Abstract
The functional role of IL-1 family member 10, recently renamed IL-38, remains unknown. In the present study we aimed to elucidate the biological function of IL-38 and to identify its receptor. Heat-killed Candida albicans was used to stimulate memory T-lymphocyte cytokine production in freshly obtained human peripheral blood mononuclear cells from healthy subjects. The addition of recombinant IL-38 (152 amino acids) inhibited the production of T-cell cytokines IL-22 (37% decrease) and IL-17 (39% decrease). The reduction in IL-22 and IL-17 caused by IL-38 was similar to that caused by the naturally occurring IL-36 receptor antagonist (IL-36Ra) in the same peripheral blood mononuclear cells cultures. IL-8 production induced by IL-36γ was reduced by IL-38 (42% decrease) and also was reduced by IL-36Ra (73% decrease). When human blood monocyte-derived dendritic cells were used, IL-38 as well as IL-36Ra increased LPS-induced IL-6 by twofold. We screened immobilized extracellular domains of each member of the IL-1 receptor family, including the IL-36 receptor (also known as “IL-1 receptor-related protein 2”) and observed that IL-38 bound only to the IL-36 receptor, as did IL-36Ra. The dose–response suppression of IL-38 as well as that of IL-36Ra of Candida-induced IL-22 and IL-17 was not that of the classic IL-1 receptor antagonist (anakinra), because low concentrations were optimal for inhibiting IL-22 production, whereas higher concentrations modestly increased IL-22. These data provide evidence that IL-38 binds to the IL-36R, as does IL-36Ra, and that IL-38 and IL-36Ra have similar biological effects on immune cells by engaging the IL-36 receptor.
Keywords: inflammation, IL-1F10, IL-1F5, IL-1Rrp2
The IL-1 family (IL-1F) consists of 11 members (1). IL-1F5, IL-1F6, IL-1F8, IL-1F9, and IL-1F10 recently have been renamed IL-36 receptor antagonist (IL-36Ra), IL-36α, IL-36β, IL-36γ, and IL-38, respectively (2). Several reports have described effector pathways and biological functions of IL-36 (α, β, and γ) and IL-36Ra (3–9); however, the biological function(s) of IL-38 remain unknown. IL-38 was cloned and characterized as a member of the IL-1 family of ligands in 2001 (10, 11). The IL-38 gene is located in the IL1 family cluster on chromosome 2 next to the gene encoding the IL-1 receptor antagonist (IL-1Ra) and IL-36Ra (12). IL-38 shares 41% homology with IL-1Ra and 43% homology with IL-36Ra (11). The primary translated product is the IL-38 precursor, which is 152 amino acids in length but, as is typical of the IL-1 family, lacks a signal peptide. The natural N terminus is unknown (11). Also, there is no caspase-1 consensus cleavage site for IL-38. IL-38 is expressed mostly in the skin and in proliferating B-cells of the tonsil (10), and often it is speculated that IL-38 acts as an IL-1 receptor antagonist, based on its amino acid homology to the naturally occurring IL-1Ra and on the observation that IL-38 binds to the soluble IL-1 receptor type I (10). However, the binding affinity of recombinant IL-38 is significantly lower than that of IL-1Ra and IL-1β (10).
The allele combinations that include IL-38 polymorphisms are associated with psoriatic arthritis and ankylosing spondylitis (13–15). These reports suggest that IL-38 plays a role in the pathogenesis of these inflammatory diseases. Increased numbers of circulating T-helper 17 (Th17) cells were found in the peripheral blood of patients with psoriatic arthritis and ankylosing spondylitis (16–22). Furthermore, the frequency of Th17 cells and level of serum IL-17 correlated strongly with systemic disease activity both at the onset and during the progression of psoriatic arthritis and ankylosing spondylitis (22). Th17 lymphocytes preferentially produce IL-17A, IL-21, and IL-22 (6). Because of the role that Th17 cells play in the pathogenesis of these and other autoimmune diseases, we hypothesize that IL-38 could be involved in the regulation of IL-17 and IL-22 production. In the present study, we aimed to identify the biological effects of IL-38 and to elucidate the receptor pathway(s) through which IL-38 exerts its effects.
Results
IL-38 Inhibits Candida-Induced Th17 Cytokine Production.
