Abstract
Glioblastoma, the most common primary malignant cancer of the brain, is characterized by rapid tumor growth and infiltration of tumor cells throughout the brain. These traits cause glioblastomas to be highly resistant to current therapies with a resultant poor prognosis. Although aberrant oncogenic signaling driven by signature genetic alterations, such as EGF receptor (EGFR) gene amplification and mutation, plays a major role in glioblastoma pathogenesis, the responsible downstream mechanisms remain less clear. Here, we report that EGFRvIII (also known as ΔEGFR and de2-7EGFR), a constitutively active EGFR mutant that is frequently co-overexpressed with EGFR in human glioblastoma, promotes tumorigenesis through Src family kinase (SFK)-dependent phosphorylation of Dock180, a guanine nucleotide exchange factor for Rac1. EGFRvIII induces phosphorylation of Dock180 at tyrosine residue 722 (Dock180Y722) and stimulates Rac1-signaling, glioblastoma cell survival and migration. Consistent with this being causal, siRNA knockdown of Dock180 or expression of a Dock180Y722F mutant inhibits each of these EGFRvIII-stimulated activities. The SFKs, Src, Fyn, and Lyn, induce phosphorylation of Dock180Y722 and inhibition of these SFKs by pharmacological inhibitors or shRNA depletion markedly attenuates EGFRvIII-induced phosphorylation of Dock180Y722, Rac1 activity, and glioblastoma cell migration. Finally, phosphorylated Dock180Y722 is coexpressed with EGFRvIII and phosphorylated SrcY418 in clinical specimens, and such coexpression correlates with an extremely poor survival in glioblastoma patients. These results suggest that targeting the SFK-p-Dock180Y722-Rac1 signaling pathway may offer a novel therapeutic strategy for glioblastomas with EGFRvIII overexpression.
Keywords: invasion, Akt
Oncogenic signaling stimulated by overexpressed genes, such as EGF receptor (EGFR), renders human brain glioblastomas malignant and resistant to combination therapies (1). Amplification of EGFR is the most frequent genetic alteration in World Health Organization (WHO) grade IV glioblastoma multiforme (GBM) (2, 3) and is associated with poor prognosis (1). About half of GBMs with EGFR amplification also express the mutant form, EGFRvIII/ΔEGFR/de2-7EGFR, that lacks a portion of the extracellular ligand-binding domain (encoded by exons 2 through 7), leading to constitutively activated oncogenic signaling (3, 4). Expression of EGFRvIII enhances glioblastoma tumorigenicity in vivo (5) and promotes glioblastoma cell motility in vitro (6). Although EGFRvIII activates PI3K/Akt signaling, other signaling cascades are also likely involved in mediating EGFRvIII-driven tumorigenesis (3, 4).
Dedicator of cytokinesis 1 (Dock1 or Dock180) is a guanine nucleotide exchange factor (GEF) that activates Rac1 and controls several cellular functions, including cell motility, survival, and proliferation (7). Dock180 facilitates nucleotide exchange on Rac1 through its Dock-homology region-2 (DHR-2) domain, but requires binding to engulfment and cell motility protein 1 (ELMO1) through its N-terminal SH3 domain to achieve full activation of Rac1 (8). Adjacent to the SH3 region resides a DHR-1 domain which interacts with phosphatidyl-inositol(3,4,5)P3 (PIP3), and thereby mediates the localization of Dock180 to the cell membrane sites of PIP3 production where Dock180 subsequently activates Rac1 through its DHR-2 domain (8). Although genomic studies have revealed no genetic alterations in Rac1, Dock180, or ELMO1 in various human cancers, including glioblastoma, it remains possible, given its central role in regulating cellular functions, that GEF-Rac1 signaling is stimulated by signals emanating from activated oncogenes, such as EGFRvIII.
GEF activation by receptor tyrosine kinases (RTK) stimulates Rac1 (9) and may be important in EGFRvIII-driven tumorigenesis (3). Dock180 activates Rac1 (8) and is involved in RTK-induced cell migration in Drosophila (10), and Dock180 plays a role in glioblastoma cell invasion through the activation of Rac1 (11). Here, we report that EGFRvIII induces tyrosine phosphorylation (p-Y) at tyrosine residue 722 (Y722) of Dock180, and that Dock180 and its phosphorylation are required for EGFRvIII-promoted glioblastoma cell growth, survival, and invasion. Correspondingly, ectopic expression of an unphosphorylatable Dock180Y722F mutant inhibited EGFRvIII-induced Rac1 activation, cell migration, and survival in vitro, and glioblastoma growth and invasion in the brain. We also report that EGFRvIII-induced p-Dock180Y722 is dependent on Src family kinases (SFKs), that p-Dock180Y722 is coexpressed with EGFRvIII and pan-p-SrcY418 in clinical glioblastoma specimens, and that such coexpression correlates with an extremely poor prognosis.
Results
Dock180 Is Required for EGFRvIII-Promoted Glioblastoma Cell Migration and Survival in Vitro.
