Abstract
We examined the effects of an inhibitor of PI3K, XL147, against human breast cancer cell lines with constitutive PI3K activation. Treatment with XL147 resulted in dose-dependent inhibition of cell growth and levels of pAKT and pS6, signal transducers in the PI3K/AKT/TOR pathway. In HER2-overexpressing cells, inhibition of PI3K was followed by up-regulation of expression and phosphorylation of multiple receptor tyrosine kinases, including HER3. Knockdown of FoxO1 and FoxO3a transcription factors suppressed the induction of HER3, InsR, IGF1R, and FGFR2 mRNAs upon inhibition of PI3K. In HER2+ cells, knockdown of HER3 with siRNA or cotreatment with the HER2 inhibitors trastuzumab or lapatinib enhanced XL147-induced cell death and inhibition of pAKT and pS6. Trastuzumab and lapatinib each synergized with XL147 for inhibition of pAKT and growth of established BT474 xenografts. These data suggest that PI3K antagonists will inhibit AKT and relieve suppression of receptor tyrosine kinase expression and their activity. Relief of this feedback limits the sustained inhibition of the PI3K/AKT pathway and attenuates the response to these agents. As a result, PI3K pathway inhibitors may have limited clinical activity overall if used as single agents. In patients with HER2-overexpressing breast cancer, PI3K inhibitors should be used in combination with HER2/HER3 antagonists.
Keywords: signaling, targeted therapy
PI3K transmits signals from ligand-activated receptor tyrosine kinases (RTKs) to intracellular molecules that regulate growth, metabolism, cell size, motility, and survival. In turn, PI3K catalyzes the phosphorylation of phosphatidylinositol 4,5-bisphosphate to produce the second messenger phosphatidylinositol-3,4,5-trisphosphate (PIP3) (1, 2). Several pleckstrin homology domain-containing proteins, including AKT and PDK1, bind to PIP3 at the plasma membrane. Phosphorylation of AKT at T308 by PDK1 and at S473 by a complex involving mTOR/Rictor (i.e., TORC2) results in the full activation of this enzyme. AKT facilitates survival and cell cycle entry by phosphorylation of proteins including GSK3α/β, FoxO transcription factors, MDM2, BAD, and p27KIP1 (3). In addition, AKT regulates protein synthesis and cell growth via activation of the mTOR/Raptor (i.e., TORC1) complex (4, 5).
PI3K/AKT is arguably the most commonly altered pathway in human cancers (6, 7). Gain-of-function mutations in PIK3CA, the gene encoding the class IA PI3K catalytic subunit p110α, are frequently present in multiple human tumors (8). Second, the PIP3 phosphatase PTEN is a tumor suppressor frequently inactivated by mutation, gene deletion, and promoter methylation (9). Further, PI3K is potently activated by oncogenes like mutant Ras and tyrosine kinases such as Bcr-Abl, HER2 (ErbB2), MET, and KIT, among others (1). Therefore, a large group of tumors with molecular alterations in the PI3K/AKT pathway is therapeutically targetable with PI3K inhibitors.
Several PI3K pathway antagonists have been developed. These include ATP mimetics that bind reversibly in the ATP pocket of the kinase domain of WT and mutant p110 (10, 11). Herein, we examined the effect of XL147 (an ATP-competitive reversible PI3K inhibitor; Exelixis) against a panel of human breast cancer cell lines harboring molecular alterations indicative of PI3K dependence, such as HER2 gene amplification, PIK3CA mutation, and/or loss of PTEN. XL147 has recently completed phase I clinical development; it exhibits an IC50 against WT and mutant p110α of approximately 40 nM (12).
In a panel of HER2-overexpressing human breast cancer cell lines, treatment with XL147 abrogated AKT and S6 phosphorylation but also induced the expression and phosphorylation of HER3 and other RTKs. The increase in mRNA of these RTKs depended on the Forkhead transcription factors FoxO1 and FoxO3a, which are negatively regulated by AKT (13). In HER2+ cells, phosphorylation of HER3 was maintained by the HER2 tyrosine kinase, resulting in partial recovery of phosphorylated AKT (pAKT) and thereby limiting the antitumor action of XL147. Knockdown of HER3 or treatment with the anti-HER2 agents trastuzumab or lapatinib sensitized HER2+ breast cancer cells to XL147 in vitro and in vivo. These data suggest that because of relief of FoxO-mediated feedback, therapeutic inhibitors of PI3K will have limited clinical activity if used as single agents. Thus, to maximally disable PI3K/AKT signaling, therapies targeted against HER2/HER3 should be added to PI3K inhibitors in HER2-dependent cells.
Results
Inhibition of PI3K Is Associated with Induction of HER3 and pHER3.
