenous estrogen supplementation, MCF-7-B7 cells formed faster growing and larger tumors compared with the MCF-7-Vec cells (Fig. 1E). Tumors formed by MCF-7-Vec cells were grossly well defined and loosely attached to surrounding tissue whereas MCF-7-B7 cells grew as highly invasive tumors firmly attached to surrounding tissues, infiltrating the underlying skeletal muscle and fat tissue (Fig. S1B). Thus, HOXB7 overexpression promoted invasive and aggressive growth of MCF-7-B7 cells.
Fig. 1.
Effect of HOXB7 expression in breast cancer cells. (A and B) (A) Immunoblot analysis of HOXB7 expression in MCF-7-Vec and MCF-7-B7 cells (three clones: MCF-7-B7 1, 2, and 3) and growth curve of MCF-7-Vec and MCF-7-B7 cells grown in monolayer culture and (B) soft agar colony formation by MCF-7-Vec and MCF-7-B7 cells. (C) T1-weighted 1H MR imaging of MCF-7-B7 cells, and MCF-7-Vec cells visualizing the degradation of ECM over a period as indicated. (D) Representative 3D reconstructed images of vascular volume maps (row 1), permeability-surface area product (row 2), and combined vascular volume and permeability-surface area product (row 3) for MCF-7 parental (column 1), MCF-7-Vec (column 2), and MCF-7-B7 (column 3) tumors in mice. (E) Tumor growth curves of MCF-7-Vec and MCF-7-B7 cells implanted s.c. in athymic mice in the presence of an exogenous slow release, estrogen implant and (F) in the absence of exogenous estrogen supplementation.
One of the hallmarks of cancer is self-sufficiency in growth signals (10). A reduced need for estrogen by ER+ cells is often linked to their resistance to TAM treatment (11). HOXB7 overexpression in MCF-7 cells resulted in a much reduced dependence on nutrients. Although MCF10A-B7 cells proliferated in low growth factor-supplemented medium and MCF-7-B7 cells grew in estrogen-deprived medium, vector-transfected cells barely survived (Fig. S1 C and E). In vivo, even in the absence of exogenous estrogen supplementation, MCF-7-B7 cells formed rapidly growing tumors in athymic nude mice (Fig. 1F), whereas MCF-7-Vec cells did not form palpable tumors. Thus, HOXB7 overexpression enabled MCF-7 cells to largely circumvent the need for exogenous estrogen for growth.
HOXB7 in the Acquisition of Anti-Estrogen Resistance.
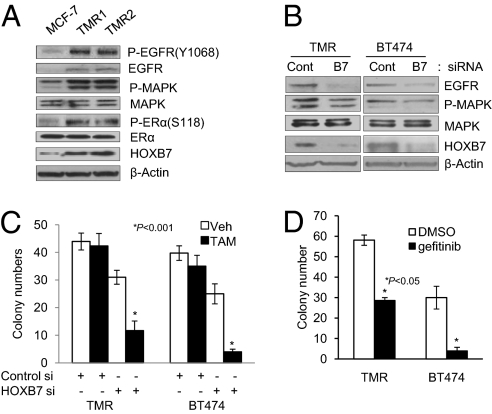
Prolonged endocrine therapy is often associated with an increase in expression of receptor tyrosine kinases such as EGFR/HER2, which, together with activation of ER-dependent gene transcription and aberrant growth/apoptosis, leads to endocrine resistance (12). In addition, epithelial–mesenchymal transition (EMT) has been observed in tamoxifen-resistant MCF-7 cells (13), similar to our report of EMT in MCF10A-HOXB7 cells (6). Because of the striking similarity in phenotype between prolonged endocrine therapy-primed TAM-resistant models and our HOXB7-overexpressing cells, we explored the hypothesis that HOXB7 is a mediator of anti-estrogen resistance. We therefore derived MCF-7 cell lines exposed to either vehicle or TAM (0.1 μM or 1 μM) for >12 mo (designated MCF-7-TMR1 or MCF-7-TMR2, respectively). MCF-7-TMR1 cells exhibited significant resistance to TAM treatment as determined by colony formation assay (Fig. S2D). Long-term TAM treatment caused elevated expression of EGFR and activated MAPK and ERα, but no significant change of total ERα. Interestingly, we found that HOXB7 was up-regulated in MCF-7-TMR cells (Fig. 2A and Fig. S2 A and B). In addition, over time (0, 2, 4, and 6 mo) MCF-7 cells treated with 0.1 μM TAM showed progressively increasing levels of HOXB7 expression, accompanied by concomitant increase in expression of EGFR (Fig. S2A).
Fig. 2.