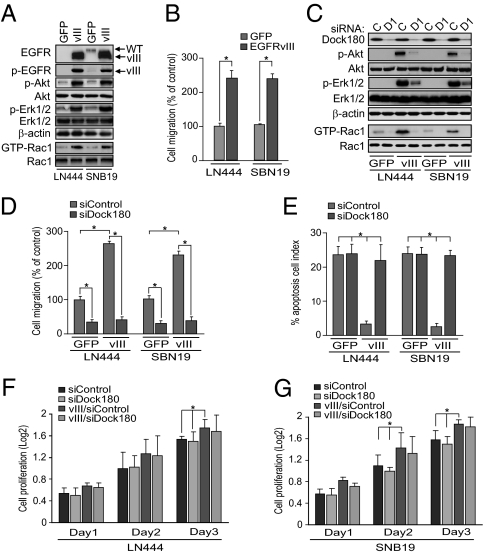
To determine if EGFRvIII signaling engages Dock180 as part of its oncogenic mechanism, we stably expressed exogenous EGFRvIII in glioblastoma LN444/GFP and SNB19/GFP cells that have high levels of endogenous Dock180 (11). Expression of EGFRvIII in LN444 and SNB19 glioblastoma cells induced p-EGFRvIII, p-Akt, p-Erk1/2, and Rac1 activity (Fig. 1A), increased in vitro cell migration (Fig. 1B), proliferation (Fig. S1 A and B), and markedly inhibited cell apoptosis (Fig. S1C).
Fig. 1.
Dock180 is required for EGFRvIII-induced Rac1 activity, glioblastoma cell migration, and survival in vitro. (A) IB analyses. (B and D) In vitro cell migration assays. Data are presented as percentage of control cells. (C) IB analyses. C, control siRNA; D1, Dock180 siRNA pool. In A and C, β-actin, Akt, Erk1/2, and Rac1 were used as loading controls. (E) Cell apoptosis. Data are presented as percentage of apoptotic cells. (F and G) Cell proliferation; data were calculated by dividing the total cell number by 50,000 and converting it to a log2 value. Data in B and D–G were from six replicates per pair per cell line. Data are representative from three independent experiments with similar results. *P < 0.05. (Scale bars, ± SD.)
We recently reported that Dock180 promotes glioblastoma cell invasion through activation of Rac1 (11). To determine whether this function of Dock180 is required for EGFRvIII-stimulated glioblastoma tumorigenesis, we knocked down endogenous Dock180 using siRNAs (11) in each of LN444/GFP, LN444/GFP/EGFRvIII, SNB19/GFP, and SNB19/GFP/EGFRvIII cells. As shown in Fig. 1C, knockdown of Dock180 in EGFRvIII-expressing cells inhibited EGFRvIII-induced p-Akt, p-Erk1/2, and Rac1 activity. Depletion of Dock180 also suppressed basal Rac1 activity in GFP control cells (11). Knockdown of Dock180 attenuated EGFRvIII-promoted cell migration and survival in EGFRvIII-expressing cells and basal levels of cell migration in GFP control cells (Fig. 1 D and E). However, depletion of Dock180 had only moderate effects on cell proliferation in both EGFRvIII- and GFP-expressing cells (Fig. 1 F and G). These data suggest that Dock180 is critical for EGFRvIII-stimulated p-Akt, p-Erk1/2, and Rac1 activity, as well as for glioblastoma cell migration and survival in vitro.
EGFRvIII Induces p-Y of Dock180 at Y722.
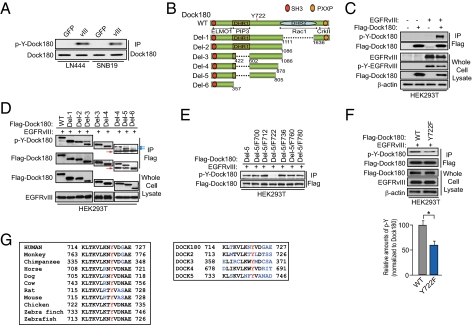
We examined whether EGFRvIII phosphorylates Dock180 at Y residues in glioblastoma cells. As shown in Fig. 2A, p-Y of endogenous Dock180 was evident in both LN444/EGFRvIII and SNB19/EGFRvIII cells, but not in GFP-expressing cells. To identify the Y residues of Dock180 that are phosphorylated by EGFRvIII, we generated Flag-tagged WT and six different Dock180 mutants that lack DHR-1, -2, or other regions (Fig. 2B). When EGFRvIII and WT Dock180 were coexpressed in HEK293T cells, EGFRvIII induced p-Y of WT Dock180, whereas expression of either protein alone did not result in p-Y of Dock180 (Fig. 2C). Next, we coexpressed EGFRvIII with WT or the six individual mutants of Dock180 and found a marked reduction of EGFRvIII-induced p-Y of the Dock180 Del-6 mutant but not the Dock180 WT or other Del mutants (Fig. 2D, blue arrows), suggesting that the p-Y sites are located between amino acid residues 602 and 805 (Fig. 2B). In this region, there are six Y residues: Y700, Y712, Y722, Y736, Y760, and Y780. To identify which Y residue is phosphorylated by EGFRvIII, we individually mutated each of these six Y residues to a phenylalanine (F) in the Dock180 Del-5 mutant. When these six Del-5 YF mutants or the Del-5 mutant were separately coexpressed with EGFRvIII in HEK293T cells, EGFRvIII induced p-Y of all Dock180 mutants except Del-5/F722, suggestive of Y722 as a potential p-Y site by EGFRvIII (Fig. 2E). To further validate this finding, we generated an Y722F mutation in the full-length Dock180 protein (Dock180Y722F). Coexpression of EGFRvIII with Dock180Y722F showed a more than 40% reduction in p-Y levels compared with that of Dock180WT (Fig. 2F). These data suggest that Y722 is a major p-Y site induced by EGFRvIII, and that there are additional p-Y sites within Dock180 because of EGFRvIII activity to a lesser extent. Next, we compared amino acid sequences surrounding Y722 in Dock180 in various species and the other four members of the Dock family, and found that Y722 and most of its surrounding residues are highly conserved among them (Fig. 2G).
