Abstract
Early maternal support has been shown to promote specific gene expression, neurogenesis, adaptive stress responses, and larger hippocampal volumes in developing animals. In humans, a relationship between psychosocial factors in early childhood and later amygdala volumes based on prospective data has been demonstrated, providing a key link between early experience and brain development. Although much retrospective data suggests a link between early psychosocial factors and hippocampal volumes in humans, to date there has been no prospective data to inform this potentially important public health issue. In a longitudinal study of depressed and healthy preschool children who underwent neuroimaging at school age, we investigated whether early maternal support predicted later hippocampal volumes. Maternal support observed in early childhood was strongly predictive of hippocampal volume measured at school age. The positive effect of maternal support on hippocampal volumes was greater in nondepressed children. These findings provide prospective evidence in humans of the positive effect of early supportive parenting on healthy hippocampal development, a brain region key to memory and stress modulation.
Keywords: depression, parental support, nurturance, neurodevelopment
The suggestion that environmental enrichment early in life results in enhanced brain development was first proposed and investigated by Hebb in the late 1940s and was empirically validated in rodents 20 y later (1). These findings provided some of the first quantitative evidence supporting the tangible role of environmental experience on structural brain development. More recent research has extended these findings by investigating the biological mechanisms by which psychosocial factors influence neuronal development. Of critical importance to the study of risk for psychopathology, animal models have elucidated the mechanisms by which maternal nurturance, a uniquely powerful form of early enrichment, promotes adaptive programming of the hypothalamic–pituitary–adrenal axis stress response and hippocampal development (2, 3). Improvements in the capacity for stress modulation have been shown to be related to epigenetic modifications whereby methylation of multiple genes results in changes in gene expression for glucocorticoid and mineralcorticoid receptors (4, 5). These changes are associated with increases in dendritic branching and neurogenesis and related increases in hippocampal volumes (6–9). Consistent with this phenomenon and conversely, the stress of early maternal deprivation has been shown to have negative effects on this cascade (10, 11). A similar relationship between early nurturance and stress modulation has also been reported in nonhuman primates (12, 13). This work has shown that early nurturing in nonhuman animals facilitates the offspring's enduring capacity for adaptive stress modulation, a phenomenon with potentially powerful public health implications if also operational in humans.
Building on these animal findings, there has been a surge of interest in the effects of early experience on brain development in humans (14). Numerous studies have documented a relationship between key early environmental factors and later cognitive and socio-emotional outcomes. Studies of Romanian orphans, using the naturalistic stress of institutional care, have shown that enhanced early caregiving through placement in therapeutic foster care had a robust and positive impact on cognitive, social, and emotional outcomes (15, 16). Differential patterns of DNA methylation in children raised in institutions compared with those raised by their biological parents have recently been provided, suggesting that the epigenetic phenomenon known in animals might also be operative in human development (17). Prospective evidence of the impact of early nurturance on structural brain development has been provided in a few studies to date. Tottenham et al. (18) reported that institutionalized orphans who experienced environmental enrichment in the form of earlier adoption displayed smaller amygdala volumes (an anatomical variation associated with better emotion regulation), but they did not find a relationship with hippocampal volumes. Lupien et al. (19) also reported larger amygdala but no change in hippocampal volumes in a sample of children exposed since birth to maternal depression, the latter a condition known to be associated with decreased parenting sensitivity and responsiveness (20). In another small study group, larger right amygdala volumes were found in institutionalized children compared with noninstitutionalized control subjects (21). Additionally, a study of a small group of premature infants demonstrated that early environmental enhancement in the neonatal intensive care unit was associated with positive changes in brain structure, evidenced by higher relative anisotropy in several specific white matter tracts (22). Rao et al. (23) found a relationship between increased early higher-quality parental care and smaller hippocampal volumes in a sample of children exposed to cocaine in utero, findings in the opposite direction of that expected from the animal data. These studies, although suggestive, have not yet provided findings in humans analogous to the animal literature and are limited by the use of populations also exposed to multiple perinatal and postnatal stressors and traumas known to impact brain development.
