Abstract
Leptin-deficient (Lepob/ob) mice are obese, diabetic, and infertile. Ablation of neurons that make agouti-related protein (AgRP) in moderately obese adult Lepob/ob mice caused severe anorexia. The mice stopped eating for 2 wk and then gradually recovered. Their body weight fell to within a normal range for WT mice, at which point food intake and glucose tolerance were restored to that of WT mice. Remarkably, both male and female Lepob/ob mice became fertile. Ablation of neurons that express melanin-concentrating hormone (MCH) in adult Lepob/ob mice had no effect on food intake, body weight, or fertility, but resulted in improved glucose tolerance. We conclude that AgRP-expressing neurons play a critical role in mediating the metabolic syndrome and infertility of Lepob/ob mice, whereas MCH-expressing neurons have only a minor role.
Keywords: diphtheria toxin, norepinephrine, neuropeptide Y, gonadotropin releasing protein, kisspeptin
The spontaneous nonsense mutation in the leptin gene that generated Lepob/ob mice results in obesity, hyperphagia, diabetes, and infertility (1, 2). These phenotypes can be reversed by chronic delivery of recombinant leptin (3, 4). The neural circuitry underlying these phenotypes has been the subject of intense investigation (5–7). Mice lacking leptin have elevated levels of neuropeptide Y (NPY) and melanin-concentrating hormone (MCH) in the hypothalamus (8, 9), and genetic experiments have demonstrated that Lepob/ob mice lacking either of these peptides have an improved metabolic phenotype (10, 11).
Because hypothalamic NPY-expressing neurons send projections to the vicinity of MCH-expressing neurons (12), which have electrophysiological responses to NPY (13), the two neuronal populations may be part of the same circuitry mediating the phenotype of Lepob/ob mice. NPY-expressing neurons in the arcuate nucleus also express agouti-related protein (AgRP), which exerts the same orexigenic effects as NPY but by a different mechanism (14).
We take advantage of the restricted expression of AgRP and MCH to hypothalamic neurons by targeting the human diphtheria toxin (DT) receptor to the genes encoding those neuropeptides, thereby allowing their selective ablation by administration of DT (15). Ablation of AgRP-expressing neurons (AgRP neurons) in adult mice results in severe anorexia and death in ∼6 d (15, 16). In this study, we applied this same methodology to MCH-expressing neurons (MCH neurons) and asked whether ablation of either of these neuronal populations in adult Lepob/ob mice is beneficial (as predicted from the constitutive loss of the neuropeptides) or lethal (as predicted from the loss of AgRP neurons in a WT background).
Results
Young Lepob/ob Mice Do Not Survive Ablation of AgRP Neurons.
Lepob/+;AgrpDTR/+ mice were bred with Lepob/+ mice to generate Lepob/ob;AgrpDTR/+ mice, and Lepob/ob;Agrp+/+ or Lepob/+;AgrpDTR/+ controls. After treatment of young male and female mice weighing ∼27 g with DT, all of the mice carrying the AgrpDTR allele (whether Lepob/ob or Lepob/+) gradually stopped feeding and lost 20% of their body weight during the next 6 d and would have succumbed, whereas mice without the AgrpDTR allele maintained their feeding and body weight (Fig. 1). Statistical analyses of our results are reported in the figure legends. Analysis of the brains of DT-treated mice at the end of the experiment revealed a virtual absence of AgRP neurons in AgrpDTR/+ mice, as measured by immunocytochemistry or quantitative PCR for Agrp transcripts (Fig. S1). We conclude that the absence of leptin does not prevent ablation of AgRP neurons by DT and does not protect these young mice from severe anorexia.
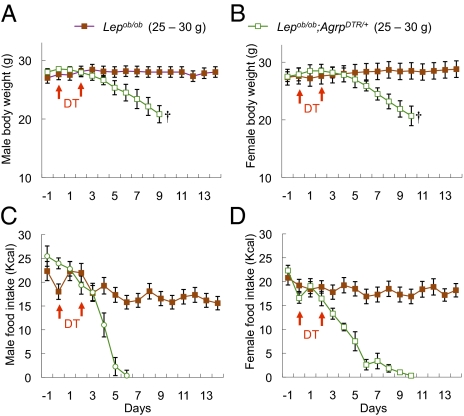
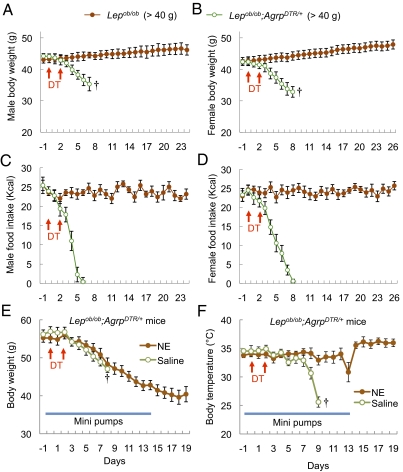
Fig. 1.