Fig. 2.
EGFRvIII induces p-Y of Dock180 at Y722. (A) IP and IB analyses. (B) Schematic of deletion mutants of Dock180. (C) EGFRvIII induces p-Y of Dock180 in HEK293T cells. (D) IB analyses. Red arrows, IgG; blue arrows, p-Y of Del mutant. (E) Mutation of Y722F in Del-5 decreased p-Y of Dock180. (F) Y722 is a major EGFRvIII-induced p-Y site in Dock180. (Scale bars, ± SD.) Bar graph underneath: relative amount of p-Y of Dock180 was determined from three separate in IP-IB blots by ImageJ and normalized to the amount of Dock180. *P < 0.05. (G) Y722 is conserved in Dock180 of various species and in Dock protein family. Black, conserved amino acids; blue, nonconserved amino acids. In A and C–F, a pan-phospho-tyrosine antibody was used to detect p-Y-Dock180. Data are representative of three independent experiments with similar results.
Phosphorylation of Dock180Y722 Is Required for EGFRvIII-Promoted Glioblastoma Tumorigenesis.
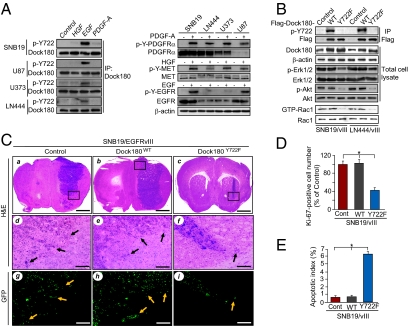
We generated a rabbit polyclonal antibody that specifically recognizes the p-Dock180Y722 protein. This anti–p-Dock180Y722 antibody detected EGF (but not PDGF or HGF) induced p-Y of endogenous Dock180 in SNB19, U87, U373, and LN444 glioblastoma cells at various levels (Fig. 3A). We then knocked down Dock180 in SNB19 or SNB19/EGFRvIII cells using an siRNA pool for Dock180 or a control siRNA (11), and found that depletion of endogenous Dock180 by the siRNA pool, but not control siRNA, significantly diminished the EGFRvIII-induced p-Dock180Y722 in SNB19/EGFRvIII cells whereas no signal was seen in SNB19 cells (Fig S2 A and B). These results validate the specificity of this antibody in detecting EGFRvIII-induced p-Y722 of endogenous Dock180 in glioblastoma cells. Next, we stably transfected Flag-tagged Dock180WT or Dock180Y722F into EGFRvIII-expressing SNB19 and LN444 glioblastoma cells. As shown in Fig. 3B, ectopic expression of Dock180WT did not affect EGFRvIII stimulation of p-Akt, p-Erk1/2, and Rac1 activity in SNB19/EGFRvIII and LN444/EGFRvIII cells. In contrast, expression of Dock180Y722F markedly reduced EGFRvIII-induced p-Akt, p-Erk1/2, and Rac1 activity. Additionally, Dock180Y722F but not Dock180WT significantly attenuated EGFRvIII-stimulated glioblastoma cell survival and migration in vitro (Fig. S3 A and B). However, expression of Dock180WT or Dock180Y722F had only a moderate impact on in vitro proliferation in both LN444/EGFRvIII and SNB19/EGFRvIII cells (Fig. S3 C and D).
Fig. 3.
Phosphorylation of Dock180Y722 is critical for EGFRvIII-driven glioblastoma growth and invasion. (A) EGF, but not PDGF-A or HGF, induces p-Y of endogenous Dock180Y722 (detected with a specific anti–p-Dock180Y722 antibody) in glioblastoma cells. (B) Effect of Dock180WT, Dock180Y722F, or a vector control on p-Dock180Y722, p-Akt, p-Erk1/2, and Rac1 activity in EGFRvIII-expressing cells. Dock180, Akt, Erk1/2, Rac1, and β-actin were used as loading controls. (C) Dock180Y722F inhibits EGFRvIII-promoted glioblastoma growth and invasion in the brain. Representative H&E and IHC images of brain sections of mice receiving various SNB19 cells (8 wk postinjection, five mice per group). (a–c) H&E staining. (Scale bars, 1 mm.) (d–f) Enlarged areas in a to c marked with squares. (Scale bars, 200 μm.) (g–i) GFP images of the same areas in d to f. (Scale bars, 200 μm.) Arrows indicate invasive tumor cells (d–i). (D and E) Quantification of Ki-67 and TUNEL staining, respectively. *P < 0.05. (Scale bars, ± SD.) Data represent three independent experiments with similar results.
We then separately implanted SNB19/EGFRvIII/Dock180WT, SNB19/EGFRvIII/Dock180Y722F, or the control SNB19/EGFRvIII/GFP cells into the brains of mice. As described previously (12), SNB19/GFP cells formed small but invasive tumors in the brains of mice. Moreover, mice that received SNB19/EGFRvIII/GFP cells showed markedly enhanced tumor growth and invasion, whereas mice that received SNB19/EGFRvIII/Dock180WT cells also developed brain tumors with large volumes and similar invasiveness (Fig. 3C and Fig. S4 A–C), suggesting no further enhancement by Dock180WT expression. In contrast, mice that received SNB19/EGFRvIII/Dock180Y722F cells developed much smaller and less invasive tumors (Fig. 3C and Fig. S4 A–C). In addition, expression of Dock180WT had no significant effect on glioblastoma cell proliferation and survival compared with the controls (Fig. 3 D and E, and Fig. S4A). However, expression of Dock180Y722F significantly suppressed EGFRvIII-stimulated glioblastoma cell proliferation and survival compared with SNB19/EGFRvIII/GFP or SNB19/EGFRvIII/Dock180WT tumors (Fig. 3 D and E and Fig. S4A). These data suggest that p-Dock180Y722 is important for EGFRvIII-promoted glioblastoma tumorigenesis in vivo and that Dock180Y722F acts in a dominant negative fashion to inhibit EGFRvIII-driven tumorigenicity.