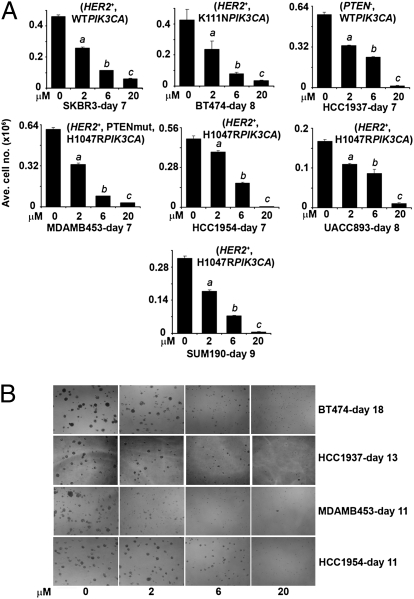
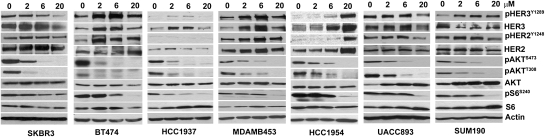
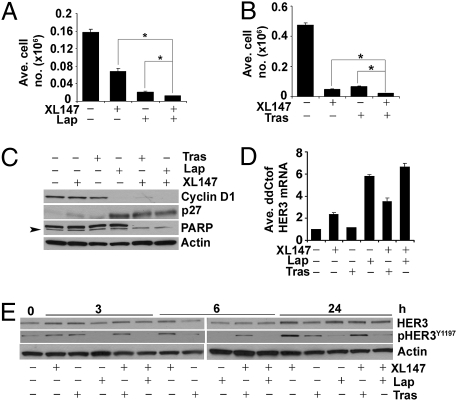
We treated with XL147 a panel of breast cancer cell lines with dysregulated PI3K activity. As XL147 binds to serum proteins with high affinity, we conducted most studies in 2.5% FBS-containing media. Treatment with XL147 inhibited the monolayer growth of all cell lines in a dose-dependent manner (Fig. 1A). A similar IC50 of 6 μM or less was observed in cells tested in 3D (Fig. 1B). Analysis of the growth curves by using the initial amount of cells at the start of treatment as baseline indicated that at the IC50 of approximately 6 μM, the main effect of XL147 was inhibition of cell proliferation. At 20 μM, however, XL147 induced cell death as revealed by the reduction in cell number below the baseline (Fig. S1A) (14). This was further confirmed by immunoblot of biomarkers of cell death and G1–S transition in cells treated with XL147 for 24 h (Fig. S1B). In all cell lines, treatment with XL147 resulted in dose-dependent inhibition of PI3K as measured by pAKTS473. Consistent with the inhibition of cell proliferation, XL147 induced a reduction in cyclin D1 and pRB and an increase in levels of the CDK inhibitor p27KIP1 but no detectable change in levels of total or cleaved poly (ADP-ribose) polymerase (PARP), a biomarker of cell death (Fig. S1B). Focusing on molecules in the PI3K and mTOR pathways, treatment with XL147 resulted in a dose-dependent reduction in pAKTS473/T308 and pS6S240/244. Surprisingly, in six of seven cell lines, XL147 also caused up-regulation of total HER3 and/or pHER3Y1289 levels. In five of six HER2-overexpressing cell lines, total HER2 and/or pHER2Y1248 were also modestly up-regulated upon inhibition of PI3K (Fig. 2).
Fig. 1.
XL147 inhibits cell growth in a dose-dependent manner. (A) Breast cancer cell lines with different lesions in the PI3K pathway (as noted within parentheses) were treated with 0 to 20 μM XL147 and counted on the days indicated. Each bar represents the mean ± SE of six replicates (a,b,cP < 0.05 vs. 0 μM XL147, paired t test). (B) Cells were cultured in Matrigel with or without 0 to 20 μM XL147 and photographed (magnification 10×) on indicated days.
Fig. 2.
XL147-mediated inhibition of PI3K associates with induction of HER3 and pHER3. Cells lines were harvested after overnight treatment with 0 to 20 μM XL147 in serum-free medium followed by immunoblot analysis with indicated antibodies.
XL147-Induced Up-Regulation of HER3 Depends on FoxO-Mediated Transcription.
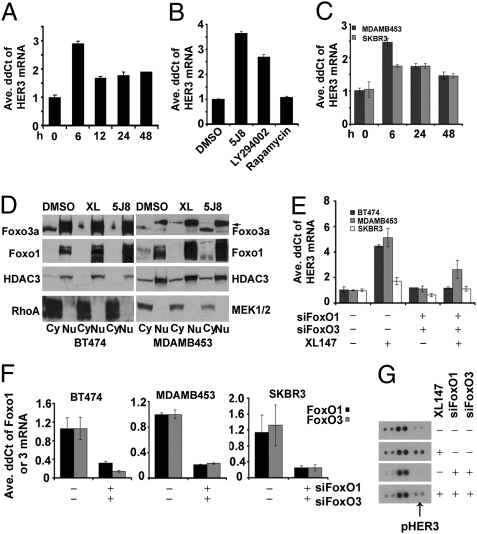
We next determined the half-life of the HER3 protein by using cycloheximide, an inhibitor of protein synthesis. The rate of decay of HER3 was not different in BT474 cells with or without 6 μM XL147. We then quantified the levels of HER3 mRNA upon inhibition of PI3K over a time course in XL147-treated cells. BT474, SKBR3, and MDA453 cells depicted a maximal increase in HER3 mRNA at 6 h, which was maintained as long as 48 h after the addition of the inhibitor (Fig. 3 A and C). The allosteric inhibitor of AKT1/2 5J8 (15) and the PI3K inhibitor LY294002 (16), but not the mTOR inhibitor rapamycin, also up-regulated HER3 mRNA (Fig. 3B).
Fig. 3.