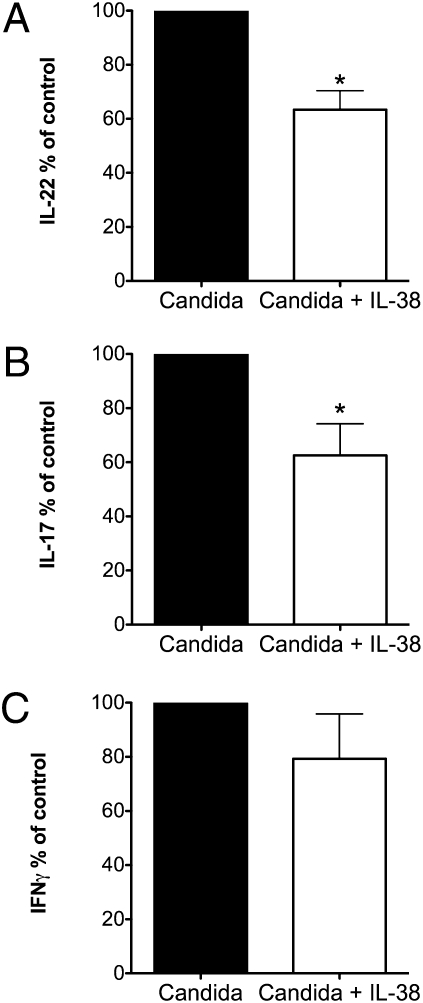
To investigate the effects of IL-38 on memory T-helper cell responses, we cultured freshly obtained human peripheral blood mononuclear cells (PBMCs) from healthy volunteers with heat-killed Candida albicans. In these cultures, antigen-presenting cells are required together with memory T lymphocytes for C. albicans to induce T-helper cytokines (23). As shown in Fig. 1, PBMCs exposed to C. albicans show a clear Th17 and Th1 response reflected by a consistently significant production of IL-17A (499 ± 124 pg/mL, mean ± SEM, n = 8 PBMC donors) and IL-22 (2,162 ± 597 pg/mL, n = 6) as well as IFN-γ (476 ± 85 pg/mL, n = 6). Baseline production of cytokines in unstimulated cultures was below the limit of detection in each experiment. However, in the presence of IL-38, the production of IL-17A induced by C. albicans was reduced by 37% (Fig. 1A), and the production of IL-22 was reduced by 39% (Fig. 1B). IL-38 alone did not induce cytokine production. There was no significant effect on IFN-γ production (Fig. 1C).
Fig. 1.
IL-38 inhibits Candida-induced Th17 cytokine production. Mean ± SEM percent change in supernatant cytokines from human PBMCs exposed to C. albicans in the presence or absence of IL-38 at 100 ng/mL. (A) IL-22 (n = 8). *P < 0.05. (B) IL-17 (n = 6). *P < 0.05. (C) IFN-γ (n = 6).
Similar to IL-38, Blocking IL-1 Suppresses Candida-Induced IL-17 and IL-22 Production.
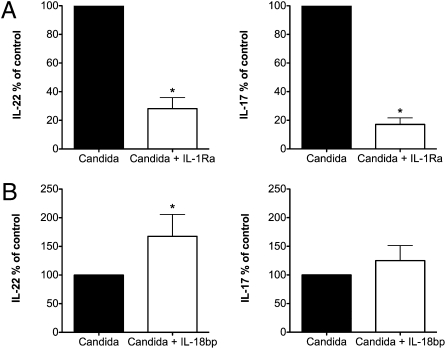
To elucidate further the pathway through which IL-38 exerts its effects, we investigated whether blocking IL-1 receptor activity affected IL-17 or IL-22 production. In the presence of IL-1Ra, there was a marked reduction of IL-17A and IL-22 by Candida-stimulated PBMC (82% and 71%, respectively) (Fig. 2A).
Fig. 2.
Effect of IL-1 and IL-18 blockade on Th17 responses driven by Candida antigen. Mean ± SEM percent change in IL-22 and IL-17 in the supernatant cytokines from human PBMCs exposed to C. albicans in the presence or absence of inhibitors. (A) IL-1Ra at 1,000 ng/mL (n = 8). (B) IL-18BP at 1,000 ng/mL (n = 6). *P < 0.05 using paired t test.
Effect of IL-18 Blockade on Th17 Responses Driven by Candida Antigen.
We next assessed the effect of IL-18, because IL-18 is required for the induction of IFN-γ. To inhibit the biological activity of endogenous IL-18, the naturally occurring IL-18 binding protein (IL-18BP), which binds native IL-18 with a high affinity and neutralizes IL-18–induced IFN-γ production (24, 25), was added to the cultures. In contrast to IL-38 and IL-1Ra, IL-18BP increased rather than decreased IL-17A and IL-22 production induced by C. albicans (165% and 120%, respectively) (Fig. 2B). These data suggest that IL-38 could act by blocking the IL-1 receptor, but it is unlikely that IL-38 suppresses IL-17 by affecting IL-18 or its receptor.
Similar to IL-38, IL-36Ra Reduces Candida-Induced Th17 Responses.