Ablation of AgRP neurons in young, leptin-deficient mice leads to anorexia. (A and B) Body weight of DT-treated male (A) and female (B) Lepob/ob mice (∼6 wk old, 25–30 g) and Lepob/ob;AgrpDTR/+ mice (∼6 wk old, 25–30 g). (C and D) Daily food intake by the mice described in A and B. All mice were raised on standard chow pellets. Error bars represent SEM. P < 0.01, ANOVA on body weight and food intake between the two groups (days 5–9). †Mice removed from the study because they became moribund.
Young Lepob/ob Mice Survive Ablation of MCH Neurons.
Mice with the DT receptor targeted to the Pmch locus, which encodes MCH, were produced as described in Materials and Methods. Young Lepob/ob;PmchDTR/+ mice and Lepob/+;PmchDTR/+ controls were treated with DT or saline. DT treatment did not alter food intake of Lepob/ob;PmchDTR/+ mice, but it slightly reduced the rate at which male (but not female) Lepob/ob;PmchDTR/+ mice gained weight (Fig. 2). Analysis of the brains from Lepob/ob;PmchDTR/+ mice and controls at the end of the experiment revealed that in both groups of mice, >95% of the MCH neurons were gone, as measured by immunocytochemistry or quantitative PCR for Pmch transcripts (Fig. S2). DT treatment of similarly aged control mice lacking the DT receptor had no effect on body weight, food intake, or glucose tolerance. Older, more obese (35–45 g body weight) Lepob/ob;PmchDTR/+ mice also survived DT treatment, with a modest effect on body weight and improvement in glucose tolerance. This experiment reaffirms that DT-mediated ablation of neurons with the attendant gliosis does not promote anorexia; thus, the effects of AgRP neuron ablation are likely specific to that neuronal population.
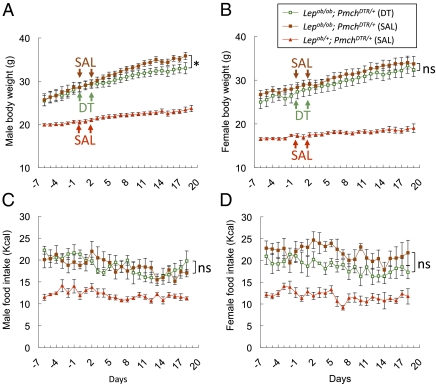
Fig. 2.
Body weight and food intake after ablation of MCH neurons in Lepob/ob mice. (A and B) Body weight was measured in obese male (A) and female (B) Lepob/ob; PmchDTR/+ and lean Lepob/+; PmchDTR/+ mice that had been injected with either saline (SAL) or DT on the days indicated by arrows. (C and D) Daily food intake by the mice described in A and B. Error bars represent SEM (n = 9–11 per group). *P = 0.03 for the interaction of time and DT treatment in two-way repeated-measures ANOVA. NS, not significant. P > 0.05.
Moderately Obese Lepob/ob;AgrpDTR/+ Mice Survive AgRP Neuron Ablation.
We previously demonstrated that mice can adapt to the loss of AgRP neurons, but this adaptation takes ∼10 d (16). Thus, we tested the hypothesis that older, more obese Lepob/ob mice also might be able to adapt by using their greater energy reserves. Moderately obese (35–40 g body weight) Lepob/ob;Agrp+/+ and lean (20–25 g) Lepob/+;AgrpDTR/+ controls were treated with DT. The lean control mice gradually stopped eating, lost 20% of their body weight, and became moribund over the subsequent 6 d, in agreement with previous results. However, both male and female Lepob/ob;AgrpDTR/+ mice weighing 35–40 g ceased eating within 6 d, and did not eat over the next 12 d. During that time, their body weight fell to ∼22–25 g. They then began to eat again at a rate similar to that of WT mice, and their body weight gradually increased but remained within the normal range (Fig. 3). The obese mice (Lepob/ob;Agrp+/+) that did not express the DT receptor were unaffected by DT administration (Fig. 3).
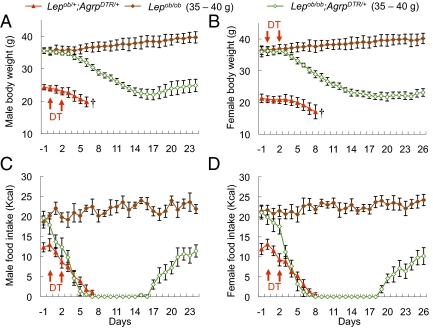
Fig. 3.