XL147-induced up-regulation of HER3 transcription is dependent on FoxO. (A) BT474 cells were treated with 6 μM XL147 for the indicated times before RNA isolation and real-time qPCR with HER3-specific primers. (B) BT474 cells were treated with 2 μM 5J8, 20 μM LY294002, and 50 nM rapamycin for 10 h before RNA isolation and qPCR for HER3. (C) MDA453 and SKBR3 cells were treated with 6 μM XL147 for as long as 48 h before RNA isolation and qPCR for HER3. For A–C, each bar represents the mean ± SE of three wells. (D) BT474 and MDA453 cells were treated with DMSO, 6 μM XL147, or 2 μM 5J8 for 4 h. Nuclear and cytoplasmic extracts were subjected to immunoblot analysis with FoxO1 and FoxO3a antibodies. Loading controls for nuclear extracts: HDAC3; for cytoplasmic extracts: RhoA (BT474) and MEK1/2 (MDA453). Arrow indicates the FoxO3a-specific band. (E and F) BT474, MDA453, and SKBR3 cells were transfected with control or FoxO1- and FoxO3a-specific siRNA duplexes followed by treatment with XL147 for 6 h before harvesting, RNA isolation and qPCR for HER3 (E) or FoxO1 and FoxO3a (F). Each bar represents the mean ± SE of triplicate wells. (G) BT474 cells were transfected with control or FoxO1 and FoxO3a siRNA and treated with XL147 for 6 h. Cell lysates were used for hybridization with pRTK arrays.
To delineate the mechanisms of HER3 transcriptional activation, we focused on the FoxO transcription factors FoxO1, FoxO3a, and FoxO4. AKT regulates the subcellular localization of these molecules by phosphorylation, thereby preventing them from translocating to the nucleus and regulating transcription (13). In absence of AKT activity, FoxO factors are predominantly nuclear and therefore can activate transcription (13). Of note, by using the PROMO database, we identified multiple putative FoxO-binding sites in the HER3 promoter (up to 5,000 bp upstream of the transcription start site) (17).
We next determined the subcellular distribution of FoxO proteins following inhibition of PI3K and AKT with XL147 and 5J8, respectively. FoxO4 was almost undetectable; thus, we focused on FoxO1 and FoxO3a. Treatment with XL147 and 5J8 resulted in accumulation of both FoxO factors in the nucleus of BT474 and MDA453 cells, sometimes accompanied by a reduction in the baseline levels in the cytosol (Fig. 3D). This was not observed with rapamycin (Fig. S2A). To determine if FoxO is involved in the increase of HER3 transcription, we transfected BT474, SKBR3, and MDA453 cells with FoxO1- and FoxO3a-specific siRNA duplexes. In all three cell lines, the siRNAs reduced both FoxO mRNAs by 70% to 80% (Fig. 3F) and abrogated the XL147-induced increase in HER3 mRNA as measured by quantitative PCR (qPCR; Fig. 3E). To further confirm this FoxO-mediated effect, we transfected BT474 cells with FoxO1 and FoxO3a siRNA duplexes and then treated them with DMSO or XL147 for 24 h. Cell lysates were then hybridized to arrays containing probes for 42 different phosphorylated receptor tyrosine kinases (pRTKs). There was a decrease in pHER3 signal in XL147-treated cells in which FoxO1 and FoxO3a had been knocked down compared with cells transfected with control oligonucleotides (Fig. 3G).
Knockdown of Compensatory Feedback to HER3 Sensitizes to PI3K Inhibition.
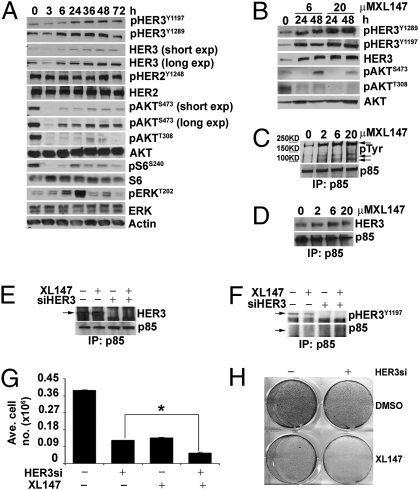
A time course of BT474 cells treated with XL147 for 0 to 72 h revealed time-dependent up-regulation of total HER3, HER3 phosphorylated at Y1197 and Y1289 (two PI3K binding sites (18)], T308 and S473 pAKT, and pS6 levels (Fig. 4A), implying partial recovery of PI3K/AKT/mTOR signaling. Although pHER3 is known to activate ERK/MAPK via its interaction with Shc (18), we did not detect consistent recovery of pERK upon reactivation of HER3 (Fig. 4A). Even though recovery of pAKT was less with a suprapharmacological dose of 20 μM, feedback up-regulation of total HER3 and pHER3 was more noticeable with this dose of XL147, further suggesting inhibition of PI3K was causal to the reactivation of HER3 (Fig. 4B). These data imply that, upon inhibition of PI3K, cells partially restore HER3 phosphorylation to maintain membrane-bound p85/p110 and some level of PIP3 which, in turn, may limit the net inhibitory effect of the PI3K inhibitor.
Fig. 4.
Knockdown of compensatory feedback to HER3 sensitizes to PI3K inhibitor. (A) BT474 cells were treated with 6 μM XL147 for as long as 72 h and subjected to immunoblot analysis. (B) Immunoblot of lysates from BT474 cells treated with 6 or 20 μM XL147 for 0 to 48 h. (C and D) BT474 cells were treated with 0 to 20 μM XL147 for 24 h and lysed. Cell lysates (0.5 mg) were subjected to immunoprecipitation with a p85 antibody followed by immunoblot for p85 and pTyr (C) or p85 and HER3 (D). Arrows in C indicate p85-associated pTyr bands. (E and F) BT474 cells were transfected with control or HER3-specific siRNA duplexes and treated with 6 μM XL147 for 24 h. Cell lysates (0.5 mg) were subjected to immunoprecipitation with a p85 antibody followed by immunoblot with HER3 (E), pHER3Y1197 (F), and p85 (E and F) antibodies. (G and H) BT474 cells were transfected with HER3-specific siRNA and treated with DMSO or 2 μM XL147. Cells were harvested for counting (G) or crystal violet staining (H) on day 6. In G, each bar represents the mean ± SE of six replicates (*P < 0.05, paired t test).