HOXB7 promotes acquired TAM resistance. (A) Immunoblot analysis of Phospho-EGFR (Y1068), EGFR, Phospho-MAPK, MAPK, Phospho-ERα (S118), ERα, and HOXB7 expression in MCF-7 cells treated long term with vehicle or 0.1 μM TAM (TMR1) or 1 μM TAM (TMR2). (B) Immunoblot analysis of EGFR, Phospho-MAPK, MAPK, and HOXB7 expression in MCF-7-TMR cells and BT474 transfected with either scrambled sequence siRNA or HOXB7-specific siRNA. (C) Soft agar colony formation in BT474 cells and MCF-7-TMR cells transfected with either scrambled sequence siRNA or HOXB7-specific siRNA treated with vehicle or 1 μM TAM. (D) Soft agar colony formation by BT474 cells and MCF-7-TMR cells treated with 1 μM gefitinib and 1 μM TAM.
To investigate whether HOXB7 overexpression is a key event in TAM resistance through EGFR expression, we depleted HOXB7 expression with HOXB7 siRNA in MCF-7-TMR cells. Remarkably, abrogation of HOXB7 expression by siRNA reversed each of the observed molecular events in the MCF-7-TMR cells and direct evidence of a role for HOXB7 was sought in an unmanipulated, TAM-resistant breast cancer cell line, BT474 (Fig. 2B). Here, reduction of endogenous HOXB7 expression using HOXB7 siRNAs was sufficient to reduce the expression levels of EGFR and P-MAPK with regained sensitivity to TAM (Fig. 2C). Whether HOXB7 overexpression is a molecular feature shared by other anti-estrogen resistance models is an interesting question. Factors contributing to SERM resistance have been previously studied using at least two well-known model systems: first, an in vitro, long-term estrogen deprivation (LTED) model, MCF-7-LTED (14), and second, an in vivo, long-term TAM-treated xenograft model, MCF-7-TAMLT (15). We therefore examined expression of HOXB7 and EGFR in cell lysates of these two models along with our MCF-7-TMR model. In line with our previous observations, HOXB7 and EGFR expression was elevated in both models (Fig. S2C). Abrogation of the EGFR-dependent pathway is apparently critical for HOXB7 siRNA's elicited effect, because an EGFR-specific inhibitor, gefitinib, dramatically converted TAM to a potent antagonist in MCF-7-TMR and BT474 cells (Fig. 2D). Thus, HOXB7 might be an important drug target, whose functional antagonism impinges on the EGFR pathway important to TAM resistance.
HOXB7 Promotes Tamoxifen Resistance.
Receptor tyrosine kinases (RTKs) are major mediators of the signaling network that transmits extracellular signals into cells and controls cellular differentiation and proliferation (16). The ErbB/HER receptors including HER1/EGFR, HER2/Neu, HER3, HER4, and their cognate ligands are involved in the pathogenesis of different types of carcinomas including breast cancer (17). Because we found that expression of HER1/EGFR was up-regulated in HOXB7-expressing breast cancer cells, we investigated the mechanism by which HOXB7 might regulate HER1/EGFR.
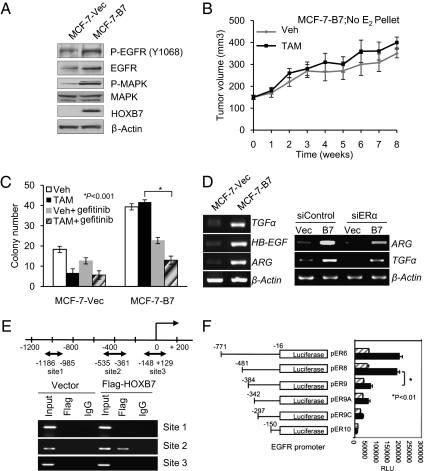
Stable overexpression of HOXB7 in MCF-7 cells resulted in increased expression of EGFR and elevated tyrosine phosphorylation at the kinase domain (Y1068) of EGFR, and, as previously observed (17), the major downstream effector, p44/42 MAPK, was activated as well (Fig. 3A).
Fig. 3.
HOXB7 promotes TAM resistance. (A) Immunoblot analysis of Phospho-EGFR, EGFR, P-MAPK, MAPK, and HOXB7 expression levels in MCF-7-Vec and MCF-7-HOXB7 cells. (B) Tumor growth curve of MCF-7-HOXB7 cells implanted s.c. in athymic Swiss female mice and treated with either vehicle or TAM (83.3 μg/d), in the absence of an exogenous estrogen supplement. (C) Soft agar colony formation by MCF-7-Vec and MCF-7-B7 cells treated with vehicle, estrogen (10 nM), or TAM (1 μM) and combination with 1 μM gefitinib as indicated. (D) Semiquantitative RT-PCR analysis of mRNA expression levels of TGF-α, HB-EGF, and Amphiregulin in HOXB7-expressing MCF-7 cells and their vector controls (Left) and Amphiregulin or TGF-α mRNA expression in MCF-7-Vec and MCF-7-B7 cells treated with either scrambled sequence siRNA or ERα-specific siRNA (Right). (E) Diagram representing the HOXB7 binding sites in the EGFR promoter region. MCF-7 cells were transfected with pcDNA3-Flag-HOXB7 and vector plasmid, and ChIP was performed by IP with either anti-Flag M2 antibody or control IgG. (F) Luciferase activity of deletion/truncation constructs of the EGFR promoter, with (solid bars) and without (hatched bars) transfected HOXB7 plasmid, to map the minimal region necessary for activation by HOXB7.