Moderately obese leptin-deficient mice survived acute ablation of AgRP neurons and exhibited normal body weight with significantly reduced food intake. (A and B) Body weight of DT-treated male (A) and female (B) mice from three groups (1) Lepob/+;AgrpDTR/+ mice (∼8 wk old, 20–25 g); (2) Lepob/ob mice (∼8 wk old, 35–40 g); and (3) Lepob/ob;AgrpDTR/+ mice (∼8 wk old, 35–40 g). (C and D) Daily food intake by the mice described in A and B. All mice were raised on standard chow pellets. Error bars represent SEM. P < 0.01, ANOVA on body weight and food intake between group 2 and group 3 (days 5–24). †Mice removed from study because they became moribund.
Improved Glucose Homeostasis After Ablation of AgRP or MCH Neurons.
Lepob/ob mice become diabetic as they gain weight, exhibiting hyperglycemia, hyperinsulinemia, and glucose intolerance (17). By 26 d after ablation of AgRP neurons in female and male Lepob/ob;AgrpDTR/+ mice weighing 35–40 g, fasting blood glucose levels had returned to normal. Their response to glucose tolerance testing was not statistically different from that of Lepob/+ controls and significantly better than that of saline-treated Lepob/ob;AgrpDTR/+ mice (Fig. 4 A and B).
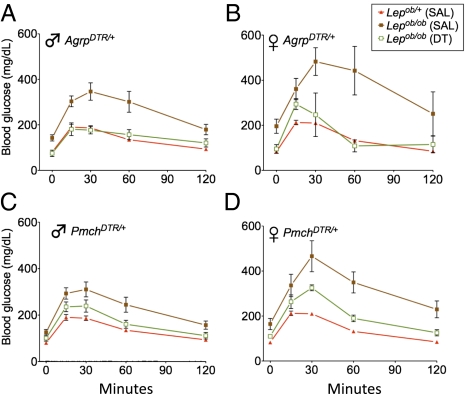
Fig. 4.
Glucose tolerance tests after ablation of AgRP or MCH neurons. (A and B) Obese Lepob/ob and lean Lepob/+ mice carrying the AgrpDTR allele were injected with either saline (SAL) or DT at 2 wk before glucose tolerance testing. Both males (A) and females (B) were tested. Error bars represent mean ± SEM (n = 5–11 per group). Area under the curve (AUC) measurements revealed significantly lower values in mice that received DT compared with obese Lepob/ob controls (P < 0.002, one-way ANOVA with Dunnett's posttest), but not significantly greater values than those in leptin-replete, lean mice. (C and D) Glucose tolerance tests were performed as in A and B, but with the PmchDTR allele instead of the AgrpDTR allele present. Error bars represent mean ± SEM (n = 3–8 per group). P < 0.004 for AUC analyses.
Ablation of MCH neurons in the Lepob/ob;PmchDTR/+ mice also improved glucose tolerance in both females and males compared with saline-treated obese controls (Fig. 4 C and D). This improvement was independent of a decrease in body weight, unlike that occurring after ablation of AgRP neurons.
Restoration of Fertility to Lepob/ob Mice After Ablation of AgRP but Not MCH Neurons.
Male Lepob/ob mice occasionally sire a litter when they are young, but female Lepob/ob mice are infertile (3, 6). The fertility of DT-treated mice was tested by placing individual males and females with WT mice of the opposite sex and recording the birth and size of litters. Both male (5/5) and female (6/6) Lepob/ob;AgrpDTR/+ mice weighing 35–40 g became fertile after ablation of AgRP neurons; some of the males sired several litters, and some females bore more than one litter within 4 mo after DT treatment (Table 1). Based on these data, we calculate that the hypothalamic-pituitary-gonadal axis becomes active within 10–16 d after the first DT injection in female leptin-deficient mice and 11–23 d in male leptin-deficient mice (Discussion). Table 1 also shows that the Lepob/ob;AgrpDTR/+ males used in these breeding experiments had a mean body weight of 31.4 g, compared with ∼55 g in untreated male Lepob/ob;AgrpDTR/+ mice of the same age. Thus, the ablated mice remained relatively lean for several months.
Table 1.
