Abstract
Mg2+ is essential for the proper folding and function of RNA, though the effect of Mg2+ concentration on the free energy, enthalpy, and entropy landscapes of RNA folding is unknown. This work exploits temperature-controlled single-molecule FRET methods to address the thermodynamics of RNA folding pathways by probing the intramolecular docking/undocking kinetics of the ubiquitous GAAA tetraloop−receptor tertiary interaction as a function of [Mg2+]. These measurements yield the barrier and standard state enthalpies, entropies, and free energies for an RNA tertiary transition, in particular, revealing the thermodynamic origin of [Mg2+]-facilitated folding. Surprisingly, these studies reveal that increasing [Mg2+] promotes tetraloop–receptor interaction by reducing the entropic barrier (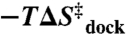 ) and the overall entropic penalty (
) and the overall entropic penalty (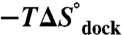 ) for docking, with essentially negligible effects on both the activation enthalpy (
) for docking, with essentially negligible effects on both the activation enthalpy (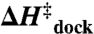 ) and overall exothermicity (
) and overall exothermicity (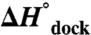 ). These observations contrast with the conventional notion that increasing [Mg2+] facilitates folding by minimizing electrostatic repulsion of opposing RNA helices, which would incorrectly predict a decrease in
). These observations contrast with the conventional notion that increasing [Mg2+] facilitates folding by minimizing electrostatic repulsion of opposing RNA helices, which would incorrectly predict a decrease in 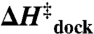 and
and 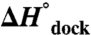 with [Mg2+]. Instead we propose that higher [Mg2+] can aid RNA folding by decreasing the entropic penalty of counterion uptake and by reducing disorder of the unfolded conformational ensemble.
with [Mg2+]. Instead we propose that higher [Mg2+] can aid RNA folding by decreasing the entropic penalty of counterion uptake and by reducing disorder of the unfolded conformational ensemble.
The folding of RNA proceeds hierarchically, whereby secondary structure is formed rapidly and subsequent slow helical packing is mediated by tertiary interactions (1, 2). RNA secondary structure prediction from the known thermodynamics is quite reliable (3), though correspondingly accurate prediction of tertiary structure remains a major challenge (1). Static tertiary structure data alone are also not enough to predict RNA functionality, as time-dependent conformational dynamics occur during biochemical processes (4, 5). As a result, one needs the full free energy, enthalpy, and entropy landscapes for folding. A major road block in achieving a predictive understanding of RNA folding landscapes is that they are often “rugged,”, i.e., with alternative conformations acting as kinetic traps (6, 7). Moreover, the electrostatic challenge of folding a charged biopolymer highlights the particularly critical role of Mg2+ and other counterions in the folding process.
Characterization of folding transition states—and the role of Mg2+ in stabilizing transition states—remains a crucial bottleneck for reconciling the kinetics and thermodynamics of RNA folding (8–13). Some insight into the free energy landscapes for RNA folding can be obtained from temperature-dependent stopped-flow kinetic studies, which offer the ability to deconstruct free energy barriers (ΔG‡) into enthalpic (ΔH‡) and entropic (-TΔS‡) components. However, with such methods, only the net rate constant (i.e., ktotal = kfold + kunfold) for approach to equilibrium can be observed, which requires strong assumptions (e.g., that kfold≫kunfold or kunfold is temperature independent) to permit accurate extraction of transition-state barrier heights (11, 14–16). Single-molecule fluorescence resonance energy transfer methods (smFRET) avoid such kinetic restrictions by providing both folding and unfolding rate constants under equilibrium conditions, though smFRET transition-states studies of RNA folding are scarce (9, 13, 17). Furthermore, despite the well known role of Mg2+ in promoting the structural assembly of RNA, there is limited information even from ensemble studies on the enthalpic vs. entropic contribution of [Mg2+] to tertiary structure stabilization (18) and no studies whatsoever addressing the thermodynamic origin of Mg2+-accelerated folding.
In this work, we exploit temperature-controlled smFRET microscopy to explore the [Mg2+]-dependent thermodynamics of RNA folding/unfolding by characterizing enthalpy and entropy changes associated with the elementary formation of an isolated tertiary interaction. Specifically, we measure the temperature dependence of the equilibrium and rate constants for intramolecular docking/undocking of a GAAA tetraloop with its 11 nucleotide receptor via a flexible U7 linker as function of [Mg2+] (Fig. 1A). The tetraloop–receptor (TL-R) interaction is a ubiquitous modular motif (19–21). Mg2+-RNA interactions can be through site-specific coordination or a diffuse ion atmosphere (22). This work focuses on the latter, more common interaction of fully hydrated Mg2+ with RNA, as it was previously shown that TL-R docking does not require direct Mg2+ coordination (16). The structures of the docked and undocked forms of the tetraloop and receptor are known (21, 23–25), allowing for direct correlation of structure with folding thermodynamics (26, 27). We show that the free energy barrier (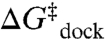 ) for docking is entropic (
) for docking is entropic (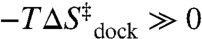 ) with an “early” (i.e.,
) with an “early” (i.e.,  ) transition state, whereas the overall reaction is exothermic (
) transition state, whereas the overall reaction is exothermic ( ) and entropically disfavored (
) and entropically disfavored (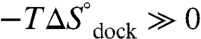 ). These observations support a paradigm that RNA folding transition states lack significant formation of the tertiary interaction (8, 9, 12, 13). Most importantly, we show that [Mg2+]-based promotion of TL-R docking is of an entropic origin—reduction of the entropic barrier (
). These observations support a paradigm that RNA folding transition states lack significant formation of the tertiary interaction (8, 9, 12, 13). Most importantly, we show that [Mg2+]-based promotion of TL-R docking is of an entropic origin—reduction of the entropic barrier ( ) and decrease in the overall entropic cost of folding (
) and decrease in the overall entropic cost of folding (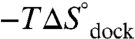 ). This result is in opposition with the traditional notion that increasing [Mg2+] aids RNA folding via electrostatic screening of the phosphate backbone, which would incorrectly predict an enthalpic origin of Mg2+-facilitated folding—by decreasing
). This result is in opposition with the traditional notion that increasing [Mg2+] aids RNA folding via electrostatic screening of the phosphate backbone, which would incorrectly predict an enthalpic origin of Mg2+-facilitated folding—by decreasing 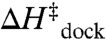 and
and  (SI Text).
(SI Text).