Next, we tested the biological properties of MCF-7-B7 cells. In culture, TAM treatment of MCF-7-Vec cells led to increased apoptosis and decreased cell viability, whereas MCF-7-B7 cells were minimally sensitive (Fig. S3 A and B). The TAM-resistant property of MCF-7-B7 cells was also verified by the estrogen-stimulated ERE-luc reporter activity in these cells (Fig. S3C). Further, MCF-7-B7 cells formed colonies in soft agar in the presence of tamoxifen at frequencies similar to those of vehicle-treated cells (Fig. S3D). Notably, unlike vector control cells, MCF-7-B7 xenografts in immunodeficient mice failed to respond to the inhibitory effects of TAM (Fig. 3B and Fig. S3 E and F). Thus, by all these growth criteria, the behavior of the MCF-7-B7 cells was very similar to that of the MCF-7-TMR cells described above.
Consistent with our predictions of HOXB7–EGFR crosstalk, abrogation of EGFR activity in MCF-7-B7 cells by the EGFR-specific inhibitor, gefitinib, significantly resensitized them to TAM treatment (Fig. 3C). To determine whether the HOXB7–EGFR crosstalk is rapid and direct, we treated estrogen-deprived MCF-7-B7 cells with 1 μM TAM for 0–30 min, which led to elevated phosphorylation of EGFR, activation of p44 MAPK, and ER phosphorylation at Ser-118 (Fig. S3G). These observations suggest that under these treatment conditions, nongenomic ERα action occurred to activate EGFR signaling. This effect was sustained for longer periods of time: MCF-7-B7 cells grown in estrogen-deprived conditions exposed to TAM for 24 h also led to the expression of significantly higher levels of active forms of EGFR, increased p44/42 MAPK activity, and ERα phosphorylation in contrast to control MCF-7-Vec cells (Fig. S3H).
MCF-7-TMR cells are known to use an autocrine signaling pathway with EGFR ligands through activated EGFR and MAPK for growth (18–20). Elevated activation of EGFR as a result of HOXB7 overexpression prompted us to examine the possible overproduction of known EGFR ligands (i.e., TGF-α, ARG, and HB-EGF). Indeed, increased mRNA expression of the three EGFR ligands was observed in MCF-7-B7 cells (Fig. 3D), MCF-7-TMR (Fig. S3I) cells, and MCF10A-B7 cells (Fig. S3J). Consistent with increased mRNA levels, a significant increase of TGF-α and HB-EGF expression was detected at the protein level in MCF10A-B7 cells (Fig. S3K). The elevated expression of TGF-α and HB-EGF was significantly abrogated by the pharmacological inhibition of EGFR activity using the EGFR-kinase inhibitor, AG1478 and gefitinib. This result suggests the possible existence of a positive feedback mechanism for the synergistic activation of the EGFR pathway as a result of HOXB7 expression in MCF10A cells (Fig. S3 K and L). We also found increased expression of TGF-α and HB-EGF upon overexpression of exogenous HOXB7 in ER-negative breast cancer cell lines, SKBR3, MDA-MB-231, and MDA-MB-435. Conversely, the expression level of these ligands was decreased by depletion of HOXB7 by siRNA (Fig. S3M). However, it is likely that an alternative pathway in MCF-7-B7 cells, such as overexpressed HOXB7 acting through ERα, regulates the overproduction of autocrine/paracrine EGFR ligands. To further examine possible cross-talk between EGFR and ER signaling as a result of HOXB7 overexpression, the ER down-regulator, fulvestrant (ICI 182780; Faslodex), was used. Fulvestrant treatment modestly reduced increases in EGFR and p44/42 MAPK activity upon HOXB7 overexpression. Consistent with reduced EGFR activity, a significant reduction in levels of the autocrine/paracrine EGFR ligands (ARG and TGF-α) was observed in fulvestrant-treated MCF-7-B7 cells (Fig. S3N). A positive role for ERα in these effects of HOXB7 was indicated by partial reduction in levels of ARG and TGF-α upon ERα-specific siRNA transfection of MCF-7-B7 cells (Fig. 3D). Thus, ER-dependent pathways might be responsible for elevated levels of the autocrine/paracrine EGFR ligands in MCF-7-B7 cells, which may result in elevated EGFR signaling.
To examine whether the interaction between HOXB7 and EGFR is direct, ChIP assays were performed. A single putative HOXB7-binding site was identified in the 800-bp EGFR promoter (Fig. 3E and Fig. S3O). To provide supportive evidence, luciferase reporter constructs containing serial deletions of the EGFR promoter (21) were cotransfected into MCF-7 and MCF10A cells, along with the HOXB7 expression plasmids. As shown in Fig. 3F, both pER6-luc–containing nucleotides −771 to −16 and pER8-luc–containing nucleotides −481 to −16 were activated 2.5- to 3-fold by HOXB7, whereas pER9-luc–containing nucleotides −342 to −16, −297 to −16, and −150 to −16 were activated at much lower levels in MCF-7 cells (Fig. 3F). These results were consistent with the ChIP assay data localizing sequences necessary for response of EGFR to HOXB7.