SFKs Are Responsible for EGFRvIII-Induced p-Dock180Y722.
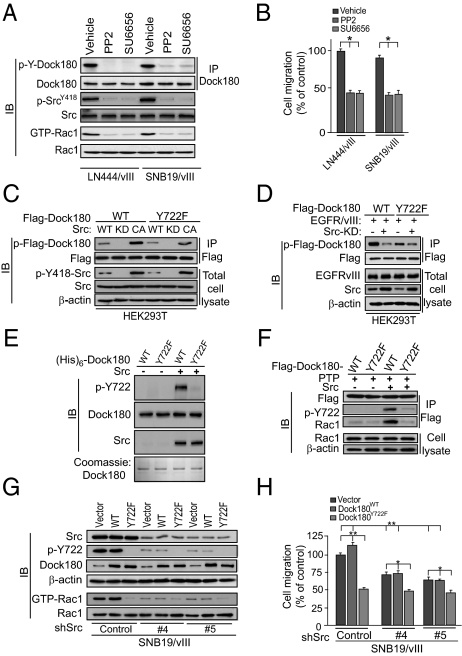
We next performed in silico analyses (http://scansite.mit.edu) and found that Y722 of Dock180 is a potential p-Y site for Src. To determine whether Src and other SFKs are involved in EGFRvIII-stimulated p-Dock180Y722 and cell migration, we first treated LN444/EGFRvIII and SNB19/EGFRvIII cells with two pharmacological inhibitors of SFKs (SU6656, PP2) or a vehicle control. As shown in Fig. 4 A and B, both PP2 and SU6656 effectively inhibited EGFRvIII-induced p-Y of Dock180, Rac1 activity, pan p-Y of SrcY418, and EGFRvIII-stimulated cell migration.
Fig. 4.
EGFRvIII-induced p-Dock180Y722 is Src-dependent. (A) Inhibition of Src by PP2 (2 μM) or SU6656 (2 μM) attenuates EGFRvIII-stimulated p-Y of Dock180 (detected with a pan-phospho-tyrosine antibody, 4G10) and Rac1 activity. (B) In vitro cell migration. (C) Src phosphorylates Dock180 at Y722 by Src. Dock180WT or Dock180Y722F and WT, a KD or a CA Src were separately coexpressed in HEK293T cells. (D) Src-KD inhibits EGFRvIII-induced p-Dock180Y722. (E) In vitro Src kinase assay. Various proteins were visualized by Coomassie brilliant blue staining. (F) Src-dependent p-Y of Dock180 at Y722 enhances association of Dock180 with Rac1. (G) Knockdown of Src inhibits EGFRvIII-induced p-Dock180Y722 and Rac1 activation. (H) In vitro cell migration. In A and C–G, Dock180, Src, Rac1, and β-actin were used as loading controls. In A, C, and D, a pan anti–p-Y antibody (4G10) was used to detect p-Y of Dock180. (E–G) A specific anti–p-Dock180Y722 antibody was used to detect p-Y722 of Dock180. In B and H, data are presented as percentage of the control from six replicates per pair per cell line. *P < 0.05 and **, P < 0.01. (Scale bars, ± SD.) Data represent three independent experiments with similar results.
Next, we coexpressed WT, kinase dead (KD) or constitutively activated (CA) Src with flag-tagged Dock180WT or Dock180Y722F in HEK293T cells. WT or CA Src induced p-Y of Dock180WT to higher levels compared with that of Dock180Y722F, whereas KD Src had no effect on p-Y of Dock180WT or Dock180Y722F. As expected, CA Src displayed higher kinase activity on p-Y of Dock180 than did WT Src (Fig. 4C). We then tested whether the dominant negative KD Src mutant inhibits the EGFRvIII-induced p-Y of Dock180. As shown in Fig. 4D, coexpression of KD Src with EGFRvIII and Dock180 partially blocked EGFRvIII-induced p-Y of both Dock180WT and Dock180Y722F compared with controls. These results are consistent with partial attenuation of EGFRvIII-induced p-Y by the Dock180Y722F mutant, suggesting that there are other p-Y sites on Dock180 stimulated by EGFRvIII through other kinases.
To validate direct Src phosphorylation of Dock180Y722, we performed in vitro p-Y assays by incubating purified recombinant (His)6-Dock180WT or (His)6-Dock180Y722F proteins with a recombinant active Src followed by immunoblot (IB) using the specific anti–p-Dock180Y722 antibody. As shown in Fig. 4E, a recombinant Src effectively induced p-Y of Dock180WT but not Dock180Y722F in vitro. Next, we evaluated the impact of Src-induced p-Y of Dock180 on its interaction with Rac1 using an in vitro reconstitution assay. In the absence of the recombinant Src, when immunoprecipitated Dock180WT or Dock180Y722F from HEK293T was dephosphorylated by a protein tyrosine phosphatase, p-Y of Y722 of Dock180 was undetectable and minimal Dock180–Rac1 interaction was observed. However, when a recombinant Src was added, p-Dock180WT but not p-Dock180Y722F was significantly induced, accompanied with an increase in association of Dock180 with Rac1 (Fig. 4F).