An increasing body of retrospective data also suggests a relationship between early nurturance, or the lack of nurturance based on the experience of trauma, and later stress reactivity and hippocampal volumes in a variety of human populations, including those with depression (24, 25). Importantly, numerous studies have reported that major depressive disorder (MDD) is associated with smaller hippocampal volumes in adults (26, 27). The findings in children and adolescents are less consistent than in adults, with some investigators reporting no hippocampal volume differences (28, 29) and others reporting decreased volume (30–32). Importantly, numerous developmental studies have demonstrated that poor-quality parenting (e.g., nonsupportive or harsh) is a risk factor for childhood MDD (33). Further, a few studies suggest that smaller hippocampal volumes in adolescents at risk for MDD are associated with increased susceptibility to the effects of psychosocial stress and subsequent risk for recurrence or development of MDD (23, 34). A similar process has also been identified in a twin sample, in which smaller hippocampal volumes increased the risk for development of stress-related psychopathology (35). Taken together, these findings suggest intriguing, although potentially complex, relationships among experiences of early stress, low nurturance, stress reactivity, and hippocampal volumes in humans, opening the possibility that the well-characterized phenomena identified in animals may also be operative in humans.
Given these converging lines of evidence, one hypothesis is that impairments in early maternal nurturing contribute to enhanced and maladaptive stress reactivity, reduced hippocampal volumes, and increased risk for depression. As described above, there is some prospective evidence in humans for the effects of maternal nurturance on structural brain development of the amygdala in high-risk samples, as well as evidence for nurturance-dependent alterations in DNA methylation in similar samples. In addition, retrospective data have established a link between early interruptions of maternal care, depression, and hippocampal volumes. However, to date there has been no prospective data documenting the positive relationship between early maternal nurturance and structural development of the hippocampus in either typically developing children or groups at risk for MDD, despite a well-documented relationship in animals and a putative mechanism of the developmental psychopathology hypothesis stated above. The hippocampus is of particular interest given the compelling findings on the role of early nurturance and its structural development from animal studies described above. If a relationship between early nurturance and hippocampal development is present in humans, it would fill a key gap in the literature and open the door to prospective investigation of healthy brain development as well as mechanisms for the development of depression in certain at-risk groups as outlined. To address this question, we investigated the relationship between maternal nurturance objectively measured through structured observation during the preschool period of development and later hippocampal volume measured at school age. These data were derived from a sample of depressed preschoolers and age-matched healthy and “other psychiatric” comparison groups, followed prospectively into school age, at which time neuroimaging was conducted. Examining the relationship between early maternal nurturance and later hippocampal volume in both healthy and depressed children allowed us to secondarily examine mediators and/or moderators in the relationships between hippocampal volume, maternal nurturance, and childhood depression.
Results
Subjects were 92 children participating in a longitudinal study of preschool depression who met all inclusion/exclusion criteria for magnetic resonance brain imaging at ages 7–13 y. These children were non–left-hand-dominant children with usable left and/or right hippocampus T1-weighted MRI volume data, who also had parent–child interaction data acquired at ages 3–5 y, allowing us to examine the relationship between early maternal support and later childhood hippocampal volume. Demographic characteristics of the study sample divided by depression severity scores are listed in Tables 1 and 2.
Table 1.
Characteristics of the sample, part 1
| Characteristic | Preschool depression severity score 4–9 (n = 41) |
Preschool depression severity score 0–3 (n = 51) |
χ2 | P | ||
| % | n | % | N | |||
| Sex | 0.46 | 0.499 | ||||
| Female | 56.1 | 23 | 49.0 | 25 | ||
| Male | 43.9 | 18 | 51.0 | 26 | ||
| Age (y) | FE | 0.560 | ||||
| 6 | 2.4 | 1 (1 M, 0 F) | 0.0 | 0 (0 M, 0 F) | ||
| 7 | 4.9 | 2 (0 M, 2 F) | 7.8 | 4 (1 M, 3 F) | ||
| 8 | 9.8 | 4 (1 M, 3 F) | 13.7 | 7 (5 M, 2 F) | ||
| 9 | 34.1 | 14 (4 M, 10 F) | 39.2 | 20 (9 M, 11 F) | ||
| 10 | 24.4 | 10 (6 M, 4 F) | 27.5 | 14 (10 M, 4 F) | ||
| 11 | 19.5 | 8 (5 M, 3 F) | 11.8 | 6 (1 M, 5 F) | ||
| 12 | 4.9 | 2 (1 M, 1 F) | 0.0 | 0 (0 M, 0 F) | ||
| Parental education | 2.87 | 0.412 | ||||
| High school diploma | 14.6 | 6 | 7.8 | 4 | ||
| Some college | 41.5 | 17 | 33.3 | 17 | ||
| 4-y college degree | 17.1 | 7 | 29.4 | 15 | ||
| Graduate education | 26.8 | 11 | 29.4 | 15 | ||
| Psychotropic medication use | 1.74 | 0.187 | ||||
| Yes | 29.3 | 12 | 17.6 | 9 | ||
| No | 70.7 | 29 | 82.4 | 42 | ||
| Maternal history of depression | 0.80 | 0.370 | ||||
| Yes | 42.5 | 17 | 33.3 | 17 | ||
| No | 57.5 | 23 | 66.7 | 34 | ||
| Maternal prenatal tobacco use | 1.05 | 0.307 | ||||
| Yes | 27.8 | 10 | 18.2 | 8 | ||
| No | 72.2 | 26 | 81.8 | 36 | ||
| Maternal prenatal alcohol use | 4.31 | 0.038 | ||||
| Yes | 36.1 | 13 | 15.9 | 7 | ||
| No | 63.9 | 23 | 84.1 | 37 | ||
F, female; FE, Fisher's exact test; M, male.