Restoration of fertility in Lepob/ob;AgrpDTR/+ mice after ablation of AgRP neurons
| DT treatment starts |
Mating starts |
When first litter born |
When second litter born |
|||||||
| Sex | Day | Body weight, g | Day | Body weight, g | Day | Body weight, g | Litter size | Day | Body weight, g | Litter size |
| Male | 1 | 38.6 | 43 | 24.4 | 78 | 28.1 | 5 | 106 | 32.8 | 6 |
| 1 | 37.4 | 43 | 24.7 | 66 | 27.5 | 3 | 88 | 30.8 | 7 | |
| 1 | 35.4 | 43 | 26 | 73 | 28.5 | 4 | 99 | 32.2 | 7 | |
| 1 | 35.5 | 39 | 22.3 | 76 | 25.6 | 6 | 103 | 28.7 | 7 | |
| 1 | 35.1 | 39 | 23.9 | 66 | 28.1 | 6 | 93 | 32.5 | 7 | |
| Average | 36.4 | 24.3 | 71.8 | 27.6 | 4.8 | 97.8 | 31.4 | 6.8 | ||
| SD | ±1.5 | ±1.3 | ±5.6 | ±1.2 | ±1.3 | ±7.3 | ±1.7 | ±0.4 | ||
| Female | 1 | 35.4 | 39 | 25.1 | 64 | ND | 3 | 90 | ND | 5 |
| 1 | 37.2 | 39 | 28.6 | 68 | ND | 3 | 94 | ND | 5 | |
| 1 | 35.9 | 39 | 27.4 | 70 | ND | 4 | 93 | ND | 6 | |
| Average | 36.2 | 27.0 | 67.3 | 3.3 | 92.3 | 5.3 | ||||
| SD | ±0.9 | ±1.8 | ±3.1 | ±0.6 | ±2.1 | ±0.6 | ||||
Results shown are for those mice (35–40 g; males and females) where a complete record of body weight and breeding record were obtained. Three additional females were fertile. After a litter was born, the original mates and pups were removed, and fresh mates were provided to score continued fertility for a total of 120 d after DT treatment. One of the males sired a third litter within 120 d.
The fertility of Lepob/ob;PmchDTR/+ mice that had been treated with DT was tested as well. Only one of nine males sired a litter, but none of the six females became pregnant. Therefore, ablation of MCH neurons did not improve the fertility of Lepob/ob;PmchDTR/+ mice. Ablation of MCH neurons in Lepob/+;PmchDTR/+ controls did not disrupt fertility.
Severely Obese Lepob/ob Mice Do Not Survive Ablation of AgRP Neurons.
We also ablated AgRP neurons in Lepob/ob mice that weighed > 40 g; however, unlike the moderately obese mice, these >40 g mice became moribund despite their greater energy reserves. Like the lighter male and female Lepob/ob;AgrpDTR/+ mice, they stopped eating after ∼7 d and began to lose weight; however, they appeared to be unhealthy, and thus the experiment was terminated (Fig. 5 A–D). One distinguishing feature of these older, more obese mice is that their body temperature plummeted to slightly above ambient temperature as they became moribund; in comparison, the body temperature of the Lepob/ob;AgrpDTR/+ mice weighing <40 g dropped slightly but then returned to that of the Lepob/ob mice (Fig. S3). Lepob/ob;AgrpDTR/+ mice weighing >40 g and that were pair-fed with the group that received DT survived and did not exhibit a drop in body temperature. Thus, the drop in body temperature in the severely obese mice appeared to be a consequence of AgRP neuron ablation, not of reduced food intake.
Fig. 5.
Severely obese leptin-deficient mice do not survive ablation of AgRP neurons due to hypothermia that can be prevented by chronic treatment with norepinephrine. (A and B) Body weight of DT-treated male (A) and female (B) Lepob/ob mice (∼10 wk old, >40 g) and Lepob/ob;AgrpDTR/+ mice (∼10 wk old, >40 g). (C and D) Daily food intake by the mice described in A and B. All mice were raised on standard chow pellets. Error bars represent SEM. P < 0.01, ANOVA on body weight and food intake between the two groups (days 4–8). (E) Body weight of DT-treated Lepob/ob;AgrpDTR/+ mice (∼14 wk old, ∼55 g) subjected to chronic administration of norepinephrine (1.5 mg/kg/d) through a 14-d osmotic pump implanted s.c. (F) Body temperature of the mice described in E. †Mice removed from the study because they became moribund.
We suspected that decreased output of the sympathetic nervous system (SNS) might contribute to the mortality of these severely obese mice. Thus, before ablation of AgRP neurons in Lepob/ob;AgrpDTR/+ mice weighing ∼55 g, we implanted osmotic minipumps to deliver norepinephrine, one of the principle neurotransmitters of the SNS, to potentially promote thermogenesis by brown adipose tissue, stimulate vasoconstriction to conserve heat, and enhance lipolysis (18). This norepinephrine treatment prevented the severe drop in body temperature (Fig. 5F) and prolonged the survival of the obese mice after AgRP neuron ablation (Fig. 5E) compared with control obese mice that received only the vehicle.