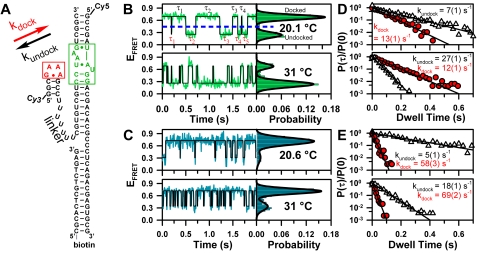
Fig. 1.
Single-molecule observation of GAAA tetraloop and receptor docking/undocking via a U7 linker. (A) TL-R construct, in which docking and undocking rate constants (kdock and kundock) are monitored by FRET between the donor (Cy3) and acceptor (Cy5). (B and C) Temperature-dependent EFRET trajectories and probability histograms at 0 mM and 1 mM MgCl2, respectively, with corresponding dwell time (τ) probability densities (D–E) from many molecules, which yield kdock and kundock from single exponential fits of the undocked (circles) and docked (triangles) dwell times, respectively.
Results
Mg2+ Increases the Melting Temperature of TL-R Interaction.
Temperature and [Mg2+]-dependent docking of an isolated GAAA tetraloop with its 11 nucleotide receptor are explored using the RNA construct shown in Fig. 1A. Linked by a flexible single-stranded poly(U) junction, the GAAA tetraloop facilely and specifically docks into its receptor, modulating the energy transfer efficiency (EFRET) between the donor (Cy3) and acceptor (Cy5) fluorophores (16, 28). EFRET is monitored by single-molecule confocal microscopy—calculated ratiometrically from the donor and acceptor emission intensities (Materials and Methods) (28, 29). The RNA vacillates between two well-resolved states of high (docked) and low (undocked) EFRET, as seen by the superimposed fits in Fig. 1B and C and Fig. S1 (30). The U7 linker (Fig. 1A) behaves as a random coil at the moderate ionic strengths and temperature ranges explored in this work (31), permitting isolation of the thermodynamic effects of [Mg2+] on the TL-R tertiary interaction alone (16).
Both [Mg2+] and temperature affect the TL-R docking equilibrium, as seen in the EFRET probability histograms for single (Fig. 1B and C) and many molecules (Figs. S2 and S3). These data reveal that increasing [Mg2+] favors the docked state, whereas increasing temperature favors the undocked state. In the absence of Mg2+, increasing from 20.1 °C to 31 °C shifts the equilibrium from the docked to undocked state, whereas at 1 mM Mg2+ this same temperature excursion only modestly affects the population distribution. Thus, increasing [Mg2+] both (i) enhances TL-R docking and (ii) increases the melting temperature of the interaction.
Dwell Time Analysis Yields kdock and kundock as a Function of [Mg2+].
TL-R interaction can be further explored by determining the rates constants for tetraloop docking and undocking (kdock and kundock, Fig. 1A) as a function of [Mg2+]. Dwell times for the TL-R RNA in the docked and undocked states are defined in the time trajectory by crossings of a threshold set at the minimum of the bimodal EFRET distribution (28) (Fig. 1B). To achieve a large statistical accuracy in rate constant determination, we calculate probability densities from the cumulative histograms (approximately 30 molecules and > 300 transitions) of docked and undocked dwell times under each experimental condition, i.e., P(τi) ≈ H(τi)/[0.5(τi+1 - τi-1)], where H(τi) is the histogram value and τi represents an ordered list of nonzero time bins (28). The normalized probability densities, P(τ)/P(0), are well described by single-exponential decays over > 3 orders of magnitude, yielding high quality rate constants, kdock and kundock, from the undocked and docked dwell times, respectively. Hidden Markov modeling is also pursued as method for determining rate constants and yields identical values within uncertainties (30). This analysis reveals that kdock is (i) largely insensitive to temperature yet increases with [Mg2+], whereas kundock (ii) increases with temperature and decreases with [Mg2+] (Fig. 1D and E).
The [Mg2+] dependence of kdock and kundock is shown at 20 ± 1 °C in Fig. 2A. Increasing [Mg2+] dramatically accelerates kdock (approximately 12-fold) and slightly decelerates kundock (approximately 1.6-fold) , leading to a steep increase of the docking equilibrium constant (Kdock = kdock/kundock, Fig. 2B), echoing trends observed in an A7 linked TL-R construct (28). These trends are fit to a four-state kinetic model with Mg2+-dependent and -independent docking/undocking pathways, where Mg2+ association/dissociation is in rapid equilibrium (Fig. 2B). In this model, the monoexponential dwell time decays (Fig. 1D and E) described by kdock or kundock represent the transition state for docking or undocking in a weighted average of k1 and k2 or k-1 and k-2, respectively (Fig. 2) (28, 29, 32). An increase in kdock and decrease in kundock with [Mg2+] implies that k1 < k2 and k-2 < k-1. We exploit the temperature dependence of kdock, kundock, and Kdock over a large dynamic range (0 to 1 mM Mg2+, highlighted in Fig. 2A) to elucidate the origin of this observed Mg2+-facilitated docking, i.e., how the overall and barrier enthalpies, entropies, and free energies of folding are influenced by [Mg2+].
Fig. 2.
[Mg2+]-dependence of TL-R RNA docking at 20 ± 1 °C: (A) kdock, kundock and (B) Kdock = kdock/kundock described by a four-state model allowing for [Mg2+]-dependent and independent docking pathways (U = undocked, D = docked) and Mg2+ binding is not resolved by FRET. From this model, kdock = {k1(Kd)n + k2[Mg2+]n}/{(Kd)n + [Mg2+)} and  . Fits of the kdock and kundock titrations with the detailed balance constraint that
. Fits of the kdock and kundock titrations with the detailed balance constraint that 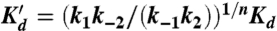 , yield n = 1.8 ± 0.2, k1 = 12.6 ± 0.9 s-1, k2 = 156 ± 23 s-1, k-1 = 8.6 ± 0.7 s-1, k-2 = 5.4 ± 0.2 s-1, Kd = 1.3 ± 0.3 mM, and
, yield n = 1.8 ± 0.2, k1 = 12.6 ± 0.9 s-1, k2 = 156 ± 23 s-1, k-1 = 8.6 ± 0.7 s-1, k-2 = 5.4 ± 0.2 s-1, Kd = 1.3 ± 0.3 mM, and 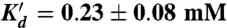 .
.
Enthalpy ( ) and Entropy (
) and Entropy (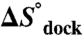 ) of TL-R Docking as Function of [Mg2+].
) of TL-R Docking as Function of [Mg2+].