Then, we stably knocked down endogenous Src using two different shRNAs in SNB19/EGFRvIII cells that expressed either Dock180WT or Dock180Y722F. An ∼75% reduction of Src in SNB19/EGFRvIII cells markedly attenuated EGFRvIII-stimulated p-Y722 of Dock180, Rac1 activity (Fig. 4G) and cell migration (Fig. 4H) in vector control and Dock180WT-expressing cells, but had a minimal impact on Dock180Y722F-expressing cells. Additionally, expression of Dock180WT had minimal impact, whereas Dock180Y722F suppressed EGFRvIII stimulation of p-Dock180Y722, Rac1 activity, and cell migration (Fig. 4 G and H).
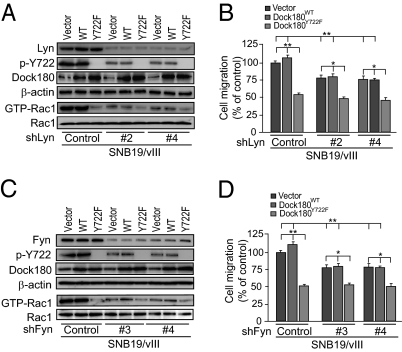
Finally, we determined whether two other SFKs, Fyn and Lyn (13, 14), are involved in EGFRvIII-stimulated Dock180 phosphorylation. We knocked down endogenous Fyn or Lyn using two separate shRNAs for each protein in SNB19/EGFRvIII cells that express Dock180WT, Dock180Y722F, or a vector control. As shown in Fig. 5 A–C, shRNA knockdown of Fyn or Lyn markedly decreased EGFRvIII-induced p-Y722 of Dock180WT, Rac1 activity, and cell migration in SNB19/EGFRvIII/vector and SNB19/EGFRvIII/Dock180WT cells, but did not affect EGFRvIII stimulation of SNB19/EGFRvIII/Dock180Y722F cells (Fig. 5). These data demonstrate that SFKs, Src, Fyn, and Lyn largely mediate EGFRvIII stimulation of Rac1 activity and glioblastoma cell migration through p-Y722 of Dock180.
Fig. 5.
EGFRvIII-induced p-Dock180Y722 is also dependent on SFKs, Fyn and Lyn. (A and C) Knockdown of Lyn or Fyn inhibits EGFRvIII-induced p-Dock180Y722 and Rac1 activation. Dock180, Rac1, and β-actin were used as loading controls. (B and D) In vitro cell migration assays; data are presented as percentage of the control from six replicates per pair per cell line. *P < 0.05 and **, P < 0.01. (Scale bars, ± SD.) Data represent three independent experiments with similar results.
SFKs Stimulate p-Dock180Y722, Rac1 Activity, and Cell Migration of Primary Human GBM Cells That Overexpress EGFRvIII.
Next, we determined whether SFKs also induce p-Y of Dock180Y722, Rac1 activity, and cell migration in primary human GBM cells. To this end, we examined cells from four different serially transplanted human GBMs, GBM6, GBM39, GBM12, and GBM14 cells that retain the EGFR status of the primary tumor from which they were derived (15). In GBM6 and GBM39 that retained EGFRvIII overexpression, strong p-Y of Dock180Y722 and Rac1 activity were found (Fig. S5A). In contrast, without EGF stimulation, neither p-Y722 of Dock180 nor increased Rac1 activity was detected in GBM12 cells that express WT EGFR or GBM14 cells that have nondetectable WT EGFR or EGFRvIII. We then treated GBM6 and GBM39 cells with the EGFR inhibitors AG1478 and Erlotinib, the SFK inhibitors SU6656, PP2, its inactive stereoisomer PP3, or vehicle control. These inhibitors markedly attenuated EGFRvIII-induced pan-p-SrcY418, p-Dock180Y722, p-Akt, p-Erk1/2, Rac1 activity, and cell migration compared with GBM cells treated with PP3 or vehicle control (Fig. S5 B and C). These results further suggest that SFK-dependent p-Dock180Y722 is critical for EGFRvIII-stimulated p-Akt, p-Erk1/2, Rac1 activity, and cell migration in glioblastoma cells.
Coexpression of EGFRvIII, p-Dock180Y722 and pan-p-SrcY418 in Clinical Glioblastoma Specimens Correlates with an Extremely Poor Prognosis.