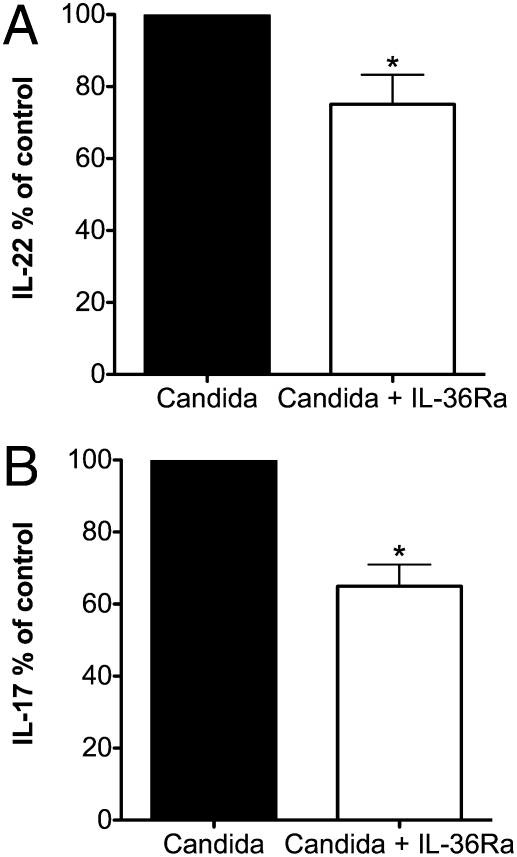
To assess a role for IL-36γ in Candida-induced IL-17 and IL-22, we specifically blocked the IL-36 receptor, IL-1 receptor-related protein type 2 (IL-1Rrp2), with IL-36Ra. When IL-36Ra was added to cultures of PBMCs stimulated with Candida, the production of IL-17 and IL-22 was decreased significantly, 23% (P < 0.01) and 32% (P < 0.01), respectively (Fig. 3). IL-36Ra alone did not induce cytokine production. These data suggest that IL-38 might act by inhibiting the IL-36R pathway.
Fig. 3.
IL-36Ra reduces Candida-induced Th17 responses. Mean ± SEM percent change in C. albicans-induced cytokines in the presence or absence of IL-36Ra at 100 ng/mL. (A) IL-22 (n = 10). (B) IL-17 (n = 7). *P < 0.05 using paired t test.
IL-38 Binds to IL-1Rrp2.
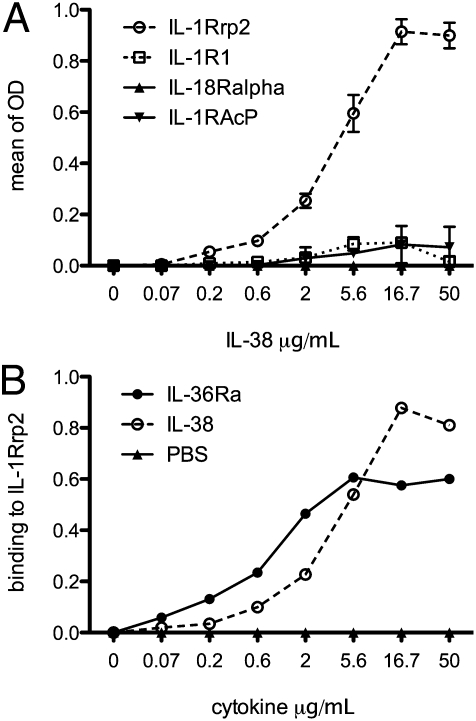
We next used a panel of soluble members of the IL-1 receptor family to determine the receptor(s) to which IL-38 binds. In this assay, the extracellular domains of each receptor were immobilized in plastic wells, and increasing concentrations of IL-38 were added. After washing, antibodies specific to IL-38 were used to detect bound IL-38 using a colorometric read-out. Receptors investigated were IL-1R type I, IL-1R accessory protein-Fc, IL-18 receptor α chain-Fc, and IL-1Rrp2-Fc. IL-38 bound to the IL-1Rrp2-Fc but did not bind the other immobilized receptors (Fig. 4A). In addition, IL-38 binding to the immobilized IL-1Rrp2 was comparable to IL-36Ra binding to the same receptor. As shown in Fig. 4B, at 5 μg/mL the optical density for IL-38 and IL-36Ra was comparable. Increasing the concentration of IL-36Ra resulted in a plateau at 5 μg/mL, whereas increasing the concentration of IL-38 resulted in increased optical density, reaching a plateau at 16.7 μg/mL.
Fig. 4.
Binding curves of IL-38 to immobilized IL-1Rrp2. (A) Flat-bottomed 96-well microtiter plates were coated with 1 μg of the extracellular domain of IL-1 family receptors in 100 μL PBS (Materials and Methods). After blocking with BSA, increasing amounts of IL-38 (shown under the horizontal axis in μg/mL) were added to each well for overnight incubation. Bound IL-38 was detected using biotinylated affinity-purified goat anti–IL-38 antibody, followed by HRP-conjugated streptavidin (n = 3). (B) Comparison of IL-38 and IL-36Ra binding to IL-1Rrp2 (n = 5). The data are expressed as mean ± SD in A and as mean in B.