Discussion
Loss of leptin or its receptor results in a severe, early-onset obesity phenotype associated with hyperphagia, diabetes, hypothermia, and infertility in rodents and humans (2, 19). Genetic experiments have established that the lack of leptin signaling in several different neuronal populations in the hypothalamus and elsewhere in the brain contribute to various aspects of the metabolic phenotype (5–7). AgRP neurons express leptin receptors, but their selective removal from AgRP neurons has minimal effects on body weight or metabolism and does not perturb fertility (20). The dramatic reversal of the metabolic phenotype of Lepob/ob mice and the restoration of fertility described here suggest that these deleterious effects of leptin deficiency are mediated to a large extent by AgRP neurons. Lepob/ob mice constitutively lacking MCH also have an improved metabolic phenotype (11), similar to that of mice lacking NPY (10). Thus, we anticipated that ablating MCH neurons in adult Lepob/ob mice also would attenuate the metabolic phenotype and provide evidence that AgRP and MCH neurons could be part of the same circuitry mediating the deleterious effects of leptin deficiency. However, ablation of MCH neurons had no effect on feeding or fertility, but did slightly improve body weight in male mice and glucose tolerance in both sexes, in agreement with previous results (11). Thus, the beneficial effects of ablating AgRP neurons appear to be largely independent of their potential regulation of MCH neurons.
We initially assumed that Lepob/ob mice weighing 35–40 g survived AgRP neuron ablation because these mice could live off their energy reserves, thereby allowing sufficient time for adaptation to occur. However, Lepob/ob mice with greater energy reserves (weight >40 g) succumbed. We noticed that the heavier Lepob/ob mice demonstrated a dramatic drop in body temperature at ∼1 wk after ablation that was not observed in the leaner mice. Perhaps the phenotype of Lepob/ob mice becomes more severe with increasing age or body weight and eventually becomes irreversible. The output of the sympathetic nervous system is important for stimulating lipolysis and regulation of body temperature (21, 22) and deteriorates with leptin deficiency (21). Thus, we hypothesized that the inability to mobilize energy reserves and escape from torpor when AgRP neurons are ablated in older Lepob/ob mice might account for their morbidity. Consistent with this hypothesis, chronic infusion of norepinephrine during the ablation period prevented the severe loss of body temperature and allowed the more obese Lepob/ob mice to survive longer. Body temperature was maintained in the obese Lepob/ob mice that were pair-fed with the AgRP neuron-ablated group and survived, indicating that the loss of AgRP neurons exacerbates the dysregulation of sympathetic nervous system with severe obesity. The loss of AgRP, NPY, and/or GABA signaling by AgRP neurons may lead to excessive activity of postsynaptic cells in the paraventricular nucleus or other upstream neurons that regulate sympathetic output (23, 24).
The restoration of fertility in Lepob/ob mice after AgRP ablation is remarkable. We focused on females because they are completely infertile, whereas young male Lepob/ob mice manifest limited fertility, especially on a calorie-restricted diet (3). Leptin replacement (3) or treatment with gonadotropins and progesterone (25) can partially restore fertility to female Lepob/ob mice. We anticipated seeing some effects of AgRP neuron ablation based on a previous observation of improved glucose regulation and fertility in Lepob/ob mice lacking NPY (10); however, because NPY is widely expressed, we could not attribute these effects to loss of NPY signaling from AgRP neurons. Diet-induced obesity impairs fertility (26); thus, the loss of body weight induced by AgRP neuron ablation might help restore fertility in Lepob/ob mice (26). However, other explanations are possible as well, given that fertility can be maintained in obese Lepob/ob mice without reducing body weight by impairing STAT3 signaling through the leptin receptor (27).
Fertility depends on the pulsatile release of gonadotropin-releasing hormone (GnRH) into the portal system to stimulate follicle-stimulating hormone and luteinizing hormone production and release by the pituitary, resulting in the stimulation of oocyte maturation and ovulation (28). The neuropeptide kisspeptin stimulates GnRH release (29), and loss of kisspeptin signaling impairs fertility (30; but see ref. 31). Infertility of Lepob/ob mice is associated with low expression of kisspeptins, low circulating gonadotropins, and dysregulation of GnRH release, but leptin does not appear to act directly on the neurons that produce kisspeptin or GnRH (32–34). Thus, the ability of leptin to hasten puberty and restore fertility to Lepob/ob mice might be mediated by its actions on neurons that regulate kisspeptin- and/or GnRH-releasing neurons. AgRP neurons are not necessary for fertility (35); nevertheless, their hyperactivity might prevent fertility, as occurs in Lepob/ob mice (36–38). Elevated release of AgRP, NPY, and/or GABA by AgRP neurons may be responsible for suppression of the gonadotropic axis (10, 16).