In line with these findings, we also found an up-regulation of EGFR in HOXB7 overexpressing MCF10A and HBL-100 cells (Fig. S3P). EGFR and p44/42 MAPK activities were also reduced by HOXB7 siRNA expression in ER-negative MDA-MB-435 and MDA-MB-468 cells (Fig. S3Q). Moreover, transfection of HOXB7 siRNA into MCF-7-HOXB7 cells was also able to resensitize them to TAM treatment (Fig. S3R). These results suggest that HOXB7 might be an attractive anticancer target in both ER+ and ER− tumors. Collectively, we have provided data demonstrating overexpression of receptors and ligands, activation of downstream effectors, direct binding of HOXB7 to EGFR promoter, and reversal of these effects by siRNA directed against HOXB7. Thus, multiple lines of evidence strongly support the conclusion that HOXB7 is an important mediator of the cross-talk between EGFR and ERα signaling that is critical for maintaining resistance to TAM in MCF-7 cells.
HOXB7 Expression Is Regulated by Estrogen.
What might be an underlying mechanism of HOXB7 overexpression in TAM-resistant breast cancer cells? On the basis of a previous finding in a Swiss cohort (22), in collaboration with the same investigators (A.K.), we tested whether HOXB7 overexpression in breast tumors could be traced to gene amplification by performing FISH on tissue microarrays of primary breast tumors. Unlike the 10% incidence observed previously (23), only 2 of 172 Swedish cases and 1 of 108 Finnish cases showed amplification of HOXB7 (Fig. S4A). On the basis of these data, we concluded that gene amplification is not a major mechanism underlying elevated expression of HOXB7 in breast cancer, including tamoxifen-resistant cancer.
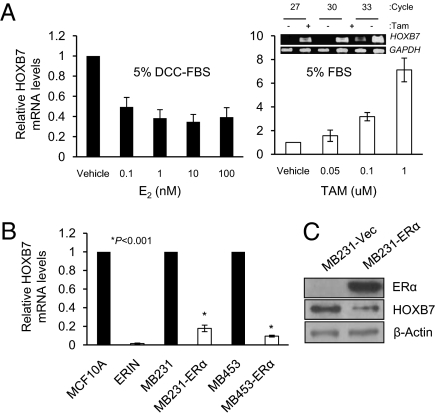
Could tamoxifen exposure, acting through ERα, be the cause of induction of HOXB7 in breast cancer cells? As predicted, HOXB7 mRNA levels increased upon treatment of MCF-7 and T47D cells with tamoxifen in medium containing 10% FBS (i.e., abundance of estrogen) (Fig. 4A and Fig. S4 B and E). Also, HOXB7 mRNA levels increased in estrogen-deprived growth conditions (5% charcoal-stripped serum) (Fig. S4C). Interestingly, HOXB7 expression was significantly reduced with estradiol stimulation under the same conditions (Fig. 4A). The down-regulation of HOXB7 expression by estradiol is abrogated by tamoxifen (Fig. S4D). To further test this concept, we overexpressed ERα in ER-negative cell lines such as MDA-MB-231, MDA-MB-453, and MCF10A. We observed that HOXB7 expression was dramatically decreased in the ERα-overexpressing cells (Fig. 4 B and C and Fig. S4F). These findings support our hypothesis that HOXB7 expression is regulated by estrogen and this action is dependent on the presence of a functional ERα.
Fig. 4.
ERα regulates HOXB7 expression. (A) Real-time quantitative PCR analysis of HOXB7 mRNA levels in MCF-7 cells. Cells were incubated in an estrogen-deprived condition for 72 h [DMEM phenol red-free medium containing 5% Dextran charcoal-stripped serum (DCC-FBS)]; cells were then treated with 0.1–100 nM estrogen (E2); and cells were treated with 0.05–1 μM of TAM in DMEM plus 5% FBS. (Inset) Semiquantitative RT-PCR analysis of HOXB7 mRNA following 1 uM TAM treatment for 24 h. (B) Real-time quantitative PCR analysis of HOXB7 mRNA levels in MDA-MB-231 and MDA-MB-453 cells were transiently transfected with ERα expression plasmid and vector control for 48 h. ERIN, the ERα overexpressed cell line, was used with MCF10A as a control. (C) Immunoblot analysis of HOXB7 and ERα expression levels in transient transfectants of MDA-MB-231-ERα.
Prognostic Significance of High HOXB7 Expression in Breast Cancer.