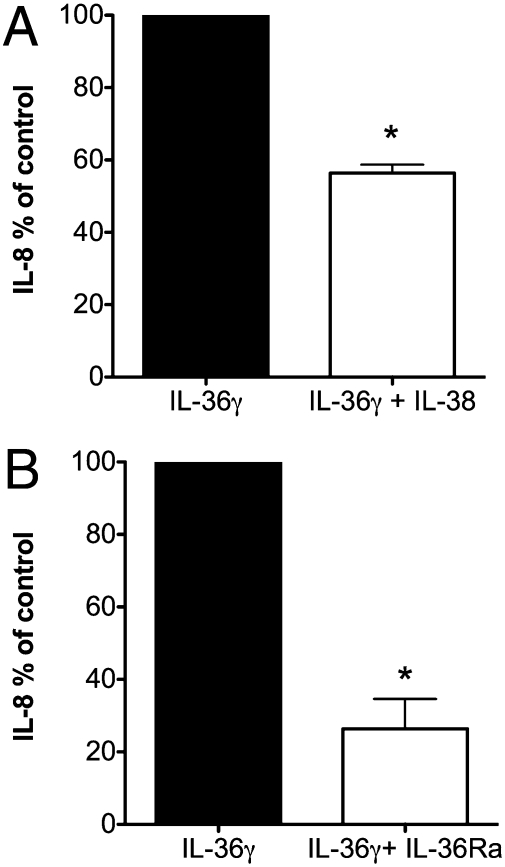
IL-38 Inhibits IL-36γ–Induced IL-8.
As shown above, we observed that IL-38 acts similarly to IL-36Ra in suppressing IL-17 and IL-22 and that IL-38 binds to immobilized IL-36R. To investigate whether IL-38 specifically inhibits a biological effect dependent on the IL-36R pathway, we stimulated PBMCs with IL-36γ in the presence of IL-38 and measured the production of IL-8. As shown in Fig. 5A, IL-8 production was reduced by 42% with IL-38 treatment. For comparison, we examined the effect of IL-36Ra on IL-36–driven IL-8; IL-8 production was decreased by 73% (Fig. 5B). These data support the concept that the biological consequence of blocking the IL-36 receptor with IL-36Ra is observed with IL-38 also.
Fig. 5.
IL-38 inhibits IL-36γ–induced IL-8. Percent change (mean ± SEM) in IL-8 from human PBMCs incubated for 24 h with either medium or IL-36γ at 100 ng/mL and in the presence or absence of (A) IL-38 at 10 ng/mL 9 (n = 5) or (B) IL-36Ra at 100 ng/mL (n = 5). *P < 0.05.
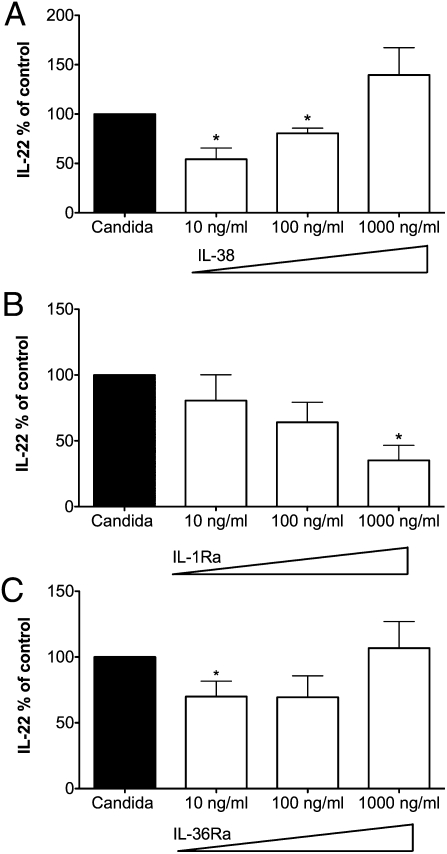
Comparison of the Dose–Response Relationship of IL-38, IL-1Ra, and IL-36Ra on Candida-Induced IL-22.
While investigating the dose-response of IL-38 in PBMCs stimulated with C. albicans, we observed that low concentrations of IL-38 (10 ng/mL) were more effective than higher concentrations in inhibiting IL-22 production (Fig. 6A). At 1,000 ng/mL of IL-38, we observed a 39% increase in IL-22 production, although this result was not statistically significant. In contrast, IL-1Ra exhibited the classic inhibitory dose-response of a pure antagonist; that is, the higher the concentration of IL-1Ra, the greater was the inhibition of IL-22 production (Fig. 6B). In the presence of increasing concentrations of IL-36Ra, we observed a dose–response relationship similar to that for IL-38; that is, low concentrations of IL-36Ra were more effective than higher concentrations in inhibiting IL-22 production (Fig. 6C).
Fig. 6.
Comparison of the dose–response relationship of IL-38, IL-1Ra, and IL-36Ra on Candida-induced IL-22. Percent change (mean ± SEM) in IL-22 released from PBMCs incubated with C. albicans in the presence or absence of increasing concentrations of (A) IL-38 (n = 4), (B) IL-1Ra (n = 4), and (C) IL-36Ra (n = 4). *P < 0.05.
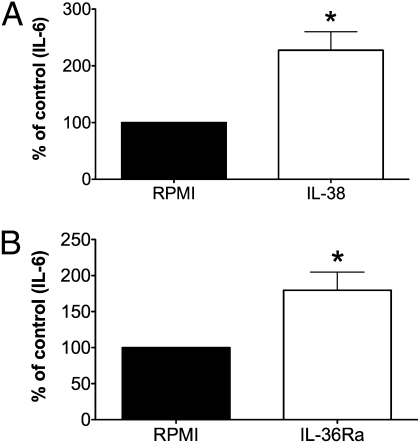
IL-38 and IL-36Ra Have Similar Effects on the Production of IL-6 by Dendritic Cells Stimulated with LPS.