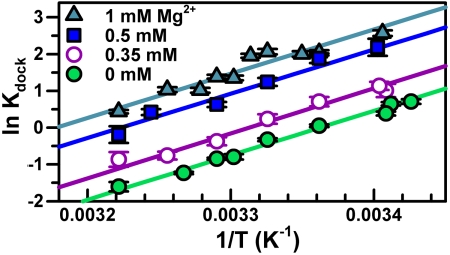
To explore how increasing [Mg2+] stabilizes TL-R interaction, we extract enthalpic and entropic information from the equilibrium constants ( ) by invoking the van’t Hoff equation,
) by invoking the van’t Hoff equation,
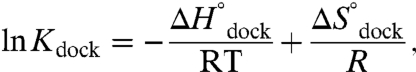 |
[1] |
where R is the ideal gas constant and  . From Eq. 1, a linear fit of ln Kdock vs. 1/T yields a slope of
. From Eq. 1, a linear fit of ln Kdock vs. 1/T yields a slope of 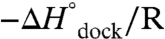 and intercept of
and intercept of  . Such an analysis assumes negligible temperature-dependent changes in
. Such an analysis assumes negligible temperature-dependent changes in 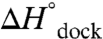 and
and 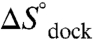 , which is supported by calorimetry measurements of a dual TL-R construct over the temperature range probed in this work (26).
, which is supported by calorimetry measurements of a dual TL-R construct over the temperature range probed in this work (26).
The TL-R van’t Hoff plots (Fig. 3 and Table 1, top section, 100 mM NaCl) demonstrate that increasing [Mg2+] has an insignificant effect on the slope, i.e., 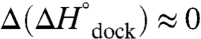 (p = 0.92, a 92% chance that fluctuations in
(p = 0.92, a 92% chance that fluctuations in 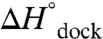 are random, SI Text), although substantially increasing the offset, i.e.,
are random, SI Text), although substantially increasing the offset, i.e., 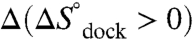 (p = 0.15, an 85% likelihood that
(p = 0.15, an 85% likelihood that  increases, SI Text). This result is quite surprising and implies that an increase in folding propensity with [Mg2+] is achieved by entropic rather than enthalpic stabilization of the docked RNA, i.e.,
increases, SI Text). This result is quite surprising and implies that an increase in folding propensity with [Mg2+] is achieved by entropic rather than enthalpic stabilization of the docked RNA, i.e.,  . Such a picture is in stark contrast with conventional expectations that increasing [Mg2+] results in electrostatic stabilization of the folded RNA by better screening of the negatively charged helices.
. Such a picture is in stark contrast with conventional expectations that increasing [Mg2+] results in electrostatic stabilization of the folded RNA by better screening of the negatively charged helices.
Fig. 3.
Temperature dependence of equilibrium constant (Kdock) for TL-R docking as a function of [Mg2+] yields the standard state enthalpy (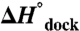 ) and entropy (
) and entropy ( ) of docking from Eq. 1 (Table 1, top, 100 mM NaCl).
) of docking from Eq. 1 (Table 1, top, 100 mM NaCl).
Table 1.
TL-R docking thermodynamics
| [MgCl2] (mM) | [NaCl] (mM) |
 (kcal/mol) (kcal/mol) |
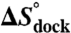 (cal/mol/K) (cal/mol/K) |
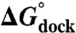 (37 °C) (kcal/mol) (37 °C) (kcal/mol) |
| TL-R docking via U7 linker | ||||
| 0.00 | 100 | −24.0 ± 0.5 | −80.7 ± 1.7 | 1.0 ± 0.7 |
| 0.35 | 100 | −24.3 ± 0.8 | −80.3 ± 2.6 | 0.6 ± 0.7 |
| 0.50 | 100 | −23.9 ± 0.9 | −77.0 ± 3.2 | −0.03 ± 1.3 |
| 1.00 | 100 | −23.9 ± 0.8 | −76.0 ± 2.6 | −0.34 ± 1.1 |
| 1.00 | 25 | −21.9 ± 1.2 | −76.8 ± 4.8 | 1.9 ± 2.3 |
| 2.00 | 25 | −16.0 ± 0.7 | −50.7 ± 2.2 | −0.3 ± 0.7 |
| TL-R docking via A7 linker | ||||
| 0.35 | 100 | −25 ± 2 | −84 ± 7 | 1.0 ± 3.0 |
| 0.50 | 100 | −23 ± 1 | −76 ± 5 | 0.2 ± 1.8 |
| 1.00 * | 100 | −15 ± 1 | −47 ± 4 | −0.4 ± 1.6 |
| 2.00 | 100 | −11 ± 1 | −34 ± 5 | −0.5 ± 1.8 |
*This condition is measured with improved precision (26).
Activation Enthalpy and Entropy for TL-R Docking and Undocking as Function of [Mg2+].
To further elucidate the thermodynamic origin of this increased folding stability with [Mg2+], we determine kdock and kundock as function of temperature. Arrhenius plots for kdock and kundock are shown in Fig. 4, revealing a steep increase in kundock and a slight decrease in kdock with temperature. As in the van’t Hoff plots (Fig. 3), the slopes of the Arrhenius plots are independent of [Mg2+], whereas the offsets increase. To extract the activation enthalpy and entropy from these plots, we invoke a transition-state analysis.
Fig. 4.
Temperature dependence of kdock and kundock as function of [Mg2+]. Transition-state analysis yields the activation enthalpy (ΔH‡) and entropy (ΔS‡) for docking and undocking from Eq. 2 (Table 2, top, 100 mM NaCl).
From generalized transition-state theory, a unimolecular reaction rate constant (e.g., kdock or kundock) can be written in terms of the enthalpic and entropic components of the activation free energy (see SI Text):
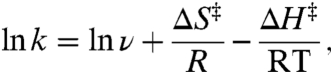 |
[2] |
where ΔS‡ and ΔH‡ are the activation entropy and enthalpy (33, 34), which are obtainable from the slope and intercept of linear fits of ln k vs. 1/T plots. Extraction of ΔH‡ is unambiguous, whereas determination of the absolute value of ΔS‡ from the experimental intercepts requires knowledge of ν. Since the dependence of the rate constant on ν is only logarithmic, an estimate of ν ≈ 1013 s-1 proves sufficient for our purposes, based on typical low frequency (approximately 300 cm-1) skeletal motions (see SI Text) (35, 36). However, the measured dependence of ΔS‡ on [Mg2+] (i.e., ΔΔS‡) is completely independent of ν and therefore obtained rigorously from experiment.