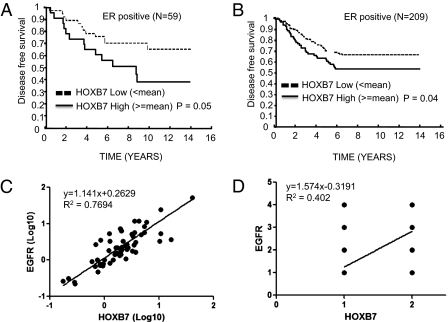
On the basis of the ability of overexpressed HOXB7 to lead to TAM resistance, we investigated whether HOXB7 overexpression in primary tumors may predict subsequent TAM resistance. We examined HOXB7 mRNA levels of hormone receptor-positive primary breast cancers in two different small sets of patients (n = 59 and n = 72) treated with adjuvant TAM monotherapy (24) and 209 patients with no adjuvant therapy after surgery by real-time PCR. In these discovery sets of tissues, the association between a higher expression level of HOXB7 and a poorer relapse-free survival rate was statistically significant (Fig. 5 A and B and Fig. S5 A and B), suggesting that elevated expression levels of HOXB7 were associated with the development of tamoxifen resistance in patients with breast cancer. To investigate whether there is a correlation between HOXB7 and EGFR expression in clinical cohorts, HOXB7 and EGFR mRNA levels were examined by qRT-PCR in 57 samples from patients with breast cancer and 48 breast cancer cell lines. We also investigated 127 samples from patients with breast cancer by immunohistochemistry (IHC), using a breast cancer tissue microarray. Interestingly, a correlation between HOXB7 and EGFR was observed both at the mRNA level (r = 0.8772, 0.8290) and under IHC detection (r = 0.6341) with statistical significance (P < 0.0001) (Fig. 5 C and D and Fig. S5 C, E, and F). Collectively, we have shown that elevated HOXB7 expression under various scenarios appears to serve as a unifying molecular hub directing the development of anti-estrogen resistance.
Fig. 5.
Kaplan–Meier plots of disease-free survival analysis of estrogen receptor-positive node-negative patients who received (A) TAM monotherapy (n = 59) and (B) no adjuvant therapy after surgery (n = 209) were stratified by HOXB7 expression level. (C) Pearson's correlation between HOXB7 and EGFR mRNA levels in samples from patients with breast cancer (n = 57). (D) Pearson's correlation between HOXB7 and EGFR protein levels by IHC in sample TMA from patients with breast cancer (n = 127).
Discussion
Molecular adaptations during acquired TAM resistance use multiple signaling pathways that are well documented in the literature. In this paper, evidence has been presented to support the hypothesis that a common mechanism leading to TAM resistance is overexpression of HOXB7. We have shown the following: (i) MCF-7 cells treated over extended periods with TAM in vitro develop TAM resistance, which is accompanied by a parallel, elevated expression of HOXB7 and EGFR, events that also occur in another two different commonly used anti-estrogen resistance models. (ii) These molecular events are largely recapitulated in MCF-7 cells by overexpression of a single gene, HOXB7, leading to acquired resistance to TAM. (iii) SiRNA-mediated silencing of HOXB7 significantly reversed many of the malignant traits and molecular changes in both the native (BT474) and engineered anti-estrogen resistance models. (iv) Preliminary evidence of potential clinical relevance was observed in a small discovery cohort of ER+ patients with breast cancer who received TAM monotherapy (24), where a higher level of HOXB7 in cancer specimens correlated significantly with poor relapse-free survival (P = 0.049). Thus, HOXB7, frequently overexpressed in breast cancer, appears to be a major upstream regulator of events leading to TAM resistance.
Mechanistically, HOXB7 acts via simultaneous up-regulation of receptor tyrosine kinase, EGFR, and EGFR ligands, each of which was efficiently reversed by HOXB7-specific siRNA. Although HOX genes have been implicated in the regulation of several pathways involved in embryogenesis and organogenesis, few target genes have been shown to be under their direct regulatory control (5). Here, we show that HOXB7 binds directly to the EGFR promoter to induce its gene transcription. Moreover, HOXB7 also up-regulated the expression of multiple EGFR ligands, presumably through cross-talk with activated ERα signaling in MCF-7 cells (Fig. 3). Coexistence of an RTK and its ligands at elevated levels presents a formidable obstacle to successful breast cancer therapy (2, 25). It is difficult to envision that the current target-specific (usually one target, one molecule) anticancer therapeutics will efficiently eradicate heterogeneous populations of cells, typical of breast cancer. Thus, in a preclinical breast cancer model, a combination treatment that simultaneously blocks multiple signals would be significantly better than single agents or dual combinations (26). Our data implicated HOXB7 as a unique target acting upstream of important RTKs and cognate ligands, whose functional antagonism might allow the attack of multiple therapeutic targets simultaneously in breast cancer.