To explore the effects of IL-38 on other immune cells, we stimulated monocyte-derived dendritic cells (DCs) with LPS in the presence or absence of IL-38. We observed that IL-6 production induced by LPS was significantly higher (twofold increase) in the presence of IL-38 (Fig. 7A). Similarly, there was a twofold increase in LPS-induced IL-6 in the presence of IL-36Ra, supporting the concept that IL-38 and IL-36Ra have similar biological activity on immune cells (Fig. 7B). IL-38 and IL-36Ra alone did not induce IL-6 production by DCs.
Fig. 7.
IL-38 and IL-36Ra similarly increase LPS-induced IL-6 in DCs. Percent change (mean ± SEM) in human blood monocyte-derived DCs incubated with either medium or LPS (10 ng/mL) in the presence or absence of (A) 100 ng/mL IL-38 (n = 7) or (B) 100 ng/mL IL-36Ra (n = 7). *P < 0.05. LPS induced 1,578 ± 596 pg/mL (mean ± SEM) in DCs.
Discussion
In the present study we demonstrate that both IL-38 and IL-36Ra reduced the production of Candida-induced IL-17 and IL-22, an assay that particularly assesses memory Th17 cell function. The reduction was in the range of 40%. In contrast, production of the characteristic Th1 cytokine IFN-γ was not affected significantly by IL-38. An unexpected finding in the present study was that IL-38 binds to IL-1Rrp2 (IL-36R), which is the ligand-binding receptor chain for IL-36. The IL-36 signaling complex is comprised of one of the three isoforms of IL-36 (IL-36α, IL-36β, or IL-36γ) together with IL-36R plus the IL-1 accessory protein (8). Consistent with the binding data as well as the suppression of IL-17 and IL-22, we observed IL-36Ra and IL-38 had similar biological effects on LPS-induced IL-6 in human DCs. IL-6 production increased twofold in the presence of either IL-38 or IL-36Ra. A direct comparison of IL-36γ activity on IL-8 production showed that IL-36Ra appears to be modestly more effective as a receptor antagonist. However, neither IL-36Ra nor IL-38 performs as a typical receptor antagonist, as IL-1Ra does.
In determining a functional role of IL-38, we hypothesized that IL-38 would affect Th17 cytokine production based on the link of IL-38 polymorphisms to the development of ankylosing spondylitis and psoriatic arthritis (21, 22). Indeed, we demonstrate here that IL-38 inhibits the production of characteristic cytokines of the Th17 response. It remains to be elucidated how IL-38 modulates Th17 responses. We have investigated the influence on IL-23 production, but we were unable to detect measurable concentrations of IL-23 in the supernatants. Second, we functionally blocked the three pathways through which IL-38 might have its effects, namely the IL-1R pathway (by blocking with IL-1Ra), the IL-18R pathway (by blocking with IL-18BP), and the IL-36R pathway (by blocking with IL-36Ra). In each experimental model we compared blocking IL-1 receptors, neutralizing IL-18, and blocking the IL-36 receptor with the effect of IL-38 treatment. Blocking the IL-36Ra or the IL-1R pathway mimicked the effects of IL-38 on the production of Th17 cytokines.
We next assessed whether IL-38 bound to members within the IL-1 receptor family. Using immobilized forms of the extracellular domains (soluble) receptors, we screened the IL-1 family of receptors. IL-38 bound only to the IL-1Rrp2. Although others have reported that IL-38 binds to soluble IL-1R type I, such binding was clearly weak (10), consistent with the binding data in the present study. From the functional data with neutralizing IL-18 with IL-18BP, IL-38 effects are inconsistent with binding to the IL-18R, as we observed in Fig. 4A. By using a ligand for the IL-36R pathway, namely IL-36γ, we show that IL-38 blocks the ability of IL-36γ to induce IL-8.
From the in silico discovery of new IL-1 family members, it was observed that IL-38 shares primary amino acid homology with IL-1Ra and IL-36Ra (10, 11). Therefore, it had been speculated repeatedly that IL-38 acts as a receptor antagonist, possibly for IL-1 (10, 11). However biological data supporting this concept were lacking. The most consistent observation from the present studies is that the effects of IL-38 are similar to those of IL-36Ra. The degree of suppression of Candida-induced IL-17 and IL-22 in human PBMC was comparable. In blood monocyte-derived DCs, the amount of enhanced LPS-induced IL-6 also was comparable. It remains to be elucidated why IL-38 and IL-36Ra decreased proinflammatory cytokines in PBMCs, whereas increased proinflammatory cytokines were observed in DCs. Relevant to the similar biological effects of IL-38 and IL-36Ra is the observation that more IL-38 bound to immobilized IL-36R than to IL-36Ra (Fig. 4B).