Fits of the Arrhenius plots in Fig. 4 to Eq. 2 (Table 2, top section, 100 mM NaCl) reveal a slight exothermicity in achieving the transition state (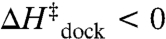 ). However, the activation entropy (
). However, the activation entropy (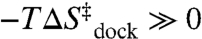 ) results in a large free energy barrier (
) results in a large free energy barrier (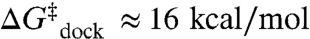 at 37 °C) that slows folding more than 10 orders of magnitude below ν. Conversely, undocking is rate limited by a large enthalpic barrier (
at 37 °C) that slows folding more than 10 orders of magnitude below ν. Conversely, undocking is rate limited by a large enthalpic barrier ( ) with a favorable entropy gain (
) with a favorable entropy gain (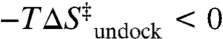 ). Most importantly, increasing [Mg2+] accelerates kdock by decreasing the entropic barrier to docking, i.e.,
). Most importantly, increasing [Mg2+] accelerates kdock by decreasing the entropic barrier to docking, i.e.,  and
and  (Table 2) with p = 0.04 and 0.56, respectively (SI Text). These trends are also apparent by the constant slopes, yet increasing intercepts of the Arrhenius plots (Fig. 4).
(Table 2) with p = 0.04 and 0.56, respectively (SI Text). These trends are also apparent by the constant slopes, yet increasing intercepts of the Arrhenius plots (Fig. 4).
Table 2.
Transition-state thermodynamics of TL-R interaction
| [Mg2+] (mM) | [NaCl] (mM) |
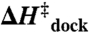 (kcal/mol) (kcal/mol) |
 (cal/mol/K) (cal/mol/K) |
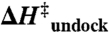 (kcal/mol) (kcal/mol) |
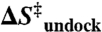 (cal/mol/K) (cal/mol/K) |
| U7 linker | |||||
| 0 | 100 | −2.9(3) * | −64(1) | 21.2(4) | 17(1) |
| 0.35 | 100 | −3.1(6) | −64(2) | 22.2(6) | 20(2) |
| 0.5 | 100 | −2.3(7) | −60(2) | 21.6(7) | 17(2) |
| 1.0 | 100 | −2.5(6) | −59(2) | 21.3(5) | 16(2) |
| 1.0 | 25 | 0.0(9) | −57(3) | 21.2(8) | 18(3) |
| 2.0 | 25 | 0.8(5) | −49(2) | 17.1(5) | 3(2) |
| A7 linker | |||||
| 0.35 | 100 | −7(4) | −76(14) | 24(4) | 25(12) |
| 0.5 | 100 | −2(2) | −59(5) | 21.4(7) | 17(3) |
| 1.0 | 100 | 2(1) | −46(4) | 16.7(8) | 1(3) |
| 2.0 | 100 | 7.4(5) | −27(2) | 19(3) | 9(4) |
*Uncertainties of the last significant digits are in parentheses.
Discussion
“Early” Transition States as a Paradigm of RNA Folding.
Characterizing RNA folding transition states has been difficult due to the ruggedness of folding landscapes (13, 15). Isolation of the TL-R interaction simplifies interpretation of the free energy landscape. The absence of an enthalpic barrier (Fig. 5A, Tables 1–2) is indicative of an early or reactant-like transition state, where enthalpic rearrangements, such as hydrogen bonding between the tetraloop and receptor (21, 24) are largely unformed. The entropic barrier to folding suggests a conformational search for the transition state (14). This interpretation can be further corroborated by Φ-analysis, whereby mutational effects on equilibrium/rate constants for folding help determine which interactions are formed in the transition state. Φ-analyses of different RNA folding systems have each concluded that tertiary interactions are largely unformed in the folding transition states (8, 9, 12, 13, 37). Mutational studies in the P4–P6 domain of the Tetrahymena ribozyme indicate that the TL-R interaction contributes only to overall thermodynamic stability of the folded domain rather than the transition state (8, 37), in agreement with this work. Indeed TL-R interaction (Table 1) can account for the entire exothermicity of the P4–P6 domain ( at 1.1. mM MgCl2, 10 mM NaCl) (18). These studies together lend strong support to an emerging paradigm that early transition states are a characteristic property of RNA folding (9).
at 1.1. mM MgCl2, 10 mM NaCl) (18). These studies together lend strong support to an emerging paradigm that early transition states are a characteristic property of RNA folding (9).
Fig. 5.
Scheme for Mg2+-facilitated TL-R folding. (A) The entropic and enthalpic reaction coordinate for TL-R docking at 1 mM Mg2+, where U, ‡, and D indicate the undocked, transition, and docked states. (B) The proposed transition state is early and compact, i.e., the tertiary interaction (red lines in docked state) is largely unformed, whereas the tetraloop and receptor are in close proximity, requiring localization of counterions (e.g., Mg2+, blue circles).
We now consider the origin of the large entropic barrier for TL-R docking and its dependence on [Mg2+]. One simple explanation is that the TL-R transition state is “compact” (i.e., tetraloop and receptor are proximal/aligned), which is achieved by a conformational search within the radius of the U7 linker (Fig. 1A). Support for a compact transition state is obtained from studies of an alternative linker, A7 vs. U7 (Fig. S4). In contrast to poly U, single-stranded poly A has a propensity for [Mg2+]-dependent helix formation, which can decrease the disorder of the unfolded RNA (38, 39). As summarized in Tables 1 and 2 (A7 data and analysis are shown in Fig. S4), the entropic barrier and cost of folding in the A7 construct decreases more dramatically with [Mg2+] than with the U7 linker. This trend supports the notion that increasing [Mg2+] orders the undocked state by encouraging base stacking in the A7 linker, limiting the conformational search of the tetraloop for the receptor. This ordering is accompanied by an enthalpic penalty (Table 2), which suggests that the tetraloop must gain proximity to the receptor in a compact transition state by breaking A-A linker base stacking (39).
In the simplest model, the search for a compact/oriented transition state is rate limited by diffusion (36). Measurements and estimates for end-to-end contact formation in similarly sized polymer systems would predict this rate to be ≈107 s-1 (see SI Text) (40–42), i.e., five orders of magnitude faster than the observed TL-R docking rate at 1 mM Mg2+ (Fig. 2). Based on a diffusion-controlled rate of 107 s-1 and an attempt frequency of ν ≈ 1013 s-1, Eq. 2 can be rearranged to estimate a diffusional contribution to the transition-state entropy of  , which is less than half of the experimental value of
, which is less than half of the experimental value of 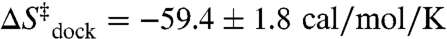 at 1 mM Mg2+. Clearly, there must be additional entropy sources beyond simple diffusion of the tetraloop to the receptor.
at 1 mM Mg2+. Clearly, there must be additional entropy sources beyond simple diffusion of the tetraloop to the receptor.