As suggested here, ERα signaling might render the cells more dependent on HOXB7-mediated pathways such as EGFR or ERα itself. It is worthwhile to note that preliminary data from clinical trials (27) of gefitinib in breast cancer suggested that contrary to predictions, patients with ER- and high EGFR-expressing tumors showed a poor response to gefitinib. On the other hand, in two separate studies, patients with TAM-resistant, ER+ breast cancer with modestly increased EGFR showed a good response (66% and 86%, respectively) to gefitinib. These observations are in line with our findings (Fig. 3C). It is likely that TAM-resistant cells use ER-dependent pathways to produce much more of EGFR-specific ligands such as HB-EGF (28), Amphiregulin (29), and TGF-α (30). These ligands bind to high-affinity EGFR in ER+ cells, rendering the cells more dependent on the EGFR pathway and therefore more vulnerable to attack by gefitinib. This premise of dual action is further supported by the clinical trial (31) where ER+ and EGFR+ patients with breast cancer treated with gefitinib and anastrozole had a greater reduction from pretreatment values in the proliferation-related Ki67 labeling index than patients treated with gefitinib alone. Thus, HOXB7 overexpression in ER+ breast cancer might have the potential to serve as a marker indicating anti-EGFR therapy.
There are three questions that remain: First, in tamoxifen resistance, HER2 and HER3 signaling pathways also play important roles. Because neither AG1478 nor gefitinib have shown a complete response to sensitizing MCF-7-HOXB7 cells to TAM treatment, we should investigate whether other RTK-signaling pathway are involved in HOXB7-mediated TAMR. Second, what connects HOXB7 action to ER function? Some hints have surfaced during our study of HOXB7 × HER2 transgenic mouse mammary tumor cells (32) that deserve attention. These tumors have elevated expression of FoxA1. FoxA1 was reported to interact with ERα to turn on the transcription of ERα-target genes, which include FoxA1 and ERα itself (33, 34). Third, is HOXB7 regulated by ERα directly or indirectly? Because we did not find ERα binding sites in the HOXB7 promoter regions, on the basis of ERα ChIP-on-ChIP profiles (35), in future studies we will need to focus our attention on indirect regulatory mechanisms in play between HOXB7 and ERα. Thus, in TAM-resistant cells, HOXB7 might orchestrate the coordinated actions of ERα- and EGFR-dependent pathways in breast tumorigenesis. Further evidence supporting this premise was elucidated here in acquired TAM resistance models (Fig. 2). Early effective antagonism of the ER pathway by use of an alternative therapeutic approach to TAM resistance might benefit those ER+ patients with higher levels of HOXB7 expression. In summary, HOXB7 might be a critical regulator on top of the hierarchy that orchestrates the process of acquisition and maintenance of anti-estrogen resistance. Thus, HOXB7 might present a unique drug target to reverse anti-estrogen resistance efficiently.
Materials and Methods
Cell Lines, Cell Culture, and Reagents.
pcDNA3 vector or pcDNA3-Flag-HOXB7 was stably transfected into MCF10A cells or MCF-7 cells by use of Effectene (Qiagen). MCF-7-LTED, the estrogen-hypersensitive MCF-7 subline, was generated from MCF-7 cells by long-term culture under estrogen-deprived conditions and thus they are called LTED cells (14) (a kind gift from Richard Santen). LTED cells are refractory to tamoxifen but sensitive to fulvestrant (11). MCF-7-TAMLT, long-term tamoxifen-stimulated tumors, were kindly provided by V. Craig Jordan and were developed by retransplanting growing estradiol-dependent MCF-7 tumors into new athymic mice and treating the mice with tamoxifen for >5 y (15). Fulvestrant and Iressa (gefitinib) were provided by Astrazeneca.
FISH Analysis.
HOXB7 copy number levels were determined by FISH on the Tissue MicroArray (TMA) as described (36). The HOXB7-specific BAC clone (RP11-361K8) was labeled with SpectrumOrange (Vysis) and SpectrumGreen-labeled chromosome 17 centromere probe (Vysis) was used as a reference. The nuclei were counterstained with DAPI. The entire tissue core was screened, with a minimum of 50 intact nuclei scored for each specimen. A total of 280 samples were successfully analyzed. Tumor samples containing a threefold or higher increase in the number of HOXB7 signals compared with centromere signals were considered to be amplified.
Real-Time PCR of HOXB7 Expression.
HOXB7 gene expression was quantified by Taqman real-time quantitative PCR in triplicate in a 96-well plate using an ABI 7900HT (Applied Biosystems), using cDNA derived from our previously published cohort of tumor samples (24). The sequences of the HOXB7 PCR primer pairs and fluorogenic minor groove binder (MGB) probe (5′–3′), respectively, are AAA ACC TAC CAC TCG CGT GTT C, GGA CGG GAA GCA AGA AGC A, and VIC-CAA GCG CCT GGC TG.
Statistical Analysis.
HOXB7 expression levels determined by RT-PCR were dichotomized into low and high groups using the median as cutoff. Kaplan–Meier analysis and log-rank tests were performed to assess the association of HOXB7 groups with distant metastasis-free survival. All statistical tests were two sided, and differences were considered statistically significant at P < 0.05. All analyses were performed using SAS (version 9.1) and R (version 2.4.1).