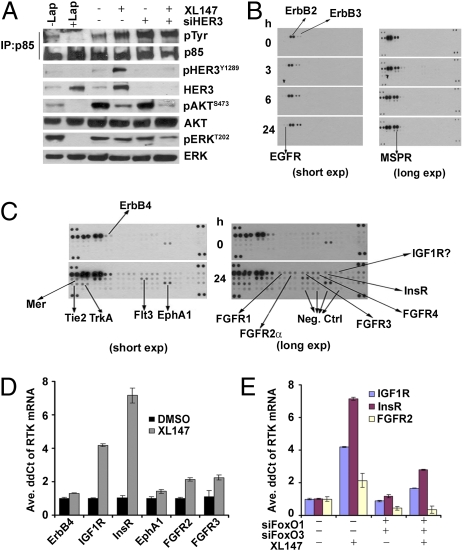
In HER2-overexpressing cells, the major mechanism of PI3K activation is the coupling of pHER3 to an N-terminal SH2 domain in p85, the regulatory subunit of PI3K (19, 20). In these cells, the main tyrosine phosphorylated protein precipitated with p85 antibodies is pHER3. This HER3 and p85 association depends on the catalytic activity of HER2 as it is disrupted by HER2 tyrosine kinase inhibitors (TKIs) (21, 22). Thus, we examined if, upon inhibition of PI3K, there was maintenance or recovery of the HER3/p85 association. BT474 cells were treated with increasing concentrations of XL147 followed by pull-down assay with p85 antibodies and subsequent pTyr and HER3 immunoblot. After XL147 treatment, there was a dose-dependent increase of an approximately 200-kDa major p85-associated pTyr band as well as other smaller and less abundant pTyr proteins (Fig. 4C, arrows). The p85-associated approximately 200-kDa band was also detected with HER3 and pHER3Y1197 antibodies (Fig. 4 D and F). Knockdown of HER3 with siRNA eliminated the HER3 and pHER3Y1197 band in the p85 pull-downs of lysates from XL147-treated cells, further confirming HER3 at least as a component of the approximately 200-kDa pTyr band (Fig. 4 E and F).
The compensatory up-regulation of total HER3 and partial maintenance of pHER3 and pAKT upon inhibition of PI3K suggested that combined inhibition of HER3 and PI3K would synergistically inhibit tumor cell viability. Therefore, we transfected BT474 cells with control or HER3 siRNA duplexes, treated them with XL147, and measured cell growth. Cell proliferation was significantly reduced by a combination of HER3 knockdown and XL147 compared with either intervention alone (Fig. 4 G and H). Consistent with a synergistic proapoptotic effect, the combination induced a greater proportion of cells in the sub-G1 phase DNA fraction as well as PARP cleavage (Fig. S3 A and B) compared with either treatment alone. Similar results were obtained with MDA453 and SKBR3 cells (Fig. S3 D and F). In both these cell lines, the combination of HER3 knockdown and XL147 inhibited pAKT and pS6 more effectively than each treatment alone (Fig. S3 C and E).
Recovery of HER3 Phosphorylation Depends on HER2.
In breast cancer cells with HER2 gene amplification, HER2 is the main kinase that phosphorylates HER3 (19, 22). As XL147 does not affect the catalytic activity of HER2 (Fig. 2), it is logical to speculate that, in HER2-overexpressing cells, HER2 remains as the kinase maintaining pHER3 upon inhibition of PI3K. Therefore, we examined the effect of XL147 in combination with the HER2 antibody trastuzumab or the HER2 TKI lapatinib. In BT474 cells, either of these combinations was significantly more effective at inhibiting cell proliferation (Fig. 5 A and B) or PARP cleavage (Fig. 5C) than XL147 or each HER2 antagonist alone. Similar results were observed with MDA453 and SKBR3 cells (Fig. S4).
Fig. 5.
Recovery of HER3 phosphorylation depends on HER2 and is limited by HER2 inhibitors. (A and B) BT474 cells were treated with 2 μM XL147 alone or in combination with 0.1 μM lapatinib (Lap) (A) or 10 μg/mL trastuzumab (Tras) (B) and counted after 6 d (A) or 8 d (B). Each bar represents mean ± SE of six replicates. (C) Immunoblot of biomarkers of apoptosis and G1–S phase transition with lysates from BT474 cells treated with the indicated inhibitors for 72 h. (D) Real-time qPCR for HER3 mRNA in cells treated with XL147 (6 μM), Lap (1 μM), Tras (10 μg/mL), or the indicated combinations for 10 h. Each bar represents mean ± SE of triplicate wells. (E) Total HER3 and pHER3 immunoblot of lysates from BT474 cells treated over a time course (0–24 h) with the indicated inhibitors at similar concentrations as in D.