Table 2.
Characteristics of the sample, part 2
| Characteristic | Preschool depression severity score 4–9 (n = 41) |
Preschool depression severity score 0–3 (n = 51) |
t | P | ||
| Mean | SD | Mean | SD | |||
| Maternal support | 12.17 | 9.13 | 12.12 | 8.91 | 0.03 | 0.978 |
| Preschool depression severity | 5.12 | 1.25 | 1.41 | 1.04 | 15.53 | <0.001 |
| Internalizing dimensional score | 6.66 | 3.21 | 3.10 | 1.97 | 6.23 | <0.001 |
| Externalizing dimensional score | 10.12 | 7.39 | 4.25 | 4.38 | 4.49 | <0.001 |
| No. of traumatic life events | 2.78 | 1.76 | 1.57 | 1.37 | 3.67 | <0.001 |
| IQ score | 104.1 | 14.1 | 108.2 | 15.9 | 1.30 | 0.197 |
| Birth weight (kg) | 3.24 | 0.55 | 3.34 | 0.63 | 0.86 | 0.392 |
| Gestational age (wk) | 38.82 | 2.09 | 39.06 | 2.38 | 0.50 | 0.620 |
| Days in NICU | 0.31 | 0.98 | 3.50 | 14.92 | 1.35 | 0.185 |
| Hippocampus volume, right (mm3) | 1,720 | 169 | 1,789 | 212 | 1.66 | 0.101 |
| Hippocampus volume, left (mm3) | 1,715 | 136 | 1,781 | 195 | 1.79 | 0.078 |
IQ, intelligence quotient; NICU, neonatal intensive care unit.
Sex, age, and parental income were examined in separate repeated-measures models to determine whether they were significantly related to hippocampal volume. Sex was significantly associated with hippocampal volume (P = 0.036), whereas age (P = 0.955) and parental income (P = 0.088) were not. Therefore, sex was included as a covariate in subsequent analyses. A repeated-measures mixed model with compound symmetric covariance structure was used to model the effects of maternal support, preschool depression severity, and their interaction on hippocampal volume (Table 3). Brain hemisphere (left or right) and sex were included as covariates in the model. The overall model was significant (χ21 = 57.84, P < 0.001), with maternal support (F1,87 = 18.58, P < 0.001) and the interaction of maternal support and preschool depression severity (F1,87 = 4.07, P = 0.047) significantly associated with hippocampal volume. The estimated increase in hippocampal volume by unit of increased maternal support (a frequency) was 13.4 mm3. A model including the three-way interaction of brain hemisphere, maternal support, and preschool depression severity was conducted to determine whether the interaction was specific to one hemisphere (Table 3). The three-way interaction was not significant (F1,74 = 0.04, P = 0.848). Finally, medication use, internalizing and externalizing symptom severity, traumatic life events, and maternal history of depression were added to the model as covariates. After controlling for these variables, maternal support (F1,80 = 18.09, P < 0.001) and the interaction of maternal support and preschool depression severity (F1,80 = 5.09, P = 0.027) were still significantly associated with hippocampal volume.
Table 3.