One possible source is organization of the tetraloop and/or receptor units in the undocked vs. transition state conformations. GAAA tetraloops are rigidly structured (21, 23, 24) and therefore not anticipated to contribute to loss of entropy in the transition state. The free receptor, by way of contrast, is considerably less organized, and must undergo rearrangement upon docking (24, 26) (Fig. 5
B). The slight exothermicity of 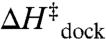 (Table 2, U7 linker, 100 mM NaCl) suggests minor tertiary contact (e.g., a hydrogen bond) in the transition state, which would add a small entropic cost (
(Table 2, U7 linker, 100 mM NaCl) suggests minor tertiary contact (e.g., a hydrogen bond) in the transition state, which would add a small entropic cost ( ). This scenario is consistent with the proposal that the free receptor becomes more “rigid” in response to the tetraloop (24), which could contribute to an entropic barrier (24, 26, 27). However, the lack of significant hydrogen bonding in the transition state (
). This scenario is consistent with the proposal that the free receptor becomes more “rigid” in response to the tetraloop (24), which could contribute to an entropic barrier (24, 26, 27). However, the lack of significant hydrogen bonding in the transition state (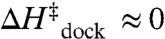 and
and 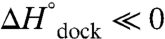 ) suggests that the receptor could still be quite dynamic and therefore not yet a dominant source of entropy loss. Furthermore, procession to the docked state from the transition state is marked by an additional entropic cost and large exothermicity (Fig. 5A), consistent with formation of the hydrogen-bonded tertiary interaction and a concomitant loss of entropy in the receptor (26, 27).
) suggests that the receptor could still be quite dynamic and therefore not yet a dominant source of entropy loss. Furthermore, procession to the docked state from the transition state is marked by an additional entropic cost and large exothermicity (Fig. 5A), consistent with formation of the hydrogen-bonded tertiary interaction and a concomitant loss of entropy in the receptor (26, 27).
A second source of the entropic docking barrier can arise from an uptake of Mg2+ and/or Na+ in the transition state. Folding increases the negative charge density of RNA and is therefore frequently accompanied by a localization of counterions (9, 43). Formation of a compact transition state (Fig. 5) requires an interface of cations to electrostatically shield the closely packed negatively charged helices (9), otherwise an enthalpic barrier to folding would be observed. For an ideal solution, the entropic cost of localizing Mg2+ on RNA can be estimated from
 |
[3] |
where R is the gas constant, n is the number ions taken up, with [Mg2+]ion atmosphere and [Mg2+]bulk representing the [Mg2+] close in and far away from the RNA, respectively (44, 45). [Mg2+]ion atmosphere can be approximated by assuming that every phosphate charge is effectively neutralized by counterions, as supported by studies of the local ion atmosphere on DNA duplexes (46). To illustrate that the penalty of counterion uptake can be quite large, we make a simple calculation. Treating the volume of the ion atmosphere as a helical rod with dimensions of 100 by 26 Å for the 84 nucleotide RNA construct predicts [Mg2+]ion atmosphere ≈ 1.3 M. This value is consistent with predictions of approximately 2 M monovalent cations on RNA helices in the absence of Mg2+ (44). From the analysis of kdock (Fig. 2), the Hill coefficient (n) of 1.8 ± 0.2 can be utilized to estimate that approximately 1.8 Mg2+ ions are taken up in the transition state (47). For this uptake and concentration gradient, Eq. 3 predicts an entropy loss of 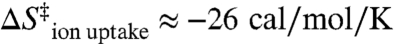 at 1 mM Mg2+. Thus, the entropy from diffusion and cation uptake (
at 1 mM Mg2+. Thus, the entropy from diffusion and cation uptake (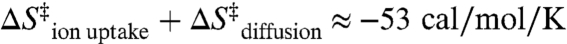 ) is on the order of the experimentally observed
) is on the order of the experimentally observed 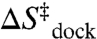 of -59.4 ± 1.8 cal/mol/K, even neglecting contributions from
of -59.4 ± 1.8 cal/mol/K, even neglecting contributions from  . Thereby, as schematically depicted in Fig. 5B, (i) cation localization and (ii) intramolecular diffusion of the tetraloop to the receptor are likely predominant sources for the large entropic rather than enthalpic barrier to docking.
. Thereby, as schematically depicted in Fig. 5B, (i) cation localization and (ii) intramolecular diffusion of the tetraloop to the receptor are likely predominant sources for the large entropic rather than enthalpic barrier to docking.
The above analysis reveals that the entropic cost of intramolecular diffusion (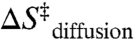 ) in the TL-R RNA accounts for nearly half of the overall entropic penalty for achieving the transition state; this contribution is not an intrinsic property of the TL-R interaction. We argue that the remaining portion of this entropic barrier arises from features of the tertiary interaction: (i) need for ion uptake to stabilize a compact TL-R transition state (
) in the TL-R RNA accounts for nearly half of the overall entropic penalty for achieving the transition state; this contribution is not an intrinsic property of the TL-R interaction. We argue that the remaining portion of this entropic barrier arises from features of the tertiary interaction: (i) need for ion uptake to stabilize a compact TL-R transition state (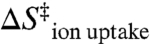 ) and (ii) ordering of the receptor (
) and (ii) ordering of the receptor ( ).
).
An Entropic Origin of Mg2+-Facilitated RNA Folding.
Dissecting the free energy of the TL-R docking reaction coordinate (ΔG° and ΔG‡) into the enthalpic and entropic components (ΔH°, ΔH‡, ΔS°, and ΔS‡) as a function of [Mg2+] provides insight into the mechanism of Mg2+-promoted docking. The overall docking reaction is exothermic and entropically costly, which can be attributed to a predominant contribution of hydrogen-bonding and base-stacking interactions (27, 48). The free energy barrier (e.g.,  at 1 mM MgCl2, 100 mM NaCl, 37 °C) for achieving the transition state is due to entropy. Increasing [Mg2+] increases the docking rate constant and the overall equilibrium constant (Fig. 2) by a reduction in the entropic barrier (
at 1 mM MgCl2, 100 mM NaCl, 37 °C) for achieving the transition state is due to entropy. Increasing [Mg2+] increases the docking rate constant and the overall equilibrium constant (Fig. 2) by a reduction in the entropic barrier (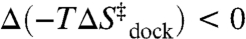 ) and entropic cost of docking (
) and entropic cost of docking (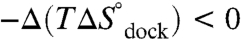 ), whereas
), whereas 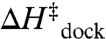 and
and  are unchanged (Tables 1 and 2).
are unchanged (Tables 1 and 2).