We speculated that the synergistic action of XL147 in combination with lapatinib or trastuzumab on cell growth was caused by a reduction in the recovery of pHER3 but not inhibition of HER3 mRNA transcription. Thus, we performed qPCR for HER3 in RNA from BT474 cells treated with XL147 plus lapatinib, trastuzumab, or both. Treatment with trastuzumab alone did not have any significant effect on HER3 mRNA levels, but in combination with XL147, it enhanced the up-regulation of HER3 RNA mediated by the PI3K inhibitor (Fig. 5D). As shown previously, lapatinib induced HER3 mRNA (23). However, the effect of lapatinib was more prominent when used in combination with XL147 (Fig. 5D), possibly because of a more pronounced inhibition of PI3K/AKT compared with either agent alone. Treatment with XL147 plus lapatinib or plus trastuzumab attenuated the recovery of pHER3 compared with cells treated with XL147 alone (Fig. 5E), suggesting that inhibition of the HER2 kinase with lapatinib or of ligand-independent HER2/HER3 dimers with trastuzumab limits the HER2-mediated activation of HER3 upon inhibition of PI3K/AKT.
Combined Inhibition of HER2 and PI3K Is Synergistic in Vivo.
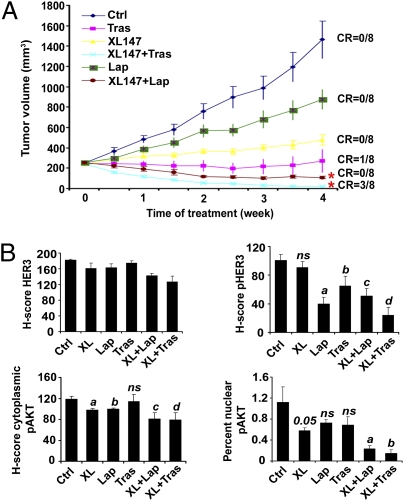
Based on the in vitro data, we proposed that addition of trastuzumab or lapatinib would enhance the antitumor effect of XL147 against HER2-dependent xenografts. Athymic mice bearing BT474 xenografts were randomized to therapy with XL147, lapatinib, trastuzumab, or XL147 plus each HER2 antagonist. Each monotherapy significantly delayed tumor growth with trastuzumab being the only agent that induced a complete tumor regression in one of eight mice. Both combinations were superior to the respective drugs given alone. Of note, the combination of trastuzumab and XL147, but not lapatinib and XL147, induced a complete tumor response in three of eight mice (P < 0.05; Fig. 6A). There was no significant drug-related toxicity in any of the treatment arms.
Fig. 6.
Combined inhibition of HER2 and PI3K is synergistic in vivo. (A) Tumor growth curve from nude mice transplanted with BT474 cells and treated with vehicle (Ctrl), XL147, lapatinib, trastuzumab, or the indicated drug combinations for 28 d. Each data point represents the mean tumor volume (in mm3) ± SE of eight mice per treatment (CR, complete response to treatment; *P < 0.05 for trastuzumab vs. trastuzumab plus XL147 and lapatinib vs. lapatinib plus XL147, two-way ANOVA). (B) Histoscore (H-score) analysis of immunohistochemical sections. Each bar represents mean ± SE (*P < 0.05 vs. control, unpaired t test).
We next examined pharmacodynamic biomarkers of target inactivation after 28 d of treatment. Treatment with XL147 for 28 d did not change pHER3 levels, whereas lapatinib was more effective than trastuzumab in reducing pHER3. The combination of XL147 plus trastuzumab inhibited pHER3 more potently than any of the other treatments (Fig. 6B and Fig. S5). The oncogenic action of AKT has been shown to correlate with cytoplasmic and nuclear pAKTS473 levels (24). Therefore, we quantitated pAKTS473 in both cellular compartments. Consistent with differences in tumor growth among treatment arms, nuclear pAKT was lower in tumors treated with XL147 plus lapatinib or XL147 plus trastuzumab compared with tumors treated with single agents. Of all three single drugs, XL147 was the only one shown statistically to inhibit nuclear pAKT levels. There were no detectable changes in cytoplasmic pAKT levels (Fig. 6B and Fig. S5). These results suggest that combined inhibition of HER2 and PI3K in HER2-dependent xenografts is required to maximally inhibit signaling output of the PI3K/AKT pathway. The levels of total HER3 observed after 28 d of treatment (Fig. 6B and Fig. S5, top row) did not reflect the up-regulation of HER3 mRNA and protein after short-term assays in cells in culture. We speculate this could be the result of the late timing of the analysis of the xenografts.
Inhibition of PI3K Induces RTKs Other than HER3.
In Fig. 4C we showed that, after XL147 treatment of BT474 cells, there is an increase in p85-associated pTyr proteins. We also showed that a prominent pTyr band of approximately 200 kDa that coprecipitates with p85 (PI3K) is recognized by HER3 and pHER3 antibodies (Fig. 4 D–F) and is eliminated upon knockdown of HER3 with siRNAs (Fig. 4 E and F). However, the dose-dependent increase in the lower molecular weight pTyr bands when PI3K is inhibited (Fig. 4C) suggested an increase in p85-associated proteins other than HER3. In addition, results in Fig. 7A showed that, upon knockdown of HER3, there is still an increase in the p85-associated approximately 200-kDa pTyr band, leading further to speculation of the presence of other compensatory p85-associated tyrosine kinases and/or adaptors aimed at partially maintaining PI3K as active. To test this hypothesis we hybridized to pRTK arrays two different concentrations (high and low) of lysates from BT474 cells treated over a 24-h time course with XL147. Treatment with XL147 resulted in an increase in the phosphorylation of HER3 and multiple other RTKs, including EGFR, ERBB4/HER4, FGFR1, FGFR2, FGFR3, FGFR4, InsR, IGF1R, EphA1, Tie2, TrkA, Flt3, MER, and macrophage-stimulating protein receptor (Fig. 7 B and C). Several of these RTKs, such as EGFR and ERBB4, migrate at approximately 200 kDa, thus potentially explaining the persistent high molecular weight pTyr band associated with p85 in cells in which HER3 has been knocked down (Fig. 7A). We then performed qPCR analysis to determine whether these changes occurred at the transcriptional level. Following treatment of BT474 cells with XL147, there was an increase in ERBB4, IGF1R, InsR, EphA1, FGFR2, and FGFR3 mRNAs, with IGF1R and InsR being the most prominent (Fig. 7D).