Murine gestation takes ∼20 d, and it takes at least 14 d for folliculogenesis to give rise to oocytes ready to ovulate (39); therefore, adequate gonadotropin production in females probably commences ∼34 d earlier than the birth of pups. Production of mature sperm starting from spermatagonial stem cells takes ∼35 d (40); thus, adequate gonadotropin production in males probably occurs ∼55 d before the birth of pups. Therefore, we estimate that hypothalamic-pituitary-gonadal axis activation occurs within 10–16 d after the first DT injection in female leptin-deficient mice and within 11–23 d in male leptin-deficient mice.
Ablation of AgRP neurons in adult mice leads to severe anorexia, resulting in starvation within 1 wk (15). Our previous experiments demonstrated that this lethal phenotype results from the loss of GABA signaling in the parabrachial nucleus and resulting hyperactivity of neurons in this brain region (16), rather than from the resulting activation of melanocortin signaling (41). Adult mice can adapt to the loss of AgRP neurons and resume feeding if the hyperactivity within the parabrachial nucleus is prevented either pharmacologically or genetically during a critical period of ∼10 d (16). Ablation of AgRP neurons before they become functionally mature also allows adaptation (15). Here we demonstrate another paradigm that allows adaptation. Mice with sufficient energy reserves and the ability to mobilize these reserves can survive on them while the adaptation process occurs. Because AgRP neurons are hyperactive in Lepob/ob mice, we suspect that loss of GABA signaling after their ablation also leads to hyperactivity within the parabrachial nucleus, but the mice can endure the complete anorexia of 12-d duration by mobilizing their adipose reserves. After the Lepob/ob mice adapt to the loss of AgRP neurons, their phenotype resembles that of normal mice, as reflected by their feeding, body weight, glucose metabolism, and fertility.
Materials and Methods
Animals and DT Treatment.
All animal experiments were approved by the University of Washington's Animal Care and Use Committee. Lepob/+ mice on a C57BL/6 background were obtained from Jackson Laboratories. AgrpDTR mice were produced and bred onto a C57BL/6 genetic background as described previously (15). PmchDTR mice were generated by PCR amplifying a 360-bp region 5′ of the initiation codon using primers that introduced a unique Kpn1 site at the initiation codon. This fragment was cloned into a targeting vector that contained PgkDTa and HSV-TK genes for negative selection and frt-flanked SvNeo for positive selection. The 5′ arm was extended to ∼9 kb by insertion of an XbaI fragment, after which a 4-kb 3′ arm was added. After linearization, the targeting construct was electroporated into G4 ES cells. Two positive colonies (out of 44) were identified by Southern blot analysis of ES cell DNA cut with Kpn1 using a probe just outside of the 5′ arm. One clone that was injected in C57BL/6 hosts gave germline transmission. These mice were bred with FLper mice to remove the frt-flanked SvNeo gene. Mice carrying the PmchDTR allele were subsequently identified by PCR using one primer in Pmch promoter and one primer in hDTR. These mice were backcrossed to C57BL/6 mice for more than six generations before being bred with Lepob/+ mice. Lepob/ob;AgrpDTR/+ and Lepob/ob;PmchDTR/+ mice were generated by double-heterozygote crosses.
Before behavioral experiments, mice were maintained on standard laboratory chow (5053; Lab Diet) that was available ad libitum. When the Lepob/ob;AgrpDTR/+ and Lepob/ob;PmchDTR/+ mice were ∼6 wk old (weighing 20–25 g), ∼8 wk old (weighing 35–40 g), or ∼10 wk old (weighing 40–45 g), they were individually housed and switched to another standard chow diet (D12450B; Research Diets) for 7 d before being injected with DT. To ablate AgRP or MCH neurons, DT was injected twice per mouse (i.m., 2 d apart; List Biologicals). Body weight and food intake were recorded daily for several weeks. The DT dose was determined based on body weight at the time of injection, with 50 μg/kg for mice weighing <40 g and 40 μg/kg for mice weighing >40 g. For some experiments, temperature sensor chips (IPTT-300; Bio Medic Data Systems) were inserted s.c. to allow daily recording of body temperature. Experiments were terminated if the body weight of leptin-deficient mice fell to 80% of that of age-matched Lepob/+ littermates or if the leptin-deficient mice appeared moribund.
Fertility was assessed by housing each male DT-treated mouse with two C57BL/6 females (2 mo old) and each DT-treated female with a WT C57BL/6 male (2 mo old). The dates on which litters were born, litter sizes, and body weights of the parents were recorded. Original C57BL/6 mates and litters were removed before fresh mates were provided to score continued fertility.