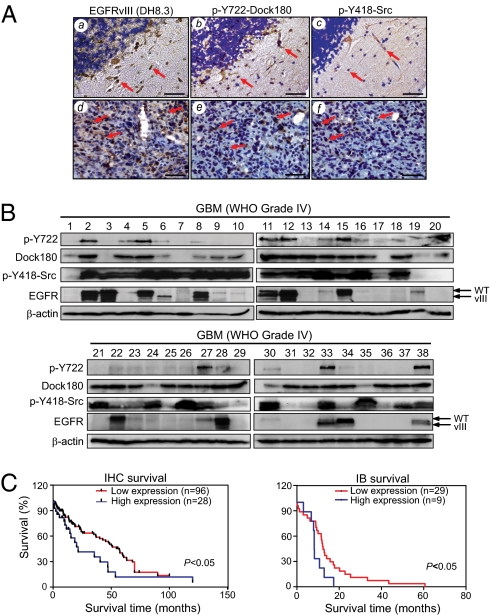
We performed immunohistochemical (IHC) analysis using antibodies against p-Dock180Y722, EGFRvIII, or p-SrcY418 (also detects p-Y of other SFKs) on a cohort of 124 clinical glioblastoma specimens with identifiable central and invasive regions (11). As shown in Table S1, EGFRvIII protein was detected by the specific anti–EGFRvIII-antibody DH8.3 (16) in 36 of 69 GBM (WHO grade IV, 52.2%) and 5 of 26 WHO grade II (19.2%), and 5 of 29 WHO grade III (17.2%) glioblastoma samples, similar to the frequency of EGFRvIII overexpression in clinical GBMs (2). Next, we stained these 46 EGFRvIII-positive tumors and an additional 11 EGFRvIII-negative samples. As shown in Tables S1 and S2, the majority of EGFRvIII-positive tumors demonstrated the presence of p-Dock180Y722 and pan-p-SrcY418. Additionally, coexpression of EGFRvIII, p-Dock180Y722 and pan-p-SrcY418 was found in tumor cells within the invasive areas, as well as in the central regions (Tables S1–S3). An example is shown in Fig. 6A, where EGFRvIII was detected in both invasive (Fig. 6Aa) and central regions (Fig. 6Ad) in a GBM specimen. Interestingly, both pan p-SrcY418 and p-Dock180Y722 were also expressed in the majority of EGFRvIII-positive tumor cells in invasive and central regions of clinical WHO grade IV and II–III specimens (Fig. 6A, b, c, e, and f, Fig. S6, and Table S1). In contrast, EGFRvIII, p-SrcY418 and p-Dock180Y722 were not detected in normal brain tissues. Spearman's rank correlation analysis of expression levels of EGFRvIII and p-Dock180Y722 in all of these IHC-stained clinical specimens showed correlation coefficients between border vs. border regions as 0.9000 (P < 0.05), center vs. center regions as 0.9747 (P < 0.05), and invasive vs. invasive areas as 0.8721 (P < 0.05), respectively (Tables S2 and S3).
Fig. 6.
Coexpression of p-Dock180Y722, EGFRvIII and p-SrcY418 correlates with an extremely poor prognosis in patients with glioblastomas. (A) IHC analysis. A total of 57 specimens that express EGFRvIII and/or p-Dock180Y722 and p-SrcY418 are listed in Table S1. Representative images of GBM (grade IV) tissue stained by anti-EGFRvIII (a and d), anti–p-SrcY418 (b and e), and anti–p-Dock180Y722 (c and f) antibodies. Arrows, positive staining for EGFRvIII, p-SrcY418, and p-Dock180Y722. (Scale bars, 50 μm.) (B) IB analysis of a separate and independent cohort of 38 snap-frozen GBM specimens. Dock180 and β-actin were used as loading controls. (C) Kaplan-Meier curves with long-rank analyses for patients with high EGFRvIII/p-Dock180Y722–expressing tumors (red line) versus low-expression tumors (blue line) of two separate cohorts of glioblastomas examined in A and B. P values were determined by using the log-rank test. Black bars, censored data. Data represent three independent experiments with similar results.
To further validate these findings, we examined expression of EGFRvIII, p-SrcY418, and p-Dock180Y722 in a separate and independent cohort of 38 clinical GBM specimens by IB analyses. As shown in Fig. 6B, overexpression of EGFR and EGFRvIII was detected in 10 of 38 (26.3%) GBMs, whereas EGFR was overexpressed in an additional two GBMs, corroborating with the genetic analyses using fluorescent in situ hybridization. Dock180 was expressed at high levels in 25 of 38 GBMs, whereas pan-p-SrcY418 was also found in 27 of 38 tumors. Significantly, p-Dock180Y722 was coexpressed with pan-p-SrcY418 in 7 of 10 EGFR/EGFRvIII-expressing GBMs (tumors 2, 5, 11, 12, 15, 33, and 38), suggestive of the presence of activated EGFR/EGFRvIII-SFK-Dock180-Rac1 signaling in these GBMs. Additionally, Kaplan-Meier analyses showed that in these two independent cohorts, patients with high expression of EGFRvIII or p-Dock180Y722 have a shorter overall survival compared with those with low expression of EGFRvIII or p-Dock180Y722 (Fig. S7 A and B). In these cases, a statistically significant correlation was found between worse prognosis of patients with high expression of p-Dock180Y722 compared with low expression in the cohort that were analyzed by IHC staining (Fig. S7A). When combining the expression status of EGFRvIII and p-Dock180Y722 in the analyses, a statistically significant worse prognosis was apparent in glioblastomas with high expression of both proteins compared with those with low expression in both cohorts (Fig. 6C). Of note, compared with prognosis of glioblastomas with individual high expression of either EGFRvIII or p-Dock180Y722, the better prognosis value of high expression of both proteins did not appear as drastic as we anticipated. However, this is probably because of the fact that overexpression of EGFRvIII is already a strong prognosis marker for malignant glioblastomas (1, 3) and a relative small number of cases (38 GBM samples) examined by IB analyses. Taken together, these data suggest that p-Dock180Y722 could be an independent, as well as an additional, clinically useful marker in the diagnosis and assessment of outcome in GBM with EGFRvIII overexpression.