One might anticipate enhanced electrostatic screening to be a relevant mechanism of [Mg2+]-assisted folding. However, such a mechanism would require a [Mg2+]-dependent decrease in the enthalpy for docking, which is not observed. This surprising result motivates us to investigate the role of ionic strength by reducing the background [NaCl] from 100 mM to 25 mM, at which charge repulsion should be amplified (21, 29). We do in fact see the correct anticipated sign of this change, i.e., with reduction in [NaCl], 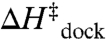 slightly increases from weakly exothermic to thermoneutral at constant 1 mM Mg2+ (Tables 1 and 2, Fig. S5). However, the change in enthalpic barrier with increasing [Mg2+] is still approximately 0. More importantly, the corresponding entropic origin of [Mg2+]-facilitated RNA folding at low [NaCl] becomes even more pronounced. Interestingly, the 25 mM NaCl, 2 mM Mg2+ condition is most similar to bulk calorimetry studies by Butcher and coworkers for bimolecular association of TL-R constructs (
slightly increases from weakly exothermic to thermoneutral at constant 1 mM Mg2+ (Tables 1 and 2, Fig. S5). However, the change in enthalpic barrier with increasing [Mg2+] is still approximately 0. More importantly, the corresponding entropic origin of [Mg2+]-facilitated RNA folding at low [NaCl] becomes even more pronounced. Interestingly, the 25 mM NaCl, 2 mM Mg2+ condition is most similar to bulk calorimetry studies by Butcher and coworkers for bimolecular association of TL-R constructs (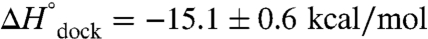 ) (48), which is in excellent agreement with our results (
) (48), which is in excellent agreement with our results ( ).
).
This work has clearly demonstrated that Mg2+-facilitated TL-R docking is of an entropic origin, a behavior reminiscent of [salt]-dependent DNA duplex formation (49). We consider possible mechanisms for this entropic effect. According to Eq. 3, increasing [Mg2+]bulk decreases the entropic penalty of counterion uptake. Increasing [Mg2+]bulk could also increase [Mg2+]ion atmosphere, and thereby decrease the number of cations (n) that need to be taken up with folding. To simply illustrate the magnitude of the decreased penalty of counterion uptake with increasing [Mg2+], we estimate that approximately 1.8 Mg2+ ion are taken up with docking. The favorable entropy gain for this uptake in higher salt (e.g., 0.35 vs. 1 mM Mg2+) yields 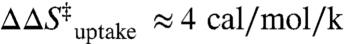 , which is sufficient to account for the observed
, which is sufficient to account for the observed 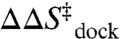 (Table 2, U7 linker, 100 mM NaCl).
(Table 2, U7 linker, 100 mM NaCl).
Other potential entropically beneficial effects of increased [Mg2+] on docking are compaction of the unfolded RNA (50) or stabilization of the receptor, both of which increase the sampling rate of the native state. The undocked EFRET of the TL-R construct (Fig. 1 and Fig. S2) shifts, but does not broaden with increasing [Mg2+], indicating compaction. However, the [Mg2+]-induced EFRET shift corresponds to only an approximately 1.2-fold increase in the diffusion-controlled collision frequency of the tetraloop and receptor (see SI Text) and therefore is only expected to make a small contribution to the 12-fold increase in kdock over the same range of [Mg2+]. Stabilization of the free receptor with increasing [Mg2+] is also not probable because even at high [Mg2+] (125 mM Mg2+), the native (bound) form of the receptor is undetectable (51). Furthermore, one would anticipate that stabilization of a nonnative receptor structure would unfavorably increase the docking enthalpy, which is not observed. Thus, we propose that the predominant mechanism for [Mg2+]-facilitated folding in the U7 TL-R RNA (Fig. 1A) is a decreased entropic penalty of counterion localization in the transition and docked states.
In natural RNAs, organization of the unfolded RNA may also contribute to the mechanism of [Mg2+]-facilitated folding. For example, junctions (linkers) limit the conformations of unfolded states (52–54) and can be rigidified by Mg2+. In support of this, the [Mg2+]-dependent decrease in the entropic cost of TL-R docking is much more pronounced in the A7 vs. U7 constructs (Tables 1 and 2)—suggesting that [Mg2+]-dependent rigidification of the linker is of large entropic benefit to the docking process. In summary, this work clearly reveals that [Mg2+]-mediated formation of an isolated TL-R tertiary interaction (i) is of an entropic origin, directly counter to traditional expectations for the role of Mg2+ in tertiary RNA folding, and (ii) involves a significantly complex interplay between the ion atmosphere and the docked vs. undocked RNA structures along the folding pathway.
Materials and Methods
RNA Preparation.
Synthetic Cy3-Cy5-labeled TL-R RNA constructs (Fig. 1) are prepared and immobilized on the glass surface of a flow cell by biotin-streptavidin chemistry (16, 28). Unless otherwise specified, experiments are performed in 50 mM hemisodium Hepes buffer (pH 7.5 at 25 °C) with 100 mM NaCl, 0.1 mM EDTA, and an oxygen scavenger of 60 nM protocatechuic acid, 5 mM protocatechuate-3,4-dioxygenase, and 2 mM Trolox (55) with the specified [Mg2+] from added MgCl2. There is kinetic heterogeneity of the TL-R constructs under all conditions; experiments are performed on the approximately 70% population of actively docking species (16, 28, 29).
Temperature-Controlled Single-Molecule FRET Measurements.
Emission from the donor (Cy3) and acceptor (Cy5) is spectrally separated for time-correlated single-photon counting detection using an inverted confocal microscope system (28, 29) with temperature control (SI Text). Fluorescence trajectories of the donor and acceptor signal from single molecules are binned at 3–10 ms integration. EFRET is calculated from the acceptor and donor signals [EFRET = IA/(IA + γID)], where γ is the quantum yield ratio of Cy5 to Cy3 and IA and ID are the acceptor and donor fluorescence intensities corrected for background, direct laser excitation of the acceptor, and collection efficiencies/crosstalk (28, 29).
Supplementary Material
ACKNOWLEDGMENTS.
We thank Drs. Samuel Butcher and J.T. Hynes for helpful discussions and Drs. Arthur Pardi and Christopher Downey for design and preparation of the RNA constructs. This work was supported by the National Science Foundation (NSF), National Institute of Standards and Technology, and the W. M. Keck Foundation Initiative in RNA Sciences at the University of Colorado at Boulder. J.L.F. was supported in part by the Optical Science and Engineering Program NSF Integrative Graduate Education Research Traineeship. J.L.F and E.D.H were supported in part by the National Institutes of Health/University of Colorado Biophysics Training Grant (T32 GM-065103).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1114859109/-/DCSupplemental.