Despite the consistent biological property of IL-38 as an inhibitor of IL-17 and IL-22 production and the binding to the IL-36 receptor, IL-38 does not behave as a classic receptor antagonist. In contrast to IL-1Ra, the inhibitory activity was lost when the concentrations of IL-38 were increased. Interestingly, IL-36Ra also did not show a classical receptor antagonistic function. Together, these data suggest that IL-38 and IL-36Ra are partial receptor antagonists; moreover, these molecules might act as agonists at higher concentrations. Furthermore, upon binding to the IL-36R, IL-38 and IL-36Ra might recruit an inhibitory coreceptor at low concentrations but a signaling coreceptor at higher concentrations. Alternatively, the fact that the N terminus of the recombinant form is not optimal for functioning as a pure receptor antagonist may cause the discrepancy in the dose-response of IL-38.
Recently, mutations in IL-36Ra resulting in a decreased ability to engage the IL-36R have been described as a cause of pustular psoriasis (26, 27). Our data support the hypothesis that the underlying mechanism of an IL-36Ra deficiency leading to pustular psoriasis results from the reduced inhibitory function of IL-36Ra on Th17 cytokine responses. This reduction would result in pathologically increased the Th17 responses in the skin, which are associated with psoriasis (28–31). That report and the present observation that IL-38 acts very similarly to IL-36Ra support a protective role for IL-38 in psoriasis, that is, as an inducer of IL-22 (32).
The importance of members within the IL-1 family functioning as natural “brakes” on the inflammatory properties of other IL-1 family members gained considerable validity with the reports of the deficiency of IL-1 receptor antagonist (DIRA) syndrome (33, 34). Recent reports that pustular psoriasis can develop because of a single amino acid mutation in IL-36Ra provide additional evidence supporting this concept. When this reasoning is extended to IL-38, it is possible that a mutation of a single amino acid can reduce the function of IL-38 and underlie a pathological skin disorder. Similarly, from mutational analyses of IL-1Ra, a single amino acid change converted a pure receptor antagonist to a functionless molecule (35). Nevertheless, although IL-1Ra, IL-36Ra, and IL-38 overlap in their ability to reduce IL-22 and IL-17 levels, the obvious duplication in function is not synonymous with redundancy in function in protecting against inflammatory processes in the skin mediated by Th17.
In conclusion, the function of IL-38 has remained elusive since its in silico discovery in 2001. Here we describe a biological function of IL-38 and provide evidence for binding to the IL-36 ligand-binding chain. The observation that IL-38 has strong effects on the Th17 response makes IL-38 an attractive cytokine to explore in Th17-related diseases.
Materials and Methods
Volunteers.
The study was approved by the Colorado Multiple Investigational Review Board. Blood was collected after informed consent from healthy volunteers who were free of infectious or inflammatory disease. Venous blood was collected in 10-mL heparin tubes and processed for PBMC as described previously (23).
Cytokines.
Recombinant GM-CSF, IL-1Ra, IL-36γ, IL-36Ra, and IL-38 were produced in Escherichia coli by R&D Systems. After extraction, refolding, and purification of IL-36Ra, the level of LPS was determined to be less that 1.0 EU/μg by the limulus amebocyte lysate method. The molecular weight of recombinant IL-36Ra is 17,000 with an N terminus at valine 2. On silver-stained SDS PAGE under reducing conditions, there is a single band, and the purity is >95%. The endotoxin level in the preparation of IL-38 used in the present article is 0.00014 EU/μg of the recombinant IL-38. Similar to recombinant IL-36Ra, the purity of recombinant IL-38 using silver-stained SDS PAGE under reducing conditions is >95%. The IL-38 used in the present study is full-length (152 amino acids).
Microorganism.
C. albicans ATCC MYA-3573 (UC 820) was grown overnight in Sabouraud's broth at 37 °C. Cells were harvested by centrifugation, washed twice, and resuspended in culture medium (RPMI-1640 Dutch modification; ICN Biomedicals) (10). C. albicans was heat-killed (1 h at 100 °C). A concentration of 1 × 10e6 heat-killed C. albicans/mL was added to the cultures.
PBMC Stimulation.
Separation and stimulation of PBMCs was performed as described previously (23). Briefly, the PBMC fraction was obtained by density-gradient centrifugation of diluted blood (one part blood to one part pyrogen-free saline) over Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden). PBMCs were washed twice in saline and suspended in RPMI 1640 supplemented with penicillin/streptomycin 1%, l-glutamine 1%, and pyruvate 1%. The cells were counted in a hemocytometer, and their number was adjusted to 5 × 106 cells/mL PBMCs (5 × 105) were cultured in RPMI 1640 supplemented with penicillin/streptomycin 1%, l-glutamine 1%, pyruvate 1%, and 10% human serum in a total volume of 200 μL per well at 37 °C in round-bottomed 96-well plates with or without C. albicans. IL-38 or IL-1Ra or IL-36Ra was added 30 min before Candida stimulation. After 5 d of incubation, supernatants were collected and stored at −20 °C until assayed.
Monocyte-Derived Dendritic Cell Stimulation.