Supplementary Material
Acknowledgments
We thank Dr. Ben H. Park for generously providing the ERIN cell line, Drs. Richard Santen (University of Virginia, Charlottesville, VA) and V. Craig Jordan (Georgetown University, Washington, DC) for the LTED and TAMLT cell lysates, and Dr. Cynthia Zahnow and Hexin Chen for reviewing the manuscript. This work was supported by Specialized Programs Of Research Excellence (SPORE) in Breast Cancer: National Institutes of Health Grant P50-CA88843 (to S.S.), Susan G. Komen Postdoctoral Fellowships (to T.Z. and K.J.), the National Key Scientific Program of China (2010CB912804, 2007CB914801, and 2011CBA01103), and the National Natural Science Foundation of China (30971492 and 30725015) (to T.Z.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. K.P. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018859108/-/DCSupplemental.
References
- 1.Brown RJ, Davidson NE. Adjuvant hormonal therapy for premenopausal women with breast cancer. Semin Oncol. 2006;33:657–663. doi: 10.1053/j.seminoncol.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Jordan VC, Brodie AM. Development and evolution of therapies targeted to the estrogen receptor for the treatment and prevention of breast cancer. Steroids. 2007;72:7–25. doi: 10.1016/j.steroids.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gutierrez MC, et al. Molecular changes in tamoxifen-resistant breast cancer: Relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J Clin Oncol. 2005;23:2469–2476. doi: 10.1200/JCO.2005.01.172. [DOI] [PubMed] [Google Scholar]
- 4.Howell A, et al. Comparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: A multinational, double-blind, randomized trial. J Clin Oncol. 2004;22:1605–1613. doi: 10.1200/JCO.2004.02.112. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Fernàndez J. The genesis and evolution of homeobox gene clusters. Nat Rev Genet. 2005;6:881–892. doi: 10.1038/nrg1723. [DOI] [PubMed] [Google Scholar]
- 6.Wu X, et al. HOXB7, a homeodomain protein, is overexpressed in breast cancer and confers epithelial-mesenchymal transition. Cancer Res. 2006;66:9527–9534. doi: 10.1158/0008-5472.CAN-05-4470. [DOI] [PubMed] [Google Scholar]
- 7.Carè A, et al. HOXB7: A key factor for tumor-associated angiogenic switch. Cancer Res. 2001;61:6532–6539. [PubMed] [Google Scholar]
- 8.Ackerstaff E, Gimi B, Artemov D, Bhujwalla ZM. Anti-inflammatory agent indomethacin reduces invasion and alters metabolism in a human breast cancer cell line. Neoplasia. 2007;9:222–235. doi: 10.1593/neo.06673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhujwalla ZM, et al. Reduction of vascular and permeable regions in solid tumors detected by macromolecular contrast magnetic resonance imaging after treatment with antiangiogenic agent TNP-470. Clin Cancer Res. 2003;9:355–362. [PubMed] [Google Scholar]
- 10.Lundberg AS, Hahn WC, Gupta P, Weinberg RA. Genes involved in senescence and immortalization. Curr Opin Cell Biol. 2000;12:705–709. doi: 10.1016/s0955-0674(00)00155-1. [DOI] [PubMed] [Google Scholar]
- 11.Martin LA, et al. The anti-oestrogen ICI 182,780, but not tamoxifen, inhibits the growth of MCF-7 breast cancer cells refractory to long-term oestrogen deprivation through down-regulation of oestrogen receptor and IGF signalling. Endocr Relat Cancer. 2005;12:1017–1036. doi: 10.1677/erc.1.00905. [DOI] [PubMed] [Google Scholar]
- 12.Nicholson RI, et al. Growth factor signalling networks in breast cancer and resistance to endocrine agents: New therapeutic strategies. J Steroid Biochem Mol Biol. 2005;93:257–262. doi: 10.1016/j.jsbmb.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Hiscox S, et al. Tamoxifen resistance in MCF7 cells promotes EMT-like behaviour and involves modulation of beta-catenin phosphorylation. Int J Cancer. 2006;118:290–301. doi: 10.1002/ijc.21355. [DOI] [PubMed] [Google Scholar]
- 14.Jeng MH, et al. Estrogen receptor expression and function in long-term estrogen-deprived human breast cancer cells. Endocrinology. 1998;139:4164–4174. doi: 10.1210/endo.139.10.6229. [DOI] [PubMed] [Google Scholar]
- 15.Osipo C, Gajdos C, Liu H, Chen B, Jordan VC. Paradoxical action of fulvestrant in estradiol-induced regression of tamoxifen-stimulated breast cancer. J Natl Cancer Inst. 2003;95:1597–1608. doi: 10.1093/jnci/djg079. [DOI] [PubMed] [Google Scholar]
- 16.Gee JM, et al. Deciphering antihormone-induced compensatory mechanisms in breast cancer and their therapeutic implications. Endocr Relat Cancer. 2006;13(Suppl 1):S77–S88. doi: 10.1677/erc.1.01274. [DOI] [PubMed] [Google Scholar]