Discussion
In this study, we report that SFK-dependent p-Dock180Y722 mediates downstream EGFRvIII-signaling and glioblastoma growth and invasion. This study highlights four important points. First, Dock180 is required for EGFRvIII-stimulated glioblastoma cell migration and survival in vitro. Second, EGFRvIII induces a specific p-Y of Dock180 at Y722 and mutation of this p-Y site inhibits EGFRvIII-promoted glioblastoma cell migration and survival in vitro and tumor growth and invasion in vivo. Third, SFKs, Src, Fyn, and Lyn mediate EGFRvIII induction of phosphorylation of Dock180Y722 in glioblastoma cells stimulating tumorigenesis. Fourth, p-Dock180Y722 and p-SrcY418 are coexpressed with EGFRvIII in clinical glioblastoma specimens. Coexpression of EGFRvIII, p-Dock180Y722 and p-SrcY418 is correlated with an extremely poor prognosis in patients with glioblastomas. Taken together, our results suggest that SFK activation of p-Dock180Y722-Rac1 signaling plays a critical role in EGFRvIII-driven glioblastoma tumorigenesis.
GEFs couple RTKs to Rac1 (9) and Dock180 is a downstream effector of EGFR-mediated cell migration in Drosophila (10). Here, we show that EGFRvIII induces SFKs-dependent p-DockY722, thereby activating Rac1-signaling and promoting glioblastoma cell growth, survival, and invasion. Rac1 is downstream of Dock180 (8) and modulates cell growth, survival, and motility (17). Consistent with this finding, inhibition of Dock180 by siRNA knockdown, overexpression of a Dock180Y722F mutant, or suppression of SFKs impaired EGFRvIII-stimulated Rac1 activity and tumorigenesis. Moreover, EGFRvIII also activates the PI3K-Akt and MAPK pathways (3, 4) and induces a cytokine circuit that stimulates EGFR-signaling in neighboring tumor cells (18). Separate disruption of these downstream pathways inhibits EGFRvIII function, underscoring the heterogeneity of clinical glioblastomas. This heterogeneity is also illustrated by the fact that activated p-SrcY418 is detected in all 38 clinical glioblastoma samples, whereas EGFRvIII and p-Dock180Y722 are only expressed in 8 of 38 specimens. Similarly, in a total of 124 clinical glioblastoma specimens analyzed by IHC, p-Dock180Y722 and p-SrcY418 were not detected in a number of tumors that express EGFRvIII, suggesting that other signaling pathways are also involved in EGFRvIII-driven tumorigenesis. The heterogeneity of glioblastomas involved p-Y of Dock180 is further demonstrated by our recent study, showing that a Src-dependent p-Y of Dock180 mediates PDGFRα, another RTK that is often overexpressed in proneural subtype of human glioblasotmas (1–3), and promoted glioblastoma tumorigenesis. Moreover, PDGFRα/Src-induced p-Y of Dock180 is at another tyrosine residue of Dock180 (19), indicating a distinct signaling from PDGFRα/Src. Taken together, our results show that SFK-dependent p-Y of Dock180 mediates EGFRvIII and PDGFRα stimulation of Rac1 signaling, cell growth, survival, and invasion in glioblastomas.
Src, Lyn, and Fyn are expressed in clinical glioblastoma samples and EGFRvIII-expressing glioblastoma cells. Inhibition of Src, Lyn, or Fyn attenuated EGFRvIII-promoted tumorigenesis and invasion (13, 14). Our data are consistent with and extend these findings. We found that Src directly induces phosphorylation of Dock180Y722 and interaction of Dock180 with Rac1 in vitro and in glioblastoma cells. Moreover, inhibition of Src, Lyn, and Fyn by pharmacological inhibitors or shRNA knockdown reduces EGFRvIII-induced p-Dock180Y722, Rac1 signaling and migration of glioblastoma cells. Coexpression of p-Dock180Y722, EGFR, and EGFRvIII and p-SrcY418 in clinical glioblastoma tumor specimens correlates with an extremely poor prognosis. Therefore, our results integrate SFK-activated p-Dock180Y722-Rac1 signaling in EGFRvIII-driven tumorigenesis.
It has been postulated that nonstimulated Dock180 assumes an inhibitory configuration in which the SH3 domain folds back and interacts with DHR-2 domain, preventing access of Rac1. Upon ELMO1 binding, folded Dock180 is opened to allow Rac1 binding to the DHR-2 domain (8). Similarly, the N terminus of a Rho GEF Vav1 interacts with its Dbl homology (DH) domain, thereby inhibiting GTPase binding (7). Moreover, a Src-induced p-Y of Vav1 at its N terminus opens the DH domain for Rac1 binding (7). Our data are consistent with this mechanism. We show that SFK-dependent p-Dock180Y722 is required for Dock180 activation of Rac1, thereby mediating EGFRvIII-promoted tumorigenesis. However, Y722 is not located in the identified functional domains but at the boundary of a helix/coil configuration in the DHR-1/DHR-2 interdomain of Dock180 (20). With the helix/coil repeats of the interdomain, the DHR-1 domain is brought into close apposition with DHR-2 and Rac1, bringing the membrane binding elements of DHR-1 and Rac1 into the same coplanarity, thereby enabling simultaneous membrane association of the ELMO1-Dock180-Rac1 complex. Additionally, a Dock180-Rac1 dimer is formed that binds to the membrane (20). Therefore, SFK-induced p-Y722 could be critical for the interdomain of Dock180 that holds DHR-1 adjacent to DHR-2 to form a dimeric complex and to achieve the activation of Rac1.