Two hundred microliters per well of 10e6/mL PBMCs were plated in a 96-well flat-bottomed plate. After 1.5 h, the plate was put on a shaker for 1 min. Nonadherent cells were removed, and medium with 10% FCS and GM-CSF (50 ng/mL) and IL-4 (20 ng/mL) was added for a total volume of 200 μL was in each well. Medium was refreshed every second day by removing 100 μL and adding back 100 μL of 20% FCS, GM-CSF (100 ng/mL), and IL-4 (40 ng/mL). Medium was removed on day 6, and DCs were stimulated with RPMI (medium) or LPS (10 ng/mL) for 24 h without serum.
Cytokine Assays.
IL-8, IL-6, IL-17A, IL-22, and IFN-γ concentrations were measured by ELISA duokits (R&D Systems) following the manufacturer's instructions.
Receptor-Binding Assays.
The wells of a 96-well ELISA plate were coated at 4 °C overnight with the extracellular domains of the IL-1 Rrp2/IL-36 receptor Fc chimera (R&D Systems) diluted in PBS. After washing, wells were blocked at 37 °C for 2 h with 1% BSA in PBS. Serial dilutions of recombinant human IL-38 or IL-36Ra/IL-1F5 (R&D Systems) were prepared using 0.5% BSA and 0.05% Tween-20 in PBS, added to the coated/blocked plate, and incubated at 4 °C overnight. Bound protein was detected by biotinylated affinity-purified goat anti-human IL-38 or biotinylated goat anti-human IL-36Ra/IL-1F5 antibody (R&D Systems), followed by HTP-conjugated streptavidin.
Statistical Analysis.
The differences between groups were analyzed by the paired Student's t test on raw cytokine data. In each donor PBMC sample, the cytokine supernatant level (pg per mL) following Candida or IL-36γ induction was set at 100%, and the percent change was calculated. The mean percent change for each experiment then was determined. The data are shown as mean percent change from the stimulated condition ± SEM.
Acknowledgments
We thank Benjamin Swartzhelder for technical assistance. These studies were supported by National Institutes of Health Grants AI15614 and AR-45584 (to C.A.D.). F.L.v.d.V. was supported by a Veni grant from the Netherlands Organization for Scientific Research and by a stipend from the Niels Stensen Foundation. M.G.N. was supported by a Vici grant from the Netherlands Organization for Scientific Research.
Footnotes
The authors declare no conflict of interest.
References
- 1.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dinarello C, et al. IL-1 family nomenclature. Nat Immunol. 2010;11:973. doi: 10.1038/ni1110-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston A, et al. IL-1F5, -F6, -F8, and -F9: A novel IL-1 family signaling system that is active in psoriasis and promotes keratinocyte antimicrobial peptide expression. J Immunol. 2011;186:2613–2622. doi: 10.4049/jimmunol.1003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chustz RT, et al. Regulation and function of the IL-1 family cytokine IL-1F9 in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2011;45:145–153. doi: 10.1165/rcmb.2010-0075OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costelloe C, et al. IL-1F5 mediates anti-inflammatory activity in the brain through induction of IL-4 following interaction with SIGIRR/TIR8. J Neurochem. 2008;105:1960–1969. doi: 10.1111/j.1471-4159.2008.05304.x. [DOI] [PubMed] [Google Scholar]
- 6.Blumberg H, et al. Opposing activities of two novel members of the IL-1 ligand family regulate skin inflammation. J Exp Med. 2007;204:2603–2614. doi: 10.1084/jem.20070157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magne D, et al. The new IL-1 family member IL-1F8 stimulates production of inflammatory mediators by synovial fibroblasts and articular chondrocytes. Arthritis Res Ther. 2006;8:R80. doi: 10.1186/ar1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Towne JE, Garka KE, Renshaw BR, Virca GD, Sims JE. Interleukin (IL)-1F6, IL-1F8, and IL-1F9 signal through IL-1Rrp2 and IL-1RAcP to activate the pathway leading to NF-kappaB and MAPKs. J Biol Chem. 2004;279:13677–13688. doi: 10.1074/jbc.M400117200. [DOI] [PubMed] [Google Scholar]
- 9.van Asseldonk EJ, et al. The effect of the interleukin-1 cytokine family members IL-1F6 and IL-1F8 on adipocyte differentiation. Obesity (Silver Spring) 2010;18:2234–2236. doi: 10.1038/oby.2010.55. [DOI] [PubMed] [Google Scholar]
- 10.Lin H, et al. Cloning and characterization of IL-1HY2, a novel interleukin-1 family member. J Biol Chem. 2001;276:20597–20602. doi: 10.1074/jbc.M010095200. [DOI] [PubMed] [Google Scholar]
- 11.Bensen JT, Dawson PA, Mychaleckyj JC, Bowden DW. Identification of a novel human cytokine gene in the interleukin gene cluster on chromosome 2q12-14. J Interferon Cytokine Res. 2001;21:899–904. doi: 10.1089/107999001753289505. [DOI] [PubMed] [Google Scholar]