- 17.Hynes NE, Lane HA. ERBB receptors and cancer: The complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 18.Knowlden JM, et al. Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology. 2003;144:1032–1044. doi: 10.1210/en.2002-220620. [DOI] [PubMed] [Google Scholar]
- 19.Knowlden JM, Hutcheson IR, Barrow D, Gee JM, Nicholson RI. Insulin-like growth factor-I receptor signaling in tamoxifen-resistant breast cancer: A supporting role to the epidermal growth factor receptor. Endocrinology. 2005;146:4609–4618. doi: 10.1210/en.2005-0247. [DOI] [PubMed] [Google Scholar]
- 20.Britton DJ, et al. Bidirectional cross talk between ERalpha and EGFR signalling pathways regulates tamoxifen-resistant growth. Breast Cancer Res Treat. 2006;96:131–146. doi: 10.1007/s10549-005-9070-2. [DOI] [PubMed] [Google Scholar]
- 21.Nishi H, Nishi KH, Johnson AC. Early Growth Response-1 gene mediates up-regulation of epidermal growth factor receptor expression during hypoxia. Cancer Res. 2002;62:827–834. [PubMed] [Google Scholar]
- 22.Hyman E, et al. Impact of DNA amplification on gene expression patterns in breast cancer. Cancer Res. 2002;62:6240–6245. [PubMed] [Google Scholar]
- 23.Rydén L, et al. South Swedish Breast Cancer Group South-East Swedish Breast Cancer Group Two years of adjuvant tamoxifen in premenopausal patients with breast cancer: A randomised, controlled trial with long-term follow-up. Eur J Cancer. 2005;41:256–264. doi: 10.1016/j.ejca.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 24.Ma XJ, et al. A two-gene expression ratio predicts clinical outcome in breast cancer patients treated with tamoxifen. Cancer Cell. 2004;5:607–616. doi: 10.1016/j.ccr.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Johnston SR, et al. Integration of signal transduction inhibitors with endocrine therapy: An approach to overcoming hormone resistance in breast cancer. Clin Cancer Res. 2003;9:524S–532S. [PubMed] [Google Scholar]
- 26.Arpino G, et al. Treatment of human epidermal growth factor receptor 2-overexpressing breast cancer xenografts with multiagent HER-targeted therapy. J Natl Cancer Inst. 2007;99:694–705. doi: 10.1093/jnci/djk151. [DOI] [PubMed] [Google Scholar]
- 27.Jones HE, et al. Development of strategies for the use of anti-growth factor treatments. Endocr Relat Cancer. 2005;12(Suppl 1):S173–S182. doi: 10.1677/erc.1.01004. [DOI] [PubMed] [Google Scholar]
- 28.Kanda N, Watanabe S. 17beta-estradiol enhances heparin-binding epidermal growth factor-like growth factor production in human keratinocytes. Am J Physiol Cell Physiol. 2005;288:C813–C823. doi: 10.1152/ajpcell.00483.2004. [DOI] [PubMed] [Google Scholar]
- 29.Ciarloni L, Mallepell S, Brisken C. Amphiregulin is an essential mediator of estrogen receptor alpha function in mammary gland development. Proc Natl Acad Sci USA. 2007;104:5455–5460. doi: 10.1073/pnas.0611647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saeki T, et al. Regulation by estrogen through the 5′-flanking region of the transforming growth factor alpha gene. Mol Endocrinol. 1991;5:1955–1963. doi: 10.1210/mend-5-12-1955. [DOI] [PubMed] [Google Scholar]
- 31.Polychronis A, et al. Preoperative gefitinib versus gefitinib and anastrozole in postmenopausal patients with oestrogen-receptor positive and epidermal-growth-factor-receptor-positive primary breast cancer: A double-blind placebo-controlled phase II randomised trial. Lancet Oncol. 2005;6:383–391. doi: 10.1016/S1470-2045(05)70176-5. [DOI] [PubMed] [Google Scholar]
- 32.Chen H, et al. Hoxb7 inhibits transgenic HER-2/neu-induced mouse mammary tumor onset but promotes progression and lung metastasis. Cancer Res. 2008;68:3637–3644. doi: 10.1158/0008-5472.CAN-07-2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carroll JS, et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell. 2005;122:33–43. doi: 10.1016/j.cell.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 34.Hurtado A, Holmes KA, Ross-Innes CS, Schmidt D, Carroll JS. FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat Genet. 2011;43:27–33. doi: 10.1038/ng.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hurtado A, et al. Regulation of ERBB2 by oestrogen receptor-PAX2 determines response to tamoxifen. Nature. 2008;456:663–666. doi: 10.1038/nature07483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pärssinen J, Kuukasjärvi T, Karhu R, Kallioniemi A. High-level amplification at 17q23 leads to coordinated overexpression of multiple adjacent genes in breast cancer. Br J Cancer. 2007;96:1258–1264. doi: 10.1038/sj.bjc.6603692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.