References
- 1.Tinoco I, Bustamante C. How RNA folds. J Mol Biol. 1999;293:271–281. doi: 10.1006/jmbi.1999.3001. [DOI] [PubMed] [Google Scholar]
- 2.Brion P, Westhof E. Hierarchy and dynamics of RNA folding. Annu Rev Biophys Biomolec Struct. 1997;26:113–137. doi: 10.1146/annurev.biophys.26.1.113. [DOI] [PubMed] [Google Scholar]
- 3.Mathews DH, Turner DH. Prediction of RNA secondary structure by free energy minimization. Curr Opin Struct Biol. 2006;16:270–278. doi: 10.1016/j.sbi.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Serganov A, Patel DJ. Ribozymes, riboswitches and beyond: Regulation of gene expression without proteins. Nat Rev Genet. 2007;8:776–790. doi: 10.1038/nrg2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhuang XW, et al. Correlating structural dynamics and function in single ribozyme molecules. Science. 2002;296:1473–1476. doi: 10.1126/science.1069013. [DOI] [PubMed] [Google Scholar]
- 6.Ditzler MA, Rueda D, Mo JJ, Hakansson K, Walter NG. A rugged free energy landscape separates multiple functional RNA folds throughout denaturation. Nucleic Acids Res. 2008;36:7088–7099. doi: 10.1093/nar/gkn871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solomatin SV, Greenfeld M, Chu S, Herschlag D. Multiple native states reveal persistent ruggedness of an RNA folding landscape. Nature. 2010;463:681–686. doi: 10.1038/nature08717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silverman SK, Cech TR. An early transition state for folding of the P4–P6 RNA domain. RNA-Publ RNA Soc. 2001;7:161–166. doi: 10.1017/s1355838201001716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bokinsky G, et al. Single-molecule transition-state analysis of RNA folding. Proc Natl Acad Sci USA. 2003;100:9302–9307. doi: 10.1073/pnas.1133280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koculi E, Thirumalai D, Woodson SA. Counterion charge density determines the position and plasticity of RNA folding transition states. J Mol Biol. 2006;359:446–454. doi: 10.1016/j.jmb.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 11.Fang XW, Thiyagarajan P, Sosnick TR, Pan T. The rate-limiting step in the folding of a large ribozyme without kinetic traps. Proc Natl Acad Sci USA. 2002;99:8518–8523. doi: 10.1073/pnas.142288399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maglott EJ, Goodwin JT, Glick GD. Probing the structure of an RNA tertiary unfolding transition state. J Am Chem Soc. 1999;121:7461–7462. [Google Scholar]
- 13.Bartley LE, Zhuang XW, Das R, Chu S, Herschlag D. Exploration of the transition state for tertiary structure formation between an RNA helix and a large structured RNA. J Mol Biol. 2003;328:1011–1026. doi: 10.1016/s0022-2836(03)00272-9. [DOI] [PubMed] [Google Scholar]
- 14.Pljevaljcic G, Klostermeier D, Millar DP. The tertiary structure of the hairpin ribozyme is formed through a slow conformational search. Biochemistry. 2005;44:4870–4876. doi: 10.1021/bi047772i. [DOI] [PubMed] [Google Scholar]
- 15.Rook MS, Treiber DK, Williamson JR. An optimal Mg2+ concentration for kinetic folding of the Tetrahymena ribozyme. Proc Natl Acad Sci USA. 1999;96:12471–12476. doi: 10.1073/pnas.96.22.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Downey CD, et al. Metal ion dependence, thermodynamics, and kinetics for intramolecular docking of a GAAA tetraloop and receptor connected by a flexible linker. Biochemistry. 2006;45:3664–3673. doi: 10.1021/bi0520941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hohng S, et al. Conformational flexibility of four-way junctions in RNA. J Mol Biol. 2004;336:69–79. doi: 10.1016/j.jmb.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Szewczak AA, Podell ER, Bevilacqua PC, Cech TR. Thermodynamic stability of the P4–P6 domain RNA tertiary structure measured by temperature gradient gel electrophoresis. Biochemistry. 1998;37:11162–11170. doi: 10.1021/bi980633e. [DOI] [PubMed] [Google Scholar]
- 19.Costa M, Michel F. Frequent use of the same tertiary motif by self-folding RNAs. EMBO J. 1995;14:1276–1285. doi: 10.1002/j.1460-2075.1995.tb07111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaeger L, Leontis NB. Tecto-RNA: One-dimensional self-assembly through tertiary interactions. Angew Chem-Int Edit. 2000;39:2521–2524. doi: 10.1002/1521-3773(20000717)39:14<2521::aid-anie2521>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 21.Cate JH, et al. Crystal structure of a group I ribozyme domain: Principles of RNA packing. Science. 1996;273:1678–1685. doi: 10.1126/science.273.5282.1678. [DOI] [PubMed] [Google Scholar]
- 22.Draper DE, Grilley D, Soto AM. Ions and RNA folding. Annu Rev Biophys Biomolec Struct. 2005;34:221–243. doi: 10.1146/annurev.biophys.34.040204.144511. [DOI] [PubMed] [Google Scholar]
- 23.Heus HA, Pardi A. Structural features that give rise to the unusual stability of RNA hairpins containing GNRA loops. Science. 1991;253:191–194. doi: 10.1126/science.1712983. [DOI] [PubMed] [Google Scholar]
- 24.Butcher SE, Dieckmann T, Feigon J. Solution structure of a GAAA tetraloop receptor RNA. EMBO J. 1997;16:7490–7499. doi: 10.1093/emboj/16.24.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis JH, et al. RNA helical packing in solution: NMR structure of a 30 kDa GAAA tetraloop-receptor complex. J Mol Biol. 2005;351:371–382. doi: 10.1016/j.jmb.2005.05.069. [DOI] [PubMed] [Google Scholar]
- 26.Vander Meulen KA, Davis JH, Foster TR, Record T, Butcher SE. Thermodynamics and folding pathway of tetraloop receptor-mediated RNA helical packing. J Mol Biol. 2008;384:702–717. doi: 10.1016/j.jmb.2008.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiore JL, Kraemer B, Koberling F, Erdmann R, Nesbitt DJ. Enthalpy-driven RNA folding: Single-molecule thermodynamics of tetraloop–receptor tertiary interaction. Biochemistry. 2009;48:2550–2558. doi: 10.1021/bi8019788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodak JH, Downey CD, Fiore JL, Pardi A, Nesbitt DJ. Docking kinetics and equilibrium of a GAAA tetraloop-receptor motif probed by single-molecule FRET. Proc Natl Acad Sci USA. 2005;102:10505–10510. doi: 10.1073/pnas.0408645102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiore JL, Hodak JH, Piestert O, Downey CD, Nesbitt DJ. Monovalent and divalent promoted GAAA tetraloop-receptor tertiary interactions from freely diffusing single-molecule studies. Biophys J. 2008;95:3892–3905. doi: 10.1529/biophysj.108.134346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKinney SA, Joo C, Ha T. Analysis of single-molecule FRET trajectories using hidden Markov modeling. Biophys J. 2006;91:1941–1951. doi: 10.1529/biophysj.106.082487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seol Y, Skinner GM, Visscher K. Elastic properties of a single-stranded charged homopolymeric ribonucleotide. Phys Rev Lett. 2004;93 doi: 10.1103/PhysRevLett.93.118102. [DOI] [PubMed] [Google Scholar]