- 12.Nicklin MJ, et al. A sequence-based map of the nine genes of the human interleukin-1 cluster. Genomics. 2002;79:718–725. doi: 10.1006/geno.2002.6751. [DOI] [PubMed] [Google Scholar]
- 13.Rahman P, et al. Association between the interleukin-1 family gene cluster and psoriatic arthritis. Arthritis Rheum. 2006;54:2321–2325. doi: 10.1002/art.21928. [DOI] [PubMed] [Google Scholar]
- 14.Chou CT, et al. Replication of association of IL1 gene complex members with ankylosing spondylitis in Taiwanese Chinese. Ann Rheum Dis. 2006;65:1106–1109. doi: 10.1136/ard.2005.046847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo ZS, et al. Association of IL-1 gene complex members with ankylosing spondylitis in Chinese Han population. Int J Immunogenet. 2010;37:33–37. doi: 10.1111/j.1744-313X.2009.00889.x. [DOI] [PubMed] [Google Scholar]
- 16.Bowness P, et al. Th17 cells expressing KIR3DL2+ and responsive to HLA-B27 homodimers are increased in ankylosing spondylitis. J Immunol. 2011;186:2672–2680. doi: 10.4049/jimmunol.1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mei Y, et al. Increased serum IL-17 and IL-23 in the patient with ankylosing spondylitis. Clin Rheumatol. 2011;30:269–273. doi: 10.1007/s10067-010-1647-4. [DOI] [PubMed] [Google Scholar]
- 18.Wendling D. IL-23 and IL-17 in ankylosing spondylitis. Rheumatol Int. 2010;30:1547. doi: 10.1007/s00296-009-1226-7. [DOI] [PubMed] [Google Scholar]
- 19.Shen H, Goodall JC, Hill Gaston JS. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009;60:1647–1656. doi: 10.1002/art.24568. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Lin Z, Wei Q, Jiang Y, Gu J. Expression of IL-23 and IL-17 and effect of IL-23 on IL-17 production in ankylosing spondylitis. Rheumatol Int. 2009;29:1343–1347. doi: 10.1007/s00296-009-0883-x. [DOI] [PubMed] [Google Scholar]
- 21.Jandus C, et al. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 2008;58:2307–2317. doi: 10.1002/art.23655. [DOI] [PubMed] [Google Scholar]
- 22.Leipe J, et al. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum. 2010;62:2876–2885. doi: 10.1002/art.27622. [DOI] [PubMed] [Google Scholar]
- 23.van de Veerdonk FL, et al. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe. 2009;5:329–340. doi: 10.1016/j.chom.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Novick D, et al. Interleukin-18 binding protein: A novel modulator of the Th1 cytokine response. Immunity. 1999;10:127–136. doi: 10.1016/s1074-7613(00)80013-8. [DOI] [PubMed] [Google Scholar]
- 25.Netea MG, et al. The role of endogenous interleukin (IL)-18, IL-12, IL-1beta, and tumor necrosis factor-alpha in the production of interferon-gamma induced by Candida albicans in human whole-blood cultures. J Infect Dis. 2002;185:963–970. doi: 10.1086/339410. [DOI] [PubMed] [Google Scholar]
- 26.Marrakchi S, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620–628. doi: 10.1056/NEJMoa1013068. [DOI] [PubMed] [Google Scholar]
- 27.Onoufriadis A, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011;89:432. doi: 10.1016/j.ajhg.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bovenschen HJ, et al. Foxp3+ regulatory T cells of psoriasis patients easily differentiate into IL-17A-producing cells and are found in lesional skin. J Invest Dermatol. 2011;131:1853–1860. doi: 10.1038/jid.2011.139. [DOI] [PubMed] [Google Scholar]
- 29.Tokura Y, Mori T, Hino R. Psoriasis and other Th17-mediated skin diseases. J UOEH. 2010;32:317–328. doi: 10.7888/juoeh.32.317. [DOI] [PubMed] [Google Scholar]
- 30.Res PC, et al. Overrepresentation of IL-17A and IL-22 producing CD8 T cells in lesional skin suggests their involvement in the pathogenesis of psoriasis. PLoS ONE. 2010;5:e14108. doi: 10.1371/journal.pone.0014108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ortega C, et al. IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines. J Leukoc Biol. 2009;86:435–443. doi: 10.1189/JLB.0109046. [DOI] [PubMed] [Google Scholar]
- 32.Wolk K, et al. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Stenerson M, et al. The first case of compound heterozygous IL1RN mutations causing deficiency of the interleukin-1-receptor antagonist. Arthritis Rheum. 2011;63:4018. doi: 10.1002/art.30565. [DOI] [PubMed] [Google Scholar]
- 34.Aksentijevich I, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. 2009;360:2426–2437. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evans RJ, et al. Mapping receptor binding sites in interleukin (IL)-1 receptor antagonist and IL-1 beta by site-directed mutagenesis. Identification of a single site in IL-1ra and two sites in IL-1 beta. J Biol Chem. 1995;270:11477–11483. doi: 10.1074/jbc.270.19.11477. [DOI] [PubMed] [Google Scholar]