In summary, our data connect the sustained activation of EGFRvIII and SFKs to the p-Dock180Y722 that stimulates Rac1 signaling and malignant behavior of human glioblastoma cells. This unique link is underscored by coexpression of EGFRvIII, p-SrcY418 and p-Dock180Y722 in clinical glioblastoma specimens and association with extremely poor prognoses. Because activation of EGFRvIII and SFKs renders an aggressive glioblastoma phenotype and the induced p-Y of Rho GEF is a common mechanism that activates Rac1 signaling, our results suggest that targeting the EGFRvIII-SFK-Dock180-Rac1 pathway could offer hope in treating malignant glioblastomas with EGFRvIII overexpression.
Materials and Methods
For descriptions of cell lines, cell cultures, reagents, antibodies, DNA constructs, IB and IP, purification of recombinant proteins, in vitro Src tyrosine phosphorylation, pull-down assays of the binding of Dock180 with Rac1, and statistical analysis, see SI Materials and Methods. Experiments using animals were performed using a protocol that was reviewed and approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Studies using human tissues were reviewed and approved by the Institutional Review Board involving Human Subjects at the University of Pittsburgh, Pittsburgh, PA.
Supplementary Material
Acknowledgments
We thank M. Matsuda, S. Courtneidge, R. Pieper, E. van Meir, and Y. Zhou for providing reagents. This work was supported by National Institutes of Health Grants CA130966 (to S.-Y.C.) and CA102583 (to K.V.); a grant from the James S. McDonnell Foundation (to B.H.); a grant from the Pennsylvania Department of Health, and Innovative Research Scholar Awards (to S.-Y.C. and B.H.); Mayo Specialized Program of Research Excellence Grants CA108961 (to J.N.S.), CA106429 (to C.K.T.), and CA95616 (to W.K.C., and F.B.F.); Grant HL070561 and the National Basic Research Program of China Grant 2011CB964801 (to T.C.); an award from the Goldhirsh Foundation (to F.B.F.); and a Clinical Fellowship from the Victorian Cancer Center (to T.G.J.). W.K.C. is a fellow of the National Foundation for Cancer Research. This project used the shared facilities at the University of Pittsburgh Cancer Institute that were supported in part by National Institutes of Health Grant P30CA047904.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1121457109/-/DCSupplemental.
References
- 1.Van Meir EG, et al. Exciting new advances in neuro-oncology: The avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60:166–193. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Research Network Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furnari FB, et al. Malignant astrocytic glioma: Genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 4.Huang PH, Xu AM, White FM. Oncogenic EGFR signaling networks in glioma. Sci Signal. 2009;2:re6. doi: 10.1126/scisignal.287re6. [DOI] [PubMed] [Google Scholar]
- 5.Nishikawa R, et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci USA. 1994;91:7727–7731. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai XM, et al. Protein phosphatase activity of PTEN inhibited the invasion of glioma cells with epidermal growth factor receptor mutation type III expression. Int J Cancer. 2005;117:905–912. doi: 10.1002/ijc.21251. [DOI] [PubMed] [Google Scholar]
- 7.Rossman KL, Der CJ, Sondek J. GEF means go: Turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 8.Côté JF, Vuori K. GEF what? Dock180 and related proteins help Rac to polarize cells in new ways. Trends Cell Biol. 2007;17:383–393. doi: 10.1016/j.tcb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiller MR. Coupling receptor tyrosine kinases to Rho GTPases—GEFs what's the link. Cell Signal. 2006;18:1834–1843. doi: 10.1016/j.cellsig.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Duchek P, Somogyi K, Jékely G, Beccari S, Rørth P. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell. 2001;107:17–26. doi: 10.1016/s0092-8674(01)00502-5. [DOI] [PubMed] [Google Scholar]
- 11.Jarzynka MJ, et al. ELMO1 and Dock180, a bipartite Rac1 guanine nucleotide exchange factor, promote human glioma cell invasion. Cancer Res. 2007;67:7203–7211. doi: 10.1158/0008-5472.CAN-07-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu B, et al. ADP-ribosylation factor 6 regulates glioma cell invasion through the IQ-domain GTPase-activating protein 1-Rac1-mediated pathway. Cancer Res. 2009;69:794–801. doi: 10.1158/0008-5472.CAN-08-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stettner MR, et al. Lyn kinase activity is the predominant cellular Src kinase activity in glioblastoma tumor cells. Cancer Res. 2005;65:5535–5543. doi: 10.1158/0008-5472.CAN-04-3688. [DOI] [PubMed] [Google Scholar]
- 14.Lu KV, et al. Fyn and Src are effectors of oncogenic epidermal growth factor receptor signaling in glioblastoma patients. Cancer Res. 2009;69:6889–6898. doi: 10.1158/0008-5472.CAN-09-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giannini C, et al. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro-oncol. 2005;7:164–176. doi: 10.1215/S1152851704000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jungbluth AA, et al. A monoclonal antibody recognizing human cancers with amplification/overexpression of the human epidermal growth factor receptor. Proc Natl Acad Sci USA. 2003;100:639–644. doi: 10.1073/pnas.232686499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 18.Inda MM, et al. Tumor heterogeneity is an active process maintained by a mutant EGFR-induced cytokine circuit in glioblastoma. Genes Dev. 2010;24:1731–1745. doi: 10.1101/gad.1890510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng H, et al. Activation of Rac1 by Src-dependent phosphorylation of Dock180Y1811 mediates PDGFRα-stimulated glioma tumorigenesis in mice and humans. J Clin Invest. 2011;121:4670–4684. doi: 10.1172/JCI58559. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Premkumar L, et al. Structural basis of membrane targeting by the Dock180 family of Rho family guanine exchange factors (Rho-GEFs) J Biol Chem. 2010;285:13211–13222. doi: 10.1074/jbc.M110.102517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.