Minipump Delivery of Norepinephrine.
At 3 d before DT administration, osmotic pumps (Alzet model 1002; Durect) were loaded with norepinephrine (30 mM; Sigma-Aldrich) and ascorbate (2%; Sigma-Aldrich) to minimize oxidation and implanted under the skin along with a temperature-monitoring chip (IPTT-300; Bio Medic Data Systems). Norepinephrine was delivered continuously at a rate of 1.5 mg/kg/day for 14 d until the pumps were depleted.
Glucose Tolerance Tests.
At various times before and after DT treatment, glucose tolerance testing was performed by fasting mice overnight for 16 h, then sampling blood from the tail vein at 0, 15, 30, 60, and 120 min after the i.p. injection of d-glucose (1 g/kg). Blood glucose was measured with a FreeStyle Lite glucometer (Abbott Laboratories).
Determining the Extent of AgRP or MCH Neuron Ablation.
At the end of the experiment, mice were perfused transcardially with 4% paraformaldehyde and brains were collected to evaluate the extent of AgRP or MCH neuron ablation by one of two methods. In one method, frozen sections (25 μm thick) containing appropriate hypothalamic regions were immunostained with antibodies against AgRP (rabbit anti-AgRP, 1:2,000 dilution; Phoenix Pharmaceuticals), NPY (rabbit anti-NPY, 1:1,000 dilution; Peninsula Laboratories), or MCH (rabbit anti-MCH, 1:1,000 dilution; Phoenix Pharmaceuticals), followed by Cy2- or Cy3-conjugated secondary antibodies (1:300 dilution; Jackson ImmunoResearch). Then the number of positive cells per section (five sections per mouse) was compared with that in control mice. Alternatively, the hypothalamus was dissected from fixed brain, RNA was isolated using a High Pure FFPE RNA Micro Kit (Roche) and reverse-transcribed with a Brilliant II One-Step SYBR Green QRT-PCR Kit (Stratagene) according to the manufacturer's protocol, and quantitative real-time PCR (Mx3000P; Stratagene) was performed using primers specific for Agrp or Pmch mRNA, with Actb as an internal control. The abundance of Agrp or Pmch mRNA was calculated relative to that of Actb and compared with that in untreated control mice.
Supplementary Material
Acknowledgments
We thank Katie Battani and Jill Wang for help with maintaining the mouse colony and genotyping; Glenda Froelick and Patricia Smith for help with histology; and Dr. Robert Steiner and his laboratory members for many helpful discussions. The work was supported in part by National Institutes of Health Grant DA24908.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 2699.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120501109/-/DCSupplemental.
References
- 1.Coleman DL. A historical perspective on leptin. Nat Med. 2010;16:1097–1099. doi: 10.1038/nm1010-1097. [DOI] [PubMed] [Google Scholar]
- 2.Friedman JM. The function of leptin in nutrition, weight, and physiology. Nutr Rev. 2002;60(10 Pt 2):S1–S14. doi: 10.1301/002966402320634878. [DOI] [PubMed] [Google Scholar]
- 3.Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet. 1996;12:318–320. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- 4.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 5.Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol. 2005;493:63–71. doi: 10.1002/cne.20786. [DOI] [PubMed] [Google Scholar]
- 6.Israel D, Chua S., Jr Leptin receptor modulation of adiposity and fertility. Trends Endocrinol Metab. 2010;21:10–16. doi: 10.1016/j.tem.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myers MG, Jr, Münzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: More complicated than a simple ARC. Cell Metab. 2009;9:117–123. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qu D, et al. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 9.Wilding JP, et al. Increased neuropeptide-Y messenger ribonucleic acid (mRNA) and decreased neurotensin mRNA in the hypothalamus of the obese (ob/ob) mouse. Endocrinology. 1993;132:1939–1944. doi: 10.1210/endo.132.5.7682936. [DOI] [PubMed] [Google Scholar]
- 10.Erickson JC, Hollopeter G, Palmiter RD. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science. 1996;274:1704–1707. doi: 10.1126/science.274.5293.1704. [DOI] [PubMed] [Google Scholar]
- 11.Segal-Lieberman G, et al. Melanin-concentrating hormone is a critical mediator of the leptin-deficient phenotype. Proc Natl Acad Sci USA. 2003;100:10085–10090. doi: 10.1073/pnas.1633636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elias CF, et al. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 1998;402:442–459. [PubMed] [Google Scholar]
- 13.van den Pol AN, Acuna-Goycolea C, Clark KR, Ghosh PK. Physiological properties of hypothalamic MCH neurons identified with selective expression of reporter gene after recombinant virus infection. Neuron. 2004;42:635–652. doi: 10.1016/s0896-6273(04)00251-x. [DOI] [PubMed] [Google Scholar]