Repeated-measures mixed models of hippocampal volume
| Model | Estimate | F | df | P |
| Model 1 | ||||
| Left brain hemisphere | −3.20 | 0.06 | 1, 75 | 0.811 |
| Female sex | −78.87 | 5.76 | 1, 87 | 0.019 |
| Preschool depression severity | 7.68 | 0.39 | 1, 87 | 0.533 |
| Maternal support | 13.38 | 18.58 | 1, 87 | <0.001 |
| Preschool depression severity × maternal support | −1.59 | 4.07 | 1, 87 | 0.047 |
| Model 2 | ||||
| Left brain hemisphere | −5.40 | 0.09 | 1, 74 | 0.761 |
| Female sex | −78.82 | 5.75 | 1, 87 | 0.019 |
| Preschool depression severity | 7.63 | 0.39 | 1, 87 | 0.536 |
| Maternal support | 13.37 | 18.55 | 1, 87 | <0.001 |
| Preschool depression severity × maternal support | −1.61 | 4.02 | 1, 87 | 0.048 |
| Preschool depression severity × maternal support × left brain hemisphere | 0.06 | 0.04 | 1, 74 | 0.848 |
| Model 3 | ||||
| Left brain hemisphere | 0.74 | 0.00 | 1, 74 | 0.954 |
| Female sex | −75.57 | 4.62 | 1, 80 | 0.035 |
| Psychiatric medication use | −49.08 | 1.26 | 1, 80 | 0.265 |
| Internalizing dimensional score | 11.24 | 1.87 | 1, 80 | 0.175 |
| Externalizing dimensional score | −4.01 | 1.42 | 1, 80 | 0.237 |
| Number of traumatic life events | −11.17 | 0.86 | 1, 80 | 0.357 |
| Maternal history of depression | 28.95 | 0.59 | 1, 80 | 0.446 |
| Preschool depression severity | 11.30 | 0.44 | 1, 80 | 0.511 |
| Maternal support | 14.25 | 18.09 | 1, 80 | <0.001 |
| Preschool depression severity × maternal support | −1.92 | 5.09 | 1, 80 | 0.027 |
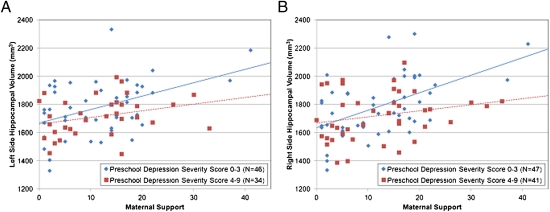
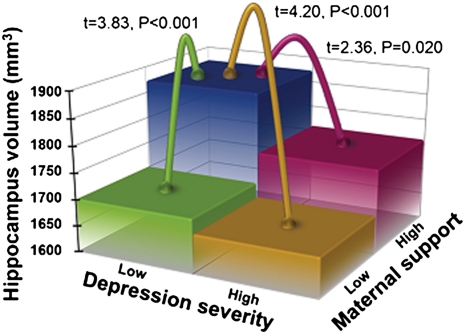
To parse the source of the interaction between maternal support and preschool depression severity, we dichotomized subjects into those with zero to three preschool depression symptoms (severity scores found in healthy samples, therefore a subgroup with no clinical depression) and those with four to nine preschool depression symptoms (high severity consistent with clinical depression) and then examined the relationship of maternal support and hippocampal volume in these groups. Fig. 1 A and B show the association of maternal support with left and right hippocampal volume in these groups. Of note, there were two subjects in the nondepressed group with maternal support scores considerably greater than the others in this group. To test whether these subjects were multivariate outliers, the Mahalanobis distance (MD) and its probability were calculated for all nondepressed subjects (36). No subjects had P(MD) < 0.001, so no subjects were considered outliers, and all data were included in the analyses (notably, even if these subjects are excluded, the effect of maternal support on hippocampal volume remains highly significant). The relationship between maternal support and hippocampal volume was significant only in low-severity/nondepressed children (F1,41 = 9.22, P = 0.004) and not in depressed/high-severity children (F1,29 = 2.37, P = 0.134). We then divided children into four groups (Fig. 2), illustrating that nondepressed children with high maternal support had significantly larger hippocampal volumes than the following three groups: nondepressed children with low maternal support (9.2% smaller volume) and depressed children with high (6.0% smaller volume) or low maternal support (10.6% smaller volume).
Fig. 1.
(A) Left side hippocampal volume by preschool depression severity and maternal support. (B) Right side hippocampal volume by preschool depression severity and maternal support.
Fig. 2.
Hippocampus volume by preschool depression severity and maternal support.
Discussion
These study findings provide evidence in humans replicating the positive relationship between early experiences of maternal nurturance and hippocampal volume well demonstrated in animal models. Using data from a prospective, longitudinal study of depressed preschoolers and comparison groups who underwent neuroimaging at school age, we found that observationally measured maternal support during a mildly stressful interactive task in early childhood was a powerful predictor of larger hippocampal volume in both hemispheres at school age. The relationship between maternal support and hippocampal volume remained significant even when other variables known to impact hippocampal volume (e.g., stressful life events, sex, and depression severity) were included in the model.