Fig. 7.
Inhibition of PI3K induces RTKs other than HER3. (A) BT474 cells were transfected with HER3 siRNA duplexes and treated with XL147 for 24 h. Cell lysates (0.5 mg) were precipitated with a p85 antibody, followed by immunoblot analysis with antibodies indicated to the right. Cell lysates from BT474 cells treated with 1 μM lapatinib for 6 h were used as positive controls (lanes 1 and 2). (B and C) BT474 cells were treated with 6 μM XL147 over a time course to 24 h as indicated. Cell lysates were prepared and 0.2 mg (lower sensitivity; B) or 0.5 mg (higher sensitivity; C) of total protein were applied to pRTK arrays. Arrows indicate RTKs whose phosphorylation was up-regulated on treatment with the PI3K inhibitor. (D) Real-time qPCR analysis of the indicated RTKs in RNA collected from cells treated with DMSO or 10 μM XL147 for 6 h. (E) Analysis of IGF1R, InsR, and FGFR2 mRNA by qPCR in RNA extracted from BT474 cells transfected with FoxO1 and FoxO3a siRNA and then treated with 10 μM XL147 for 6 h. Each bar represents the mean ± SE of triplicate wells.
Computational analyses revealed multiple putative FoxO-binding sites in the INSR, IGF1R, and FGFR2 gene promoters (17). Like for HER3 (Fig.3E), knockdown of FoxO and FoxO3a with siRNA limited the induction of IGF1R, InsR, and FGFR2 mRNAs (Fig. 7E) in cells treated with XL147. Finally, to determine the potential therapeutic relevance of this feedback, we examined whether depletion of these RTKs sensitized cells to the PI3K inhibitor. We transfected BT474, MDA453, and (low HER2) MCF7 cells with IGF1R and InsR siRNAs followed by treatment with XL147. Knockdown of both RTKs was effective in all three cell lines (Fig. S6 A and B) and depletion of either RTK sensitized all cells to XL147-mediated growth inhibition (Fig. S6 C–E). Collectively, these data suggest that cancer cells limit the inhibition of PI3K by feedback mechanisms that up-regulate multiple RTKs or adaptors capable of engaging p85 and thus activating PI3K. In turn, these molecules partially maintain PI3K/AKT signaling and counteract the antitumor effect of single-agent therapeutic inhibitors of this pathway.
Discussion
We have examined the effects of the PI3K inhibitor XL147 on a panel of breast cancer cell lines with molecular alterations indicative of PI3K dependence. In this report, we mainly focused on breast cancer cells with HER2 gene amplification. In addition to the inhibition of AKT and mTOR, XL147 treatment resulted in time-dependent feedback up-regulation of HER3 expression and phosphorylation. In turn, pHER3 engaged p85, activated PI3K, and induced partial recovery of pAKT and pS6 (Figs. 2 and 4 and Fig. S3 C and E). As AKT phosphorylates FoxO transcription factors, thus preventing their nuclear translocation (13), the inhibition of AKT by XL147 resulted in accumulation of FoxO3a and FoxO1 in the nucleus (Fig. 3D). Knockdown of FoxO1 and FoxO3a suppressed the induction of HER3 mRNA (Fig. 3E) and pHER3 (Fig. 3G) upon inhibition of PI3K/AKT with XL147. These results suggest that HER3 is down-regulated by PI3K/AKT and are consistent a recent observation in ovarian cancer cells in which the PI3K inhibitor GDC-0941 blocked down-regulation of the HER3 mRNA upon treatment with the HER3-activating ligand heregulin (25).
RNAi of HER3 sensitized to XL147-induced cell death (Fig. S3 A and B) and enhanced XL147-mediated inhibition of pAKT and pS6 (Fig. S3 C and E), suggesting that the up-regulation of total and pHER3 limited the antitumor effect of XL147. This result has particular relevance in HER2-overexpressing cells in which the kinase-deficient HER3 coreceptor, when it has been dimerized with and activated by HER2, is the key mechanism engaging p85 and activating PI3K. Indeed, breast cancer cells with HER2 amplification are particularly sensitive to apoptosis induced by PI3K inhibitors (14). In addition, this association of HER2/HER3 dimer with p85 has been found to be essential for the viability of HER2-dependent cells (22, 26, 27).
As inhibition of PI3K with XL147 did not affect the HER2 kinase, HER3 phosphorylation was maintained and further increased, remaining associated with p85 in XL147-treated cells (Fig. 4 D–F). Addition of the HER2 antibody trastuzumab or the HER2 TKI lapatinib attenuated the recovery of pHER3 upon treatment with XL147 (Fig. 5E) even though HER3 transcription was further increased by the combinations of inhibitors compared with each inhibitor alone (Fig. 5D). Also, both combinations of XL147 with each HER2 antagonist were also more effective than each inhibitor alone at reducing pHER3 and pAKT and inhibiting growth of HER2+ xenografts (Fig. 6). These data suggest that, in HER2-overexpressing cells, inhibition of the HER2 kinase with lapatinib or disruption of ligand-independent HER2/HER3 dimers with trastuzumab (27) limits the activating input of HER2 to the up-regulated HER3 coreceptor when PI3K is inhibited.