- 14.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 15.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 16.Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubuc PU. The development of obesity, hyperinsulinemia, and hyperglycemia in ob/ob mice. Metabolism. 1976;25:1567–1574. doi: 10.1016/0026-0495(76)90109-8. [DOI] [PubMed] [Google Scholar]
- 18.Thomas SA, Palmiter RD. Thermoregulatory and metabolic phenotypes of mice lacking noradrenaline and adrenaline. Nature. 1997;387:94–97. doi: 10.1038/387094a0. [DOI] [PubMed] [Google Scholar]
- 19.Farooqi IS, O'Rahilly S. Leptin: A pivotal regulator of human energy homeostasis. Am J Clin Nutr. 2009;89:980S–984S. doi: 10.3945/ajcn.2008.26788C. [DOI] [PubMed] [Google Scholar]
- 20.van de Wall E, et al. Collective and individual functions of leptin receptor-modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149:1773–1785. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bray GA, York DA. The MONA LISA hypothesis in the time of leptin. Recent Prog Horm Res. 1998;53:95––117. discussion 117–118. [PubMed] [Google Scholar]
- 22.Swoap SJ. The pharmacology and molecular mechanisms underlying temperature regulation and torpor. Biochem Pharmacol. 2008;76:817–824. doi: 10.1016/j.bcp.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stanley S, et al. Identification of neuronal subpopulations that project from hypothalamus to both liver and adipose tissue polysynaptically. Proc Natl Acad Sci USA. 2010;107:7024–7029. doi: 10.1073/pnas.1002790107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song CK, et al. Melanocortin-4 receptor mRNA expressed in sympathetic outflow neurons to brown adipose tissue: Neuroanatomical and functional evidence. Am J Physiol Regul Integr Comp Physiol. 2008;295:R417–R428. doi: 10.1152/ajpregu.00174.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pineda R, Aguilar E, Pinilla L, Tena-Sempere M. Physiological roles of the kisspeptin/GPR54 system in the neuroendocrine control of reproduction. Prog Brain Res. 2010;181:55–77. doi: 10.1016/S0079-6123(08)81005-9. [DOI] [PubMed] [Google Scholar]
- 26.Brothers KJ, et al. Rescue of obesity-induced infertility in female mice due to a pituitary-specific knockout of the insulin receptor. Cell Metab. 2010;12:295–305. doi: 10.1016/j.cmet.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bates SH, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 28.Tsutsumi R, Webster NJ. GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocr J. 2009;56:729–737. doi: 10.1507/endocrj.k09e-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gottsch ML, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 30.Gianetti E, Seminara S. Kisspeptin and KISS1R: A critical pathway in the reproductive system. Reproduction. 2008;136:295–301. doi: 10.1530/REP-08-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayer C, Boehm U. Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nat Neurosci. 2011;14:704–710. doi: 10.1038/nn.2818. [DOI] [PubMed] [Google Scholar]
- 32.Luque RM, Kineman RD, Tena-Sempere M. Regulation of hypothalamic expression of KiSS-1 and GPR54 genes by metabolic factors: Analyses using mouse models and a cell line. Endocrinology. 2007;148:4601–4611. doi: 10.1210/en.2007-0500. [DOI] [PubMed] [Google Scholar]
- 33.Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18:298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- 34.Donato J, Jr, et al. Leptin's effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest. 2011;121:355–368. doi: 10.1172/JCI45106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phillips CT, Palmiter RD. Role of agouti-related protein-expressing neurons in lactation. Endocrinology. 2008;149:544–550. doi: 10.1210/en.2007-1153. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz MW, et al. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes. 1996;45:531–535. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- 37.Shutter JR, et al. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev. 1997;11:593–602. doi: 10.1101/gad.11.5.593. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi KA, Cone RD. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology. 2005;146:1043–1047. doi: 10.1210/en.2004-1397. [DOI] [PubMed] [Google Scholar]
- 39.Barash IA, et al. Leptin is a metabolic signal to the reproductive system. Endocrinology. 1996;137:3144–3147. doi: 10.1210/endo.137.7.8770941. [DOI] [PubMed] [Google Scholar]
- 40.Clermont Y. Kinetics of spermatogenesis in mammals: Seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52:198–236. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- 41.Wu Q, Howell MP, Cowley MA, Palmiter RD. Starvation after AgRP neuron ablation is independent of melanocortin signaling. Proc Natl Acad Sci USA. 2008;105:2687–2692. doi: 10.1073/pnas.0712062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.