Further, maternal support and depression severity interacted in predicting volume. Positive maternal support was a stronger predictor of greater hippocampal volume in nondepressed children. These findings suggest that early maternal support exerts a positive influence on hippocampal development in children without depression but not in depressed children, in whom the negative effects of this risk condition seem to impede the potential benefits of maternal support. As such, our findings did not support the hypothesis that the influence of maternal support on hippocampal volume would be found among children with depression. Instead, these results suggest that either depression has an effect on hippocampal volumes that is not mediated by maternal support or that an examination of maternal support before the onset of depression is needed to adequately test this risk hypothesis. Our sample of depressed children all had very-early-onset depression that was present before our assessment of maternal support. Other limitations to the design include the fact that maternal support was measured only in early and not later in childhood and that earlier experiences of nurturing before age 3 y, shown to be important to developmental outcomes, were not prospectively studied. Prospective research in high-risk samples before the onset of depression may be needed to specifically assess whether hippocampal volume mediates a relationship between poor early nurturance and subsequent risk for depression.
The experience of a nurturing caregiver early in life has proven to be one of the most essential prerequisites for healthy development and adaptive functioning in mammals (37). The current data provide evidence that the well-established significant impact of positive parenting on enhancing and maintaining hippocampal neuroplasticity, likely through epigenetic mechanisms enhancing neurogenesis, as suggested by the work of Naumova et al. (17), may also to be operative in humans. Further, it was notable that this effect remained robust even after controlling for other factors known to impact hippocampal volume, such as stressful life events and maternal history of depression. Importantly, although 96.7% of caregivers in this study sample were mothers, we expect that this effect pertains to the primary caregiver (the provider of nurturance) whether it be mother, father, grandparent, or other.
Whether maternal support early in childhood is more or less powerful than at later periods of development is of interest and remains unclear from these data. However, sensitive periods for the importance of maternal support have been suggested by early adoption studies (15). However, given the central role of the caregiver in the life of the young child, this developmental period would seem to be an optimal time for enhancing the early maternal–child relationship. On the basis of these data, one cannot rule out that the relationship between maternal support and hippocampal volume in offspring is based on genetic factors, that supportive caregivers could have larger hippocampal volumes and then have biological children with larger volumes. However, there is no evidence in the literature of a relationship between hippocampal volume and supportive care giving in adults. The available data also suggest that heritability of hippocampal volumes is moderate and lower than many other brain regions (38). Furthermore, numerous studies have demonstrated that the hippocampus is sensitive to environmental and psychosocial influences in both animals and humans, making a purely genetic mechanism less likely (9, 39). Despite these assurances, longitudinal scan data, including scans at the preschool period, would be necessary to clarify the role of genetic factors, a conclusion underscored by previous research showing that smaller hippocampal volumes increased the risk for development of stress-related psychopathology, suggesting that complex interactive processes could be at play (35).
These data extend the current literature, establishing the crucial role of the caregiver in early childhood for healthy social and emotional development by demonstrating that the early experience of supportive caregiving also positively impacts structural development of the hippocampus, at least in children without early-onset depression. Notably, the relationships between maternal support and hippocampal volumes remained highly significant when comorbid internalizing and externalizing symptoms were controlled in the analysis. The importance of this effect is underscored by the fact that the hippocampus is a brain region central to memory, emotion regulation, and stress modulation, all areas key to healthy social adaptation. We believe these findings have potentially profound public health implications and suggest that greater public health emphasis on early parenting could be a very fruitful social investment. The finding that early parenting support, a modifiable psychosocial factor, is directly related to healthy development of a key brain region known to impact cognitive functioning and emotion regulation opens an exciting opportunity to impact the development of children in a powerful and positive fashion. This finding, when replicated, would strongly suggest enhancement of public policies and programs that provide support and parenting education to caregivers early in development.
Materials and Methods
Participants.
The study sample was originally recruited between the ages of 3 and 6 y from daycare centers and preschools in the St. Louis metropolitan area using a screening checklist to oversample children with symptoms of depression (40). Healthy preschoolers and those with other psychiatric disorders were included as comparison groups for the original study sample ascertainment. Preschoolers with neurological or chronic medical problems or those with significant developmental delays were excluded. Informed consent (or assent) was obtained from all study subjects, who were fully informed about the nature and consequences of the study procedures before participation. Children and their caregivers were assessed at four to six annual waves before the time of scanning.
Measures.
At each annual wave, parents were interviewed about their child using the Preschool Age Psychiatric Assessment (PAPA), an age-appropriate diagnostic interview addressing the child's psychiatric symptoms and stressful life events with established reliability (41). The PAPA was used to derive depression severity scores by summing all symptoms of depression, a variable previously shown to be a sensitive measure of the severity of illness (42). Further details on training and administration of the PAPA in the study sample can be found in Luby et al. (40).