These data are reminiscent of reports in which inhibition of mTOR with rapamycin or “rapalogues” relieves suppression of insulin and IGF-I receptor signaling via up-regulation of IRS-1, thereby reactivating PI3K/AKT (28, 29). In addition, dual inhibitors of TORC1 and PI3K have also been shown to induce HER3 expression (23). However, the TORC1 inhibitor rapamycin did not mirror the effect of XL147 or the AKT inhibitor 5J8 on HER3 mRNA (Fig. 3B and Fig. S2B). This suggests that the effects of AKT inhibition on FoxO-mediated feedback up-regulation of HER3 transcription are not mediated by TORC1.
These data have implications that apply to therapeutic inhibitors of RTKs that rely on PI3K/AKT, such as HER2. For example, trastuzumab, when given alone, is a weak inhibitor of AKT (26, 27, 30) (Fig. 6B and Fig. S5) and thus does not relieve AKT-mediated suppression of FoxO-induced HER3 transcription. On the contrary, the prompt and strong inhibition of pAKT with lapatinib results in strong up-regulation of HER3 expression (Fig. 5D). The combination of lapatinib and XL147 was synergistic in vitro (Fig. 5A and Fig. S4 A and C) and in vivo (Fig. 6A), but was also the most potent at inducing HER3 mRNA (Fig. 5D). Despite the weaker inhibition of pAKT by trastuzumab, the combination of XL147 and trastuzumab appeared to be superior to the combination of XL147 and lapatinib at inhibiting pAKT and tumor growth (Fig. 6 and Fig. S5). These differences suggest that the inability of trastuzumab to derepress compensatory HER3 expression is a possible advantage of trastuzumab versus lapatinib. Current trials comparing both inhibitors may shed light on the clinical relevance of these differences.
We detected an increase in p85-associated pTyr proteins on inhibition of PI3K with XL147. Indeed, a p85-associated P-Tyr band of approximately 200 kDa that comigrates with HER3 was still detectable in XL147-treated BT474 cells after HER3 had been knocked down with siRNA (Fig. 7A), suggesting the presence of other compensatory RTKs and/or adaptors aimed at partially maintaining PI3K active. By using RTK arrays and siRNA knockdown, we found FoxO-dependent up-regulation of InsR, IGF1R, and FGFR2 after inhibition of PI3K/AKT with XL147 (Fig. 7 C–E). In several cell lines, knockdown of InsR and IGF1R sensitized to the PI3K inhibitor (Fig. S6 C–E). One of these, the MCF-7 cell line, harbors an activating mutation in PIK3CA but not HER2 gene amplification. These data imply that PI3K inhibition could be associated with the compensatory induction of a group of RTKs over a range of tumor cells. Similar changes were observed with a second pan-PI3K small molecule inhibitor, BKM120,* suggesting these changes are not secondary to off-target effects of XL147 (Fig. S7).
These findings have several clinical implications. In cancer cells, therapeutic antagonists of the PI3K pathway will inhibit AKT and relieve suppression of RTK expression and their activity. Relief of this feedback limits the sustained inhibition of the pathway and attenuates the therapeutic response to these agents. We propose that, as a result, if used as single agents, PI3K pathway inhibitors will have limited clinical activity. Relief of this feedback is commensurate with the magnitude and intensity of inhibition of AKT and cannot be applied to all types of PI3K pathway antagonists (i.e., trastuzumab vs. lapatinib vs. XL147). In HER2-overexpressing cells, up-regulated expression of HER3- and HER2-induced phosphorylation of HER3 are the main mechanisms that counteract inhibition of PI3K/AKT. Therefore, PI3K inhibitors should be used in combination with HER2/HER3 antagonists in HER2+ breast cancers. The most appropriate anticancer agents to combine with PI3K/AKT inhibitors in other PI3K-dependent cancers without HER2 gene amplification will depend on the major compensatory feedback mechanisms that are activated on inhibition of this pathway. These will require additional investigation in clinical trials.
Materials and Methods
Cell Lines.
SI Materials and Methods includes information on cell lines.
Inhibitors.
The following inhibitors were used: lapatinib and rapamycin (LC Laboratories), trastuzumab (Vanderbilt Medical Center Pharmacy), LY294002 (Calbiochem), the AKT1/2 inhibitor 5J8 (provided by Craig Lindsley, Vanderbilt University, Nashville, TN) (15), BKM-120 (Active Biochemical), and XL147 (Exelixis).
Growth and Crystal Violet Assay, Immunoprecipitation, Immunoblotting, RNAi, RNA Isolation, and Real-Time qPCR.
Growth and crystal violet assay, immunoprecipitation, immunoblotting, RNAi, RNA isolation, and real-time qPCR were performed as described previously (31, 32). Sequences for mismatch control and human HER3 siRNA duplexes were described by Wang et al. (32). Human FoxO1, FoxO3a, IGF1R, and InsR siRNA duplexes were obtained from Ambion and Qiagen, respectively. Primer sequences can be found in SI Materials and Methods.
Cell Cycle Analysis.
SI Materials and Methods includes information on cell cycle analysis.
Cytoplasmic, Nuclear Fractionation, and Proteome Profiler Human Phospho-RTK Arrays.
Cytoplasmic and nuclear extracts were prepared using the Nuclear Extract Kit from Active Motif. The pRTK arrays were performed according to the manufacturer's instructions (R&D Systems).