- 32.Kim HD, et al. Mg2+-dependent conformational change of RNA studied by fluorescence correlation and FRET on immobilized single molecules. Proc Natl Acad Sci USA. 2002;99:4284–4289. doi: 10.1073/pnas.032077799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou HX. Rate theories for biologists. Q Rev Biophys. 2010;43:219–293. doi: 10.1017/S0033583510000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanggi P, Talkner P, Borkovec M. Reaction-rate theory: 50 years after Kramers. Rev Mod Phys. 1990;62:251–341. [Google Scholar]
- 35.Frauenfelder H, Wolynes PG. Rate theories and puzzles of Heme protein kinetics. Science. 1985;229:337–345. doi: 10.1126/science.4012322. [DOI] [PubMed] [Google Scholar]
- 36.Zhou HX. Theory for the rate of contact formation in a polymer chain with local conformational transitions. J Chem Phys. 2003;118:2010–2015. [Google Scholar]
- 37.Young BT, Silverman SK. The GAAA tetraloop-receptor interaction contributes differentially to folding thermodynamics and kinetics for the P4–P6 RNA domain. Biochemistry. 2002;41:12271–12276. doi: 10.1021/bi0264869. [DOI] [PubMed] [Google Scholar]
- 38.Dewey TG, Turner DH. Laser temperature-jump study of stacking in adenylic acid polymers. Biochemistry. 1979;18:5757–5762. doi: 10.1021/bi00593a002. [DOI] [PubMed] [Google Scholar]
- 39.Seol Y, Skinner GM, Visscher K, Buhot A, Halperin A. Stretching of homopolymeric RNA reveals single-stranded helices and base-stacking. Phys Rev Lett. 2007;98 doi: 10.1103/PhysRevLett.98.158103. [DOI] [PubMed] [Google Scholar]
- 40.Porschke D, Uhlenbec Oc, Martin FH. Thermodynamics and kinetics of helix-coil transition of oligomers containing GC base pairs. Biopolymers. 1973;12:1313–1335. [Google Scholar]
- 41.Thirumalai D, Hyeon C. RNA and protein folding: Common themes and variations. Biochemistry. 2005;44:4957–4970. doi: 10.1021/bi047314+. [DOI] [PubMed] [Google Scholar]
- 42.Hagen SJ, Hofrichter J, Szabo A, Eaton WA. Diffusion-limited contact formation in unfolded cytochrome c: Estimating the maximum rate of protein folding. Proc Natl Acad Sci USA. 1996;93:11615–11617. doi: 10.1073/pnas.93.21.11615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Misra VK, Draper DE. The linkage between magnesium binding and RNA folding. J Mol Biol. 2002;317:507–521. doi: 10.1006/jmbi.2002.5422. [DOI] [PubMed] [Google Scholar]
- 44.Heilman-Miller SL, Pan J, Thirumalai D, Woodson SA. Role of counterion condensation in folding of the Tetrahymena ribozyme II. Counterion-dependence of folding kinetics. J Mol Biol. 2001;309:57–68. doi: 10.1006/jmbi.2001.4660. [DOI] [PubMed] [Google Scholar]
- 45.Record MT, Anderson CF, Lohman TM. Thermodynamic analysis of ion effects on binding and conformational equilibria of proteins and nucleic acids: Roles of ion association or release, screening, and ion effects on water activity. Q Rev Biophys. 1978;11:103–178. doi: 10.1017/s003358350000202x. [DOI] [PubMed] [Google Scholar]
- 46.Bai Y, et al. Quantitative and comprehensive decomposition of the ion atmosphere around nucleic acids. J Am Chem Soc. 2007;129:14981–14988. doi: 10.1021/ja075020g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leipply D, Draper DE. Dependence of RNA tertiary structural stability on Mg2+ concentration: Interpretation of the Hill equation and coefficient. Biochemistry. 2010;49:1843–1853. doi: 10.1021/bi902036j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meulen KAV, Davis JH, Foster TR, Record MT, Butcher SE. Thermodynamics and folding pathwayof tetraloop receptor-mediated RNA helical packing. J Mol Biol. 2008;384:702–717. doi: 10.1016/j.jmb.2008.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.SantaLucia J. A unified view of polymer, dumbbell, and oligonucleotide DNA nearest-neighbor thermodynamics. Proc Natl Acad Sci USA. 1998;95:1460–1465. doi: 10.1073/pnas.95.4.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bai Y, et al. Critical assessment of nucleic acid electrostatics via experimental and computational investigation of an unfolded state ensemble. J Am Chem Soc. 2008;130:12334–12341. doi: 10.1021/ja800854u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qin PZ, Feigon J, Hubbell WL. Site-directed spin labeling studies reveal solution conformational changes in a GAAA tetraloop receptor upon Mg2+-dependent docking of a GAAA tetraloop. J Mol Biol. 2005;351:1–8. doi: 10.1016/j.jmb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 52.Bailor MH, Sun XY, Al-Hashimi HM. Topology links RNA secondary structure with global conformation, dynamics, and adaptation. Science. 2010;327:202–206. doi: 10.1126/science.1181085. [DOI] [PubMed] [Google Scholar]
- 53.Chu VB, et al. Do conformational biases of simple helical junctions influence RNA folding stability and specificity? RNA-Publ RNA Soc. 2009;15:2195–2205. doi: 10.1261/rna.1747509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klostermeier D, Millar DP. Helical junctions as determinants for RNA folding: Origin of tertiary structure stability of the hairpin ribozyme. Biochemistry. 2000;39:12970–12978. doi: 10.1021/bi0014103. [DOI] [PubMed] [Google Scholar]
- 55.Aitken CE, Marshall RA, Puglisi JD. An oxygen scavenging system for improvement of dye stability in single-molecule fluorescence experiments. Biophys J. 2008;94:1826–1835. doi: 10.1529/biophysj.107.117689. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.