At the second annual wave, when subjects were between the ages of 4 and 7 y, parent and child were also observed interacting in a mildly stressful challenging task in the laboratory, “the waiting task,” during which maternal support was measured. The waiting task is a parent–child interaction paradigm designed to elicit mild stress for both members of the dyad (43). The task requires the child to wait for 8 min before opening a brightly wrapped gift, which is sitting within arm's reach. Concurrently the child's primary caregiver completes questionnaires. The supportive and/or nonsupportive care giving strategies that the parent uses to help regulate the child's impulse and desire to open the gift immediately are coded by staff trained to reliability. Each display of specific types of supportive strategies by the caregiver is counted as 1 unit. Several research groups have previously reported acceptable psychometric properties for the task, and it is a well-validated approach to measuring parenting strategy during which maternal support and responsiveness was evaluated (43, 44).
Maternal history of depression was measured using the Family Interview for Genetic Studies (45), a widely used and well-validated measure of family history of psychiatric disorders. Detailed methods of training and administration are described by Luby et al. (40).
Structural Imaging Methods.
Parents of either healthy or depressed study subjects who were participants in a longitudinal study of preschool-onset depression were screened by phone to determine whether any exclusion criteria for MRI scanning were present. Exclusion criteria included (i) contraindications for MRI scanning; (ii) head injury with loss of consciousness >5 min; (iii) stroke, seizure disorder, or other chronic neurological or medical illness with known neurological impact; (iv) diagnosis of a pervasive developmental disorder; and (v) treatment for lead poisoning.
All MR scans were performed on a Siemens 3.0-T Tim Trio dedicated research scanner. Subjects were scanned without sedation in a semistandardized position. Two 3D T1-weighted magnetization prepared rapid gradient echo research-tailored MR scans (1-mm isotropic voxels) were acquired sagittally (repetition time 2,400 ms, echo time 3.16 ms, inversion time 1,200 ms, flip angle 8°, slab 160 mm, 160 partitions, 256 × 256 matrix, field of view 256, total scanning time 12:36). Images were coregistered and averaged to increase signal to noise ratio (46), then 16-bit image data were linearly interpolated to 0.5-mm3 voxels and converted to 8-bit using ANALYZE (47).
Hippocampal volumes were derived from a well-established template-based automated segmentation using high-dimensional transformation methods (48–50). Briefly, hippocampal segmentations and volumes were derived from an atlas- or template-based automated segmentation using a high-dimensional transformation after placement of standardized landmarks by an experienced rater (C.B.) (51) and preliminary transformations using these landmarks. Hippocampus boundary definitions were standardized as detailed in previous reports (48, 52). As specified by these definitions, a template segmentation was created by hand (C.B.) and based on one subject with typical anatomy and reviewed by neuroanatomical gold standard experts (K.N.B. and Mohktar Gado). This gold standard hippocampal segmentation was converted to a 3D tessellated surface. Using the landmarks, the target (subject) images were oriented to the template image by initially applying a rigid landmark transformation algorithm, followed by a more precise target to template nonlinear, large deformation landmark matching algorithm (LDL). Voxel intensities in each target image were scaled to more closely match those of the template image. A high-dimensional transformation, large deformation diffeomorphic metric mapping (51), was then used to generate a transformed template surface. After inverting the LDL algorithm described above, the transformed template surface represented the target (subject) hippocampus. The reliability of this process is equivalent or superior to manual outlining by experts (52). All segmentation results were blindly reviewed for accuracy (C.B.), and inadequately defined hippocampi were not included in analyses. Hippocampal volumes included all hippocampal gray matter and white matter contained within the segmented structure.