Xenograft Experiments and Immunohistochemistry.
Protocols for xenografts studies and immunohistochemistry can be found in SI Materials and Methods. Animal experiments were approved by the institutional animal care committee of Vanderbilt University Medical Center (VUMC).
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant R01 CA80195, American Cancer Society Clinical Research Professorship Grant CRP-07-234, the Lee Jeans Translational Breast Cancer Research Program (C.L.A.), Breast Cancer Specialized Program of Research Excellence (SPORE) Grant P50 CA98131, and Vanderbilt–Ingram Cancer Center Support Grant P30 CA68485. A.C. is partially supported by postdoctoral fellowship Award KG091215 from the Susan G. Komen Breast Cancer Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
*Voliva CF, et al. The 101st Annual Meeting of the American Association for Cancer Research, Apr 17–21, 2010, Washington, DC, abstr 4498.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018001108/-/DCSupplemental.
References
- 1.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 2.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 3.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol. 2005;15:702–713. doi: 10.1016/j.cub.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 5.Shor B, Gibbons JJ, Abraham RT, Yu K. Targeting mTOR globally in cancer: Thinking beyond rapamycin. Cell Cycle. 2009;8:3831–3837. doi: 10.4161/cc.8.23.10070. [DOI] [PubMed] [Google Scholar]
- 6.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28:1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 8.Samuels Y, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 9.Keniry M, Parsons R. The role of PTEN signaling perturbations in cancer and in targeted therapy. Oncogene. 2008;27:5477–5485. doi: 10.1038/onc.2008.248. [DOI] [PubMed] [Google Scholar]
- 10.Folkes AJ, et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem. 2008;51:5522–5532. doi: 10.1021/jm800295d. [DOI] [PubMed] [Google Scholar]
- 11.Maira SM, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 12.Edelman G, et al. A phase I dose-escalation study of XL147 (SAR245408), a PI3K inhibitor administered orally to patients (pts) with advanced malignancies. J Clin Oncol. 2010;28(15s):3004. [Google Scholar]
- 13.Myatt SS, Lam EW-F. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–859. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 14.Brachmann SM, et al. Specific apoptosis induction by the dual PI3K/mTor inhibitor NVP-BEZ235 in HER2 amplified and PIK3CA mutant breast cancer cells. Proc Natl Acad Sci USA. 2009;106:22299–22304. doi: 10.1073/pnas.0905152106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindsley CW, et al. Allosteric Akt (PKB) inhibitors: Discovery and SAR of isozyme selective inhibitors. Bioorg Med Chem Lett. 2005;15:761–764. doi: 10.1016/j.bmcl.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 17.Messeguer X, et al. PROMO: Detection of known transcription regulatory elements using species-tailored searches. Bioinformatics. 2002;18:333–334. doi: 10.1093/bioinformatics/18.2.333. [DOI] [PubMed] [Google Scholar]
- 18.Hellyer NJ, Kim M-S, Koland JG. Heregulin-dependent activation of phosphoinositide 3-kinase and Akt via the ErbB2/ErbB3 co-receptor. J Biol Chem. 2001;276:42153–42161. doi: 10.1074/jbc.M102079200. [DOI] [PubMed] [Google Scholar]
- 19.Holbro T, et al. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci USA. 2003;100:8933–8938. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee-Hoeflich ST, et al. A central role for HER3 in HER2-amplified breast cancer: Implications for targeted therapy. Cancer Res. 2008;68:5878–5887. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- 21.Engelman JA, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 22.Ritter CA, et al. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res. 2007;13:4909–4919. doi: 10.1158/1078-0432.CCR-07-0701. [DOI] [PubMed] [Google Scholar]
- 23.Amin DN, et al. Resiliency and vulnerability in the HER2-HER3 tumorigenic driver. Sci. Transl. Med. 2010;2:16ra7. doi: 10.1126/scitranslmed.3000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martelli AM, et al. Intranuclear 3′-phosphoinositide metabolism and Akt signaling: New mechanisms for tumorigenesis and protection against apoptosis? Cell Signal. 2006;18:1101–1107. doi: 10.1016/j.cellsig.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Makhija S, et al. Clinical activity of gemcitabine plus pertuzumab in platinum-resistant ovarian cancer, fallopian tube cancer, or primary peritoneal cancer. J Clin Oncol. 2010;28:1215–1223. doi: 10.1200/JCO.2009.22.3354. [DOI] [PubMed] [Google Scholar]
- 26.Yakes FM, et al. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62:4132–4141. [PubMed] [Google Scholar]
- 27.Junttila TT, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–440. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 28.Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: Of feedbacks and cross-talks. Oncogene. 2008;27:5527–5541. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- 29.O'Reilly KE, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohsin SK, et al. Neoadjuvant trastuzumab induces apoptosis in primary breast cancers. J Clin Oncol. 2005;23:2460–2468. doi: 10.1200/JCO.2005.00.661. [DOI] [PubMed] [Google Scholar]
- 31.Chakrabarty A, et al. H1047R phosphatidylinositol 3-kinase mutant enhances HER2-mediated transformation by heregulin production and activation of HER3. Oncogene. 2010;29:5193–5203. doi: 10.1038/onc.2010.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang SE, et al. Transforming growth factor beta engages TACE and ErbB3 to activate phosphatidylinositol-3 kinase/Akt in ErbB2-overexpressing breast cancer and desensitizes cells to trastuzumab. Mol Cell Biol. 2008;28:5605–5620. doi: 10.1128/MCB.00787-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.