Acknowledgments
The authors thank Michael J. Miller and Tilak Ratnanather of the Center for Imaging Science, The Johns Hopkins University, for their technical assistance and insights with the implementation of the large deformation diffeomorphic metric mapping to quantify the hippocampus volumes. Funding for this study was provided by National Institute of Mental Health Grants MH64769 (to J.L.L.) and MH090786 (to J.L.L., D.M.B., and K.N.B.). The data reported in this paper are archived at the Washington University School of Medicine.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Greenough WT, Black JE, Wallace CS. Experience and brain development. Child Dev. 1987;58:539–559. [PubMed] [Google Scholar]
- 2.Liu D, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 3.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 4.Fish EW, et al. Epigenetic programming of stress responses through variations in maternal care. Ann N Y Acad Sci. 2004;1036:167–180. doi: 10.1196/annals.1330.011. [DOI] [PubMed] [Google Scholar]
- 5.Szyf M, Weaver IC, Champagne FA, Diorio J, Meaney MJ. Maternal programming of steroid receptor expression and phenotype through DNA methylation in the rat. Front Neuroendocrinol. 2005;26:139–162. doi: 10.1016/j.yfrne.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16:250–260. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- 7.Sapolsky RM. Mothering style and methylation. Nat Neurosci. 2004;7:791–792. doi: 10.1038/nn0804-791. [DOI] [PubMed] [Google Scholar]
- 8.Duffy SN, Craddock KJ, Abel T, Nguyen PV. Environmental enrichment modifies the PKA-dependence of hippocampal LTP and improves hippocampus-dependent memory. Learn Mem. 2001;8:26–34. doi: 10.1101/lm.36301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: Structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62:3–12. doi: 10.1016/j.neuropharm.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francis DD, Meaney MJ. Maternal care and the development of stress responses. Curr Opin Neurobiol. 1999;9:128–134. doi: 10.1016/s0959-4388(99)80016-6. [DOI] [PubMed] [Google Scholar]
- 11.Meaney MJ, Aitken DH, Viau V, Sharma S, Sarrieau A. Neonatal handling alters adrenocortical negative feedback sensitivity and hippocampal type II glucocorticoid receptor binding in the rat. Neuroendocrinology. 1989;50:597–604. doi: 10.1159/000125287. [DOI] [PubMed] [Google Scholar]
- 12.Parker KJ, Buckmaster CL, Sundlass K, Schatzberg AF, Lyons DM. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proc Natl Acad Sci USA. 2006;103:3000–3005. doi: 10.1073/pnas.0506571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozorovitskiy Y, et al. Experience induces structural and biochemical changes in the adult primate brain. Proc Natl Acad Sci USA. 2005;102:17478–17482. doi: 10.1073/pnas.0508817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shonkoff JP, Phillips D. From Neurons to Neighborhoods: The Science of Early Childhood Development. Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- 15.Nelson CA, 3rd, et al. Cognitive recovery in socially deprived young children: The Bucharest Early Intervention Project. Science. 2007;318:1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- 16.Fox SE, Levitt P, Nelson CA., 3rd How the timing and quality of early experiences influence the development of brain architecture. Child Dev. 2010;81:28–40. doi: 10.1111/j.1467-8624.2009.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naumova OY, et al. Differential patterns of whole-genome DNA methylation in institutionalized children and children raised by their biological parents. Dev Psychopathol. 2011;Nov 29:1–13. doi: 10.1017/S0954579411000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tottenham N, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. 2010;13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lupien SJ, et al. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc Natl Acad Sci USA. 2011;108:14324–14329. doi: 10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lovejoy MC, Graczyk PA, O'Hare E, Neuman G. Maternal depression and parenting behavior: A meta-analytic review. Clin Psychol Rev. 2000;20:561–592. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- 21.Mehta MA, et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: The English and Romanian Adoptees study pilot. J Child Psychol Psychiatry. 2009;50:943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- 22.Als H, et al. Early experience alters brain function and structure. Pediatrics. 2004;113:846–857. doi: 10.1542/peds.113.4.846. [DOI] [PubMed] [Google Scholar]
- 23.Rao H, et al. Early parental care is important for hippocampal maturation: Evidence from brain morphology in humans. Neuroimage. 2010;49:1144–1150. doi: 10.1016/j.neuroimage.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vythilingam M, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002;159:2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaufman J, Charney D. Effects of early stress on brain structure and function: Implications for understanding the relationship between child maltreatment and depression. Dev Psychopathol. 2001;13:451–471. doi: 10.1017/s0954579401003030. [DOI] [PubMed] [Google Scholar]
- 26.Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: A meta-analysis. Am J Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- 27.Videbech P, Ravnkilde B. Hippocampal volume and depression: A meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 28.Rosso IM, et al. Amygdala and hippocampus volumes in pediatric major depression. Biol Psychiatry. 2005;57:21–26. doi: 10.1016/j.biopsych.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 29.MacMillan S, et al. Increased amygdala: Hippocampal volume ratios associated with severity of anxiety in pediatric major depression. J Child Adolesc Psychopharmacol. 2003;13:65–73. doi: 10.1089/104454603321666207. [DOI] [PubMed] [Google Scholar]
- 30.Caetano SC, et al. Medial temporal lobe abnormalities in pediatric unipolar depression. Neurosci Lett. 2007;427:142–147. doi: 10.1016/j.neulet.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 31.McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- 32.MacMaster FP, et al. Amygdala and hippocampal volumes in familial early onset major depressive disorder. Biol Psychiatry. 2008;63:385–390. doi: 10.1016/j.biopsych.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kerns KA, Brumariu LE, Seibert A. Multi-method assessment of mother-child attachment: Links to parenting and child depressive symptoms in middle childhood. Attach Hum Dev. 2011;13:315–333. doi: 10.1080/14616734.2011.584398. [DOI] [PubMed] [Google Scholar]
- 34.Whittle S, et al. Hippocampal volume and sensitivity to maternal aggressive behavior: A prospective study of adolescent depressive symptoms. Dev Psychopathol. 2011;23:115–129. doi: 10.1017/S0954579410000684. [DOI] [PubMed] [Google Scholar]
- 35.Gilbertson MW, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevens JP. Outliers and influential data points in regression analysis. Psychol Bull. 1984;95:334–344. [Google Scholar]
- 37.Suomi SJ, van der Horst FC, van der Veer R. Rigorous experiments on monkey love: An account of Harry F. Harlow's role in the history of attachment theory. Integr Psychol Behav Sci. 2008;42:354–369. doi: 10.1007/s12124-008-9072-9. [DOI] [PubMed] [Google Scholar]
- 38.Peper JS, Brouwer RM, Boomsma DI, Kahn RS, Hulshoff Pol HE. Genetic influences on human brain structure: A review of brain imaging studies in twins. Hum Brain Mapp. 2007;28:464–473. doi: 10.1002/hbm.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front Hum Neurosci. 2010;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luby JL, Si X, Belden AC, Tandon M, Spitznagel E. Preschool depression: Homotypic continuity and course over 24 months. Arch Gen Psychiatry. 2009;66:897–905. doi: 10.1001/archgenpsychiatry.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Egger HL, et al. Test-retest reliability of the Preschool Age Psychiatric Assessment (PAPA) J Am Acad Child Adolesc Psychiatry. 2006;45:538–549. doi: 10.1097/01.chi.0000205705.71194.b8. [DOI] [PubMed] [Google Scholar]
- 42.Luby JL, Mrakotsky C, Heffelfinger A, Brown K, Spitznagel E. Characteristics of depressed preschoolers with and without anhedonia: Evidence for a melancholic depressive subtype in young children. Am J Psychiatry. 2004;161:1998–2004. doi: 10.1176/appi.ajp.161.11.1998. [DOI] [PubMed] [Google Scholar]
- 43.Carmichael-Olson H, Greenberg M, Slough N. Manual for the Waiting Task. Seattle: University of Washington; 1985. [Google Scholar]
- 44.Cole PM, Teti LO, Zahn-Waxler C. Mutual emotion regulation and the stability of conduct problems between preschool and early school age. Dev Psychopathol. 2003;15:1–18. [PubMed] [Google Scholar]
- 45.Maxwell ME. Manual for the Family Interview for Genetic Studies (FIGS) Bethesda, MD: Clinical Neurogenetics Branch, Intramural Research Program, National Insititute of Mental Health; 1992. [Google Scholar]
- 46.Holmes CJ, et al. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- 47.Robb RA. The biomedical imaging resource at Mayo Clinic. IEEE Trans Med Imaging. 2001;20:854–867. doi: 10.1109/42.952724. [DOI] [PubMed] [Google Scholar]
- 48.Csernansky JG, et al. Hippocampal deformities in schizophrenia characterized by high dimensional brain mapping. Am J Psychiatry. 2002;159:2000–2006. doi: 10.1176/appi.ajp.159.12.2000. [DOI] [PubMed] [Google Scholar]
- 49.Miller MI. Computational anatomy: Shape, growth, and atrophy comparison via diffeomorphisms. Neuroimage. 2004;23(Suppl 1):S19–S33. doi: 10.1016/j.neuroimage.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 50.Posener JA, et al. High-dimensional mapping of the hippocampus in depression. Am J Psychiatry. 2003;160:83–89. doi: 10.1176/appi.ajp.160.1.83. [DOI] [PubMed] [Google Scholar]
- 51.Beg MF, Miller MI, Trouvé A, Younes L. Computing large deformation metric mappings via geodesic flows of diffeomorphisms. Int J Comput Vis. 2005;61:139–157. [Google Scholar]
- 52.Haller JW, et al. Three-dimensional hippocampal MR morphometry with high-dimensional transformation of a neuroanatomic atlas. Radiology. 1997;202:504–510. doi: 10.1148/radiology.202.2.9015081. [DOI] [PubMed] [Google Scholar]